Chemical Variability of the Essential Oil of Origanum ehrenbergii Boiss. from Lebanon, Assessed by Independent Component Analysis (ICA) and Common Component and Specific Weight Analysis (CCSWA)
Abstract
1. Introduction
2. Results and Discussion
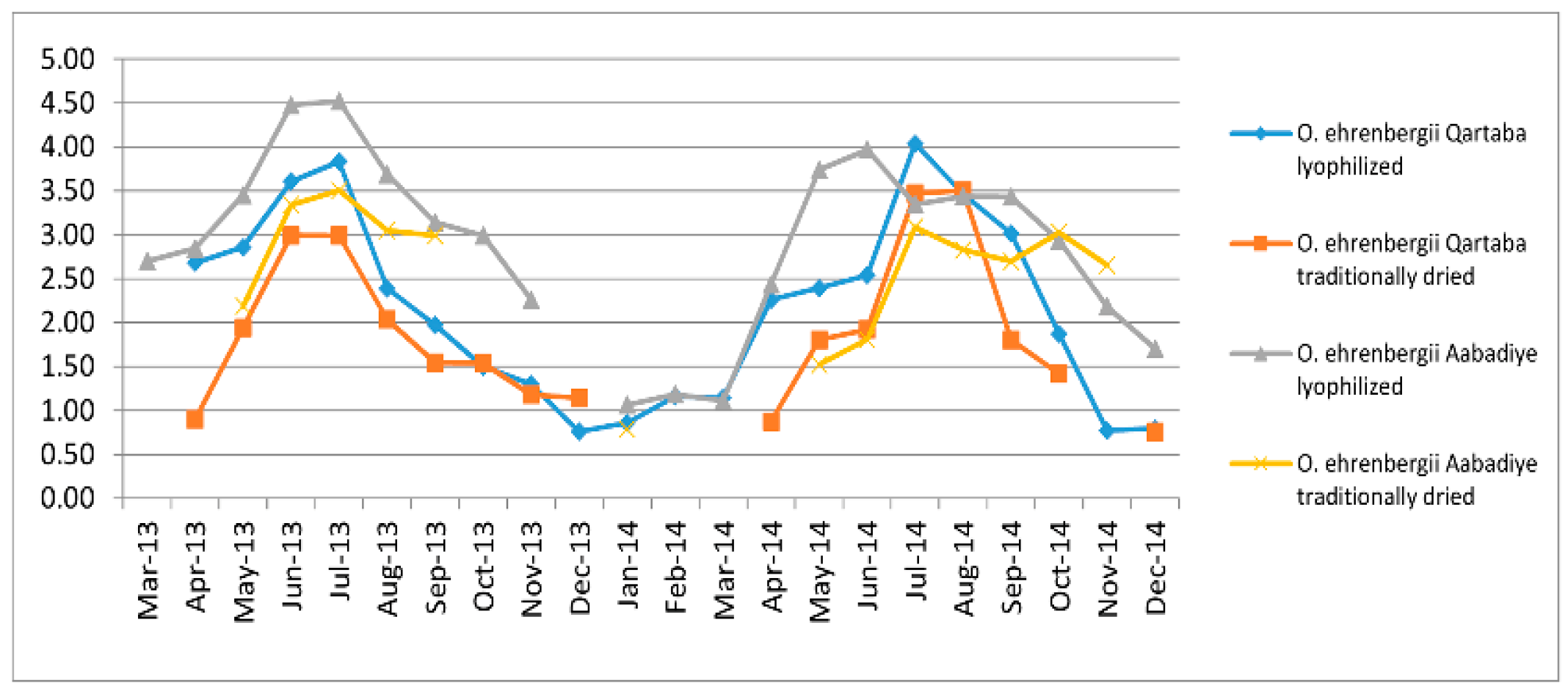
2.1. Effect of Time of Harvest, Altitude (m.a.s.l.), Drying Methods and Soil Composition on O. ehrenbergii EO Yield
2.2. Chemical Composition of the Essential Oils
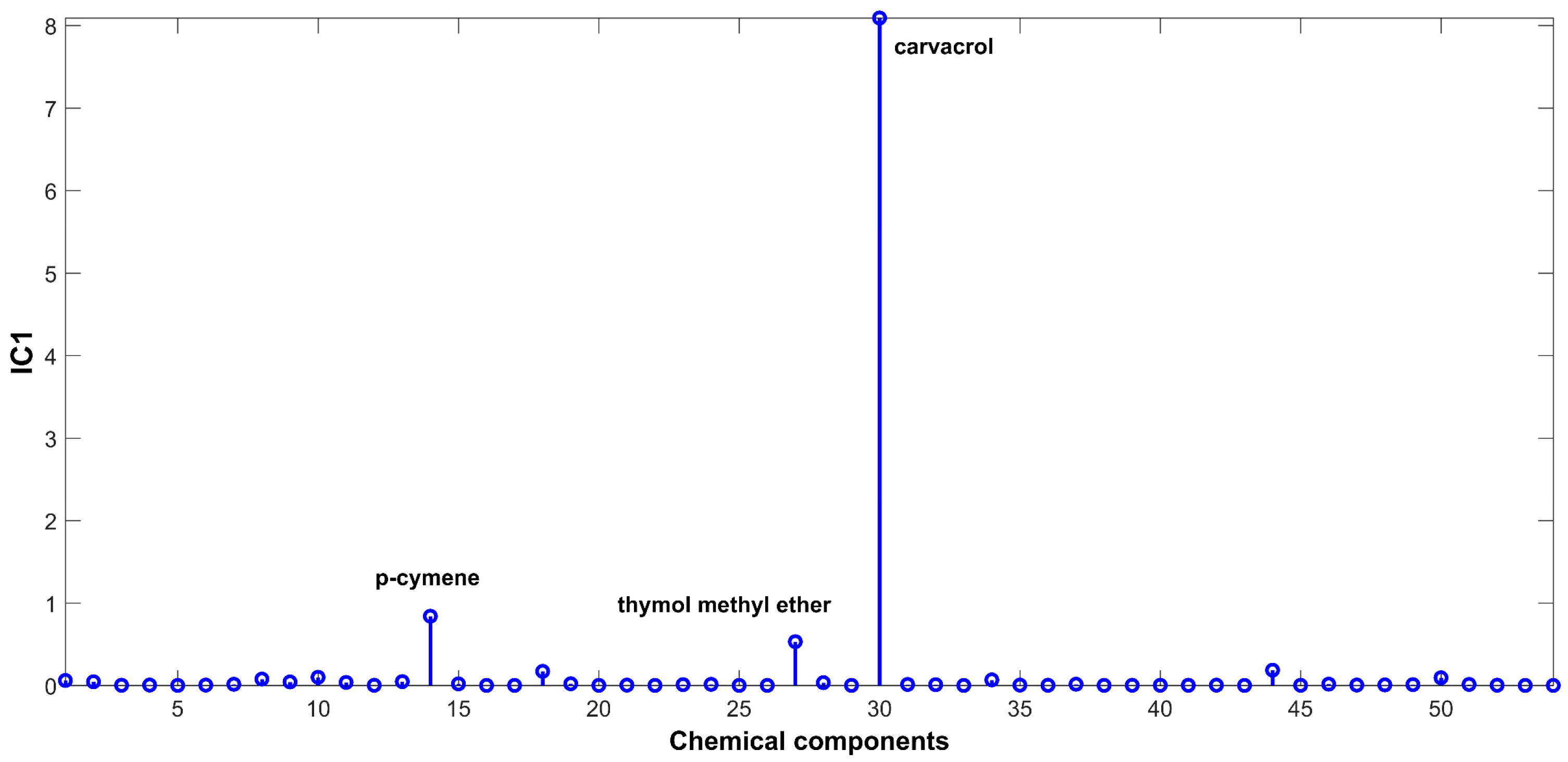
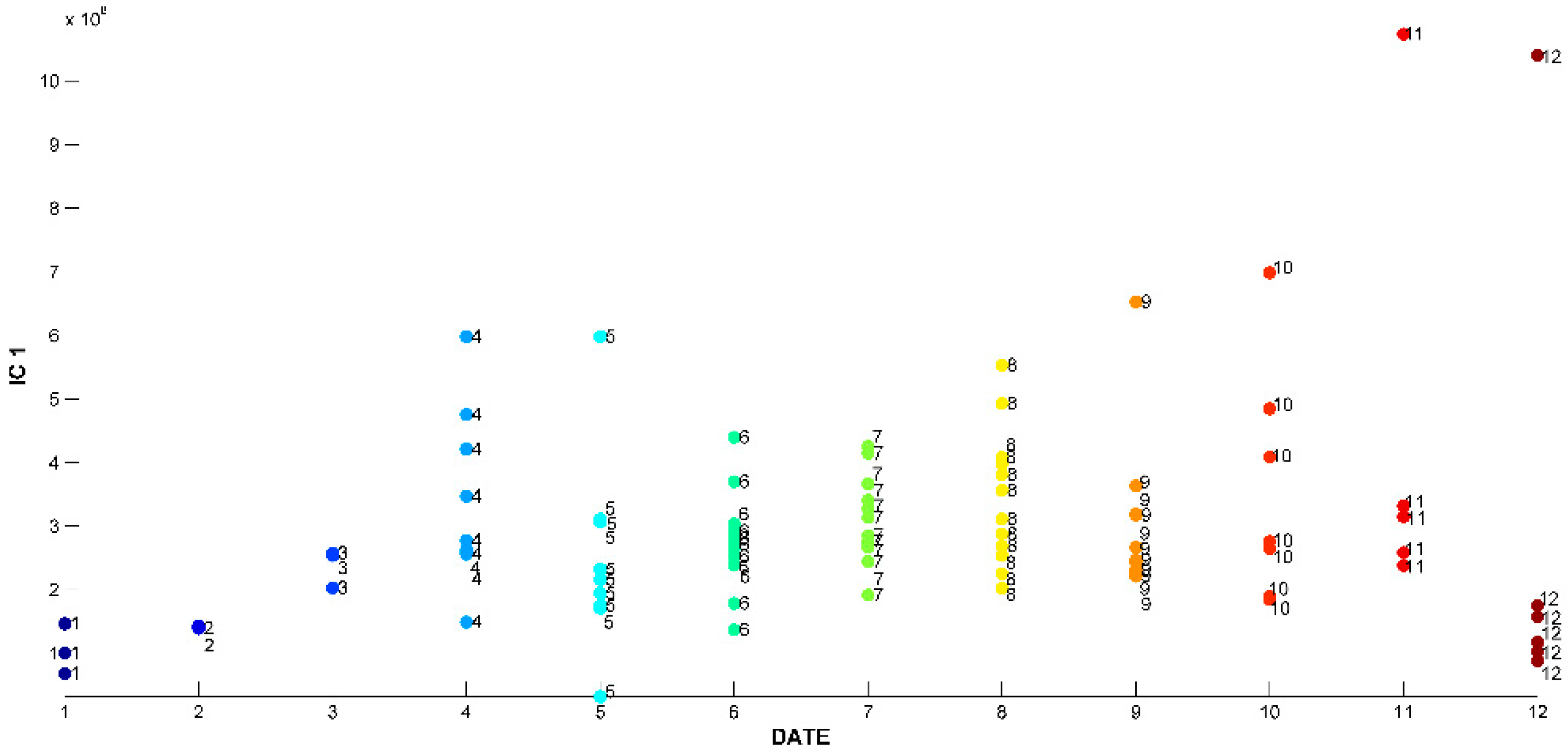
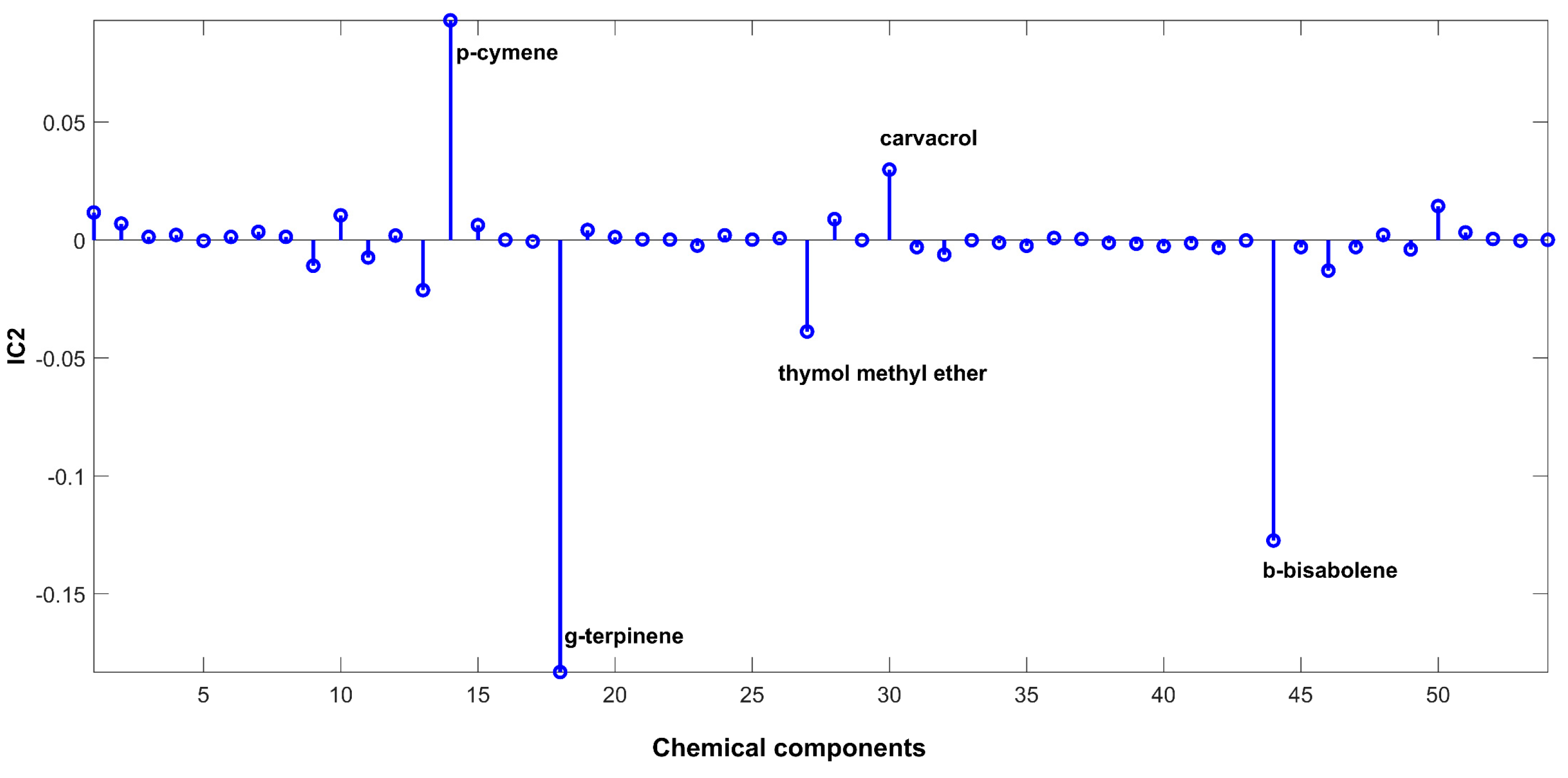
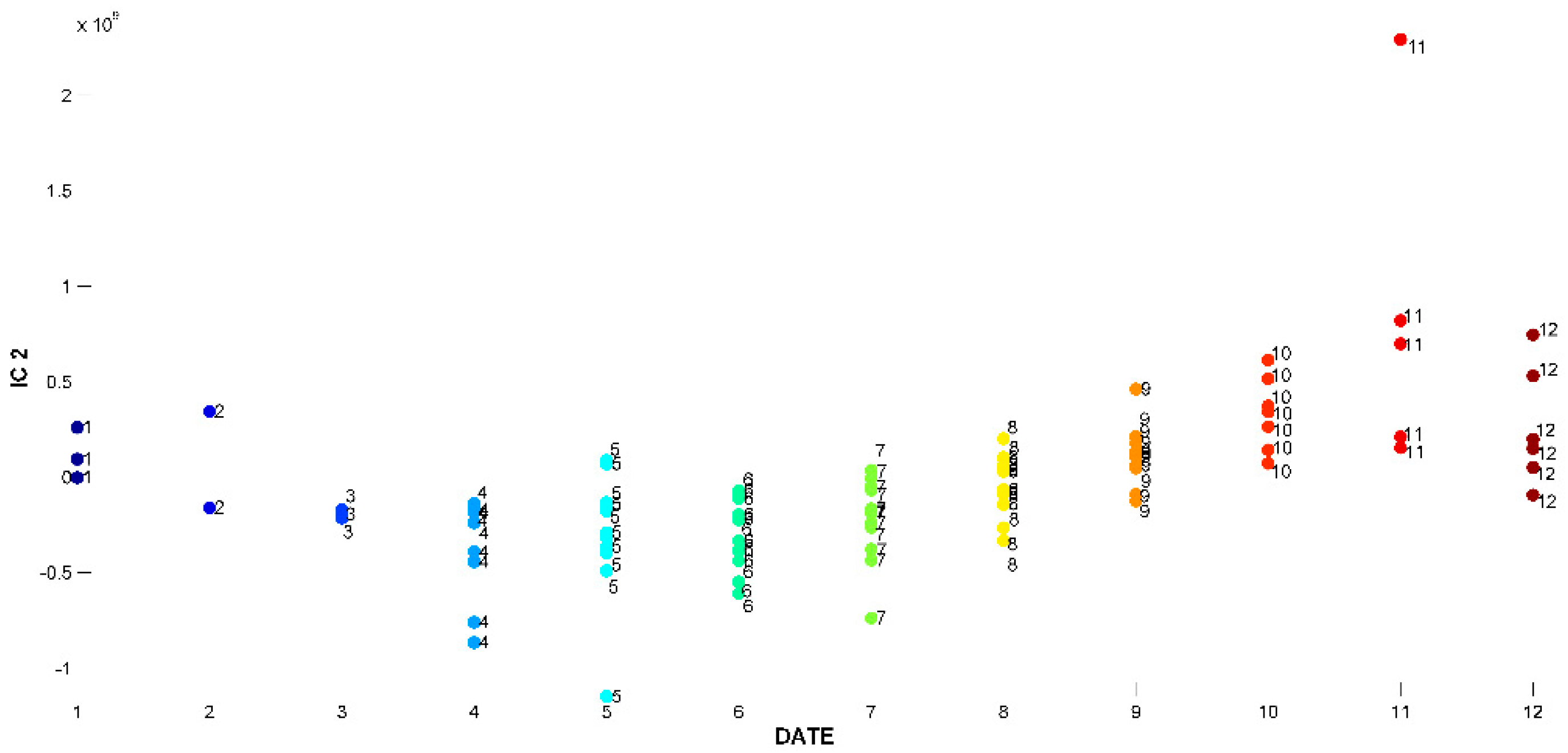
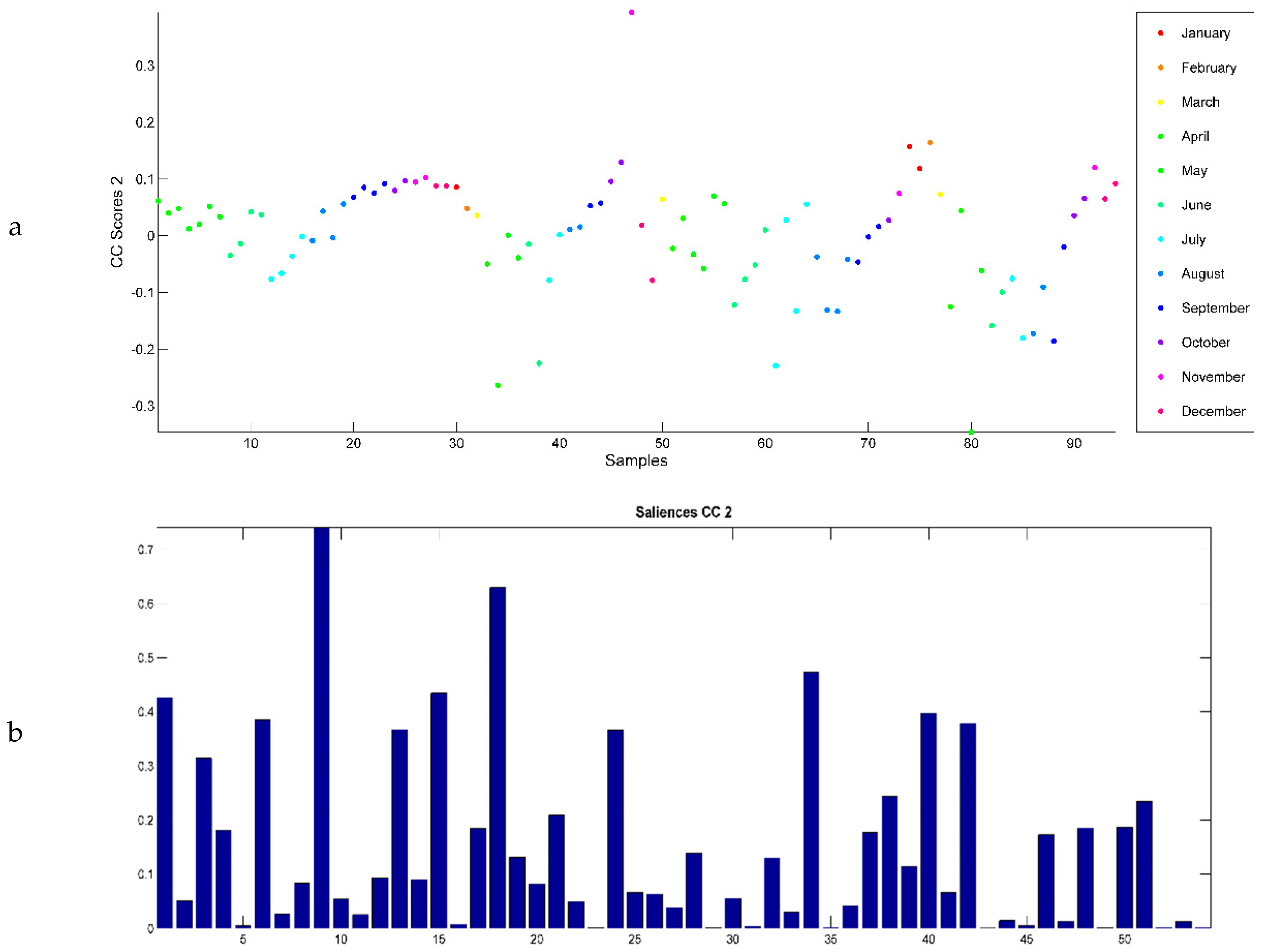
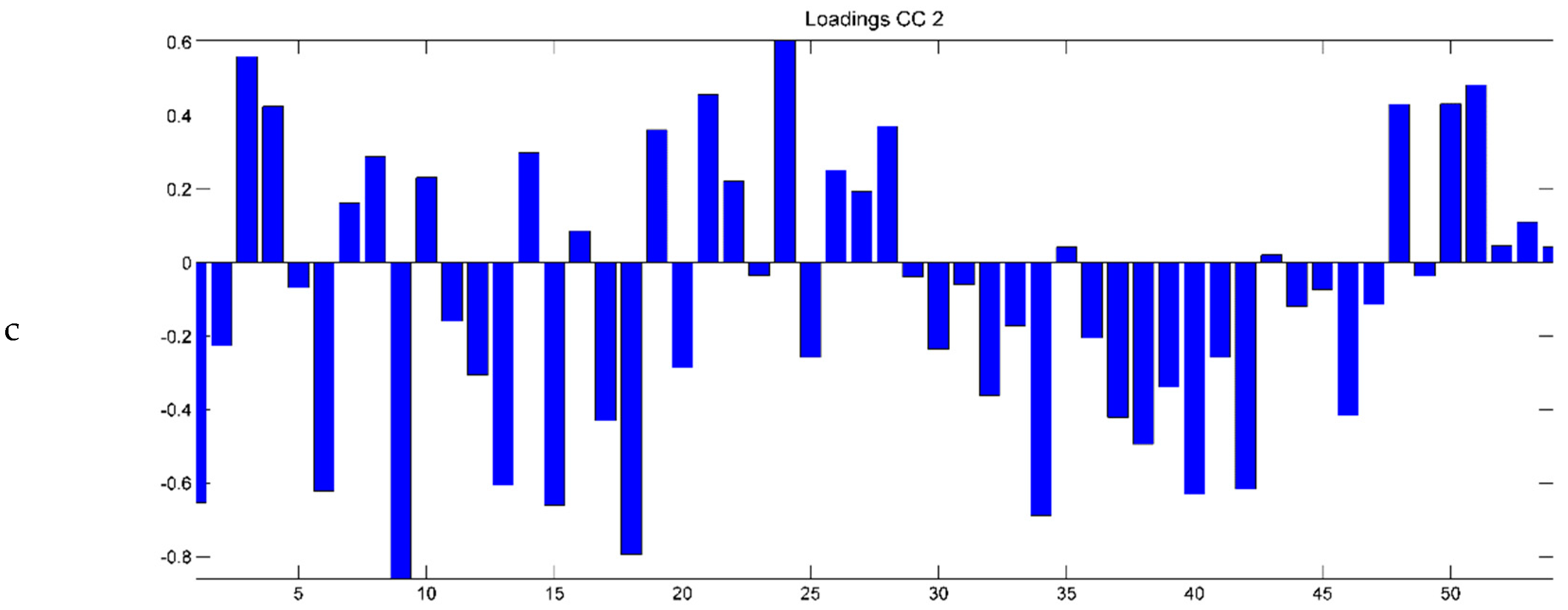
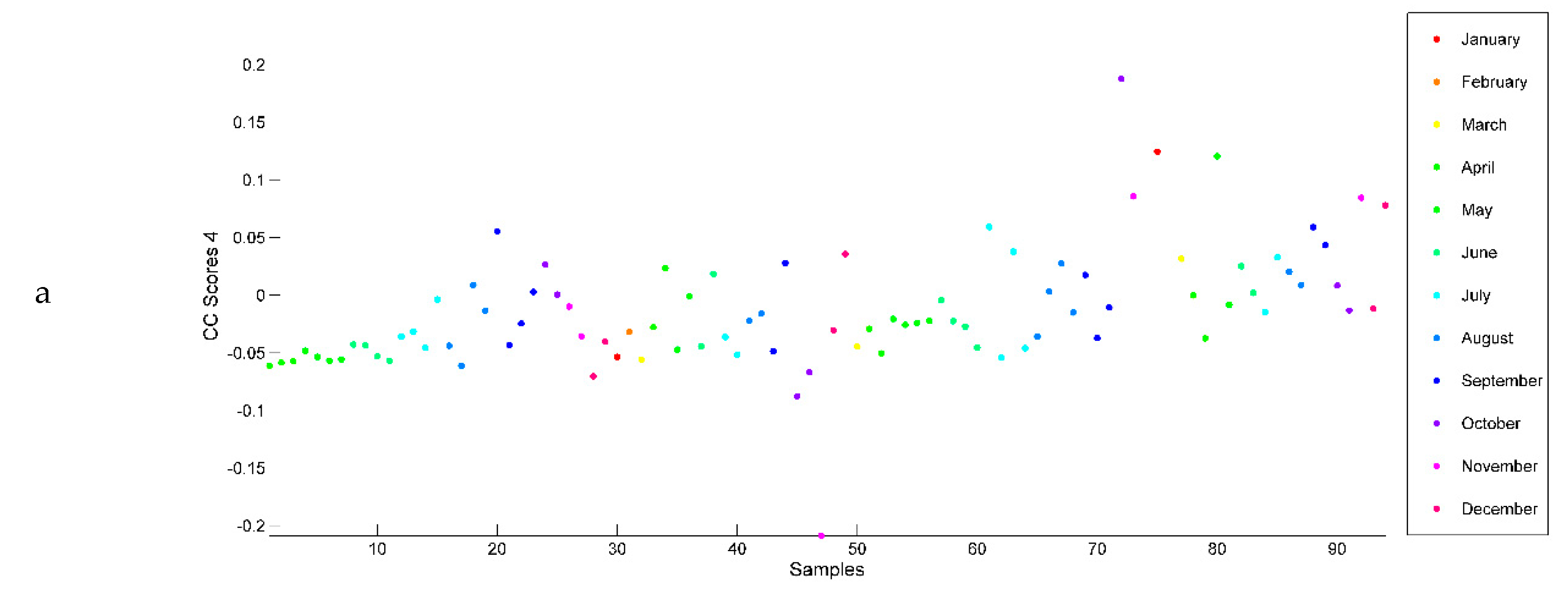
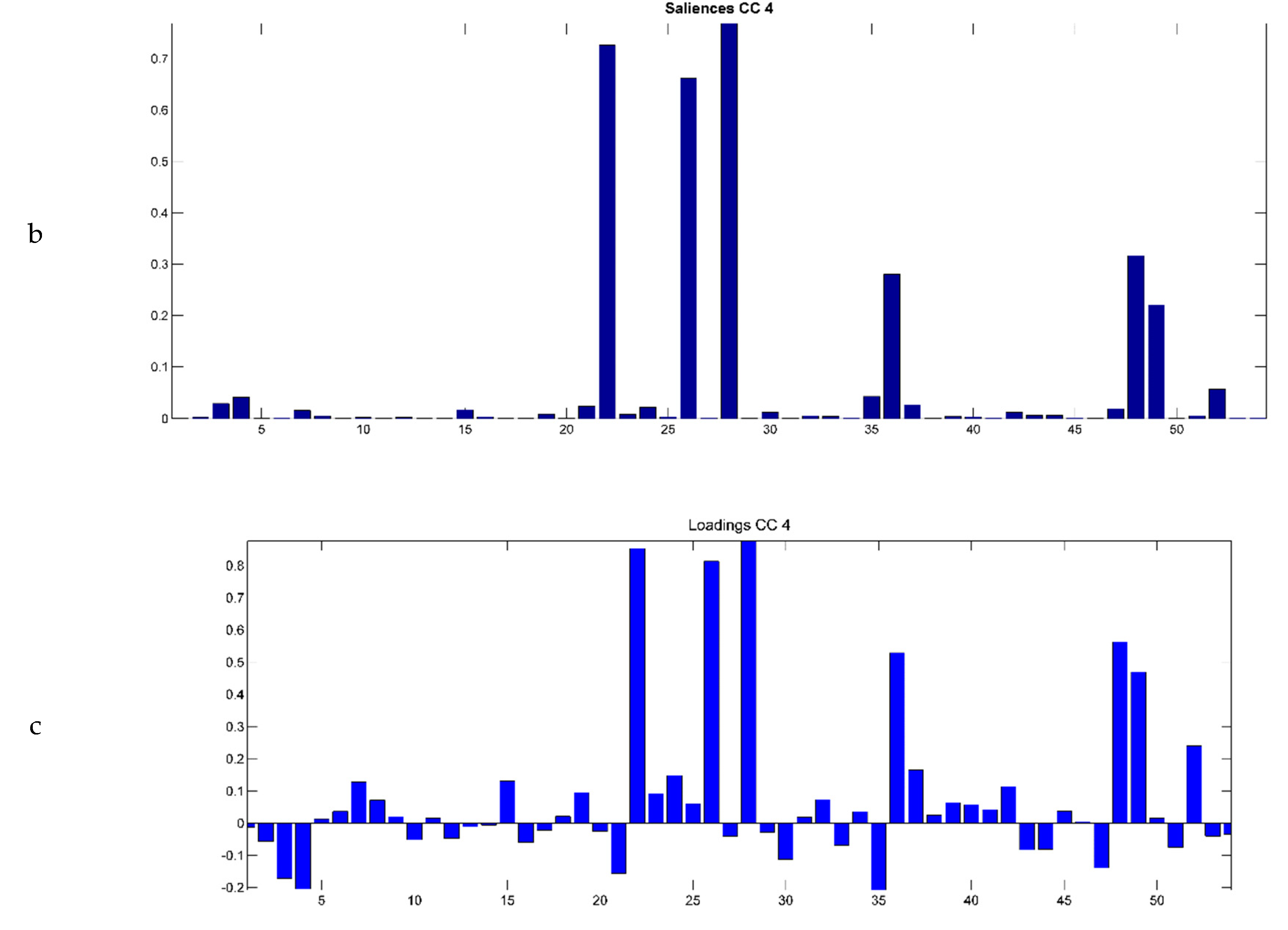
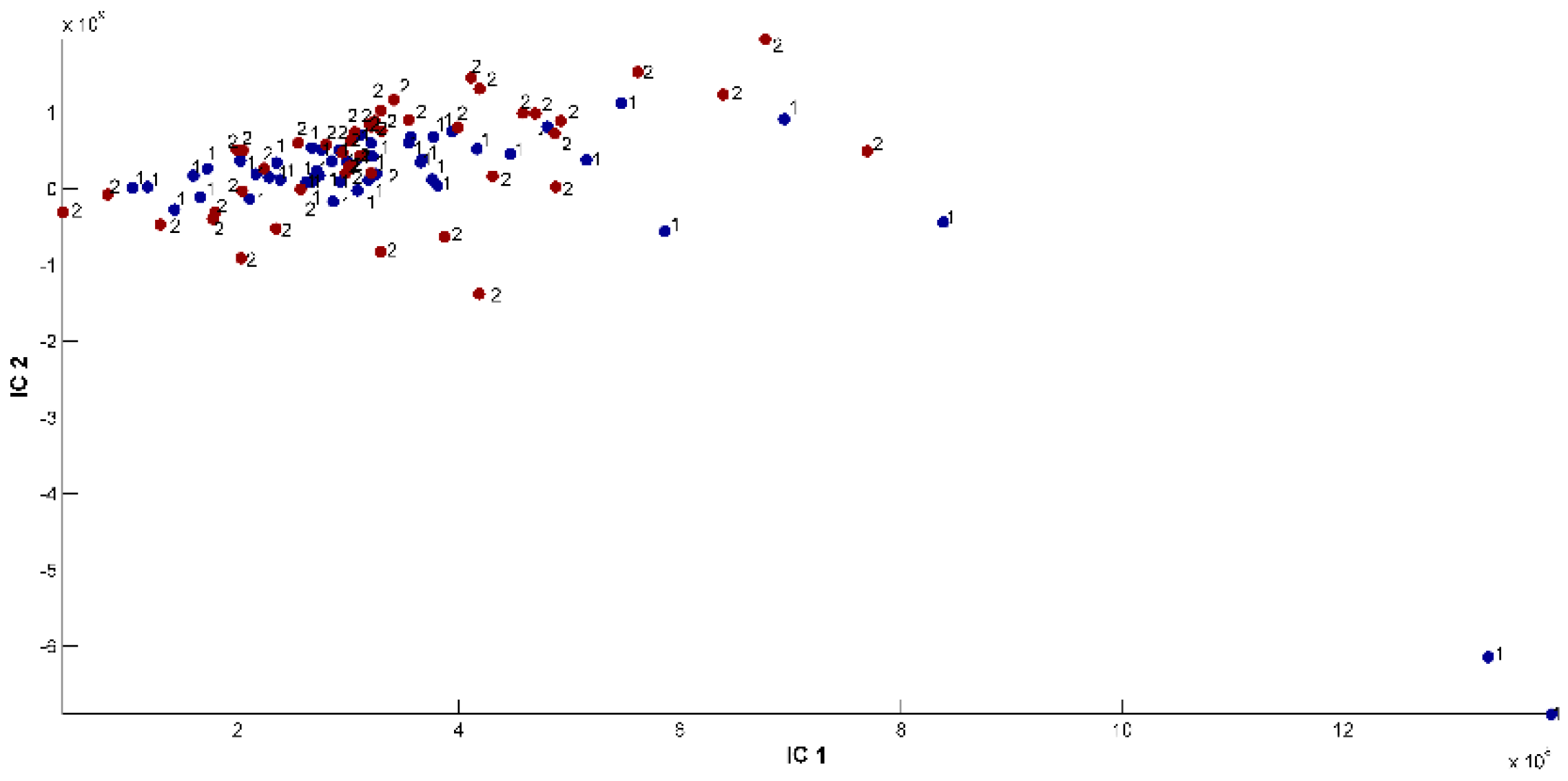
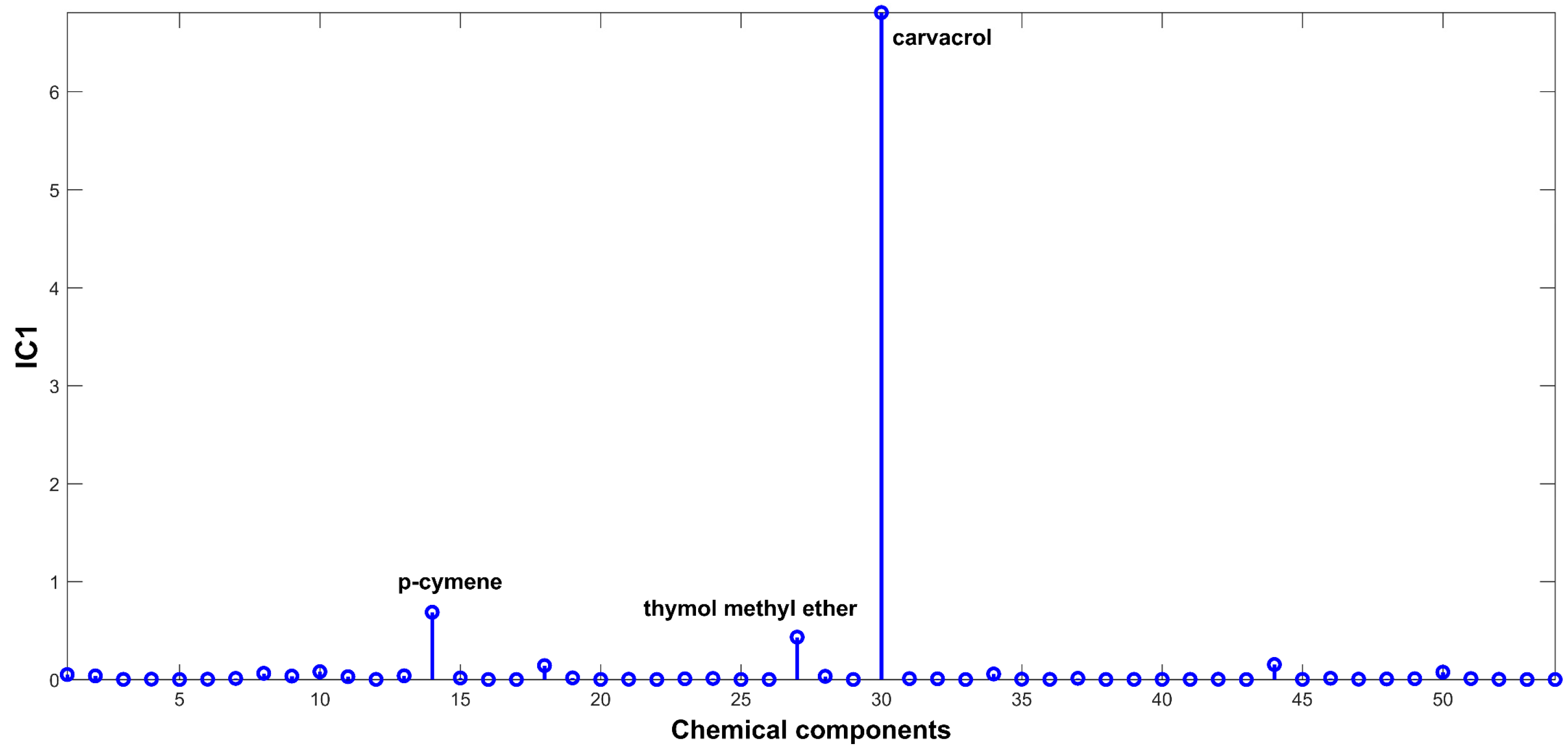
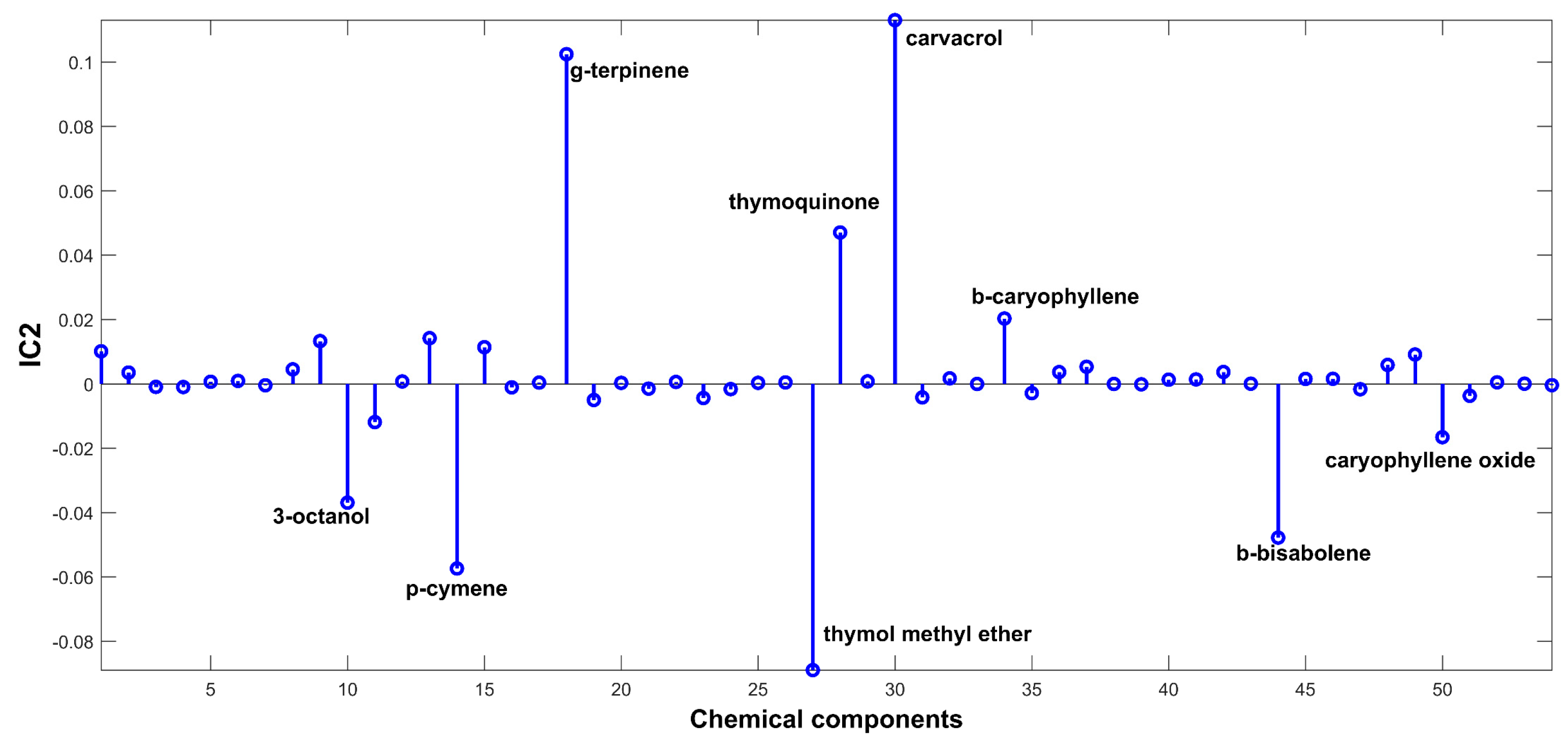
2.3. Evaluation of EO Chemical Variability by Independent Component Analysis (ICA) and Common Component and Specific Weight Analysis (CCSWA)
2.3.1. Effect of Time of Harvest on EO Chemical Composition
2.3.2. Effect of Drying Method on EO Chemical Composition
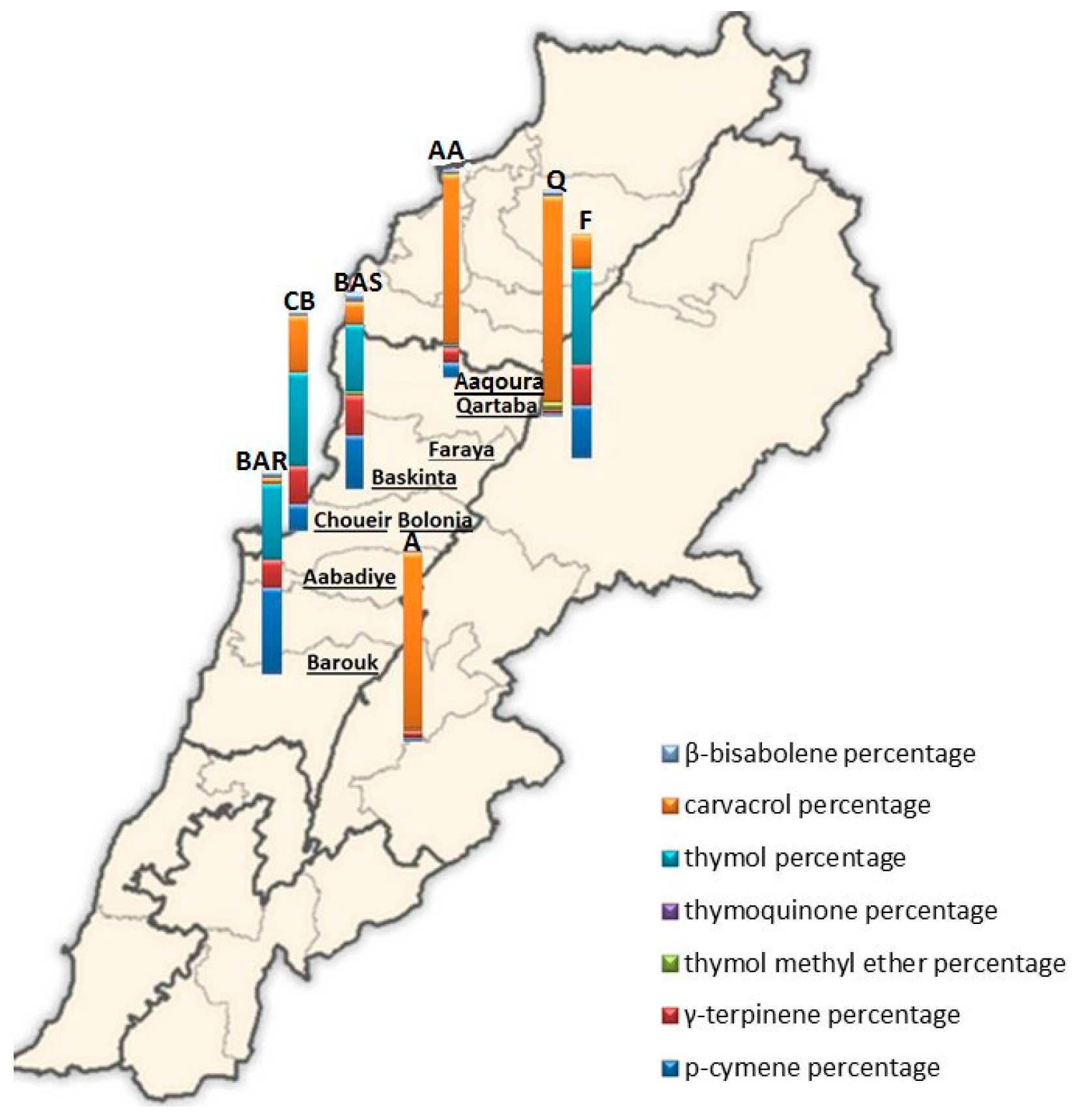
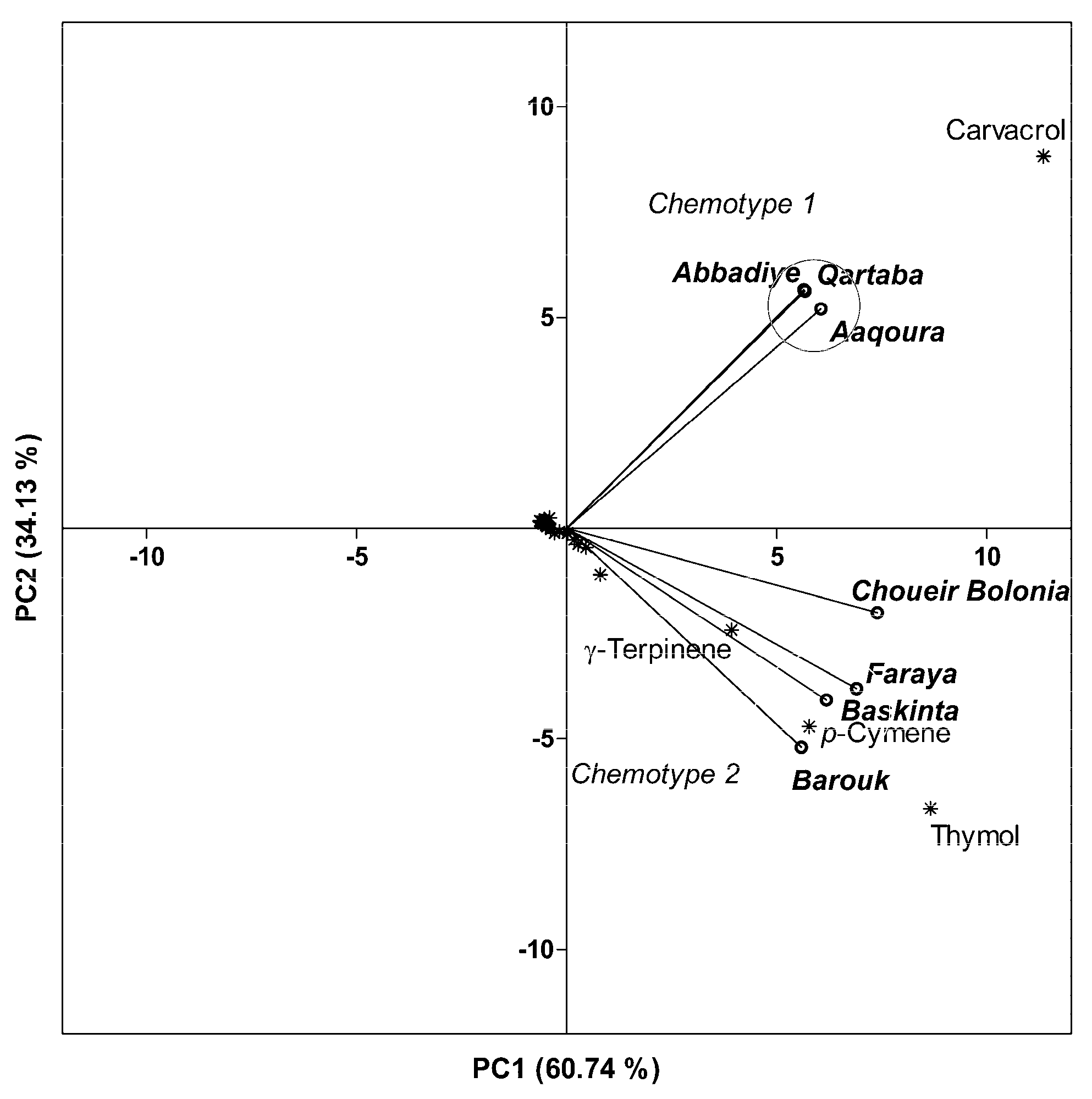
2.3.3. Effect of Geographical Location and Soil Composition on EO Chemical Composition
2.4. Comparison of EOs of O. ehrenbergii Samples Collected at Qartaba and Aabadiye with Results from other Lebanese Regions
3. Materials and Methods
3.1. Plant Material and Essential Oil Extraction
3.2. Soil Analysis
3.3. Essential Oil Analysis
3.3.1. GC Analyses
3.3.2. GC-MS Analyses
3.3.3. Identifications and Quantifications
3.4. Essential Oil Analysis
3.4.1. Analysis of Variance
3.4.2. Principal Component Analysis (PCA)
3.4.3. Independent Component Analysis (ICA)
3.4.4. Common Components and Specific Weight Analysis Method (CCSWA)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| EO | Essential oil |
| PCA | Principal component analysis |
| ICA | Independent component analysis |
| CCSWA | Common component and specific weight analysis |
References
- Myers, N.; Mittermeier, R.A.; Mittermeier, C.G.; Da Fonseca, G.A.; Kent, J. Biodiversity hotspots for conservation priorities. Nature 2000, 403, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Hilan, C.; Sfeir, R.; Aitour, S. Chimiotypes des plantes communes au Liban du genre Origanum et du genre Micromeria (Lamiaceae). Lebanese Sci. J. 2011, 12, 79–91. [Google Scholar]
- Figueredo, G.; Chalchat, J.; Pasquier, B.A. Study of the mediterranean oregano populations chemical composition of essential oils of Origanum ehrenbergii Boiss. from two populations in Lebanon. J. Essent. Oil Res. 2005, 17, 593–596. [Google Scholar] [CrossRef]
- Mouterde, P. Nouvelle flore du Liban et de la Syrie; Distribution Librairie Orientale: Beyrouth, Liban, 1983. [Google Scholar]
- Ahmad, A.; Alkarkhi, A.F.; Hena, S.; Khim, L.H. Extraction, separation and identification of chemical ingredients of Elephantopus scaber L. using factorial design of experiment. Int. J. Chem. 2009, 1, 36. [Google Scholar] [CrossRef]
- Charles, D.J.; Simon, J.E. Comparison of extraction methods for the rapid determination of essential oil content and composition of basil. J. Am. Soc. Hortic. Sci. 1990, 115, 458–462. [Google Scholar] [CrossRef]
- Haoui, I.E.; Derriche, R.; Madani, L.; Oukali, Z. Analysis of the chemical composition of essential oil from Algerian Inula viscosa (L.) Aiton. Arabian J. Chem. 2015, 8, 587–590. [Google Scholar] [CrossRef]
- Hassanpouraghdam, M.B.; Hassani, A.; Vojodi, L.; Farsad-Akhtar, N. Drying method affects essential oil content and composition of basil (Ocimum basilicum L.). J. Essent. Oil Bear. Pl. 2010, 13, 759–766. [Google Scholar] [CrossRef]
- Negreiros, J.R.S.; Miqueloni, D.P.; Cartaxo, C.B.C. Yield of essential oil and safrole content based on fresh and dry biomass of long pepper in the Brazilian amazon. Acta Amaz. 2015, 45, 75–80. [Google Scholar] [CrossRef]
- Zgheib, R.; Yassine, C.; El Beyrouthy, M. Investigation of essential oil chemical polymorphism of Salvia fruticosa naturally growing in Lebanon. J. Essent. Oil Bear. Pl. 2018, in press. [Google Scholar]
- Zgheib, R.; Chaillou, S.; Ouaini, N.; Kassouf, A.; Rutledge, D.N.; Azzi, D.; El Beyrouthy, M. Chemometric tools to highlight the variability of the chemical composition and yield of Lebanese Origanum syriacum L. essential oil. Chem. Biodivers. 2016, 13, 1326–1347. [Google Scholar] [CrossRef] [PubMed]
- Courtois, E.A.; Dexter, K.G.; Paine, C.E.T.; Stien, D.; Engel, J.; Baraloto, C.; Chave, J. Evolutionary patterns of volatile terpene emissions across 202 tropical tree species. Ecol. Evol. 2015, 6, 2854–2864. [Google Scholar] [CrossRef] [PubMed]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential oil and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crops Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Rohloff, J.; Dragland, S.; Mordal, R.; Iversen, T. Effect of harvest time and drying method on biomass production, essential oil yield, and quality of peppermint (Mentha× piperita L.). J. Agric. Food. Chem. 2005, 53, 4143–4148. [Google Scholar] [CrossRef] [PubMed]
- Sefidkon, F.; Abbasi, K.; Jamzad, Z.; Ahmadi, S. The effect of distillation methods and stage of plant growth on the essential oil content and composition of Satureja rechingeri Jamzad. Food Chem. 2007, 100, 1054–1058. [Google Scholar] [CrossRef]
- Kizil, S.; Ipek, A.; Arslan, N.; Khawar, K.M. Effect of different developing stages on some agronomical characteristics and essential oil composition of oregano (Origanum onites). N. Z. J. Crop Hortic. Sci. 2008, 36, 71–76. [Google Scholar] [CrossRef]
- Verdian-rizi, M. Phenological variation of Laurus nobilis L. essential oil from Iran. Electron. J. Environ. Agric. Food Chem. 2008, 7, 3321–3325. [Google Scholar]
- Tanu; Prakash, A.; Adholeya, A. Effect of different organic manures/composts on the herbage and essential oil yield of Cymbopogon winterianus and their influence on the native AM population in a marginal alfisol. Bioresour. Technol. 2004, 92, 311–319. [Google Scholar] [CrossRef]
- Homer, L.E.; Leach, D.N.; Lea, D.; Lee, L.S.; Henry, R.J.; Baverstock, P.R. Natural variation in the essential oil content of Melaleuca alternifolia Cheel (Myrtaceae). Biochem. Syst. Ecol. 2000, 28, 367–382. [Google Scholar] [CrossRef]
- Laborde, J.; Traboulsi, M. Cartographie automatique des précipitations: Application aux précipitations moyennes annuelles du Moyen-Orient. Publ. Assoc. Int. Climatol. 2002, 14, 296–303. [Google Scholar]
- Avci, A.B. Chemical variation on the essential oil of Thymus praecox ssp. scorpilii var. laniger. Int. J. Agric. Biol. 2011, 13, 607–610. [Google Scholar]
- Baydar, H.S.D. Isparta koşullarında izmir kekiğinin (Origanum onites L.) verimi ve uçucu yağ kalitesi üzerine araştırmalar. Ünv. Fen Bilimleri Ens. Der. 2002, 6, 17–24. [Google Scholar]
- Giuliani, C.; Maggi, F.; Papa, F.; Maleci Bini, L. Congruence of phytochemical and morphological profiles along an altitudinal gradient in Origanum vulgare ssp. vulgare from Venetian region (NE Italy). Chem. Biodivers. 2013, 10, 569–583. [Google Scholar] [CrossRef] [PubMed]
- Awada, F.; Kobaissi, A.; Chokr, A.; Hamze, K.; Hayar, S.; Mortada, A. Factors affecting quantitative and qualitative variation of thyme (Origanum syriacum L.) essential oil in Lebanon. Adv. Environ. Biol. 2012, 6, 1509–1514. [Google Scholar]
- Amarti, F.; Satrani, B.; Aafi, A.; Ghanmi, M.; Farah, A.; Aberchane, M.; Chaouch, A. Composition chimique et activité antimicrobienne des huiles essentielles de T. capitatus et de T. bleicherianus du Maroc. Phytothérapie 2008, 6, 342–347. [Google Scholar] [CrossRef]
- Amarti, F.; Satrani, B.; Ghanmi, M.; Farah, A.; Aafi, A.; Aarab, L.; Chaouch, A. Composition chimique et activité antimicrobienne des huiles essentielles de T. algeriensis Boiss. & Reut. et T. ciliatus (Desf.) Benth. du Maroc. Biotechnol. Agron. Soc. Environ. 2010, 14, 141–148. [Google Scholar]
- Baydar, H.; Sağdiç, O.; Özkan, G.; Karadoğan, T. Antibacterial activity and composition of essential oils from Origanum, Thymbra and Satureja species with commercial importance in Turkey. Food Control. 2004, 15, 169–172. [Google Scholar] [CrossRef]
- Bertrand, D.; Coredella, C. SAISIR Package. Free Toolbox for Chemometrics in the Matlab, Octave or Scilab Environments. 2011. Available online: http://www.chimiometrie.fr/saisir_webpage.html (accessed on 24 March 2015).
- Badary, O.A.; Taha, R.A.; Gamal El-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003, 26, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A. Thymoquinone attenuates ifosfamide-induced fanconi syndrome in rats and enhances its antitumor activity in mice. J. Ethnopharmacol. 1999, 67, 135–142. [Google Scholar] [CrossRef]
- Badary, O.A.; Nagi, M.N.; Al-Shabanah, O.A.; Al-Sawaf, H.A.; Al-Sohaibani, M.O.; Al-Bekairi, A.M. Thymoquinone ameliorates the nephrotoxicity induced by cisplatin in rodents and potentiates its antitumor activity. Can. J. Physiol. Pharmacol. 1997, 75, 1356–1361. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.U.; Kheir, W.G.A.; Kheir, L.A.; Darwiche, N.; Crooks, P.A. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs 2004, 15, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Flemming, T.; Kayser, O. Heterologous expressed human cytochromes are useful tools in synthesis of thymoquinone. Planta Med. 2009, 75. [Google Scholar] [CrossRef]
- Jukić, M.; Miloš, M. Catalytic oxidation and antioxidant properties of thyme essential oils (Thymus vulgare L.). Croat. Chem. Acta. 2005, 78, 105–110. [Google Scholar]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Langenheim, J.H. Higher plant terpenoids: A phytocentric overview of their ecological roles. J. Chem. Ecol. 1994, 20, 1223–1280. [Google Scholar] [CrossRef] [PubMed]
- Barakat, A.; Wakim, L.H.; Apostolides, N.A.; Srour, G.; El Beyrouthy, M. Variation in the essential oils of Thymbra spicata L. growing wild in Lebanon according to the date of harvest. J. Essent. Oil Res. 2013, 25, 506–511. [Google Scholar] [CrossRef]
- Paula, J.A.; Ferri, P.H.; Bara, M.T.F.; Tresvenzol, L.M.; Sá, F.A.; Paula, J.R. Infraspecific chemical variability in the essential oils of Pimenta pseudocaryophyllus (Gomes) L.R. Landrum (Myrtaceae). Biochem. Syst. Ecol. 2011, 39, 643–650. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Menichini, F.; Conforti, F.; Tundis, R.; Bonesi, M.; Saab, A.M.; Statti, G.A.; De Cindo, B.; Houghton, P.J.; Menichini, F.; et al. Chemical analysis, antioxidant, antiinflammatory and anticholinesterase activities of Origanum ehrenbergii Boiss and Origanum syriacum L. essential oils. Food Chem. 2009, 117, 174–180. [Google Scholar] [CrossRef]
- Figueredo, G. Composition Chimique des Huiles Essentielles D’origan; Editions Universitaires Européennes (EUE): Sarrebruck, Allemagne, 2010. [Google Scholar]
- European Pharmacopoeia. European Pharmacopoeia, 3rd ed.; Council of Europe Publishing: Strasbourg, France, 1997. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured Publ. Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Jennings, W.; Shibamoto, T. Qualitative Analysis of Flavour and Fragrance Volatiles by Glass Capillary Gas Chromatography; Academic Press: New York, NY, USA; London, UK, 1980. [Google Scholar]
- Davies, N.W. Gas chromatographic retention indices of monoterpenes and sesquiterpenes on methyl silicon and carbowax 20M phases. J. Chromatogr. A 1990, 503, 1–24. [Google Scholar] [CrossRef]
- Kohl, S.M.; Klein, M.S.; Hochrein, J.; Oefner, P.J.; Spang, R.; Gronwald, W. State-of-the art data normalization methods improve NMR-based metabolomic analysis. Metabolomics 2012, 8, 146–160. [Google Scholar] [CrossRef] [PubMed]
- Abdi, H.; Williams, L.J. Principal component analysis. Wiley Interdiscip. Rev. Comput. Stat. 2010, 2, 433–459. [Google Scholar] [CrossRef]
- De Lathauwer, L.; De Moor, B.; Vandewalle, J.J. An introduction to independent component analysis. J. Chemometr. 2000, 14, 123–149. [Google Scholar] [CrossRef]
- Stone, J.V. Independent component analysis: An introduction. Trends Cogn. Sci. 2002, 6, 59–64. [Google Scholar] [CrossRef]
- Comon, P. Independent component analysis, a new concept? Signal Process. 1994, 36, 287–314. [Google Scholar] [CrossRef]
- Hyvärinen, A.; Oja, E. Independent component analysis: Algorithms and applications. Neural Netw. 2000, 13, 411–430. [Google Scholar] [CrossRef]
- Rutledge, D.N.; Jouan-Rimbaud Bouveresse, D. Independent components analysis with the JADE algorithm. TrAC Trends Anal. Chem. 2013, 50, 22–32. [Google Scholar] [CrossRef]
- Wang, G.; Ding, Q.; Hou, Z. Independent component analysis and its applications in signal processing for analytical chemistry. TrAC Trends Anal. Chem. 2008, 27, 368–376. [Google Scholar] [CrossRef]
- Ammari, F.; Cordella, C.B.; Boughanmi, N.; Rutledge, D.N. Independent components analysis applied to 3D-front-face fluorescence spectra of edible oils to study the antioxidant effect of Nigella sativa L. extract on the thermal stability of heated oils. Chemom. Intell. Lab. Syst. 2012, 113, 32–42. [Google Scholar] [CrossRef]
- Jouan-Rimbaud Bouveresse, D.; Pinto, R.C.; Schmidtke, L.M.; Locquet, N.; Rutledge, D.N. Identification of significant factors by an extension of ANOVA–PCA based on multi-block analysis. Chemom. Intell. Lab. Syst. 2011, 106, 173–182. [Google Scholar] [CrossRef]
- Gustafsson, M.G. Independent component analysis yields chemically interpretable latent variables in multivariate regression. J. Chem. Inf. Model. 2005, 45, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Zgheib, R.; Chaillou, S.; Ouaini, N.; Rutledge, D.N.; Stien, D.; Kassouf, A.; El Beyrouthy, M. Investigation of Origanum libanoticum Boiss. essential oils chemical polymorphism by independent components analysis (ICA). Nat. Prod. Commun. 2018, 13, 1731–1740. [Google Scholar]
- Mazerolles, G.; Hanafi, M.; Dufour, E.; Bertrand, D.; Qannari, E. Common components and specific weights analysis: A chemometric method for dealing with complexity of food products. Chemom. Intell. Lab. Syst. 2006, 81, 41–49. [Google Scholar] [CrossRef]
- Qannari, E.M.; Wakeling, I.; Courcoux, P.; MacFie, H.J. Defining the underlying sensory dimensions. Food Qual. Prefer. 2000, 11, 151–154. [Google Scholar] [CrossRef]
- Jouan-Rimbaud Bouveresse, D.; Moya-González, A.; Ammari, F.; Rutledge, D.N. Two novel methods for the determination of the number of components in independent components analysis models. Chemom. Intell. Lab. Syst. 2012, 112, 24–32. [Google Scholar] [CrossRef]
| Qartaba | Aabadiye | |
|---|---|---|
| Altitude (m) | 1250 | 600 |
| pH | 6.53 | 6.28 |
| Organic Matter (%) | 4.11 | 4.55 |
| P2O5 (mg/kg) | 19.1 | 8.33 |
| K2O (mg/kg) | 234.32 | 53.07 |
| N (%) | 0.345 | 0.248 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zgheib, R.; El-Beyrouthy, M.; Chaillou, S.; Ouaini, N.; Rutledge, D.N.; Stien, D.; Kassouf, A.; Leonti, M.; Iriti, M. Chemical Variability of the Essential Oil of Origanum ehrenbergii Boiss. from Lebanon, Assessed by Independent Component Analysis (ICA) and Common Component and Specific Weight Analysis (CCSWA). Int. J. Mol. Sci. 2019, 20, 1026. https://doi.org/10.3390/ijms20051026
Zgheib R, El-Beyrouthy M, Chaillou S, Ouaini N, Rutledge DN, Stien D, Kassouf A, Leonti M, Iriti M. Chemical Variability of the Essential Oil of Origanum ehrenbergii Boiss. from Lebanon, Assessed by Independent Component Analysis (ICA) and Common Component and Specific Weight Analysis (CCSWA). International Journal of Molecular Sciences. 2019; 20(5):1026. https://doi.org/10.3390/ijms20051026
Chicago/Turabian StyleZgheib, Raviella, Marc El-Beyrouthy, Sylvain Chaillou, Naim Ouaini, Douglas N. Rutledge, Didier Stien, Amine Kassouf, Marco Leonti, and Marcello Iriti. 2019. "Chemical Variability of the Essential Oil of Origanum ehrenbergii Boiss. from Lebanon, Assessed by Independent Component Analysis (ICA) and Common Component and Specific Weight Analysis (CCSWA)" International Journal of Molecular Sciences 20, no. 5: 1026. https://doi.org/10.3390/ijms20051026
APA StyleZgheib, R., El-Beyrouthy, M., Chaillou, S., Ouaini, N., Rutledge, D. N., Stien, D., Kassouf, A., Leonti, M., & Iriti, M. (2019). Chemical Variability of the Essential Oil of Origanum ehrenbergii Boiss. from Lebanon, Assessed by Independent Component Analysis (ICA) and Common Component and Specific Weight Analysis (CCSWA). International Journal of Molecular Sciences, 20(5), 1026. https://doi.org/10.3390/ijms20051026






