Histological and Cytological Characterization of Anther and Appendage Development in Asian Lotus (Nelumbo nucifera Gaertn.)
Abstract
1. Introduction
2. Results
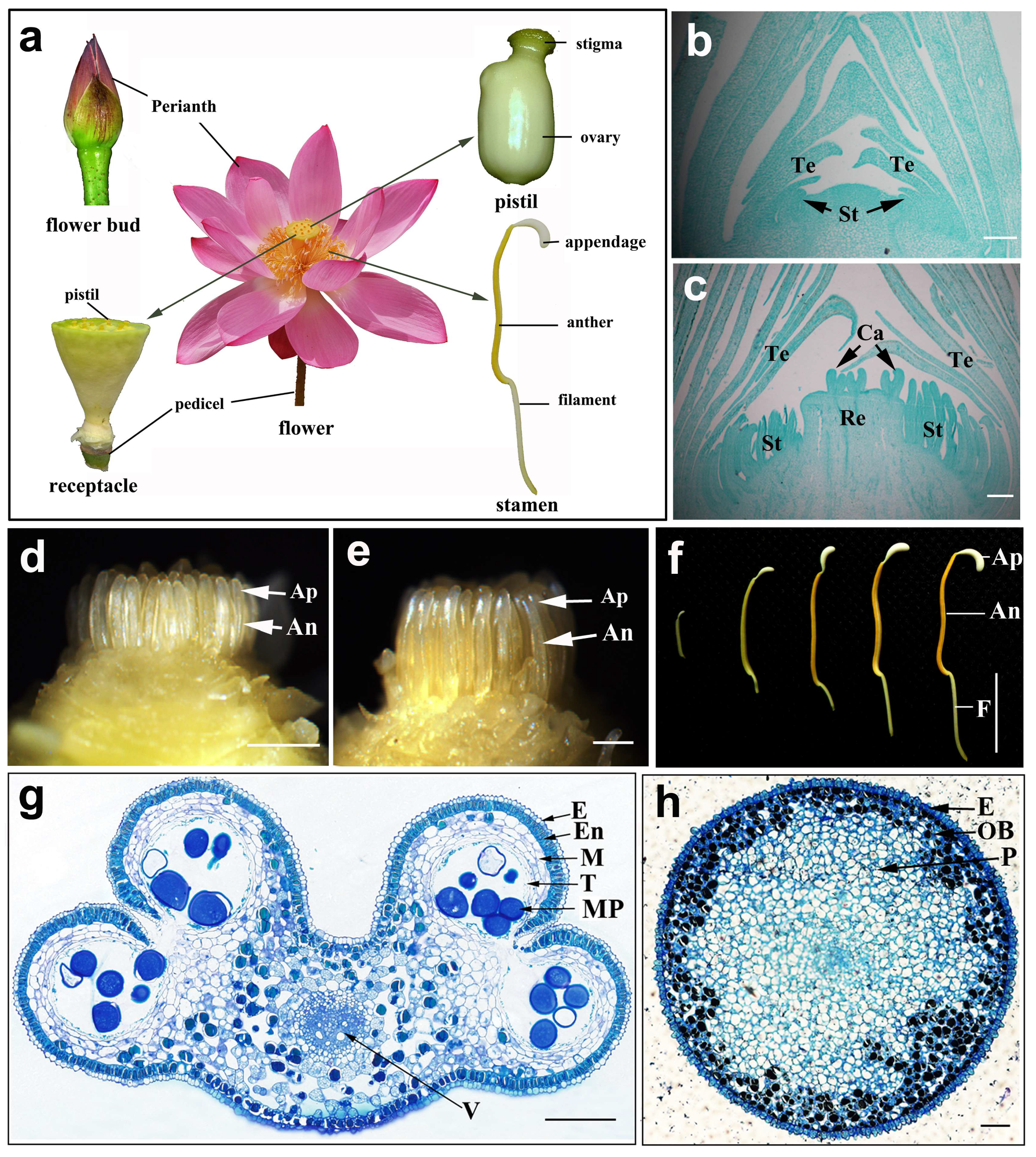
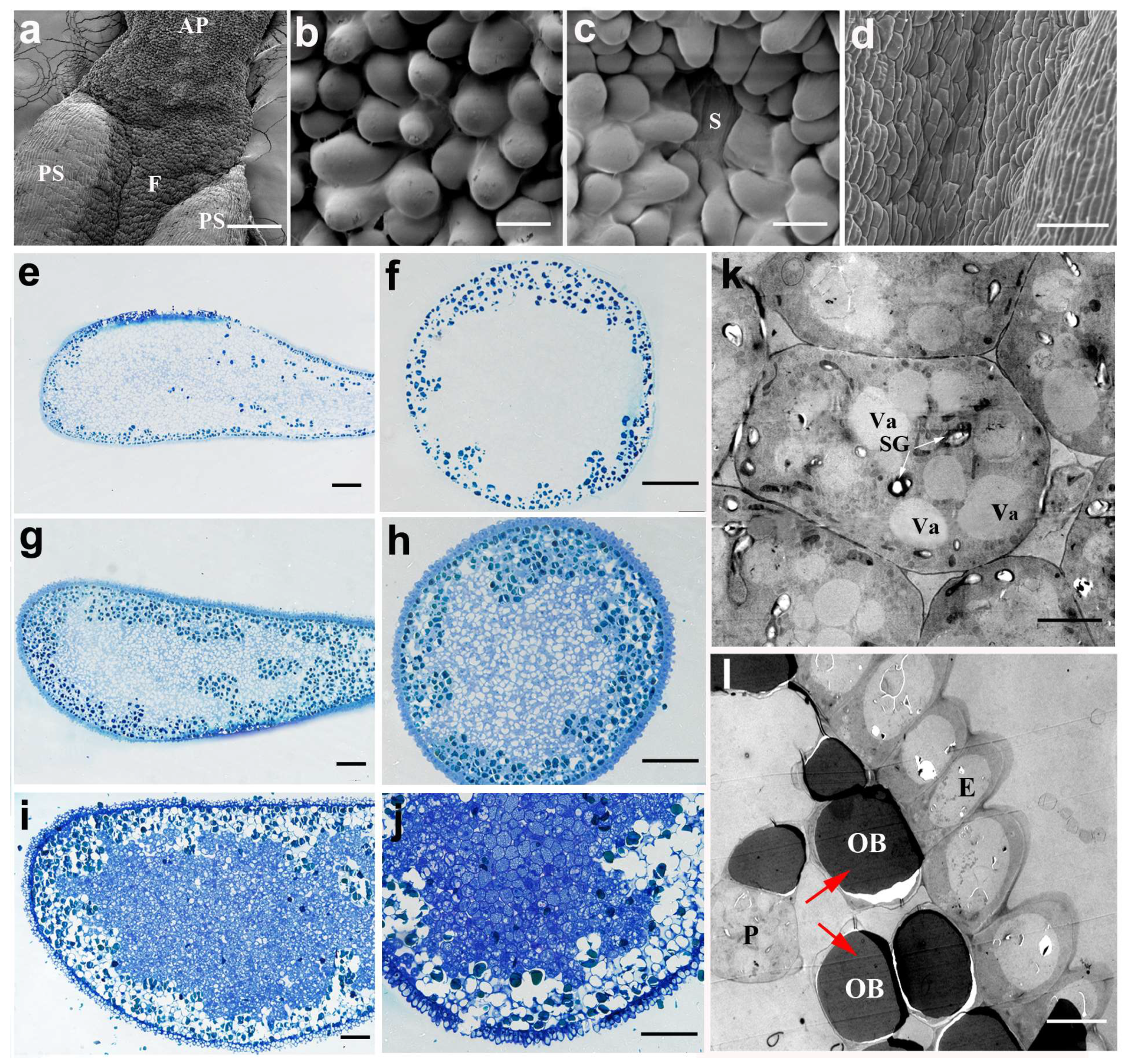
2.1. The Morphologic Characteristics of the Anther and Its Appendage in N. nucifera
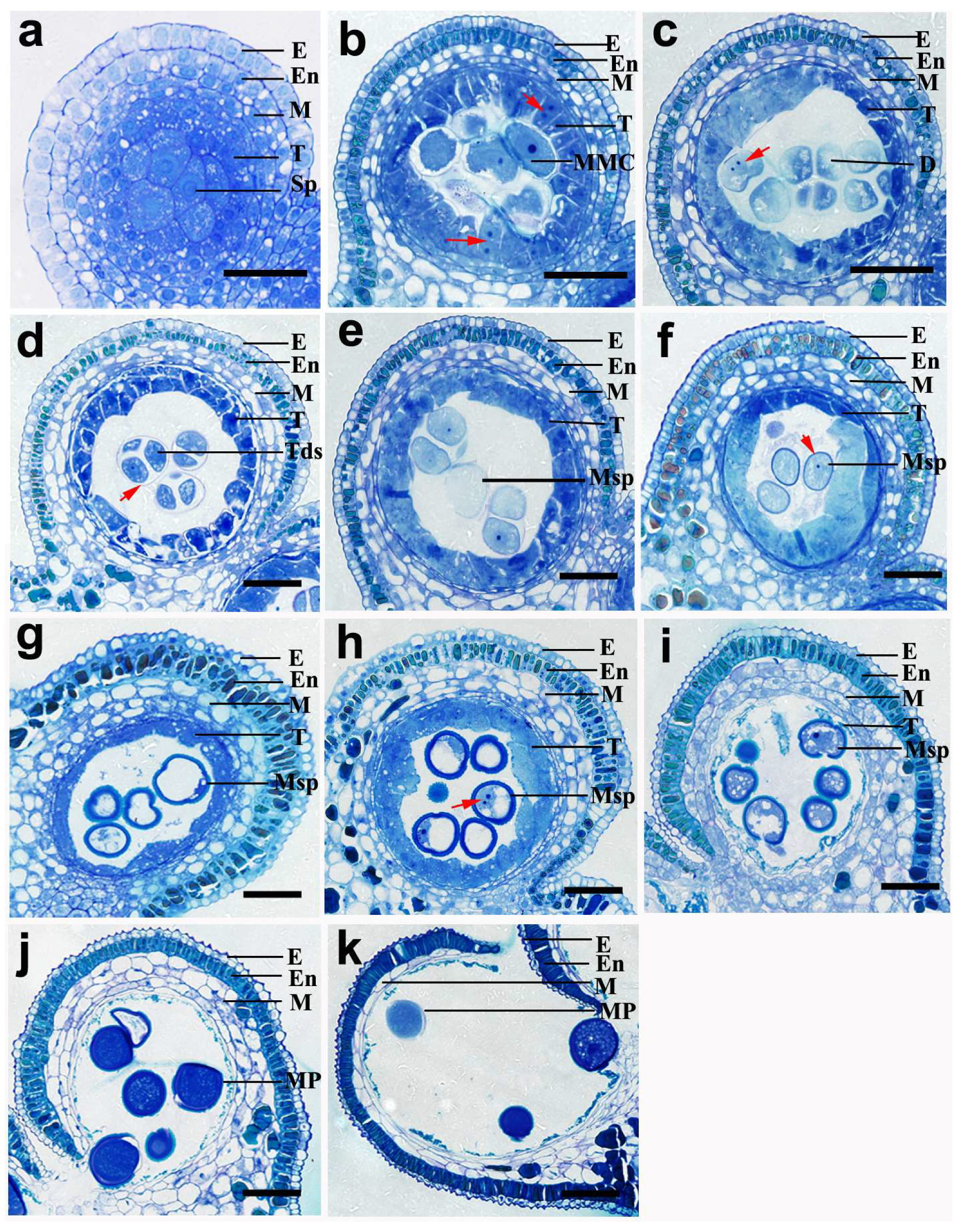
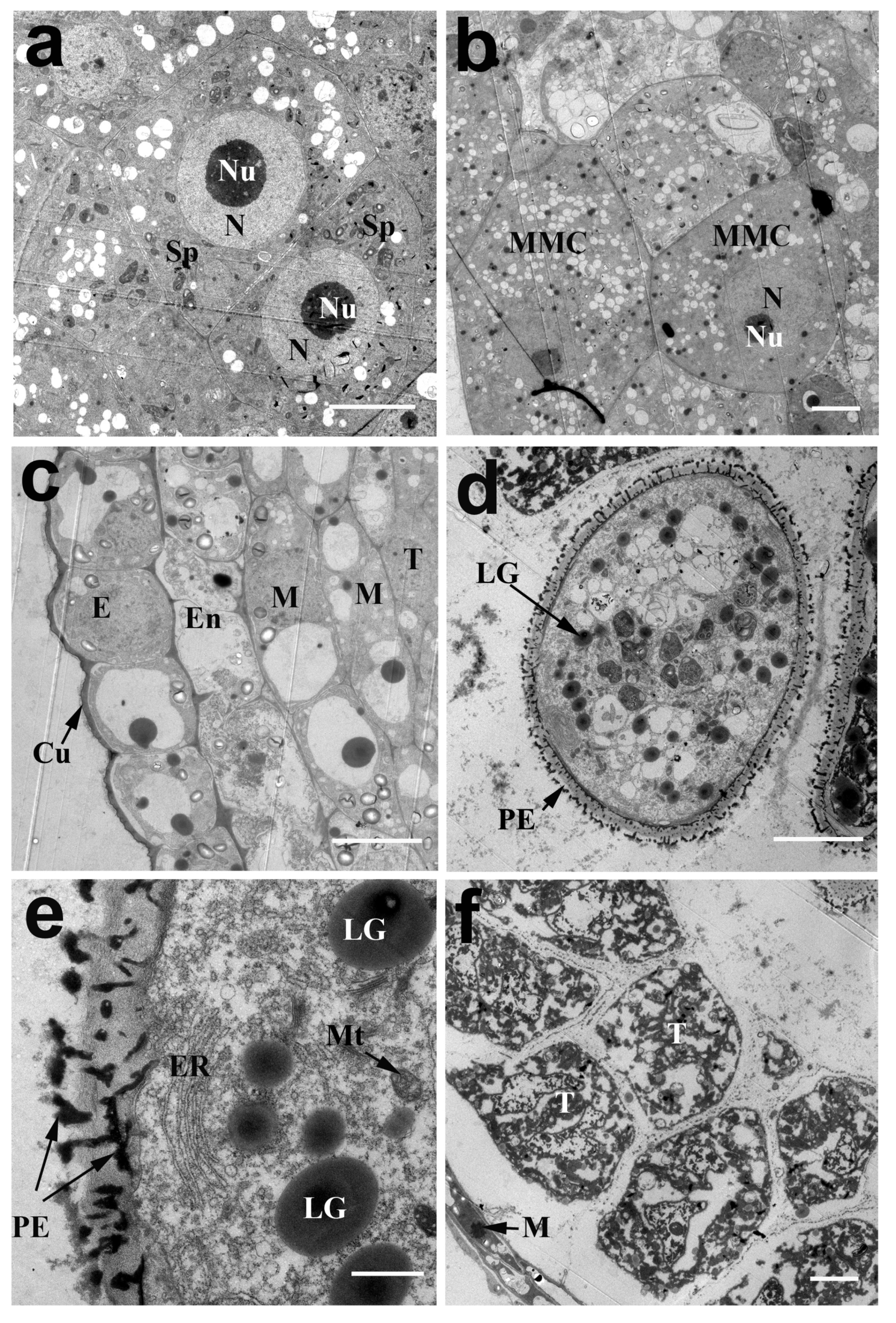
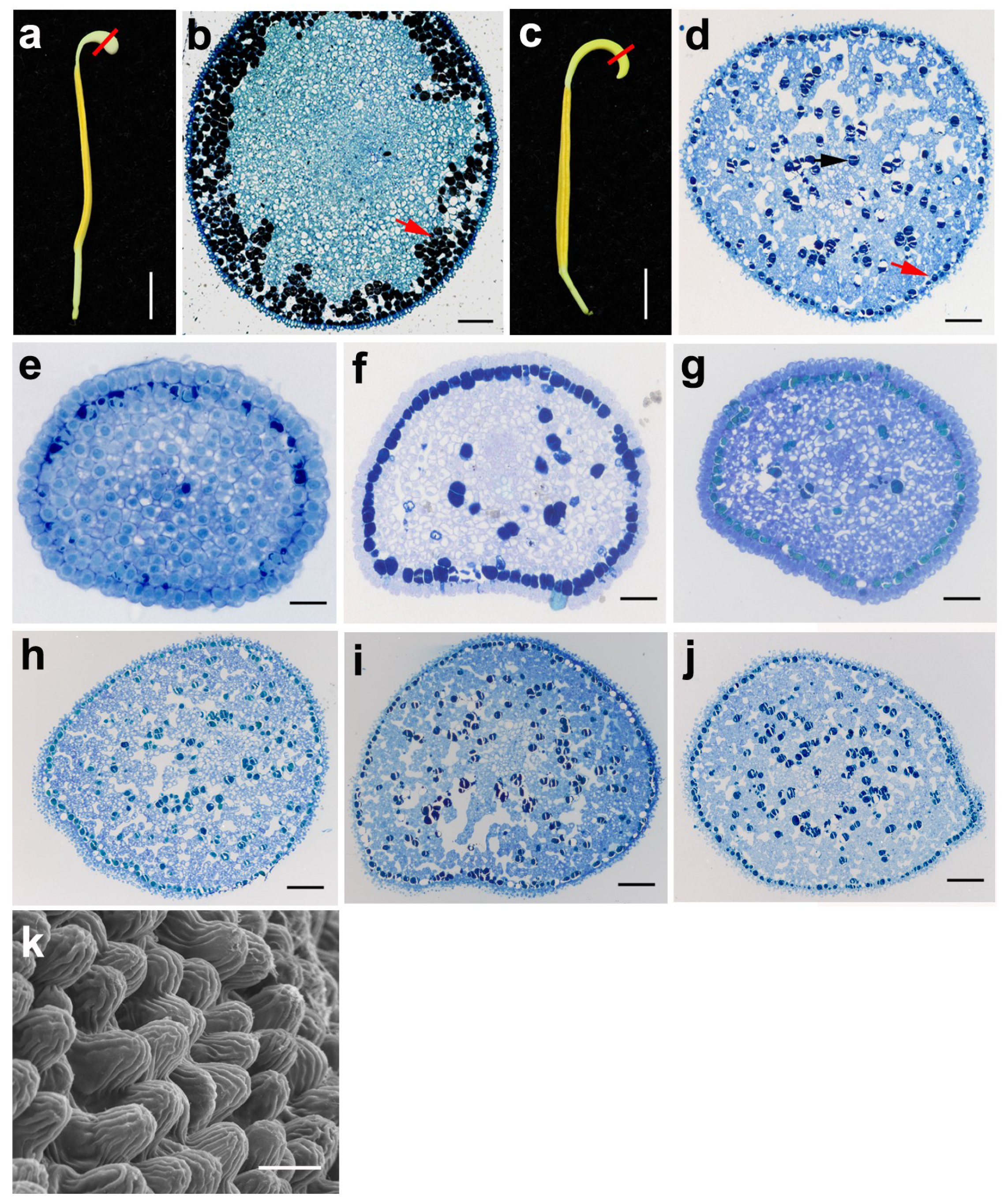
2.2. The Development of Pollen in N. nucifera
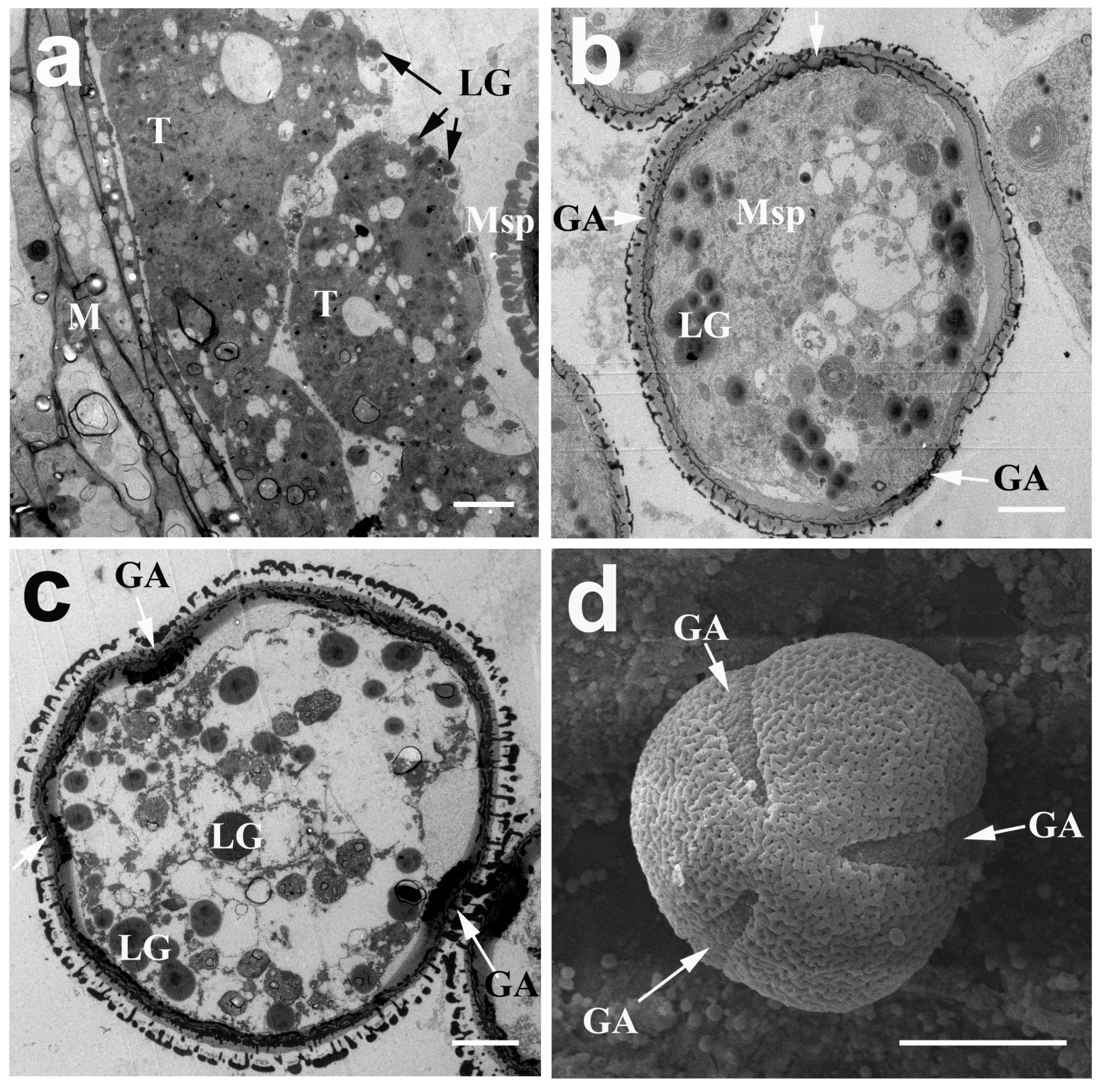
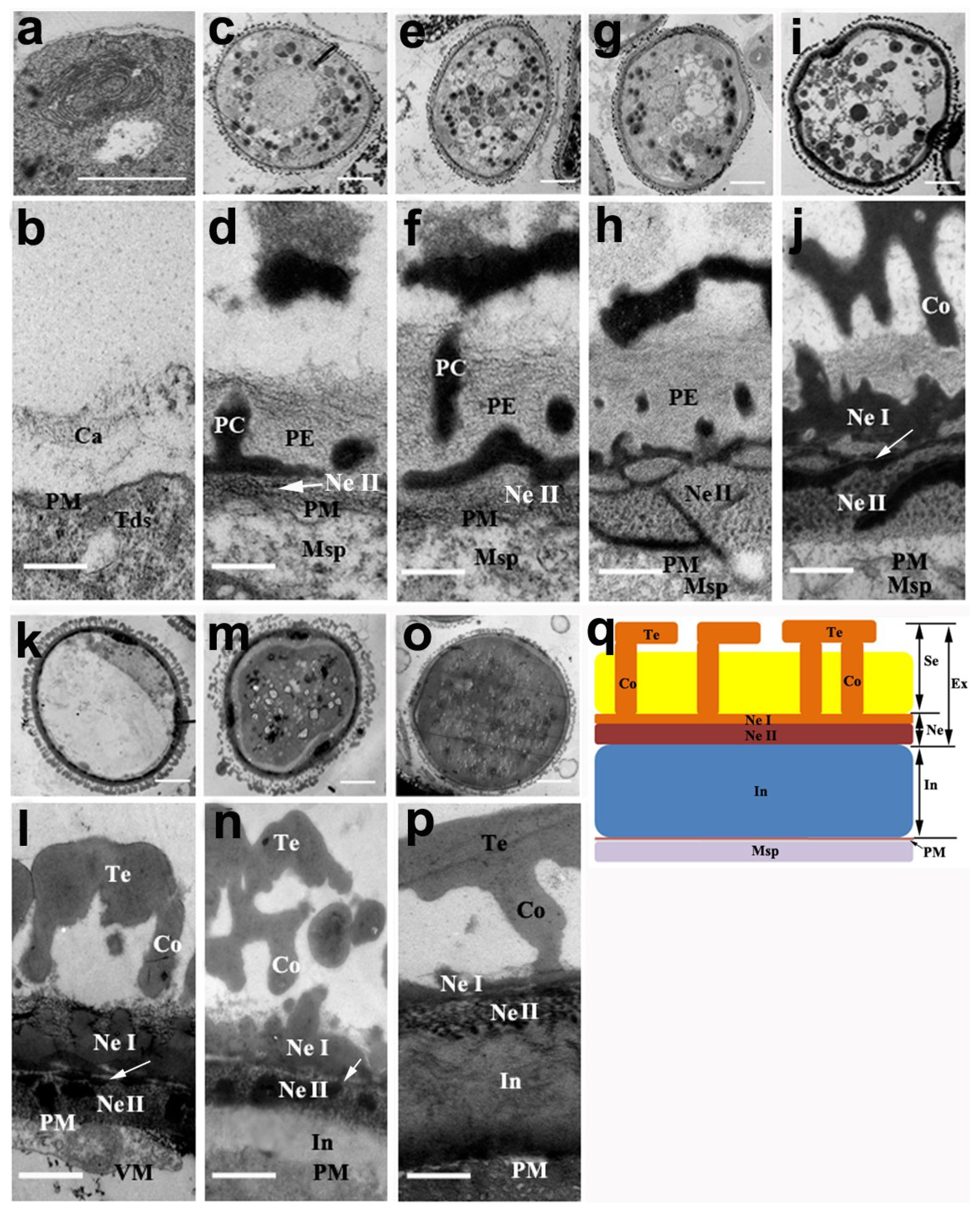
2.3. The Development of the Pollen Wall in N. nucifera
2.4. The Development and Structure of the Anther Appendage in the Lotus
3. Discussion
3.1. The Characteristics of Anther Wall Morphogenesis in N. nucifera
3.2. The Characteristics of Pollen and Pollen Wall Morphogenesis in N. nucifera
3.3. The Developmental Differences in the Anther and Appendage between N. nucifera and N. lutea
4. Materials and Methods
4.1. Preparation of Plant Material
4.2. Semi-thin Section for Morphological Observation
4.3. Section Observation by Scanning Electron Microscopy
4.4. Section Observation by Transmission Electron Microscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, M.Z.; Liu, T.; Guo, M.Q. Current advances in the metabolomics study on lotus seeds. Front. Plant Sci. 2016, 7, 891. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.R.; Gautam, L.N.S.; Adhikari, D.; Karki, R. A Comprehensive review on chemical profiling of Nelumbo nucifera: Potential for drug development. Phytother. Res. 2016. [Google Scholar] [CrossRef]
- Guo, H.B. Cultivation of lotus (Nelumbo nucifera Gaertn.ssp. nucifera) and its utilization in China. Genet. Resour. Crop Evol. 2009, 56, 323–330. [Google Scholar] [CrossRef]
- Wang, Q.C.; Zhang, X.Y. Illustrations of Lotus Cultivars in China; China Forestry Press: Beijing, China, 2004; pp. 2–7. [Google Scholar]
- Shen-Miller, J.; Schopf, J.W.; Harbottle, G.; Cao, R.J.; Ouyang, S.; Zhou, K.S.; Southon, J.R.; Liu, G.H. Long-living lotus: Germination and soil γ-irradiation of centuries-old fruits, and cultivation, growth, and phenotypic abnormalities of offspring. Am. J. Bot. 2002, 89, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Shen-Miller, J. Sacred lotus, the long-living fruits of China antique. Seed Sci. Res. 2002, 12, 131–143. [Google Scholar] [CrossRef]
- Tian, D.K.; Mo, H.B.; Zhang, W.W.; Huang, X.; Li, C.; Xu, Y.Y. Progress on international lotus registration and construction of international Nelumbo database. VI international symposium on the taxonomy of cultivated plants. ISHS Acta Hortic. 2013, 1035. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mukherjee, D.; Maji, A.K.; Rai, S.; Heinrich, M. The sacred lotus (Nelumbo nucifera)-phytochemical and therapeutic profile. J. Pharm. Pharmacol. 2009, 61, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Kreunen, S.S.; Osborn, J.M. Pollen and anther development in Nelumbo (Nelumbonaceae). Am. J. Bot. 1999, 86, 1662–1676. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Q.; Chen, J.Y.; Huang, G.Z. Preliminary studies on biosytematic relationship between the two Nelumbo species. Acta Hortic. Sin. 1992, 19, 164–170. [Google Scholar]
- Quilichini, T.D.; Douglas, C.J.; Samuels, A.L. New views of tapetum ultrastructure and pollen exine development in Arabidopsis thaliana. Ann. Bot. 2014, 114, 1189–1201. [Google Scholar] [CrossRef] [PubMed]
- Soltis, D.E.; Soltis, P.S.; Chase, M.W.; Mort, M.E.; Albach, D.C.; Zanis, M.; Savolainen, V.; Hahn, W.H.; Hoot, S.B.; Fay, M.F.; et al. Angiosperm phylogeny inferred from 18S rDNA, rbcL, and atpB sequences. Bot. J. Linn. Soc. 2000, 133, 381–461. [Google Scholar] [CrossRef]
- Aya, K.; Ueguchi-Tanaka, M.; Kondo, M.; Hamada, K.; Yano, K.; Nishimura, M.; Matsuoka, M. Gibberellin modulates anther development in rice via the transcriptional regulation of GAMYB. Plant Cell 2009, 21, 1453–1472. [Google Scholar] [CrossRef] [PubMed]
- Cecchetti, V.; Celebrin, D.; Napoli, N.; Ghelli, R.; Brunetti, P.; Costantino, P.; Cardarelli, M. An auxin maximum in the middle layer controls stamen development and pollen maturation in Arabidopsis. New Phytol. 2017, 213, 1194–1207. [Google Scholar] [CrossRef] [PubMed]
- Falasca, G.; D’Angeli, S.; Biasi, R.; Fattorini, L.; Matteucci, M.; Canini, A.; Altamura, M.M. Tapetum and middle layer control male fertility in Actinidia deliciosa. Ann. Bot. 2013, 112, 1045–1055. [Google Scholar] [CrossRef] [PubMed]
- Ariizumi, T.; Toriyama, K. Genetic regulation of sporopollenin synthesis and pollen exine development. Annu. Rev. Plant Biol. 2011, 62, 437–460. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, A.S.; Penneys, D.S.; Staedler, Y.M.; Fragner, L.; Weckwerth, W.; Schonenberger, J. A specialized bird pollination system with a bellows mechanism for pollen transfer and staminal food body rewards. Curr. Biol. 2014, 24, 1615–1619. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.L.; Li, Q.J. Tail-like anther crest aids pollination by manipulating pollinator’s behaviour in a wild ginger. Sci. Rep. 2016, 6, 22340. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Dai, C.; Yang, C.F.; Wang, Q.F.; Motley, T.J. Anther appendages of Incarvillea trigger a pollen-dispensing mechanism. Ann. Bot. 2008, 102, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Hermann, P.M.; Palser, B.F. Stamen development in the Ericaceae. I. Anther wall, microsporogenesis, inversion, and appendages. Am. J. Bot. 2000, 87, 934–957. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.J.; Kress, W.J.; Manos, P.S. The phylogeny, evolution, and classification of the genus Globba and tribe Globbeae (Zingiberaceae): Appendages do matter. Am. J. Bot. 2004, 91, 100–114. [Google Scholar] [CrossRef] [PubMed]
- Kuta, E.; Bohdanowicz, J.; Słomka, A.; Pilarska, M.; Bothe, H. Floral structure and pollen morphology of two zinc violets (Viola lutea ssp. calaminaria and V. lutea ssp. westfalica) indicate their taxonomic affinity to Viola lutea. Plant Syst. Evol. 2012, 298, 445–455. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Liang, W.Q.; Shi, J.X.; Xu, J.; Zhang, D.B. MYB56 encoding a R2R3 MYB transcription factor regulates seed size in Arabidopsis thaliana. J. Integr. Plant Biol. 2013, 55, 1166–1178. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, D.S.; Liu, H.S.; Yin, C.S.; Li, X.X.; Liang, W.Q.; Yuan, Z.; Xu, B.; Chu, H.W.; Wang, J.; et al. The rice tapetum degeneration retardation gene is required for tapetum degradation and anther development. Plant Cell 2006, 18, 2999–3014. [Google Scholar] [CrossRef] [PubMed]
- Racich, J.L.; Koutsky, J.A. Nodular structure in epoxy resins. J. Appl. Polym. Sci. 1976, 20, 2111–2129. [Google Scholar] [CrossRef]
| Stage | Bud Length (cm) | Anther Length (mm) | Appendage Length (mm) | Pollen Development Stage | Significant Events |
|---|---|---|---|---|---|
| 1 | <1.5 | <1.5 | <0.5 | Sporogenous cell | Formation of four-layered anther wall and pre-meiosis DNA synthesis |
| 2 | 1.5–2.0 | <1.5 | 0.5 | MMC | Formation of microspore mother cells and tapetum layer. Mitosis of tapetal cells |
| 3 | 2.0–2.8 | 2.0–5.0 | <1.0 | Dyads | Meiosis I |
| 4 | 2.8–4.0 | 5.0–6.0 | 1.0–1.5 | Tetrad | Formation of four haploid spores |
| 5 | 4.0–5.0 | 6.0–11.0 | 1.5–3.0 | Early young microspore | Degradation of callose wall. Formation of uninucleated gametophyte and primexine |
| 6 | 5.0–6.0 | 11.0–15.0 | 3.0–4.5 | Middle young microspore | Formation of exine |
| 7 | 6.0–6.5 | 15.0–15.5 | 4.5–5.0 | Late young microspore | Formation of large central vacuoles |
| 8 | 6.5–7.0 | 15.5–16.0 | 5.0 | Early bicellular pollen | Formation of vegetative nucleus and a generative nucleus by pollen mitosis I |
| 9 | 7.0–9.0 | 16.0 | 5.0 | Late bicellular pollen | Formation of intine |
| 10 | 9.0–10.0 | 16.0–16.5 | 5.0 | Mature pollen | Accumulation of starch grains |
| 11 | 10.0 (fully open) | 16.6 | 5.0 | Anther dehiscence | Anther dehiscence and pollen released |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Chen, Q.; Liu, Q.; Liu, F.; Cui, L.; Shao, W.; Wu, S.; Xu, J.; Tian, D. Histological and Cytological Characterization of Anther and Appendage Development in Asian Lotus (Nelumbo nucifera Gaertn.). Int. J. Mol. Sci. 2019, 20, 1015. https://doi.org/10.3390/ijms20051015
Zhang D, Chen Q, Liu Q, Liu F, Cui L, Shao W, Wu S, Xu J, Tian D. Histological and Cytological Characterization of Anther and Appendage Development in Asian Lotus (Nelumbo nucifera Gaertn.). International Journal of Molecular Sciences. 2019; 20(5):1015. https://doi.org/10.3390/ijms20051015
Chicago/Turabian StyleZhang, Dasheng, Qing Chen, Qingqing Liu, Fengluan Liu, Lijie Cui, Wen Shao, Shaohua Wu, Jie Xu, and Daike Tian. 2019. "Histological and Cytological Characterization of Anther and Appendage Development in Asian Lotus (Nelumbo nucifera Gaertn.)" International Journal of Molecular Sciences 20, no. 5: 1015. https://doi.org/10.3390/ijms20051015
APA StyleZhang, D., Chen, Q., Liu, Q., Liu, F., Cui, L., Shao, W., Wu, S., Xu, J., & Tian, D. (2019). Histological and Cytological Characterization of Anther and Appendage Development in Asian Lotus (Nelumbo nucifera Gaertn.). International Journal of Molecular Sciences, 20(5), 1015. https://doi.org/10.3390/ijms20051015






