Novel 1,3,4-Oxadiazole Derivatives Containing a Cinnamic Acid Moiety as Potential Bactericide for Rice Bacterial Diseases
Abstract
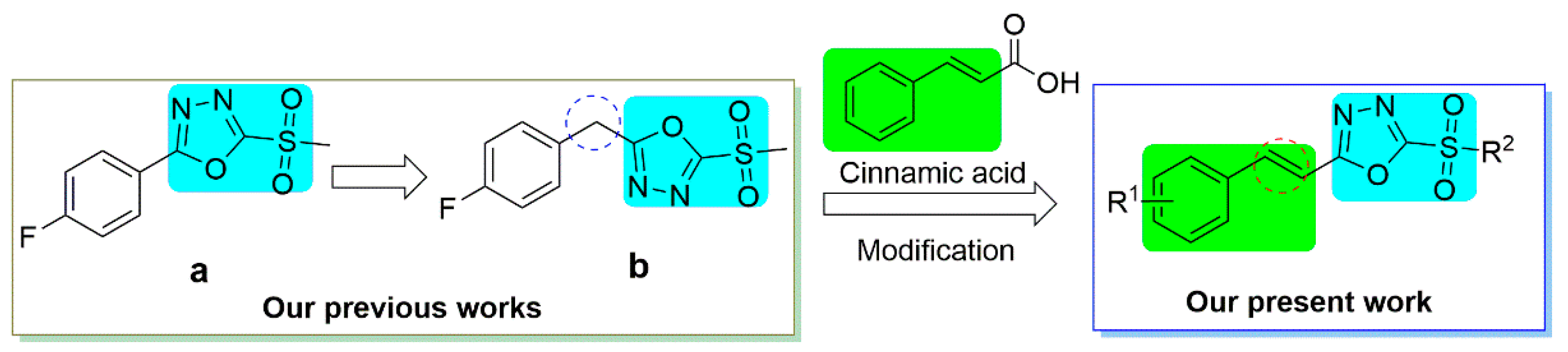
:1. Introduction
2. Results
2.1. Synthesis
2.2. In Vitro Antibacterial Activity
2.2.1. In Vitro Antibacterial Activity of Xoo
2.2.2. In Vitro Antibacterial Activity of Xoc
2.3. In Vivo Antibacterial Activity
2.3.1. In Vivo Antibacterial Activities Against Xoo
2.3.2. In Vivo Antibacterial Activities Against Xoc
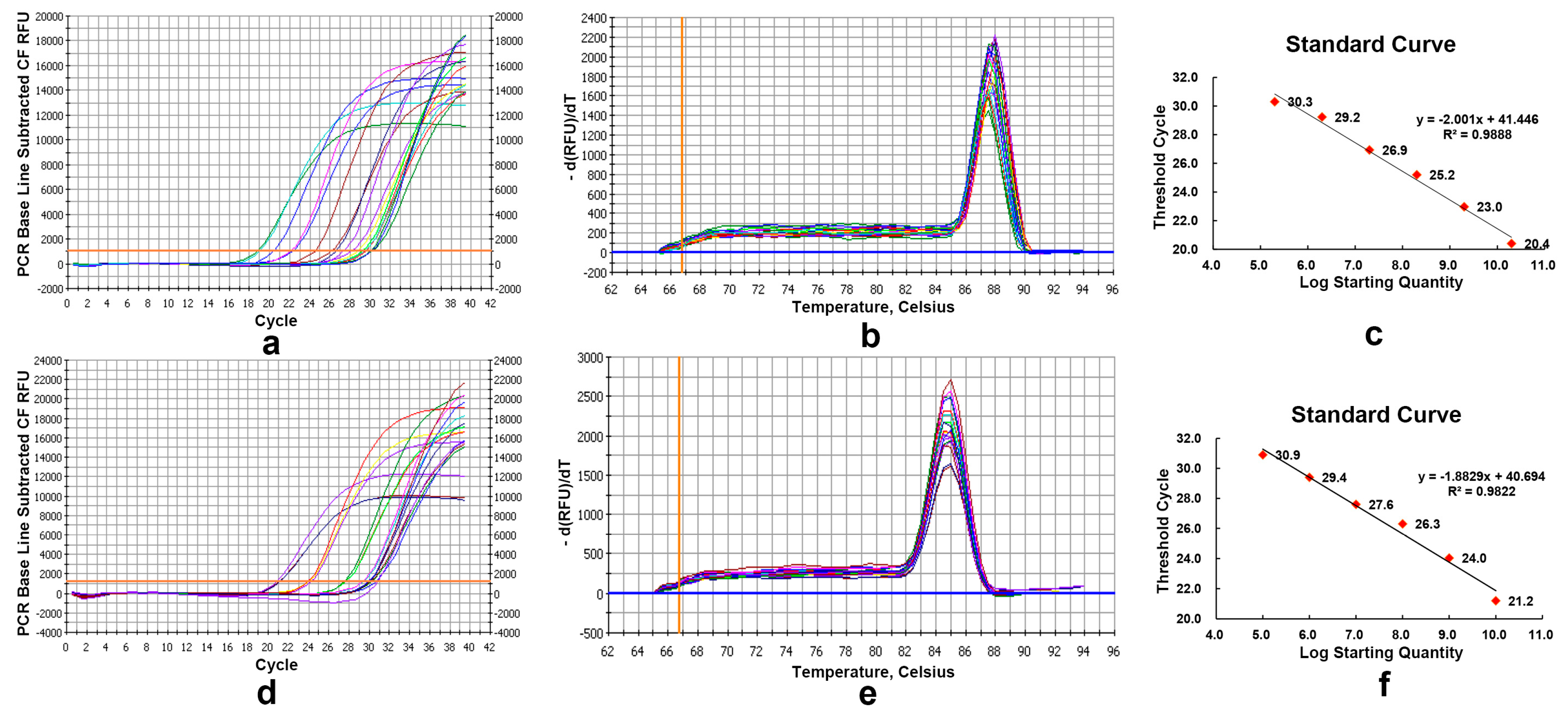
2.4. Real-Time Quantitative PCR Assays
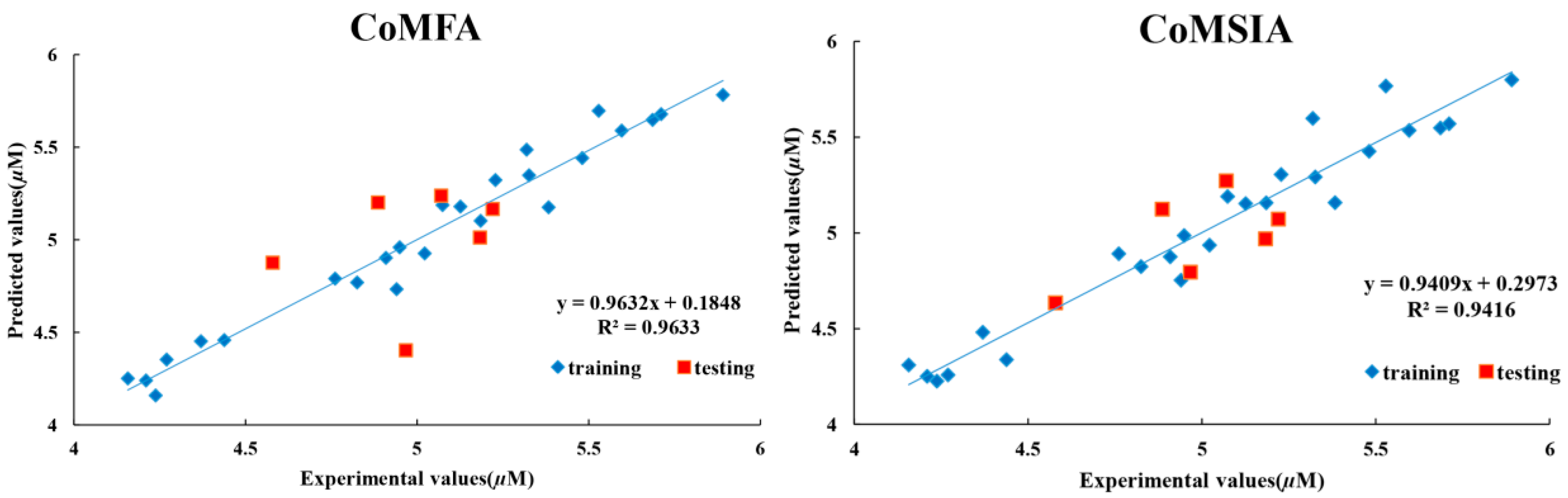
2.5. Study of 3D-QSAR Models
3. Discussion
4. Materials and Methods
4.1. Materials and Instrument
4.2. Chemistry
4.2.1. General Procedure for the Synthesis of Intermediates 2 and 3
4.2.2. General Procedure for the Synthesis of Intermediate 4
4.2.3. General Procedure for the Title Compounds 5a–5ae
4.3. In Vitro Antibacterial Activity
4.4. In Vivo Antibacterial Activity
4.4.1. In Vivo Antibacterial Activities Against Rice Bacterial Leaf Blight
4.4.2. In Vivo Antibacterial Activities Against Rice Bacterial Leaf Streak
4.5. Real-Time Quantitative PCR Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Xoo | Xanthomonas oryzae pv. Oryzae |
| Xoc | Xanthomonas oryzae pv. Oryzicola |
| EC50 | 50% effective concentration |
| CoMFA | comparative molecular field analysis |
| CoMSIA | comparative molecular similarity index analysis |
| 3D-QSAR | three-dimensional quantitative structure–activity relationship |
| PLS | partial least squares |
| ONC | optimal number of components |
| SEE | standard error of estimate |
| OD595 | optical density at 595 nm |
References
- Liu, W.D.; Liu, J.L.; Triplett, L.; Leach, J.E.; Wang, G.L. Novel insights into rice innate immunity against bacterial and fungal pathogens. Annu. Rev. Phytopathol. 2014, 52, 213–241. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Y.; Wang, C.L.; Zhao, K.J. Rice routes of countering Xanthomonas oryzae. Int. J. Mol. Sci. 2018, 19, 3008. [Google Scholar] [CrossRef] [PubMed]
- Bae, N.; Park, H.J.; Park, H.; Kim, M.; Do, E.; Han, S.W. Elucidating functions of fleQ in Xanthomonas oryzae pv. oryzae by comparative proteomic and phenotypic analyses. Int. J. Mol. Sci. 2018, 19, 3038. [Google Scholar] [CrossRef]
- Niño-Liu, D.O.; Ronald, P.C.; Bogdanove, A.J. Xanthomonas oryzae pathovars: Model pathogens of a model crop. Mol. Plant Pathol. 2006, 7, 303–324. [Google Scholar] [CrossRef] [PubMed]
- Tariq, R.; Wang, C.L.; Qin, T.F.; Xu, F.F.; Tang, Y.C.; Gao, Y.; Ji, Z.Y.; Zhao, K.J. Comparative transcriptome profiling of rice near-isogenic line carrying Xa23 under infection of Xanthomonas oryzae pv. oryzae. Int. J. Mol. Sci. 2018, 19, 717. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Yang, X.; Gu, C.Y.; Zhang, A.F.; Wang, W.X.; Gao, T.C.; Yao, J.; Yuan, S.K. Activity of a novel bactericide, zinc thiazole against Xanthomonas oryzae pv. oryzae in Anhui Province of China. Ann. Appl. Biol. 2015, 166, 129–135. [Google Scholar] [CrossRef]
- Wang, P.Y.; Zhou, L.; Zhou, J.; Wu, Z.B.; Xue, W.; Song, B.A.; Yang, S. Synthesis and antibacterial activity of pyridinium-tailored 2,5-substituted-1,3,4-oxadiazole thioether/sulfoxide/sulfone derivatives. Bioorg. Med. Chem. Lett. 2016, 26, 1214–1217. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Shi, L.; Gao, M.N.; Yang, X.; Xue, W.; Jin, L.H.; Hu, D.Y.; Song, B.A. Antibacterial activities against rice bacterial leaf blight and tomato bacterial wilt of 2-mercapto-5-substituted-1,3,4-oxadiazole/thiadiazole derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 481–484. [Google Scholar] [CrossRef] [PubMed]
- El-Seedi, H.R.; El-Said, A.M.A.; Khalifa, S.A.M.; Göransson, U.; Bohlin, L.; Borg-Karlson, A.K.; Verpoorte, R. Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J. Agric. Food Chem. 2012, 60, 10877–10895. [Google Scholar] [CrossRef] [PubMed]
- Heřmánková-Vavříková, E.; Křenková, A.; Petrásková, L.; Chambers, C.S.; Zápal, J.; Kuzma, M.; Valentová, K.; Křen, V. Synthesis and antiradical activity of isoquercitrin esters with aromatic acids and their homologues. Int. J. Mol. Sci. 2017, 18, 1074. [Google Scholar] [CrossRef] [PubMed]
- Guitard, R.; Nardello-Rataj, V.; Aubry, J.M. Theoretical and kinetic tools for selecting effective antioxidants: Application to the protection of omega-3 oils with natural and synthetic phenols. Int. J. Mol. Sci. 2016, 17, 1220. [Google Scholar] [CrossRef] [PubMed]
- Kai, Z.P.; Huang, J.; Xie, Y.; Tobe, S.S.; Ling, Y.; Zhang, L.; Zhao, Y.C.; Yang, X.L. Synthesis, biological activity, and hologram quantitative structure–activity relationships of novel allatostatin analogues. J. Agric. Food Chem. 2010, 58, 2652–2658. [Google Scholar] [CrossRef] [PubMed]
- Gravina, H.D.; Tafuri, N.F.; Silva, J.A.; Fietto, J.L.R.; Oliveira, T.T.; Diaz, M.A.N.; Almeida, M.R. In vitro assessment of the antiviral potential of trans-cinnamic acid, quercetin and morin against equid herpesvirus 1. Res. Vet. Sci. 2011, 91, 158–162. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.X.; Zhang, J.; Chen, J.X.; Pan, J.K.; Zhao, L.; Liu, D.Y.; Zhang, A.W.; Chen, J.; Hu, D.Y.; Song, B.A. Design, synthesis, antiviral bioactivity and three–dimensional quantitative structure–activity relationship study of novel ferulic acid ester derivatives containing quinazoline moiety. Pest Manag. Sci. 2017, 73, 2079–2089. [Google Scholar] [CrossRef] [PubMed]
- Lei, K.; Sun, D.W.; Hua, X.W.; Tao, Y.Y.; Xu, X.H.; Kong, C.H. Synthesis, fungicidal activity and structure–activity relationships of 3-benzoyl-4-hydroxylcoumarin derivatives. Pest Manag. Sci. 2015, 72, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Lou, J.F.; Luo, C.; Zhou, L.G.; Wang, M.G.; Wang, L. Phenolic compounds from halimodendron halodendron (pall.) voss and their antimicrobial and antioxidant activities. Int. J. Mol. Sci. 2012, 13, 11349–11364. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.F.; Zhou, Y.; Zheng, Y.; Li, C.L.; Sheng, S.; Wang, J.; Wu, F. Enzymatic modification of chitosan by cinnamic acids: Antibacterial activity against Ralstonia solanacearum. Int. J. Biol. Macromol. 2016, 87, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Vishnoi, S.; Agrawal, V.; Kasana, V.K. Synthesis and structure-activity relationships of substituted cinnamic acids and amide analogues: A new class of herbicides. J. Agric. Food Chem. 2009, 57, 3261–3265. [Google Scholar] [CrossRef] [PubMed]
- Sova, M. Antioxidant and antimicrobial activities of cinnamic acid derivatives. Mini-Rev. Med. Chem. 2012, 12, 749–767. [Google Scholar] [CrossRef] [PubMed]
- Anslow, P.A.; Stratford, M. Preservative and Flavoring System. U.S. Patent 6042861, 22 October 1998. [Google Scholar]
- Li, P.; Tian, P.Y.; Chen, Y.Z.; Song, X.P.; Xue, W.; Jin, L.H.; Hu, D.Y.; Yang, S.; Song, B.A. Novel bisthioether derivatives containing a 1,3,4-oxadiazole moiety: Design, synthesis, antibacterial and nematocidal activities. Pest Manag. Sci. 2018, 74, 844–852. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Han, F.F.; He, M.; Hu, D.Y.; He, J.; Yang, S.; Song, B.A. Inhibition of tobacco bacterial wilt with sulfone derivatives containing an 1,3,4-oxadiazole moiety. J. Agric. Food Chem. 2012, 60, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Hu, D.Y.; Xie, D.D.; Chen, J.X.; Jin, L.H.; Song, B.A. Design, synthesis, and evaluation of new sulfone derivatives containing a 1,3,4-oxadiazole moiety as active antibacterial agents. J. Agric. Food Chem. 2018, 66, 3093–3100. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.M.; Li, S.Z.; He, M.; Yang, S.; Li, X.Y.; Li, P. Synthesis and bioactivities of novel thioether/sulfone derivatives containing 1,2,3-thiadiazole and 1,3,4-oxadiazole/thiadiazole moiety. Bioorg. Med. Chem. Lett. 2013, 23, 5821–5824. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.X.; Chen, Y.Z.; Gan, X.H.; Song, B.J.; Hu, D.Y.; Song, B.A. Synthesis, nematicidal evaluation, and 3D-QSAR analysis of novel 1,3,4-oxadiazole–cinnamic acid hybrids. J. Agric. Food Chem. 2018, 66, 9616–9623. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.H.; Hu, D.Y.; Li, P.; Wu, J.; Chen, X.W.; Xue, W.; Song, B.A. Design, synthesis, antiviral activity and three-dimensional quantitative structure–activity relationship study of novel 1,4-pentadien-3-one derivatives containing the 1,3,4-oxadiazole moiety. Pest Manag. Sci. 2016, 72, 534–543. [Google Scholar] [CrossRef] [PubMed]
- Tajik, H.; Dadras, A. Synthesis and herbicidal activity of novel 5-chloro-3-fluoro-2-phenoxypyridines with a 1,3,4-oxadiazole ring. J. Pestic. Sci. 2011, 36, 27–32. [Google Scholar] [CrossRef]
- Tabanca, N.; Ali, A.; Bernier, U.R.; Khan, I.A.; Kocyigit-Kaymakcioglu, B.; Oruç-Emre, E.E.; Unsalan, S.; Rollas, S. Biting deterrence and insecticidal activity of hydrazide-hydrazones and their corresponding 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazoles against Aedes aegypti. Pest Manag. Sci. 2013, 69, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Breslav, M.; Grimm, J.; Guan, K.L.; Huang, A.H.; Liu, F.Q.; Maryanoff, C.A.; Palmer, D.; Patel, M.; Qian, Y.; et al. A new procedure for preparation of carboxylic acids hydrazides. J. Org. Chem. 2002, 67, 9471–9474. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Song, B.A.; Yang, S.; Xu, G.F.; Bhadury, P.S.; Jin, L.H.; Hu, D.Y.; Li, Q.Z.; Liu, F.; Xue, W.; et al. Synthesis and antifungal activities of 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4- thiadiazole and 5-(3,4,5-trimethoxyphenyl)-2-sulfonyl-1,3,4-oxadiazol derivatives. Bioorg. Med. Chem. Lett. 2007, 15, 3981–3989. [Google Scholar] [CrossRef] [PubMed]
- Dalgaard, P.; Ross, T.; Kamperman, L.; Neumeyer, K.; McMeekin, T.A. Estimation of bacterial growth rates from turbidimetric and viable count data. Int. J. Food Microbiol. 1994, 23, 391–404. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.; Zhu, X.F.; Hou, Y.P.; Gao, T.C.; Zhou, M.G.; Xu, Y. A molecular mechanism of resistance to streptomycin in Xanthomonas oryzae pv. oryzicola. Phytoparasitica 2011, 39, 393–401. [Google Scholar] [CrossRef]
- Csaikl, U.M.; Bastian, H.; Brettschneider, R.; Gauch, S.; Meir, A.; Schauerte, M.; Ziegenhagen, B. Comparative analysis of different DNA extraction protocols: A fast, universal maxi-preparation of high quality plant DNA for genetic evaluation and phylogenetic studies. Plant Mol. Biol. Rep. 1998, 16, 69–86. [Google Scholar] [CrossRef]
- Lu, W.; Pan, L.Q.; Zhao, H.J.; Jia, Y.L.; Wang, Y.L.; Yu, X.P.; Wang, X.Y. Molecular detection of Xanthomonas oryzae pv. oryzae, Xanthomonas oryzae pv. oryzicola, and Burkholderia glumae in infected rice seeds and leaves. Crop J. 2014, 2, 398–406. [Google Scholar] [CrossRef]



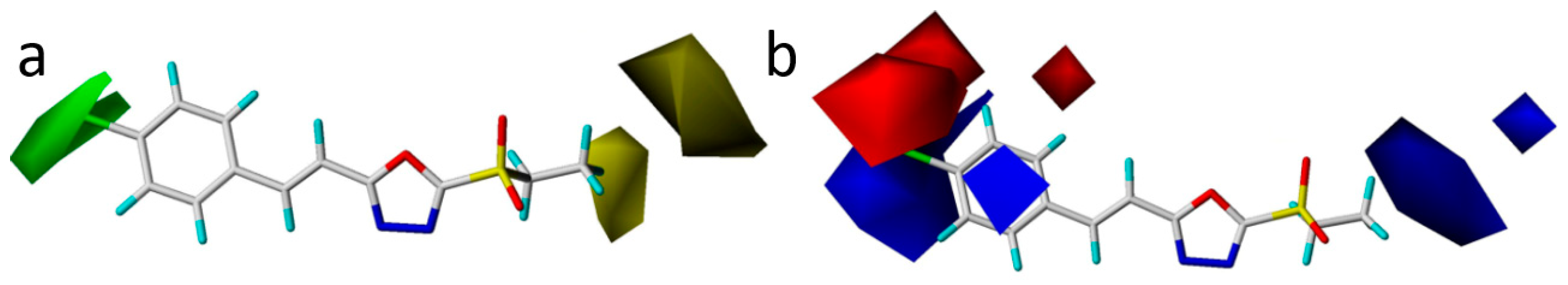
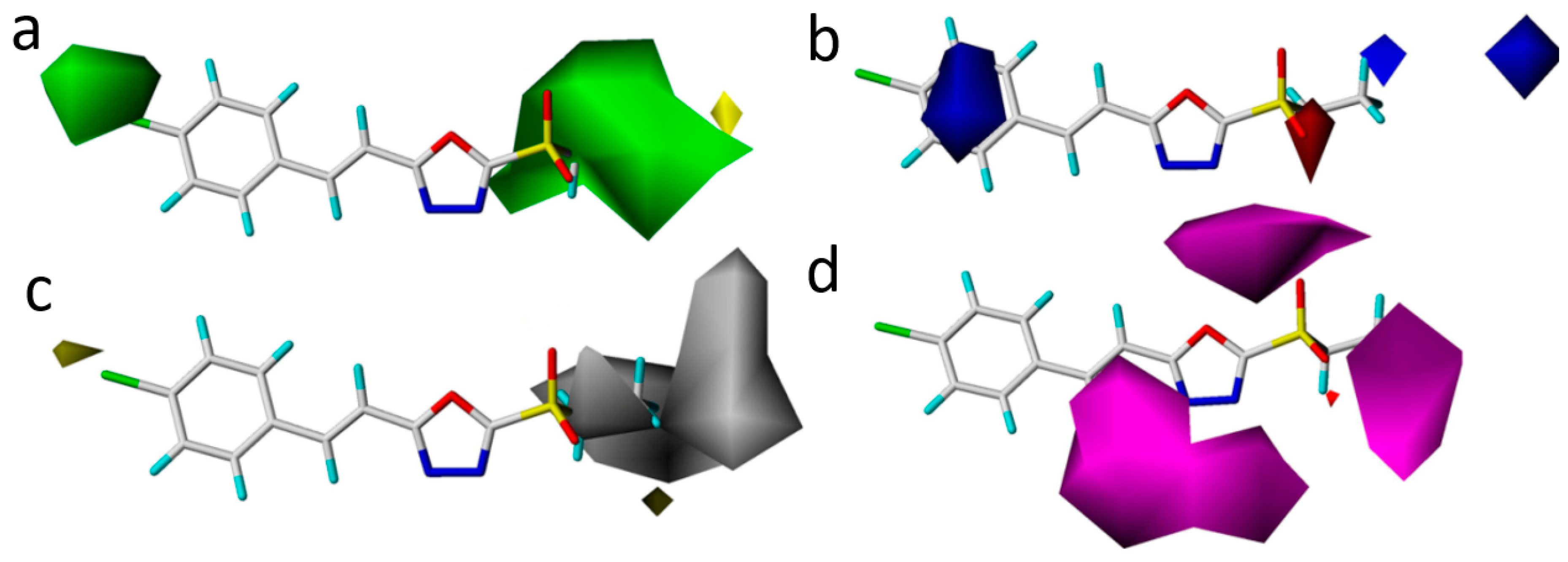
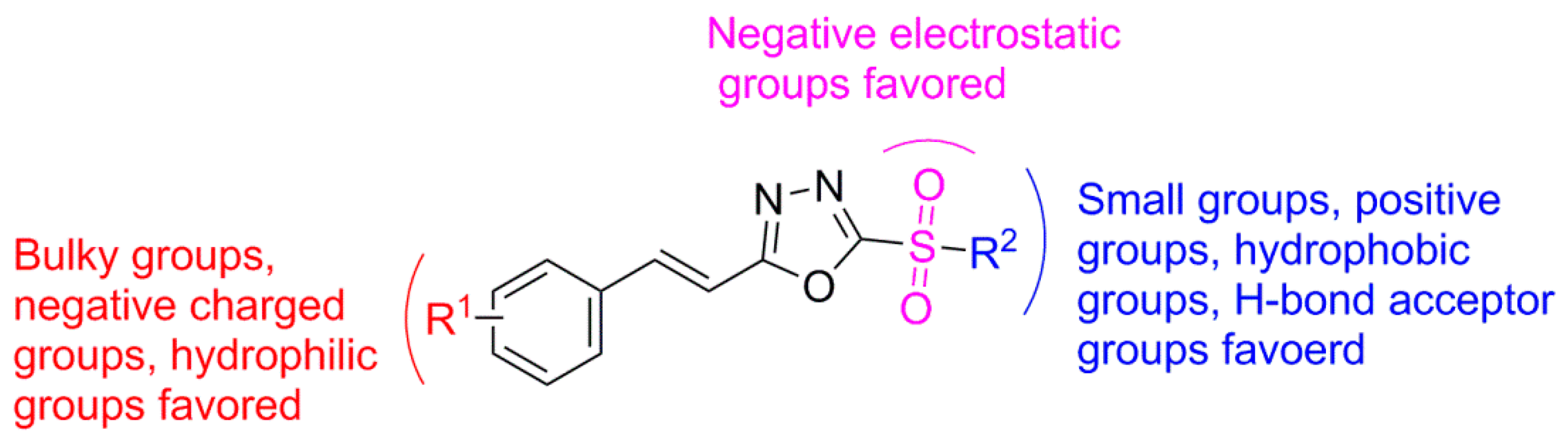
| Compound | Inhibition (%) | Toxic Regression Equation | r | EC50 (μg/mL) a | |
|---|---|---|---|---|---|
| 50 (μg/mL) | 5 (μg/mL) | ||||
| 5a | 100 | 57.1 ± 3.3 | y = 1.70x + 4.08 | 0.99 | 3.47 ± 1.47 |
| 5b | 100 | 77.2 ± 1.0 | y = 4.84x + 2.40 | 0.96 | 3.43 ± 0.77 |
| 5c | 97.7 ± 2.2 | 48.6 ± 1.8 | y = 1.82x + 4.42 | 0.98 | 2.08 ± 1.13 |
| 5d | 100 | 77.1 ± 1.4 | y = 1.39x + 4.91 | 0.99 | 1.15 ± 0.93 |
| 5e | 74.1 ± 1.0 | 71.3 ± 2.4 | y = 1.44x + 4.64 | 0.99 | 1.76 ± 0.80 |
| 5f | 92.2 ± 1.5 | 65.3 ± 0.9 | y = 1.06x + 4.40 | 0.96 | 3.60 ± 1.13 |
| 5g | 93.1 ± 3.3 | 49.9 ± 2.5 | y = 1.54x + 3.91 | 0.96 | 5.06 ± 1.71 |
| 5h | 99.1 ± 1.0 | 65.3 ± 4.9 | y = 1.67x + 3.48 | 0.96 | 8.07 ± 2.66 |
| 5i | 91.8 ± 3.0 | 79.6 ± 1.6 | y = 1.51x + 4.63 | 0.97 | 1.75 ± 0.97 |
| 5j | 97.2 ± 4.0 | 72.4 ± 4.9 | y = 1.56x + 4.41 | 0.99 | 2.38 ± 1.17 |
| 5k | 100 | 82.8 ± 3.1 | y = 1.30x + 5.28 | 0.97 | 0.61 ± 0.43 |
| 5l | 89.8 ± 1.9 | 37.3 ± 2.5 | y = 0.87x + 4.06 | 0.99 | 11.83 ± 3.43 |
| 5m | 97.6 ± 1.8 | 92.5 ± 1.4 | y = 4.56x + 3.37 | 0.97 | 2.28 ± 0.41 |
| 5n | 100 | 77.2 ± 3.3 | y = 1.49x + 4.67 | 0.91 | 1.67 ± 0.65 |
| 5o | 95.2 ± 4.3 | 88.1 ± 4.1 | y = 1.06x + 4.94 | 0.96 | 1.42 ± 0.70 |
| 5p | 88.0 ± 2.6 | 63.3 ± 3.4 | y = 0.79x + 4.85 | 0.97 | 1.53 ± 1.18 |
| 5q | 100 | 100 | y = 3.68x + 5.51 | 0.91 | 0.72 ± 0.22 |
| 5r | 100 | 97.3 ± 0.8 | y = 2.33x + 5.55 | 0.99 | 0.58 ± 0.35 |
| 5s | 100 | 76.0 ± 4.8 | y = 2.92x + 5.03 | 0.90 | 0.97 ± 0.41 |
| 5t | 100 | 99.8 ± 0.2 | y = 1.97x + 5.70 | 0.96 | 0.44 ± 0.26 |
| 5u | 93.8 ± 4.0 | 57.9 ± 3.2 | y = 2.80x + 3.81 | 0.99 | 2.66 ± 0.92 |
| 5v | 96.7 ± 2.3 | 67.3 ± 1.0 | y = 1.89x + 4.46 | 0.95 | 1.93 ± 0.85 |
| 5w | 100 | 55.9 ± 1.0 | y = 2.20x + 2.00 | 0.93 | 22.74 ± 3.62 |
| 5x | 82.2 ± 4.1 | 65.4 ± 3.0 | y = 1.22x + 4.27 | 0.94 | 3.95 ± 1.11 |
| 5y | 100 | 54.0 ± 2.7 | y = 1.43x + 3.12 | 0.91 | 20.95 ± 4.32 |
| 5z | 63.3 ± 5.0 | 32.5 ± 3.7 | y = 1.24x + 3.38 | 0.98 | 19.90 ± 4.84 |
| 5aa | 82.6 ± 1.3 | 44.3 ± 1.5 | y= 1.00x + 3.80 | 0.97 | 15.94 ± 3.03 |
| 5ab | 67.9 ± 4.3 | 37.9 ± 2.7 | y = 0.80x + 3.98 | 0.99 | 19.34 ± 3.24 |
| 5ac | 90.1 ± 1.3 | 50.5 ± 2.3 | y = 0.63x + 4.60 | 0.98 | 4.43 ± 1.74 |
| 5ad | 95.2 ± 2.0 | 58.7 ± 3.1 | y = 0.62x + 4.92 | 0.97 | 1.36 ± 0.69 |
| 5ae | 100 | 59.3 ± 0.3 | y = 0.60x + 4.10 | 0.96 | 4.00 ± 2.73 |
| Cinnamic acid | 10.3 ± 4.3 | 1.4 ± 0.5 | y = 4.57x − 5.85 | 0.98 | 236.07 ± 6.29 |
| a | 100 | 90.1 ± 3.7 | y = 2.65x + 4.85 | 0.97 | 1.41 ± 0.64 |
| b | 100 | 84.8 ± 2.9 | y = 6.78x + 5.50 | 0.97 | 0.84 ± 0.33 |
| Bismerthiazol | 38.9 ± 1.8 | 4.2 ± 1.4 | y = 2.35x + 0.53 | 0.95 | 85.66 ± 4.10 |
| Thiodiazole copper | 34.3 ± 2.6 | 1.9 ± 0.5 | y = 1.67x + 1.49 | 0.95 | 123.10 ± 4.74 |
| Compound | Inhibition (%) | Toxic Regression Equation | r | EC50 (μg/mL) a | |
|---|---|---|---|---|---|
| 50 (μg/mL) | 5 (μg/mL) | ||||
| 5a | 98.1 ± 1.5 | 58.5 ± 1.9 | y = 1.20x + 4.50 | 0.98 | 2.65 ± 1.87 |
| 5b | 91.3 ± 2.2 | 39.1 ± 1.2 | y = 1.62x + 3.69 | 0.98 | 6.42 ± 1.93 |
| 5c | 97.6 ± 2.7 | 38.5 ± 3.0 | y = 1.90x + 3.48 | 0.99 | 6.27 ± 2.79 |
| 5d | 97.9 ± 2.5 | 31.3 ± 4.4 | y = 2.33x + 3.33 | 0.96 | 5.23 ± 3.96 |
| 5e | 74.8 ± 4.7 | 11.0 ± 3.9 | y = 3.63x + 0.27 | 0.93 | 19.86 ± 3.26 |
| 5f | 90.4 ± 1.3 | 47.8 ± 1.8 | y = 1.31x + 4.08 | 0.97 | 4.94 ± 1.81 |
| 5g | 89.3 ± 2.2 | 64.4 ± 1.4 | y = 1.03x + 4.49 | 0.97 | 3.06 ± 1.87 |
| 5h | 46.9 ± 2.3 | 7.7 ± 0.9 | y = 1.79x + 2.20 | 0.96 | 36.44 ± 2.90 |
| 5i | 97.9 ± 1.8 | 34.4 ± 4.5 | y = 2.15x + 4.65 | 0.95 | 1.45 ± 1.77 |
| 5j | 98.6 ± 1.1 | 69.5 ± 4.3 | y = 1.35x + 4.75 | 0.99 | 1.51 ± 1.18 |
| 5k | 100 | 92.5 ± 2.5 | y = 3.04x + 4.26 | 0.94 | 1.76 ± 0.74 |
| 5l | 90.7 ± 3.8 | 69.0 ± 4.4 | y = 0.82x + 4.95 | 0.99 | 1.16 ± 0.93 |
| 5m | 100 | 83.0 ± 2.8 | y = 1.49x + 4.89 | 0.98 | 1.17 ± 0.60 |
| 5n | 100 | 77.4 ± 1.3 | y = 1.49x + 4.67 | 0.91 | 1.49 ± 0.93 |
| 5o | 75.7 ± 4.2 | 46.5 ± 3.3 | y = 0.78x + 4.35 | 0.97 | 6.71 ± 3.23 |
| 5p | 67.7 ± 3.8 | 50.9 ± 0.8 | y = 0.41x + 4.73 | 0.91 | 4.24 ± 2.02 |
| 5q | 100 | 72.8 ± 1.8 | y = 0.79x + 5.00 | 0.93 | 0.97 ± 0.49 |
| 5r | 100 | 96.6 ± 1.3 | y = 2.33x + 6.09 | 0.99 | 0.34 ± 0.20 |
| 5s | 100 | 83.7 ± 0.8 | y = 0.67x + 5.05 | 0.97 | 0.82 ± 0.32 |
| 5t | 100 | 98.2 ± 2.0 | y = 2.09x + 6.47 | 0.98 | 0.20 ± 0.13 |
| 5u | 94.4 ± 4.7 | 77.6 ± 1.0 | y = 0.62x + 5.26 | 0.98 | 0.38 ± 0.14 |
| 5v | 77.1 ± 2.8 | 64.8 ± 2.3 | y = 0.46x + 4.97 | 0.93 | 1.14 ± 0.66 |
| 5w | 66.9 ± 4.4 | 32.4 ± 2.2 | y = 0.97x + 3.81 | 0.98 | 16.44 ± 2.98 |
| 5x | 91.7 ± 4.9 | 59.2 ± 1.3 | y = 1.16x + 4.26 | 0.97 | 2.82 ± 1.51 |
| 5y | 83.5 ± 3.1 | 58.6 ± 3.1 | y = 0.66x + 4.61 | 0.94 | 3.87 ± 2.33 |
| 5z | 67.0 ± 2.6 | 36.2 ± 3.7 | y = 0.72x + 4.13 | 0.96 | 16.37 ± 3.72 |
| 5aa | 77.1 ± 2.6 | 44.7 ± 2.5 | y=1.11x + 3.91 | 0.97 | 9.57 ± 4.36 |
| 5ab | 70.4 ± 0.8 | 40.9 ± 2.8 | y = 0.82x + 4.11 | 0.98 | 12.20 ± 2.03 |
| 5ac | 74.3 ± 1.1 | 41.4 ± 2.3 | y = 0.71x + 4.25 | 0.99 | 11.54 ± 3.44 |
| 5ad | 99.0 ± 1.0 | 78.2 ± 4.8 | y = 1.94x + 4.16 | 0.97 | 2.69 ± 1.10 |
| 5ae | 78.3 ± 2.5 | 54.8 ± 3.2 | y = 0.80x + 4.55 | 0.95 | 3.52 ± 1.25 |
| Cinnamic acid | 16.9 ± 3.0 | 2.2 ± 1.2 | y = 1.07x + 1.89 | 0.96 | 270.45 ± 6.51 |
| a | 100 | 98.1 ± 0.9 | y = 3.67x + 3.88 | 0.98 | 2.01 ± 0.76 |
| b | 100 | 93.0 ± 2.2 | y = 1.63x + 4.89 | 0.96 | 1.16 ± 0.57 |
| Bismerthiazol | 24.4 ± 4.0 | 2.6 ± 1.8 | y = 2.04x + 0.81 | 0.97 | 110.96 ± 2.79 |
| Thiodiazole copper | 18.7 ± 3.2 | 1.6 ± 0.6 | y = 1.79x + 1.04 | 0.95 | 161.52 ± 4.84 |
| Treatment | 14 Days after Spraying | ||
|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) 2 | |
| 5t | 100 | 36.40 | 66.62 ± 10.78 a |
| Bismerthiazol | 100 | 46.89 | 51.71 ± 6.90 b |
| Thiodiazole copper | 100 | 44.50 | 57.33 ± 7.26 b |
| CK 1 | 100 | 86.70 | - |
| Treatment | 14 Days after Spraying | ||
|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) 2 | |
| 5t | 100 | 43.83 | 56.05 ± 8.73 a |
| Bismerthiazol | 100 | 56.31 | 43.65 ± 7.98 b |
| Thiodiazole copper | 100 | 49.60 | 48.60 ± 5.14 b |
| CK 1 | 100 | 89.11 | - |
| Treatment | 14 Days after Spraying | ||
|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) 2 | |
| 5t | 100 | 38.11 | 58.22 ± 9.93 a |
| Bismerthiazol | 100 | 48.75 | 46.08 ± 6.62 c |
| Thiodiazole copper | 100 | 42.75 | 53.77 ± 6.89 b |
| CK 1 | 100 | 81.50 | - |
| Treatment | 14 Days after Spraying | ||
|---|---|---|---|
| Morbidity (%) | Disease Index (%) | Control Efficiency (%) 2 | |
| 5t | 100 | 46.20 | 55.92 ± 10.34 a |
| Bismerthiazol | 100 | 57.35 | 45.25 ± 5.26 c |
| Thiodiazole copper | 100 | 53.80 | 42.83 ± 9.55 c |
| CK 1 | 100 | 83.30 | - |
| Compound | Protective Treatment | Curative Treatment | ||
|---|---|---|---|---|
| Ct 1 | Titer 2 (CFU/mL) | Ct 1 | Titer 2 (CFU/mL) | |
| 5t | 18.67 ± 1.76 | 2.42 × 1011 | 18.47 ± 1.19 | 3.05 × 1011 |
| Thiodiazole copper | 18.47 ± 1.27 | 3.05 × 1011 | 18.30 ± 1.21 | 3.69 × 1011 |
| Bismerthiazol | 18.37 ± 1.68 | 3.42 × 1011 | 18.07 ± 0.60 | 4.83 × 1011 |
| CK 3 | 17.40 ± 0.62 | 1.04 × 1012 | 17.23 ± 0.25 | 1.26 × 1012 |
| Compound | Protective Treatment | Curative Treatment | ||
|---|---|---|---|---|
| Ct 1 | Titer 2 (CFU/mL) | Ct 1 | Titer 2 (CFU/mL) | |
| 5t | 21.10 ± 0.66 | 2.55 × 1010 | 20.23 ± 0.25 | 7.35 × 1010 |
| Thiodiazole copper | 20.77 ± 0.21 | 3.83 × 1010 | 20.13 ± 0.81 | 8.31 × 1010 |
| Bismerthiazol | 20.70 ± 0.26 | 4.16 × 1010 | 20.17 ± 0.38 | 7.98 × 1010 |
| CK 3 | 19.83 ± 0.25 | 1.20 × 1011 | 19.70 ± 0.46 | 1.41 × 1011 |
| Compound | EC50 (μg/mL) | Exp a | CoMFA | CoMSIA | ||
|---|---|---|---|---|---|---|
| Pred. b | Res. c | Pred. d | Res. c | |||
| 5a | 3.74 | 4.825 | 4.769 | −0.056 | 4.824 | −0.001 |
| 5b * | 3.43 | 4.886 | 5.201 | 0.314 | 5.124 | 0.238 |
| 5c | 2.08 | 5.126 | 5.179 | 0.053 | 5.153 | 0.027 |
| 5d | 1.15 | 5.383 | 5.175 | −0.209 | 5.158 | −0.225 |
| 5e * | 1.76 | 5.220 | 5.166 | −0.054 | 5.072 | −0.148 |
| 5f | 3.60 | 4.909 | 4.902 | −0.007 | 4.875 | −0.034 |
| 5g | 5.06 | 4.761 | 4.790 | 0.029 | 4.891 | 0.129 |
| 5h * | 8.07 | 4.579 | 4.876 | 0.279 | 4.635 | 0.056 |
| 5i | 1.75 | 5.185 | 5.102 | −0.083 | 5.157 | −0.028 |
| 5j | 2.38 | 5.074 | 5.187 | 0.113 | 5.190 | 0.116 |
| 5k | 0.61 | 5.686 | 5.647 | −0.039 | 5.548 | −0.138 |
| 5l | 11.83 | 4.438 | 4.458 | 0.020 | 4.338 | −0.104 |
| 5m * | 2.28 | 5.070 | 5.238 | 0.168 | 5.272 | 0.201 |
| 5n | 1.67 | 5.228 | 5.322 | 0.094 | 5.305 | 0.078 |
| 5o | 1.42 | 5.319 | 5.486 | 0.167 | 5.598 | 0.279 |
| 5p | 1.53 | 5.326 | 5.348 | 0.022 | 5.292 | −0.034 |
| 5q | 0.72 | 5.596 | 5.589 | −0.007 | 5.535 | −0.061 |
| 5r | 0.58 | 5.711 | 5.678 | −0.033 | 5.570 | −0.141 |
| 5s | 0.97 | 5.529 | 5.696 | 0.167 | 5.767 | 0.238 |
| 5t | 0.44 | 5.891 | 5.782 | −0.109 | 5.799 | −0.091 |
| 5u | 2.66 | 5.022 | 4.926 | −0.096 | 4.936 | −0.087 |
| 5v * | 1.93 | 5.183 | 5.012 | −0.171 | 4.969 | −0.214 |
| 5w | 22.74 | 4.157 | 4.251 | 0.094 | 4.310 | 0.154 |
| 5x | 3.95 | 4.940 | 4.733 | −0.207 | 4.753 | −0.187 |
| 5y | 20.95 | 4.210 | 4.240 | 0.029 | 4.251 | 0.040 |
| 5z | 19.92 | 4.238 | 4.159 | −0.079 | 4.226 | −0.012 |
| 5aa | 15.94 | 4.370 | 4.452 | 0.082 | 4.481 | 0.111 |
| 5ab | 19.34 | 4.270 | 4.353 | 0.083 | 4.258 | −0.012 |
| 5ac | 4.43 | 4.949 | 4.959 | 0.010 | 4.986 | 0.037 |
| 5ad | 1.36 | 5.481 | 5.441 | −0.040 | 5.426 | −0.055 |
| 5ae * | 4.00 | 4.967 | 4.403 | −0.565 | 4.795 | −0.173 |
| Statistical parameter | CoMFA | CoMSIA | Validation criterion |
|---|---|---|---|
| q2 a | 0.725 | 0.707 | >0.5 |
| ONC b | 6 | 6 | |
| r2 c | 0.963 | 0.941 | >0.8 |
| SEE d | 0.114 | 0.144 | <0.3 |
| F-value | 78.624 | 48.186 | |
| Fraction of field contributions: | |||
| Steric | 0.651 | 0.156 | |
| Electrostatic | 0.349 | 0.329 | |
| Hydrophobic | 0.472 | ||
| H-bond donor | 0.000 | ||
| H-bond acceptor | 0.042 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Gan, X.; Wang, Y.; Li, S.; Yi, C.; Chen, J.; He, F.; Yang, Y.; Hu, D.; Song, B. Novel 1,3,4-Oxadiazole Derivatives Containing a Cinnamic Acid Moiety as Potential Bactericide for Rice Bacterial Diseases. Int. J. Mol. Sci. 2019, 20, 1020. https://doi.org/10.3390/ijms20051020
Wang S, Gan X, Wang Y, Li S, Yi C, Chen J, He F, Yang Y, Hu D, Song B. Novel 1,3,4-Oxadiazole Derivatives Containing a Cinnamic Acid Moiety as Potential Bactericide for Rice Bacterial Diseases. International Journal of Molecular Sciences. 2019; 20(5):1020. https://doi.org/10.3390/ijms20051020
Chicago/Turabian StyleWang, Shaobo, Xiuhai Gan, Yanju Wang, Shaoyuan Li, Chongfen Yi, Jixiang Chen, Fangcheng He, Yuyuan Yang, Deyu Hu, and Baoan Song. 2019. "Novel 1,3,4-Oxadiazole Derivatives Containing a Cinnamic Acid Moiety as Potential Bactericide for Rice Bacterial Diseases" International Journal of Molecular Sciences 20, no. 5: 1020. https://doi.org/10.3390/ijms20051020
APA StyleWang, S., Gan, X., Wang, Y., Li, S., Yi, C., Chen, J., He, F., Yang, Y., Hu, D., & Song, B. (2019). Novel 1,3,4-Oxadiazole Derivatives Containing a Cinnamic Acid Moiety as Potential Bactericide for Rice Bacterial Diseases. International Journal of Molecular Sciences, 20(5), 1020. https://doi.org/10.3390/ijms20051020






