Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells
Abstract
1. Introduction
2. Results
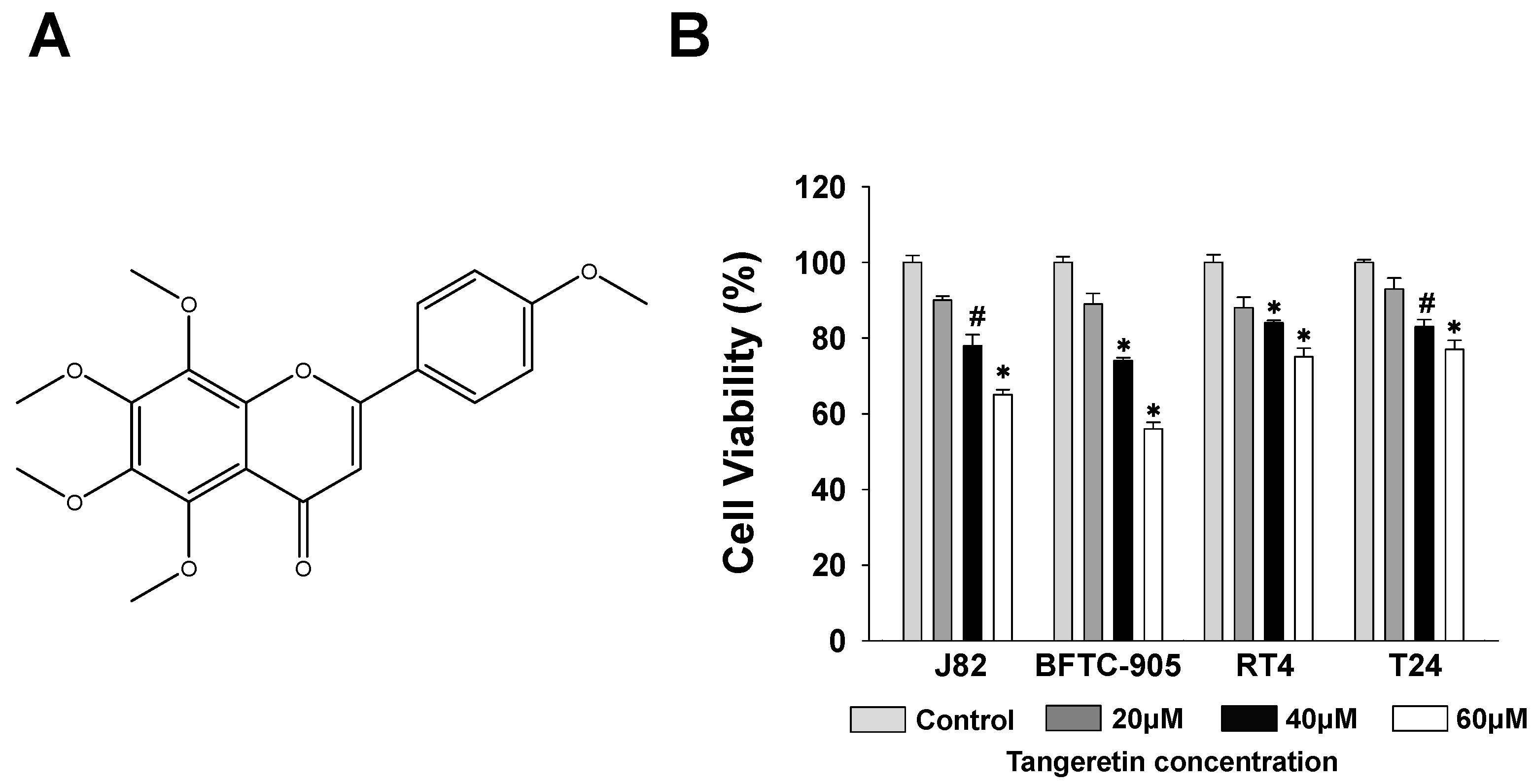
2.1. Cytotoxicity of Tangeretin towards Bladder Cancer Cells
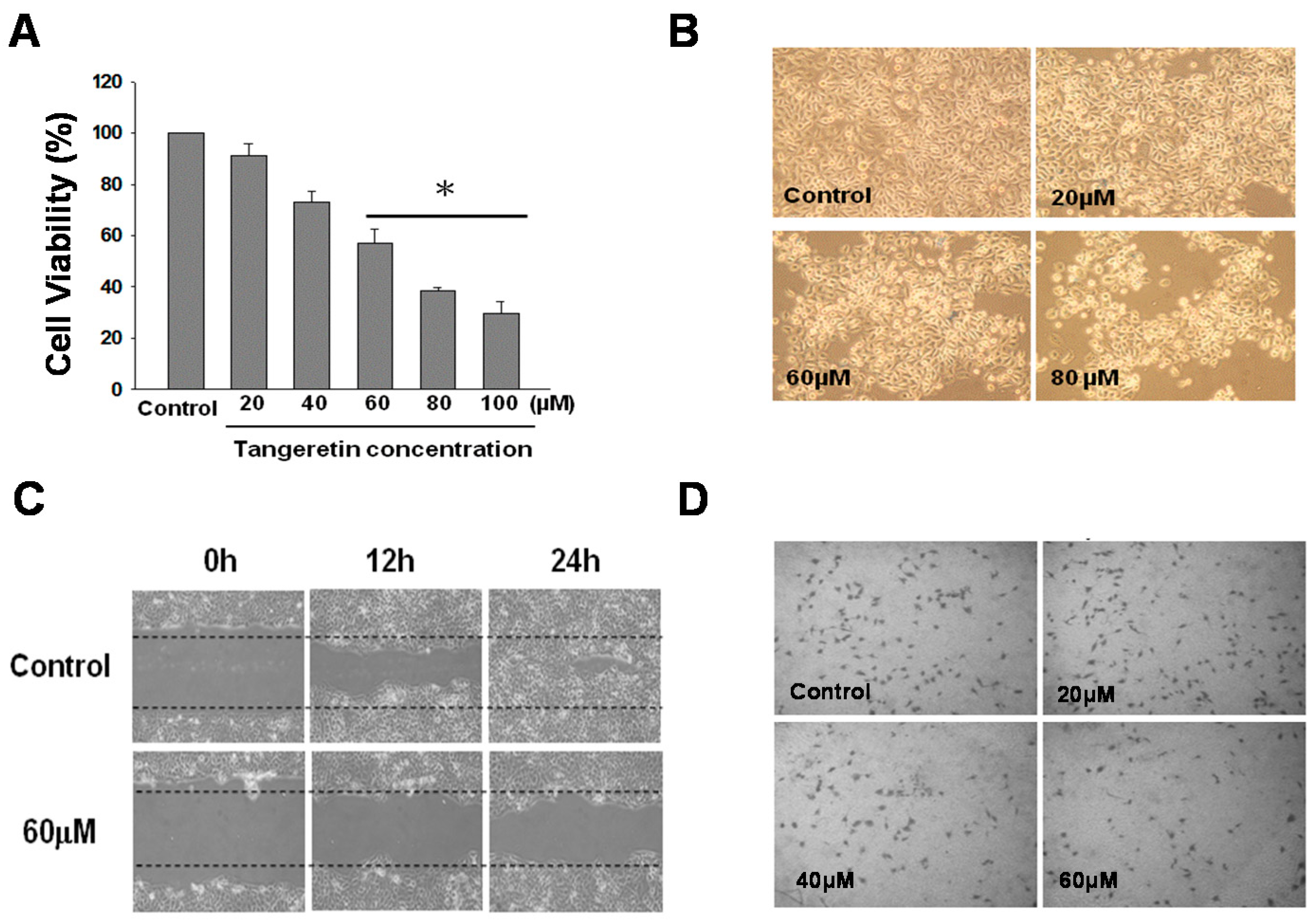
2.2. Inhibition Effect of Tangeretin on BFTC-905 Cells
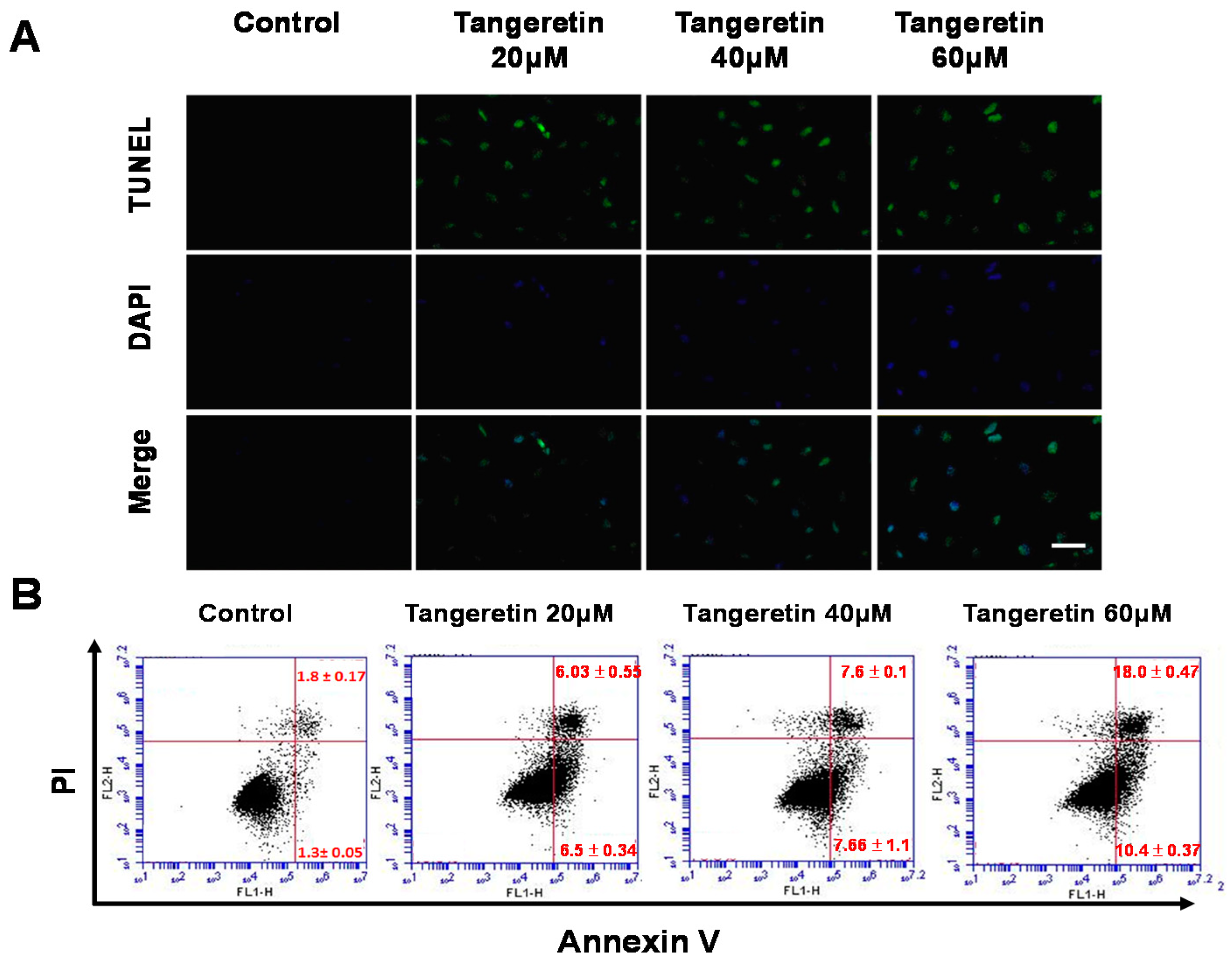
2.3. Tangeretin-Induced Apoptosis in BFTC-905 Cells
2.4. Use of Two-Dimensional Gel Electrophoresis to Measure Changes in Protein Expressions of BFTC-905 Cells after Tangeretin Treatment
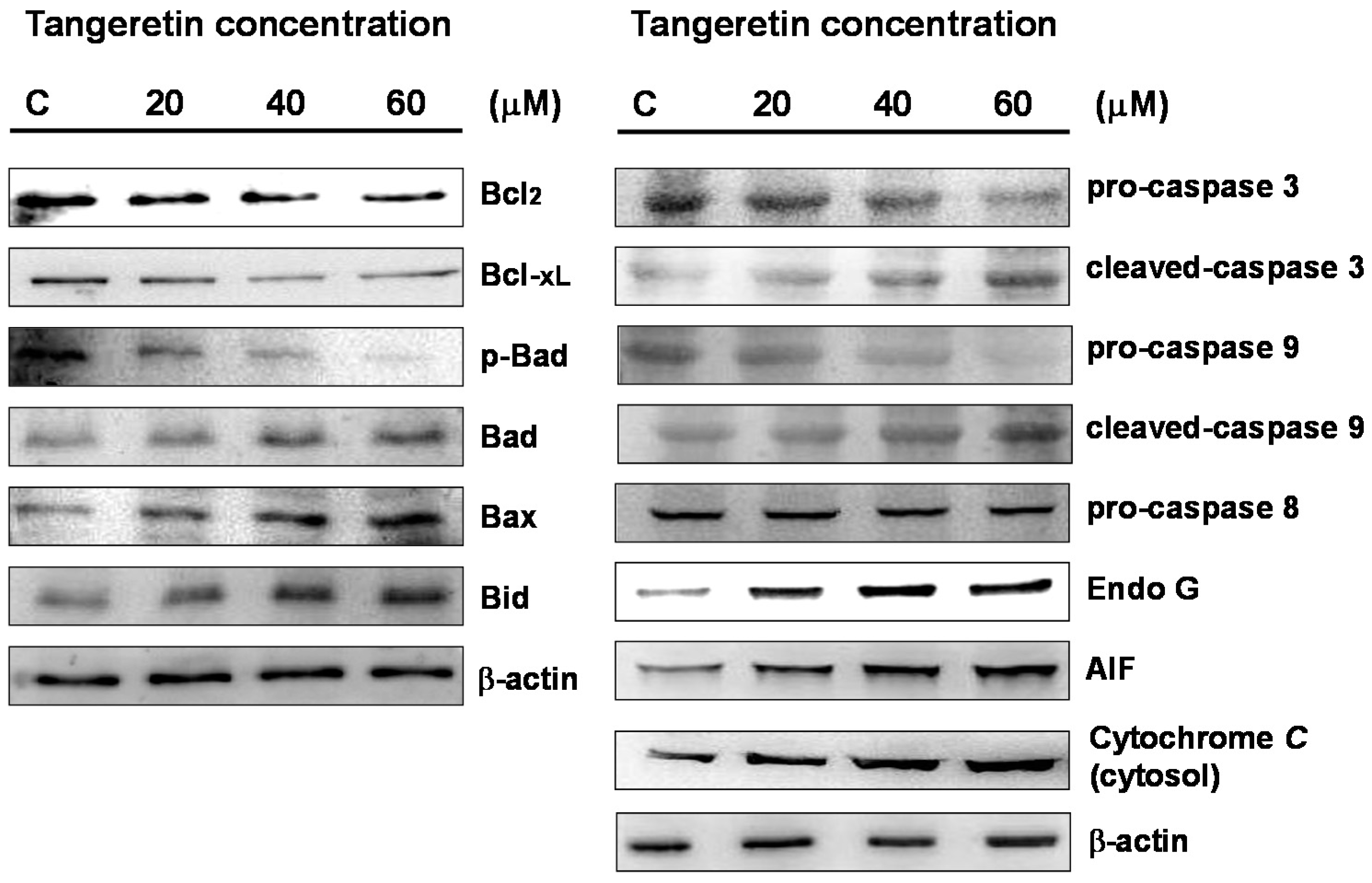
2.5. Tangeretin Induced Mitochondrial Dysfunction and Activated Caspase-Dependent Pathways in BFTC-905 Cells
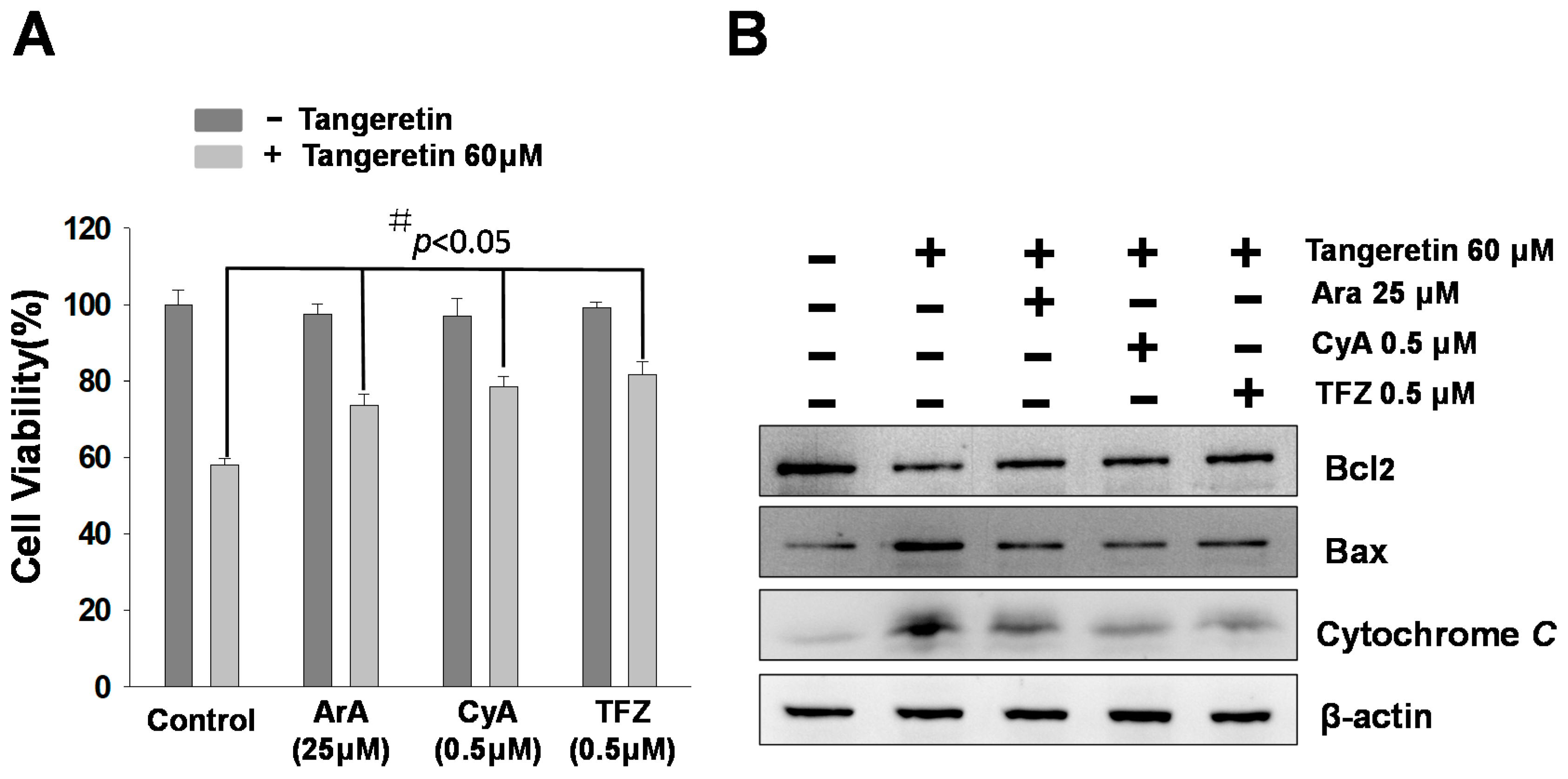
2.6. Mitochondrial Inhibitors Block Tangeretin-Induced Apoptosis
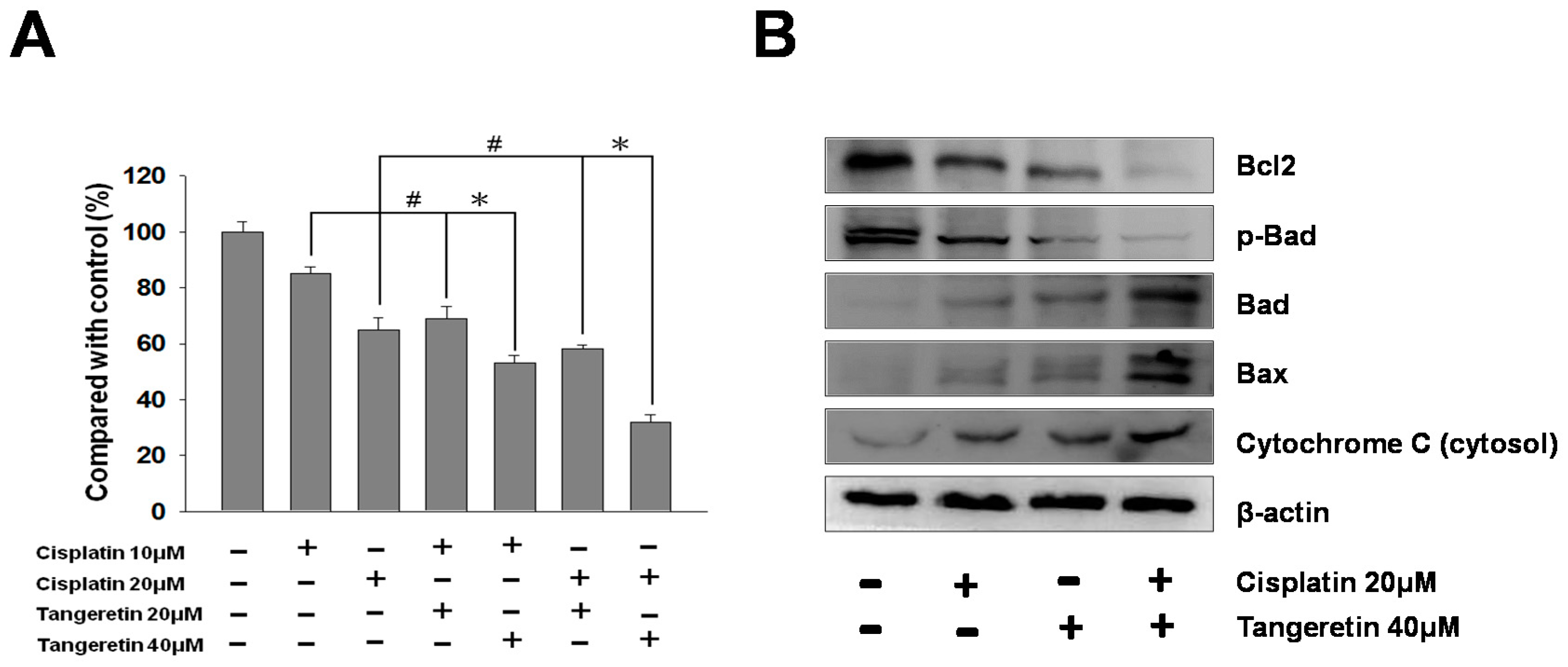
2.7. Cisplatin and Tangeretin Synergistically Enhanced the Cell Cytotoxicity of BFTC-905 Cells
3. Discussion
3.1. Tangeretin Induces Apoptosis in BFTC-905 Cells
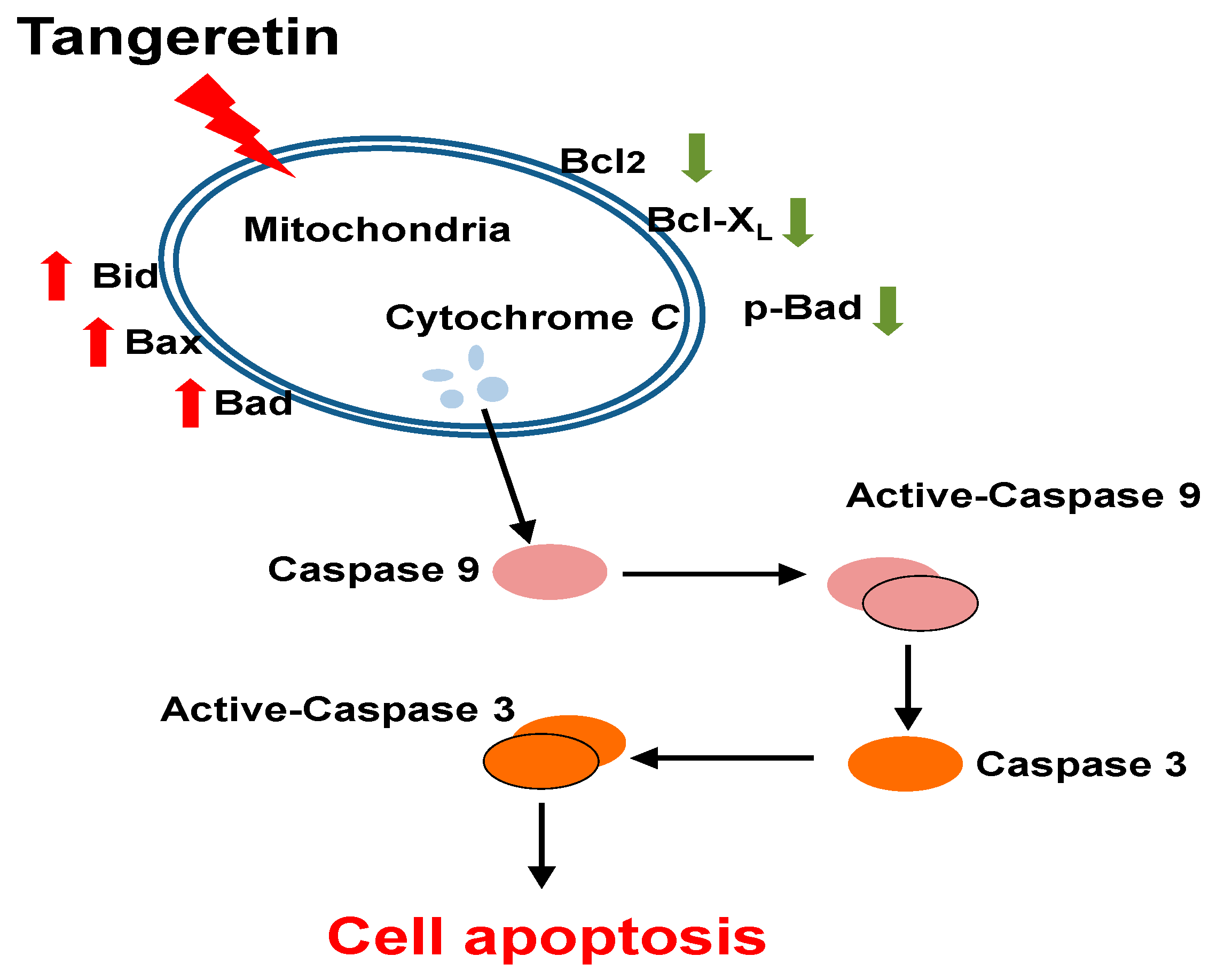
3.2. Tangeretin Induced Mitochondrial Dysfunction, Leading to Apoptosis in BFTC-905 Cells
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Tangeretin Treatment
4.3. Cell MTT Assay
4.4. Quantitative Detection of Apoptosis by Flow Cytometry
4.5. Wound-Healing and Transwell Migration Assays
4.6. DAPI and TUNEL Stain
4.7. Protein Preparation and Measurement
4.8. Two-Dimensional Gel Electrophoresis and Protein Identification by Liquid Chromatography–Tandem Mass Spectrometry
4.9. Western Blotting
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gotoh, A.; Nagaya, H.; Kanno, T.; Nishizaki, T. Antitumor action of alpha(1)-adrenoceptor blockers on human bladder, prostate and renal cancer cells. Pharmacology 2012, 90, 242–246. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.J. Recent advances in treatment of advanced urothelial carcinoma. Curr. Urol. Rep. 2012, 13, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Azemar, M.D.; Audouin, M.; Revaux, A.; Misrai, V.; Comperat, E.; Bitker, M.O.; Chartier-Kastler, E.; Richard, F.; Cussenot, O.; Roupret, M. Primary upper urinary tract tumors and subsequent location in the bladder. Progres en urologie: Journal de l’Association francaise d’urologie et de la Societe francaise d’urologie 2009, 19, 583–588. [Google Scholar] [CrossRef]
- Dzombeta, T.; Krajacic-Jagarcec, G.; Tomas, D.; Kraus, O.; Ruzic, B.; Kruslin, B. Urothelial carcinoma with an inverted growth pattern: A report of 4 cases. Acta medica Croatica: Casopis Hravatske akademije medicinskih znanosti 2010, 64, 47–50. [Google Scholar]
- Silverman, D.T.; Hartge, P.; Morrison, A.S.; Devesa, S.S. Epidemiology of bladder cancer. Hematol. Oncol. Clin. N. Am. 1992, 6, 1–30. [Google Scholar] [CrossRef]
- Kanitz, M.H.; Swaminathan, S.; Savage, R.E., Jr. Two-dimensional electrophoretic protein profile analysis following exposure of human uroepithelial cells to occupational bladder carcinogens. Cancer Lett. 2004, 205, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Malats, N.; Real, F.X. Epidemiology of bladder cancer. Hematol. Oncol. Clin. N. Am. 2015, 29, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, H.; Shimbo, T.; Yoshida, K.; Yoshikawa, N.; Uesugi, Y.; Yamamoto, K.; Azuma, H.; Narumi, Y. Treatment Results of Radiotherapy Combined with Balloon-occluded Arterial Infusion Chemotherapy for Invasive Bladder Cancer. Anticancer Res. 2016, 36, 731–736. [Google Scholar] [PubMed]
- McHugh, L.A.; Kriajevska, M.; Mellon, J.K.; Griffiths, T.R. Combined treatment of bladder cancer cell lines with lapatinib and varying chemotherapy regimens—Evidence of schedule-dependent synergy. Urology 2007, 69, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Barreca, D.; Bellocco, E.; Leuzzi, U.; Gattuso, G. First evidence of C- and O-glycosyl flavone in blood orange (Citrus sinensis (L.) Osbeck) juice and their influence on antioxidant properties. Food Chem. 2014, 149, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Cameron, R.G.; Savary, B.J.; Hotchkiss, A.T.; Fishman, M.L. Isolation, characterization, and pectin-modifying properties of a thermally tolerant pectin methylesterase from Citrus sinensis var. Valencia. J. Agric. Food Chem. 2005, 53, 2255–2260. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T.; Abe, K.; Gotoh, M.; Oka, K. Citrus flavone tangeretin inhibits leukaemic HL-60 cell growth partially through induction of apoptosis with less cytotoxicity on normal lymphocytes. Br. J. Cancer 1995, 72, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, A.; Subramanian, S.P. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7,12-dimethylbenz[a]anthracene. Toxicol. Lett. 2014, 229, 333–348. [Google Scholar] [CrossRef] [PubMed]
- Arafa el, S.A.; Zhu, Q.; Barakat, B.M.; Wani, G.; Zhao, Q.; El-Mahdy, M.A.; Wani, A.A. Tangeretin sensitizes cisplatin-resistant human ovarian cancer cells through downregulation of phosphoinositide 3-kinase/Akt signaling pathway. Cancer Res. 2009, 69, 8910–8917. [Google Scholar] [CrossRef] [PubMed]
- Chong, S.Y.; Wu, M.Y.; Lo, Y.C. Tangeretin sensitizes SGS1-deficient cells by inducing DNA damage. J. Agric. Food Chem. 2013, 61, 6376–6382. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Cao, A.; Shi, J.; Yin, P.; Wang, L.; Ji, G.; Xie, J.; Wu, D. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells through extrinsic and intrinsic signaling pathways. Oncol. Rep. 2014, 31, 1788–1794. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.R.; Li, S.; Ho, C.T.; Chang, Y.H.; Tan, K.T.; Chung, T.W.; Wang, B.Y.; Chen, Y.K.; Lin, C.C. Tangeretin derivative, 5-acetyloxy-6,7,8,4’-tetramethoxyflavone induces G2/M arrest, apoptosis and autophagy in human non-small cell lung cancer cells in vitro and in vivo. Cancer Biol. Ther. 2016, 17, 48–64. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.L.; Wang, D.W.; Yu, X.D.; Zhou, Y.L. Tangeretin induces cell cycle arrest and apoptosis through upregulation of PTEN expression in glioma cells. Biomed. Pharmacother. 2016, 81, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.B.; Xiao, N.; Liu, X.J. Dietary flavonoid tangeretin induces reprogramming of epithelial to mesenchymal transition in prostate cancer cells by targeting the PI3K/Akt/mTOR signaling pathway. Oncol. Lett. 2018, 15, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Pan, M.H.; Chen, W.J.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.K. Tangeretin induces cell-cycle G1 arrest through inhibiting cyclin-dependent kinases 2 and 4 activities as well as elevating Cdk inhibitors p21 and p27 in human colorectal carcinoma cells. Carcinogenesis 2002, 23, 1677–1684. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.W.; Lou, S.N.; Chiu, E.M.; Ho, C.T. Antioxidant activity and effective compounds of immature calamondin peel. Food Chem. 2013, 136, 1130–1135. [Google Scholar] [CrossRef] [PubMed]
- Ahadpour, M.; Eskandari, M.R.; Mashayekhi, V.; Haj Mohammad Ebrahim Tehrani, K.; Jafarian, I.; Naserzadeh, P.; Hosseini, M.J. Mitochondrial oxidative stress and dysfunction induced by isoniazid: Study on isolated rat liver and brain mitochondria. Drug Chem. Toxicol. 2016, 39, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.Y.; Wu, Y.J.; Chang, C.I.; Chiu, C.C.; Wu, M.L. The Effect of Bornyl cis-4-Hydroxycinnamate on Melanoma Cell Apoptosis Is Associated with Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2018, 19, 1370. [Google Scholar] [CrossRef] [PubMed]
- Mohan, S.; Abdelwahab, S.I.; Kamalidehghan, B.; Syam, S.; May, K.S.; Harmal, N.S.; Shafifiyaz, N.; Hadi, A.H.; Hashim, N.M.; Rahmani, M.; et al. Involvement of NF-kappaB and Bcl2/Bax signaling pathways in the apoptosis of MCF7 cells induced by a xanthone compound Pyranocycloartobiloxanthone A. Phytomedicine 2012, 19, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Biswas, R.; Rhee, Y.H.; Kim, J.; Ahn, J.C. Sulforaphene promotes Bax/Bcl2, MAPK-dependent human gastric cancer AGS cells apoptosis and inhibits migration via EGFR, p-ERK1/2 down-regulation. Gen. Physiol. Biophys. 2016, 35, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Goyal, S.; Tyagi, C.; Jamal, S.; Singh, A.; Grover, A. BIM (BCL-2 interacting mediator of cell death) SAHB (stabilized alpha helix of BCL2) not always convinces BAX (BCL-2-associated X protein) for apoptosis. J. Mol. Graph. Model. 2016, 67, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.A.; Hellman, B.E. Different roles of Fpg and Endo III on catechol-induced DNA damage in extended-term cultures of human lymphocytes and L5178Y mouse lymphoma cells. Toxicology In Vitro 2005, 19, 779–786. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.; Vikram, A.; Trivedi, P.P.; Jena, G.B. Alkaline, Endo III and FPG modified comet assay as biomarkers for the detection of oxidative DNA damage in rats with experimentally induced diabetes. Mutat. Res. 2011, 726, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Vuda, M.; Kamath, A. Drug induced mitochondrial dysfunction: Mechanisms and adverse clinical consequences. Mitochondrion 2016, 31, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Ricci, C.; Pastukh, V.; Leonard, J.; Turrens, J.; Wilson, G.; Schaffer, D.; Schaffer, S.W. Mitochondrial DNA damage triggers mitochondrial-superoxide generation and apoptosis. Am. J. Physiol. Cell Physiol. 2008, 294, C413–C422. [Google Scholar] [CrossRef] [PubMed]
- Cortelazzo, A.; Lampariello, R.L.; Sticozzi, C.; Guerranti, R.; Mirasole, C.; Zolla, L.; Sacchetti, G.; Hajek, J.; Valacchi, G. Proteomic profiling and post-translational modifications in human keratinocytes treated with Mucuna pruriens leaf extract. J. Ethnopharmacol. 2014, 151, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, P.; Yang, L.; Li, Y.; Tian, Y.; Li, S.; Bai, Y. Integrating 3-omics data analyze rat lung tissue of COPD states and medical intervention by delineation of molecular and pathway alterations. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Sun, H.; Zheng, C.; Gao, J.; Fu, Q.; Hu, N.; Shao, X.; Zhou, Y.; Xiong, J.; Nie, K.; et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018, 9, 161. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.J.; Wang, R.Y.; Chen, J.C.; Chiu, C.C.; Liao, M.H.; Wu, Y.J. Cytotoxicity of 11-epi-Sinulariolide Acetate Isolated from Cultured Soft Corals on HA22T Cells through the Endoplasmic Reticulum Stress Pathway and Mitochondrial Dysfunction. Int. J. Mol. Sci. 2016, 17, 1787. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Q.; Cao, Y.L.; Hao, F.; Yan, Z.R.; Wang, M.L.; Liu, X.W. Tangeretin alters neuronal apoptosis and ameliorates the severity of seizures in experimental epilepsy-induced rats by modulating apoptotic protein expressions, regulating matrix metalloproteinases, and activating the PI3K/Akt cell survival pathway. Adv. Med. Sci. 2017, 62, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Q.; Fang, D.C.; Wang, R.Q.; Yang, S.M. Effect of NF-kappaB, survivin, Bcl-2 and Caspase3 on apoptosis of gastric cancer cells induced by tumor necrosis factor related apoptosis inducing ligand. World J. Gastroenterol. 2004, 10, 22–25. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Su, J.H.; Lin, J.J.; Chen, C.C.; Hwang, W.I.; Huang, H.H.; Wu, Y.J. An investigation into the cytotoxic effects of 13-acetoxysarcocrassolide from the soft coral Sarcophyton crassocaule on bladder cancer cells. Mar. Drugs 2011, 9, 2622–2642. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Wu, J.G.; Jiang, Y.B.; Liu, Y.J.; Sun, T.; Wu, N.; Wu, C.J. Antitumor activity of 4-O-(2″-O-acetyl-6″-O-p-coumaroyl-beta-D-glucopyranosyl)-p-coumaric acid against lung cancers via mitochondrial-mediated apoptosis. Chem. Biol. Interact. 2015, 233, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Su, T.R.; Lin, J.J.; Chiu, C.C.; Chen, J.Y.; Su, J.H.; Cheng, Z.J.; Hwang, W.I.; Huang, H.H.; Wu, Y.J. Proteomic investigation of anti-tumor activities exerted by sinularin against A2058 melanoma cells. Electrophoresis 2012, 33, 1139–1152. [Google Scholar] [CrossRef] [PubMed]
- Su, C.C.; Chen, J.Y.; Din, Z.H.; Su, J.H.; Yang, Z.Y.; Chen, Y.J.; Wang, R.Y.; Wu, Y.J. 13-acetoxysarcocrassolide induces apoptosis on human gastric carcinoma cells through mitochondria-related apoptotic pathways: p38/JNK activation and PI3K/AKT suppression. Mar. Drugs 2014, 12, 5295–5315. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, A.; Sultana, R.; Forster, S.; Perluigi, M.; Cenini, G.; Cini, C.; Cai, J.; Klein, J.B.; Farr, S.A.; Niehoff, M.L.; et al. Antisense directed against PS-1 gene decreases brain oxidative markers in aged senescence accelerated mice (SAMP8) and reverses learning and memory impairment: A proteomics study. Free Radic. Biol. Med. 2013, 65, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Lin, J.J.; Yang, Z.Y.; Tsai, C.C.; Hsu, J.L.; Wu, Y.J. Proteomic study reveals a co-occurrence of gallic acid-induced apoptosis and glycolysis in B16F10 melanoma cells. J. Agric. Food Chem. 2014, 62, 11672–11680. [Google Scholar] [CrossRef] [PubMed]
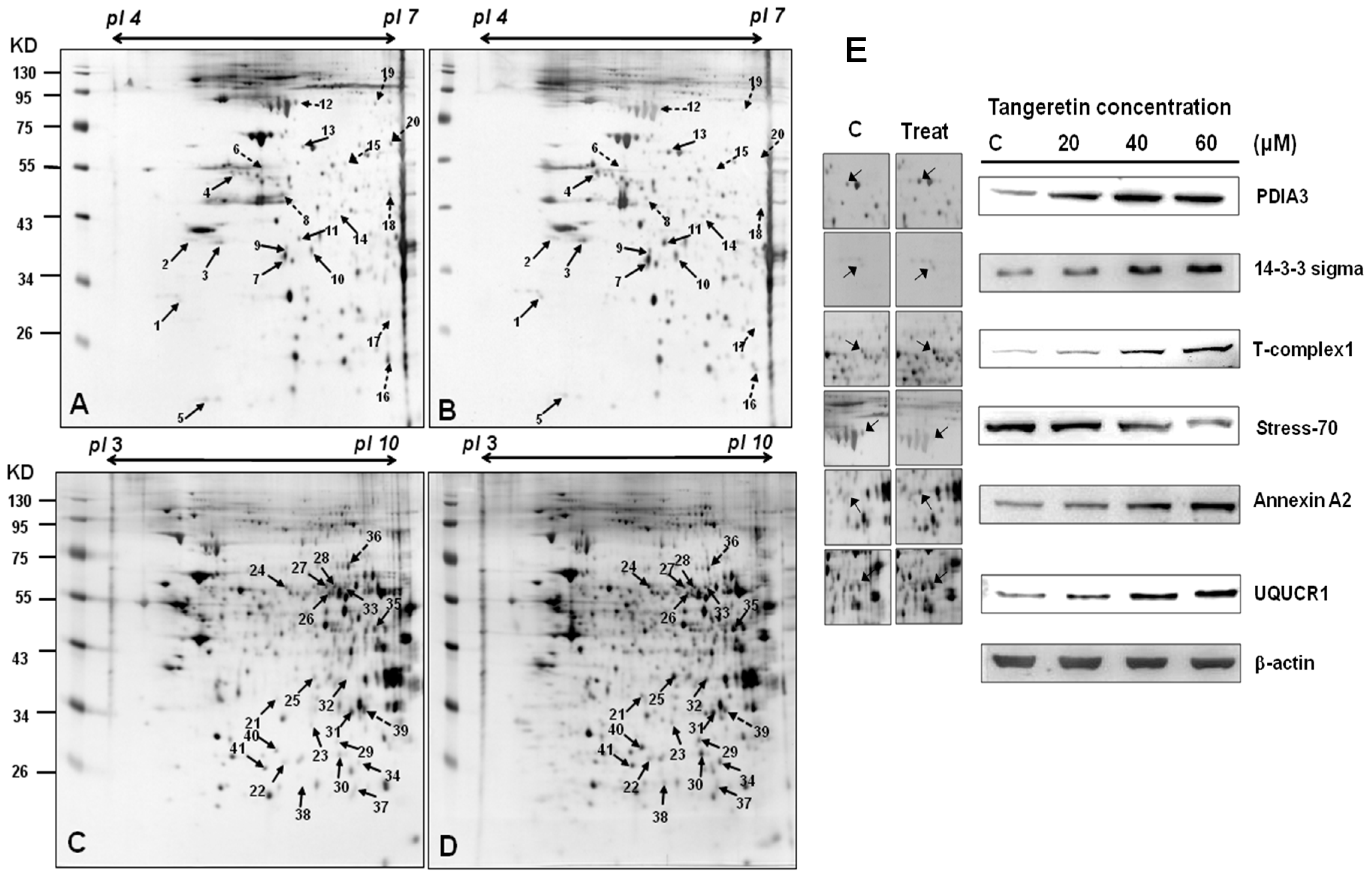
| Spot No. | Protein Name | Accession.no | Calculated Mw/pI | Peptide Matched | Sequence Covered % | MASCOT Score | Regulation (Fold- Change) * |
|---|---|---|---|---|---|---|---|
| 1 | 14-3-3 protein sigma | P31947 | 27.75/4.68 | 14 | 45 | 158 | +2.9 |
| 2 | Nucleophosmin (NPM) | P06748 | 32.55/4.64 | 7 | 25 | 87 | +3.9 |
| 3 | Heterogeneous nuclear ribonucleoproteins C1/C2 | P07910 | 33.65/4.95 | 25 | 27 | 301 | +3.9 |
| 4 | Protein disulfide-isomerase A6 precursor | Q15084 | 48.09/4.95 | 2 | 5 | 77 | +4.8 |
| 5 | ATP synthase D chain, mitochondrial | O75947 | 18.47/5.21 | 5 | 19 | 84 | +2.1 |
| 6 | Thymidine phosphorylase precursor | P19971 | 49.92/5.36 | 14 | 31 | 108 | −6.8 |
| 7 | Guanine nucleotide-binding protein subunit beta 4 | Q9HAV0 | 37.54/5.6 | 3 | 6 | 66 | +2.5 |
| 8 | Creatine kinase B-type | P12277 | 42.61/5.34 | 12 | 14 | 60 | −5.9 |
| 9 | Eukaryotic translation initiation factor 3 subunit 2 | Q13347 | 36.47/5.38 | 17 | 39 | 167 | +2.3 |
| 10 | L-lactate dehydrogenase B chain | P07195 | 36.61/5.71 | 19 | 30 | 84 | +2.2 |
| 11 | 60S acidic ribosomal protein P0 (L10E) | P05388 | 34.25/5.71 | 7 | 26 | 52 | +4.2 |
| 12 | Stress-70 protein, mitochondrial precursor | P38646 | 73.63/5.87 | 11 | 17 | 158 | −2.8 |
| 13 | Protein disulfide-isomerase A3 precursor | P30101 | 56.74/5.98 | 32 | 41 | 270 | +2.9 |
| 14 | DnaJ homolog subfamily B member 11 precursor | Q9UBS4 | 40.48/5.81 | 13 | 26 | 150 | +2.2 |
| 15 | Heterogeneous nuclear ribonucleoprotein H | P31943 | 49.19/5.89 | 15 | 11 | 136 | −2.6 |
| 16 | Dermcidin precursor (Preproteolysin) | P81605 | 11.27/6.08 | 10 | 1 | 43 | −2.6 |
| 17 | Triosephosphate isomerase | P60174 | 26.65/6.45 | 23 | 11 | 82 | +2.1 |
| 18 | Serpin B3 | P29508 | 44.53/6.35 | 37 | 13 | 176 | −3.0 |
| 19 | Serum albumin precursor | P02768 | 69.31/5.92 | 25 | 21 | 289 | −2.5 |
| 20 | D-3-phosphoglycerate dehydrogenase | O43175 | 56.61/6.29 | 22 | 14 | 234 | −3.1 |
| 21 | 26S proteasome non-ATPase regulatory subunit 14 | O00487 | 34.55/6.06 | 3 | 8 | 57 | +2.7 |
| 22 | Peroxiredoxin-6 | P30041 | 25.01/6.0 | 10 | 34 | 70 | +2.1 |
| 23 | Purine nucleoside phosphorylase | P00491 | 32.09/6.45 | 1 | 5 | 89 | +2.4 |
| 24 | T-complex protein 1 subunit beta | P78371 | 57.45/6.01 | 8 | 15 | 64 | +2.7 |
| 25 | Annexin A1 | P04083 | 38.69/6.57 | 23 | 54 | 392 | +3.4 |
| 26 | Dihydrolipoyl dehydrogenase | P09622 | 54.11/7.59 | 8 | 13 | 144 | +1.8 |
| 27 | Inosine-5’-monophosphate dehydrogenase 2 | P12268 | 55.77/6.44 | 6 | 9 | 135 | +1.7 |
| 28 | Adenylyl cyclase-associated protein 1 | Q01518 | 51.82/8.27 | 13 | 20 | 140 | +1.8 |
| 29 | Phosphoglycerate mutase 1 | P18669 | 28.78/6.67 | 11 | 39 | 89 | +2.5 |
| 30 | Triosephosphate isomerase | P60174 | 26.65/6.45 | 34 | 62 | 338 | +2.8 |
| 31 | Electron transfer flavoprotein subunit alpha | P13804 | 35.05/8.62 | 12 | 31 | 218 | +1.9 |
| 32 | Annexin A2 | P07355 | 38.58/7.57 | 9 | 25 | 133 | +2.3 |
| 33 | Adenylyl cyclase-associated protein 1 | Q01518 | 51.82/8.27 | 13 | 13 | 112 | +2.1 |
| 34 | 3-hydroxyacyl-CoA dehydrogenase type-2 | Q99714 | 26.9/7.66 | 21 | 60 | 290 | +2.9 |
| 35 | Ubiquinol-cytochrome-c reductase complex core protein 2 | P22695 | 48.41/8.74 | 22 | 28 | 222 | +3.4 |
| 36 | Heterogeneous nuclear ribonucleoprotein L | P14866 | 60.14/6.65 | 21 | 23 | 202 | −1.8 |
| 37 | Pyruvate kinase isozymes M1/M2 | P14618 | 57.9/7.96 | 13 | 21 | 178 | +2.4 |
| 38 | Acyl-protein thioesterase 1 | O75608 | 24.65/6.29 | 7 | 20 | 75 | +2.6 |
| 39 | Guanine nucleotide-binding protein subunit beta 2-like 1 | P63244 | 35.05/7.6 | 8 | 15 | 89 | −2.2 |
| 40 | Endoplasmic reticulum protein ERp29 precursor | P30040 | 28.97/6.77 | 21 | 46 | 264 | +4.1 |
| 41 | Enoyl-CoA hydratase | P30084 | 31.36/8.34 | 19 | 32 | 302 | +3.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.-J.; Huang, C.-C.; Su, Y.-L.; Luo, H.-L.; Lee, N.-L.; Sung, M.-T.; Wu, Y.-J. Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells. Int. J. Mol. Sci. 2019, 20, 1017. https://doi.org/10.3390/ijms20051017
Lin J-J, Huang C-C, Su Y-L, Luo H-L, Lee N-L, Sung M-T, Wu Y-J. Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells. International Journal of Molecular Sciences. 2019; 20(5):1017. https://doi.org/10.3390/ijms20051017
Chicago/Turabian StyleLin, Jen-Jie, Chun-Chieh Huang, Yu-Li Su, Hao-Lun Luo, Nai-Lun Lee, Ming-Tse Sung, and Yu-Jen Wu. 2019. "Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells" International Journal of Molecular Sciences 20, no. 5: 1017. https://doi.org/10.3390/ijms20051017
APA StyleLin, J.-J., Huang, C.-C., Su, Y.-L., Luo, H.-L., Lee, N.-L., Sung, M.-T., & Wu, Y.-J. (2019). Proteomics Analysis of Tangeretin-Induced Apoptosis through Mitochondrial Dysfunction in Bladder Cancer Cells. International Journal of Molecular Sciences, 20(5), 1017. https://doi.org/10.3390/ijms20051017





