Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism
Abstract
1. Introduction
2. Uptake and Translocation of AgNPs in Plants
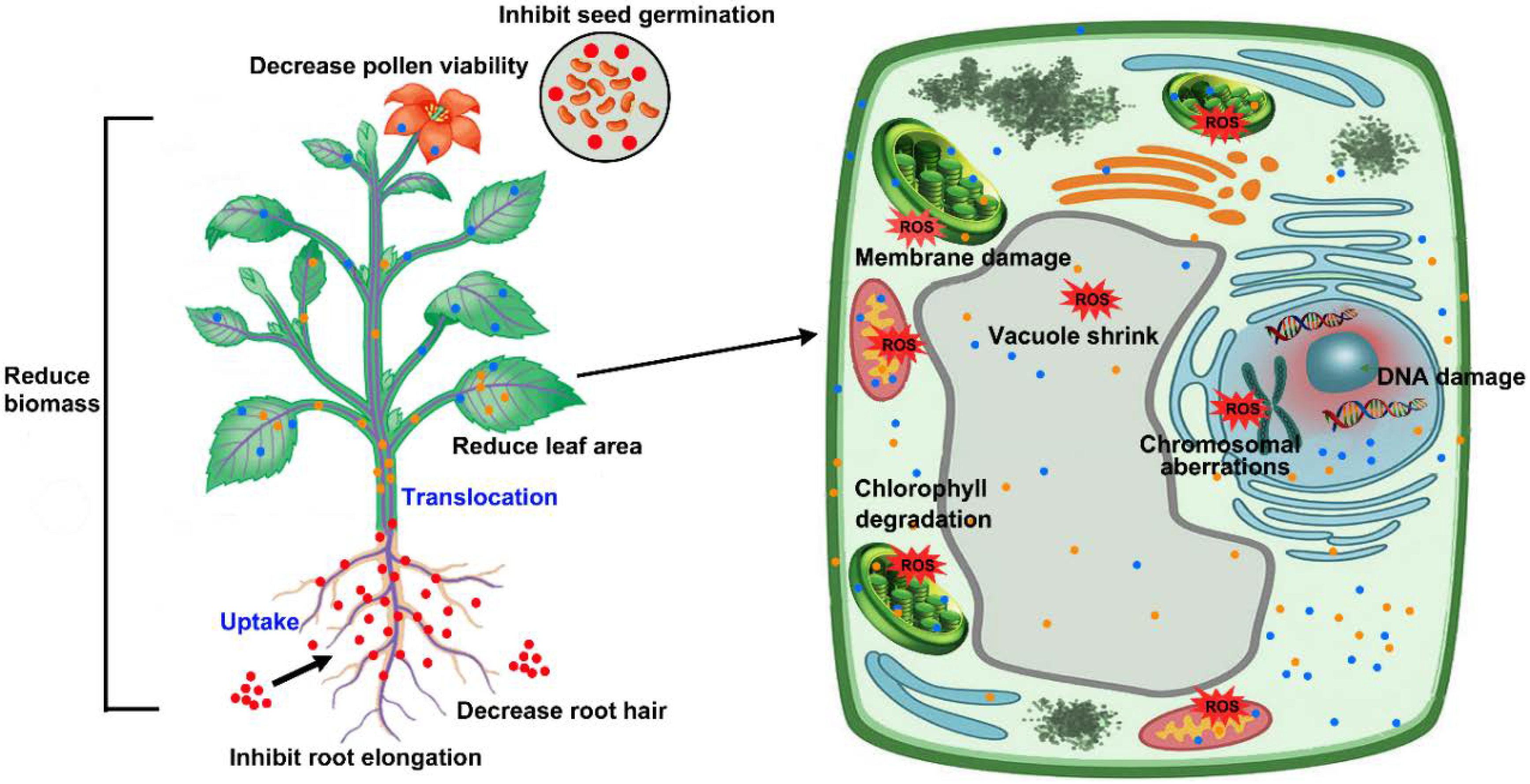
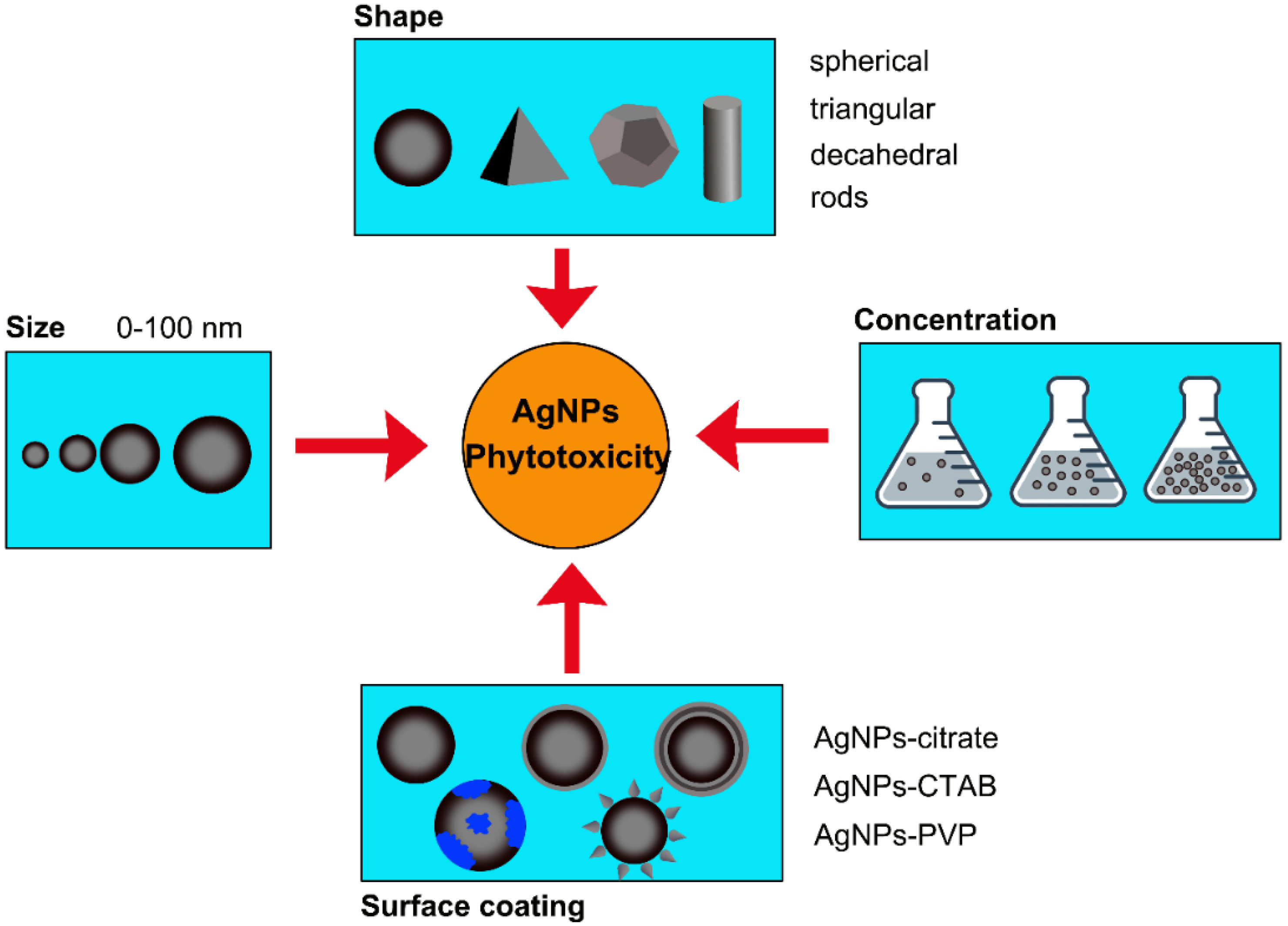
3. Phytotoxicity of AgNPs
3.1. Phytotoxicity at the Morphological Level
3.2. Phytotoxicity at Physiological Level
3.3. Cytotoxicity and Genotoxicity
4. Toxicity Mechanisms
4.1. AgNP-Induced Oxidative Stress
4.2. Silver-Specific Toxicity
4.3. AgNP-Specific Toxicity
5. Tolerance Mechanisms
6. Potential Risk in Human Health Posed by AgNPs via Food Chain
7. Conclusions and Perspectives
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Vance, M.E.; Kuiken, T.; Vejerano, E.P.; McGinnis, S.P.; Hochella, M.F., Jr.; Rejeski, D.; Hull, M.S. Nanotechnology in the real world: Redeveloping the nanomaterial consumer products inventory. Beilstein J. Nanotechnol. 2015, 6, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Posgai, R.; Gorey, T.J.; Nielsen, M.; Hussain, S.M.; Rowe, J.J. Silver nanoparticles induced heat shock protein 70, oxidative stress and apoptosis in Drosophila melanogaster. Toxicol. Appl. Pharm. 2010, 242, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Durán, N.; Marcato, P.D.; De Souza, G.I.; Alves, O.L.; Esposito, E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 2007, 3, 203–208. [Google Scholar] [CrossRef]
- Pandian, A.M.K.; Karthikeyan, C.; Rajasimman, M.; Dinesh, M.G. Synthesis of silver nanoparticle and its application. Ecotoxicol. Environ. Saf. 2015, 121, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Quang Huy, T.; Van Quy, N.; Anh-Tuan, L. Silver nanoparticles: Synthesis, properties, toxicology, applications and perspectives. Adv. Nat. Sci. Nanosci. Nanotechnol. 2013, 4, 033001. [Google Scholar] [CrossRef]
- Zhang, C.; Hu, Z.; Li, P.; Gajaraj, S. Governing factors affecting the impacts of silver nanoparticles on wastewater treatment. Sci. Total Environ. 2016, 572, 852–873. [Google Scholar] [CrossRef] [PubMed]
- Steinitz, B.; Bilavendran, A.D. Thiosulfate stimulates growth and alleviates silver and copper toxicity in tomato root cultures. Plant Cell Tissue Organ Cult. 2011, 107, 355–363. [Google Scholar] [CrossRef]
- Monica, R.C.; Cremonini, R. Nanoparticles and higher plants. Caryologia 2009, 62, 161–165. [Google Scholar] [CrossRef]
- Alavi, S.; Dehpour, A. Evaluation of the nanosilver colloidal solution in comparison with the registered fungicide to control greenhouse cucumber downy mildew disease in the north of Iran. In Proceedings of the VI International Postharvest Symposium, Antalya, Turkey, 11 November 2010; pp. 1643–1646. [Google Scholar]
- Sah, S.; Sorooshzadeh, A.; Rezazadeh, H.; Naghdibadi, H. Effect of nano silver and silver nitrate on seed yield of borage. J. Med. Plants Res. 2011, 5, 706–710. [Google Scholar]
- Vinković, T.; Novák, O.; Strnad, M.; Goessler, W.; Jurašin, D.D.; Parađiković, N.; Vrček, I.V. Cytokinin response in pepper plants (Capsicum annuum L.) exposed to silver nanoparticles. Environ. Res. 2017, 156, 10–18. [Google Scholar] [CrossRef]
- Hedberg, J.; Skoglund, S.; Karlsson, M.-E.; Wold, S.; Odnevall Wallinder, I.; Hedberg, Y. Sequential studies of silver released from silver nanoparticles in aqueous media simulating sweat, laundry detergent solutions and surface water. Environ. Sci. Technol. 2014, 48, 7314–7322. [Google Scholar] [CrossRef] [PubMed]
- Künniger, T.; Gerecke, A.C.; Ulrich, A.; Huch, A.; Vonbank, R.; Heeb, M.; Wichser, A.; Haag, R.; Kunz, P.; Faller, M. Release and environmental impact of silver nanoparticles and conventional organic biocides from coated wooden façades. Environ. Pollut. 2014, 184, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Lombi, E.; Donner, E.; Scheckel, K.G.; Sekine, R.; Lorenz, C.; Goetz, N.V.; Nowack, B. Silver speciation and release in commercial antimicrobial textiles as influenced by washing. Chemosphere 2014, 111, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.Y.; Gottschalk, F.; Hungerbühler, K.; Nowack, B. Comprehensive probabilistic modelling of environmental emissions of engineered nanomaterials. Environ. Pollut. 2014, 185, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled environmental concentrations of engineered nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for different regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Mueller, N.C.; Nowack, B. Exposure modeling of engineered nanoparticles in the environment. Environ. Sci. Technol. 2008, 42, 4447–4453. [Google Scholar] [CrossRef] [PubMed]
- Benn, T.M.; Westerhoff, P. Nanoparticle silver released into water from commercially available sock fabrics. Environ. Sci. Technol. 2008, 42, 4133–4139. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Mueller, E.; Vonbank, R.; Boller, M.; Burkhardt, M. Release of silver nanoparticles from outdoor facades. Environ. Pollut. 2010, 158, 2900–2905. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Nowack, B. The release of engineered nanomaterials to the environment. J. Environ. Monit. 2011, 13, 1145–1155. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.E.; Khosravi, K.; Newman, K.; Metcalfe, C.D. Detection and characterization of silver nanoparticles in aqueous matrices using asymmetric-flow field flow fractionation with inductively coupled plasma mass spectrometry. J. Chromatogr. A 2012, 1233, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Blaser, S.A.; Scheringer, M.; MacLeod, M.; Hungerbühler, K. Estimation of cumulative aquatic exposure and risk due to silver: Contribution of nano-functionalized plastics and textiles. Sci. Total Environ. 2008, 390, 396–409. [Google Scholar] [CrossRef] [PubMed]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Possibilities and limitations of modeling environmental exposure to engineered nanomaterials by probabilistic material flow analysis. Environ. Toxicol. Chem. 2010, 29, 1036–1048. [Google Scholar] [CrossRef] [PubMed]
- Fabrega, J.; Luoma, S.N.; Tyler, C.R.; Galloway, T.S.; Lead, J.R. Silver nanoparticles: Behaviour and effects in the aquatic environment. Environ. Int. 2011, 37, 517–531. [Google Scholar] [CrossRef] [PubMed]
- Kaegi, R.; Voegelin, A.; Sinnet, B.; Zuleeg, S.; Hagendorfer, H.; Burkhardt, M.; Siegrist, H. Behavior of metallic silver nanoparticles in a pilot wastewater treatment plant. Environ. Sci. Technol. 2011, 45, 3902–3908. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.-J.; Herth, S. Plant nanotoxicology. Trends Plant Sci. 2011, 16, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, A.; Keller, A.A. Estimating potential life cycle releases of engineered nanomaterials from wastewater treatment plants. ACS Sustain. Chem. Eng. 2014, 2, 1656–1665. [Google Scholar] [CrossRef]
- Ma, X.; Geiser-Lee, J.; Deng, Y.; Kolmakov, A. Interactions between engineered nanoparticles (ENPs) and plants: Phytotoxicity, uptake and accumulation. Sci. Total Environ. 2010, 408, 3053–3061. [Google Scholar] [CrossRef] [PubMed]
- Maynard, A.D.; Warheit, D.B.; Philbert, M.A. The new toxicology of sophisticated materials: Nanotoxicology and beyond. Toxicol. Sci. 2011, 120, S109–S129. [Google Scholar] [CrossRef] [PubMed]
- Beer, C.; Foldbjerg, R.; Hayashi, Y.; Sutherland, D.S.; Autrup, H. Toxicity of silver nanoparticles-Nanoparticle or silver ion? Toxicol. Lett. 2012, 208, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Colman, B.P.; Arnaout, C.L.; Anciaux, S.; Gunsch, C.K.; Hochella, M.F., Jr.; Kim, B.; Lowry, G.V.; McGill, B.M.; Reinsch, B.C.; Richardson, C.J.; et al. Low concentrations of silver nanoparticles in biosolids cause adverse ecosystem responses under realistic field scenario. PLoS ONE 2013, 8, e57189. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Zovko, M.; Štefanić, P.P.; Biba, R.; Tkalec, M.; Domijan, A.-M.; Vrček, I.V.; Letofsky-Papst, I.; Šikić, S.; Balen, B. Phytotoxic effects of silver nanoparticles in tobacco plants. Environ. Sci. Pollut. Res. 2018, 25, 5590–5602. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Garrido, I.; Pérez, S.; Blasco, J. Toxicity of silver and gold nanoparticles on marine microalgae. Mar. Environ. Res. 2015, 111, 60–73. [Google Scholar] [CrossRef] [PubMed]
- Ratte, H.T. Bioaccumulation and toxicity of silver compounds: A review. Environ. Toxicol. Chem. 1999, 18, 89–108. [Google Scholar] [CrossRef]
- Ma, C.; White, J.C.; Dhankher, O.P.; Xing, B. Metal-based nanotoxicity and detoxification pathways in higher plants. Environ. Sci. Technol. 2015, 49, 7109–7122. [Google Scholar] [CrossRef] [PubMed]
- Maurer-Jones, M.A.; Gunsolus, I.L.; Murphy, C.J.; Haynes, C.L. Toxicity of engineered nanoparticles in the environment. Anal. Chem. 2013, 85, 3036–3049. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Rico, C.M.; White, J.C. Trophic transfer, transformation, and impact of engineered nanomaterials in terrestrial environments. Environ. Sci. Technol. 2014, 48, 2526–2540. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Wang, Q.; Yao, Y.; Zhang, W.; Geisler, M.; Li, K.; Huang, Y.; Chen, Y.; Kolmakov, A.; Ma, X. Phytotoxicity, accumulation and transport of silver nanoparticles by Arabidopsis thaliana. Nanotoxicology 2013, 7, 323–337. [Google Scholar] [CrossRef] [PubMed]
- Geisler-Lee, J.; Brooks, M.; Gerfen, J.R.; Wang, Q.; Fotis, C.; Sparer, A.; Ma, X.; Berg, R.H.; Geisler, M. Reproductive toxicity and life history study of silver nanoparticle effect, uptake and transport in Arabidopsis thaliana. Nanomaterials 2014, 4, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Miralles, P.; Church, T.L.; Harris, A.T. Toxicity, uptake, and translocation of engineered nanomaterials in vascular plants. Environ. Sci. Technol. 2012, 46, 9224–9239. [Google Scholar] [CrossRef] [PubMed]
- Aslani, F.; Bagheri, S.; Muhd Julkapli, N.; Juraimi, A.S.; Hashemi, F.S.G.; Baghdadi, A. Effects of engineered nanomaterials on plants growth: An overview. Sci. World J. 2014, 2014. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Tripathi, A.; Singh, S.; Singh, Y.; Vishwakarma, K.; Yadav, G.; Sharma, S.; Singh, V.K.; Mishra, R.K.; Upadhyay, R.G.; et al. Uptake, accumulation and toxicity of silver nanoparticle in autotrophic plants, and heterotrophic microbes: A concentric review. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Baun, A.; Behra, R.; Hartmann, N.B.; Filser, J.; Miao, A.-J.; Quigg, A.; Santschi, P.H.; Sigg, L. Environmental behavior and ecotoxicity of engineered nanoparticles to algae, plants, and fungi. Ecotoxicology 2008, 17, 372–386. [Google Scholar] [CrossRef] [PubMed]
- Carpita, N.C.; Gibeaut, D.M. Structural models of primary cell walls in flowering plants: Consistency of molecular structure with the physical properties of the walls during growth. Plant J. 1993, 3, 1–30. [Google Scholar] [CrossRef] [PubMed]
- Heinlein, M.; Epel, B.L. Macromolecular transport and signaling through plasmodesmata. Int. Rev. Cytol. 2004. [Google Scholar] [CrossRef]
- Lucas, W.J.; Lee, J.-Y. Plasmodesmata as a supracellular control network in plants. Nat. Rev. Mol. Cell Biol. 2004, 5, 712. [Google Scholar] [CrossRef] [PubMed]
- Larue, C.; Castillo-Michel, H.; Sobanska, S.; Cécillon, L.; Bureau, S.; Barthès, V.; Ouerdane, L.; Carrière, M.; Sarret, G. Foliar exposure of the crop Lactuca sativa to silver nanoparticles: Evidence for internalization and changes in Ag speciation. J. Hazard. Mater. 2014, 264, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-C.; Dang, F.; Li, M.; Zhu, M.; Zhong, H.; Hintelmann, H.; Zhou, D.-M. Effects of exposure pathways on the accumulation and phytotoxicity of silver nanoparticles in soybean and rice. Nanotoxicology 2017, 11, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-S.; Li, M.; Chang, F.-Y.; Li, W.; Yin, L.-Y. Physiological analysis of silver nanoparticles and AgNO3 toxicity to Spirodela polyrhiza. Environ. Toxicol. Chem. 2012, 31, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Kaveh, R.; Li, Y.-S.; Ranjbar, S.; Tehrani, R.; Brueck, C.L.; Van Aken, B. Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 2013, 47, 10637–10644. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; McLean, J.E.; Martineau, N.; Britt, D.W.; Haverkamp, R.; Anderson, A.J. Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ. Sci. Technol. 2013, 47, 1082–1090. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Physiological and molecular level effects of silver nanoparticles exposure in rice (Oryza sativa L.) seedlings. Chemosphere 2014, 112, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Stampoulis, D.; Sinha, S.K.; White, J.C. Assay-dependent phytotoxicity of nanoparticles to plants. Environ. Sci. Technol. 2009, 43, 9473–9479. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Peng, X.; Han, X.; Ren, J.; Sun, L.; Fu, Z. Comparison of the toxicity of silver nanoparticles and silver ions on the growth of terrestrial plant model Arabidopsis thaliana. J. Environ. Sci. 2013, 25, 1947–1956. [Google Scholar] [CrossRef]
- Amooaghaie, R.; Tabatabaei, F.; Ahadi, A.-M. Role of hematin and sodium nitroprusside in regulating Brassica nigra seed germination under nanosilver and silver nitrate stresses. Ecotoxicol. Environ. Saf. 2015, 113, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Gubbins, E.J.; Batty, L.C.; Lead, J.R. Phytotoxicity of silver nanoparticles to Lemna minor L. Environ. Pollut. 2011, 159, 1551–1559. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-M.; Kwak, J.I.; An, Y.-J. Effect of silver nanoparticles in crop plants Phaseolus radiatus and Sorghum bicolor: Media effect on phytotoxicity. Chemosphere 2012, 86, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Cheng, Y.; Espinasse, B.; Colman, B.P.; Auffan, M.; Wiesner, M.; Rose, J.; Liu, J.; Bernhardt, E.S. More than the Ions: The effects of silver nanoparticles on Lolium multiflorum. Environ. Sci. Technol. 2011, 45, 2360–2367. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Raja, N.I.; Ahmad, M.S.; Hussain, M.; Iqbal, M. Effect of silver nanoparticles and silver nitrate on growth of rice under biotic stress. IET Nanobiotechnol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Jiang, F.; Ma, C.; Rui, Y.; Rui, M.; Adeel, M.; Cao, W.; Xing, B. Alteration of crop yield and quality of wheat upon exposure to silver nanoparticles in a life cycle study. J. Agric. Food Chem. 2018, 66, 2589–2597. [Google Scholar] [CrossRef] [PubMed]
- Al-Huqail, A.A.; Hatata, M.M.; Al-Huqail, A.A.; Ibrahim, M.M. Preparation, characterization of silver phyto nanoparticles and their impact on growth potential of Lupinus termis L. seedlings. Saudi J. Biol. Sci. 2018, 25, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, N.R.; Abdel-Megeed, A.; Ali, H.M.; Salem, M.Z.M.; Al-Hayali, M.F.A.; Elshikh, M.S. Genotoxicity effects of silver nanoparticles on wheat (Triticum aestivum L.) root tip cells. Ecotoxicol. Environ. Saf. 2018, 155, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Tang, H.; Deng, Z.; Liu, Y.; Chen, X.; Wang, H. Ag nanoparticles inhibit the growth of the bryophyte, Physcomitrella patens. Ecotoxicol. Environ. Saf. 2018, 164, 739–748. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.P.P.; Jesus, F.; Aguiar, S.; de Oliveira, R.; Fernandes, M.; Ranville, J.; Nogueira, A.J.A. Phytotoxicity of silver nanoparticles to Lemna minor: Surface coating and exposure period-related effects. Sci. Total Environ. 2018, 618, 1389–1399. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, D.K.; Singh, S.; Singh, S.; Srivastava, P.K.; Singh, V.P.; Singh, S.; Prasad, S.M.; Singh, P.K.; Dubey, N.K.; Pandey, A.C.; et al. Nitric oxide alleviates silver nanoparticles (AgNPs)-induced phytotoxicity in Pisum sativum seedlings. Plant Physiol. Biochem. 2017, 110, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Cvjetko, P.; Milošić, A.; Domijan, A.-M.; Vinković Vrček, I.; Tolić, S.; Peharec Štefanić, P.; Letofsky-Papst, I.; Tkalec, M.; Balen, B. Toxicity of silver ions and differently coated silver nanoparticles in Allium cepa roots. Ecotoxicol. Environ. Saf. 2017, 137, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Saha, N.; Dutta Gupta, S. Low-dose toxicity of biogenic silver nanoparticles fabricated by Swertia chirata on root tips and flower buds of Allium cepa. J. Hazard. Mater. 2017, 330, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Jasim, B.; Thomas, R.; Mathew, J.; Radhakrishnan, E.K. Plant growth and diosgenin enhancement effect of silver nanoparticles in Fenugreek (Trigonella foenum-graecum L.). Saudi Pharm. J. 2017, 25, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wang, L.; Li, S.; Yin, L.; Huang, J.; Chen, C. Toxicity of silver nanoparticles to Arabidopsis: Inhibition of root gravitropism by interfering with auxin pathway. Environ. Toxicol. Chem. 2017, 36, 2773–2780. [Google Scholar] [CrossRef] [PubMed]
- Vishwakarma, K.; Upadhyay, N.; Singh, J.; Liu, S.; Singh, V.P.; Prasad, S.M.; Chauhan, D.K.; Tripathi, D.K.; Sharma, S. Differential phytotoxic impact of plant mediated silver nanoparticles (AgNPs) and silver nitrate (AgNO3) on Brassica sp. Front. Plant Sci. 2017, 8, 1501. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Sun, S.; Scheckel, K.G.; Malysheva, A.; McKenna, B.A.; Menzies, N.W.; Zhao, F.-J.; Kopittke, P.M. Characterizing the uptake, accumulation and toxicity of silver sulfide nanoparticles in plants. Environ. Sci. 2017, 4, 448–460. [Google Scholar] [CrossRef]
- Abd-Alla, M.H.; Nafady, N.A.; Khalaf, D.M. Assessment of silver nanoparticles contamination on faba bean-Rhizobium leguminosarum bv. viciae-Glomus aggregatum symbiosis: Implications for induction of autophagy process in root nodule. Agric. Ecosyst. Environ. 2016, 218, 163–177. [Google Scholar] [CrossRef]
- Bagherzadeh Homaee, M.; Ehsanpour, A.A. Silver nanoparticles and silver ions: Oxidative stress responses and toxicity in potato (Solanum tuberosum L.) grown in vitro. Hortic. Environ. Biotechnol. 2016, 57, 544–553. [Google Scholar] [CrossRef]
- Mehta, C.M.; Srivastava, R.; Arora, S.; Sharma, A.K. Impact assessment of silver nanoparticles on plant growth and soil bacterial diversity. 3 Biotech 2016, 6, 254. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Armendariz, R.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Effects of silver nanoparticles on radish sprouts: Root growth reduction and modifications in the nutritional value. Front. Plant Sci. 2016, 7, 90. [Google Scholar] [CrossRef] [PubMed]
- Sosan, A.; Svistunenko, D.; Straltsova, D.; Tsiurkina, K.; Smolich, I.; Lawson, T.; Subramaniam, S.; Golovko, V.; Anderson, D.; Sokolik, A.; et al. Engineered silver nanoparticles are sensed at the plasma membrane and dramatically modify the physiology of Arabidopsis thaliana plants. Plant J. 2016, 85, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Zhang, L.; Chen, Z.; Sheng, X.; Qiu, J.; Xu, D. Co-exposure of silver nanoparticles and chiral herbicide imazethapyr to Arabidopsis thaliana: Enantioselective effects. Chemosphere 2016, 145, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Zou, X.; Li, P.; Huang, Q.; Zhang, H. The different response mechanisms of Wolffia globosa: Light-induced silver nanoparticle toxicity. Aquat. Toxicol. 2016, 176, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kohan-Baghkheirati, E.; Geisler-Lee, J. Gene expression, protein function and pathways of Arabidopsis thaliana responding to silver nanoparticles in comparison to silver ions, cold, salt, drought, and heat. Nanomaterials 2015, 5, 436–467. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, S.; Bernales, I.; Cristobal, S. Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom. 2015, 16, 341. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Menzies, N.W.; Lombi, E.; Sekine, R.; Blamey, F.P.C.; Hernandez-Soriano, M.C.; Cheng, M.; Kappen, P.; Peijnenburg, W.J.G.M.; Tang, C.; et al. Silver sulfide nanoparticles (Ag2S-NPs) are taken up by plants and are phytotoxic. Nanotoxicology 2015, 9, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-S.; Qiu, X.-N.; Li, G.-B.; Li, W.; Yin, L.-Y. Silver nanoparticles induced accumulation of reactive oxygen species and alteration of antioxidant systems in the aquatic plant Spirodela polyrhiza. Environ. Toxicol. Chem. 2014, 33, 1398–1405. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.M.G.; Chung, I.M. Assessment of silver nanoparticle-induced physiological and molecular changes in Arabidopsis thaliana. Environ. Sci. Pollut. Res. 2014, 21, 8858–8869. [Google Scholar] [CrossRef] [PubMed]
- Syu, Y.-Y.; Hung, J.-H.; Chen, J.-C.; Chuang, H.-W. Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol. Biochem. 2014, 83, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Thuesombat, P.; Hannongbua, S.; Akasit, S.; Chadchawan, S. Effect of silver nanoparticles on rice (Oryza sativa L. cv. KDML 105) seed germination and seedling growth. Ecotoxicol. Environ. Saf. 2014, 104, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, L.R.; Dubey, B. Evaluation of developmental responses of two crop plants exposed to silver and zinc oxide nanoparticles. Sci. Total Environ. 2013, 452–453, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Mirzajani, F.; Askari, H.; Hamzelou, S.; Farzaneh, M.; Ghassempour, A. Effect of silver nanoparticles on Oryza sativa L. and its rhizosphere bacteria. Ecotoxicol. Environ. Saf. 2013, 88, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Song, U.; Jun, H.; Waldman, B.; Roh, J.; Kim, Y.; Yi, J.; Lee, E.J. Functional analyses of nanoparticle toxicity: A comparative study of the effects of TiO2 and Ag on tomatoes (Lycopersicon esculentum). Ecotoxicol. Environ. Saf. 2013, 93, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Koo, Y.; Alexander, A.; Yang, Y.; Westerhof, S.; Zhang, Q.; Schnoor, J.L.; Colvin, V.L.; Braam, J.; Alvarez, P.J.J. Phytostimulation of Poplars and Arabidopsis exposed to silver nanoparticles and Ag+ at sublethal concentrations. Environ. Sci. Technol. 2013, 47, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Colman, B.P.; McGill, B.M.; Wright, J.P.; Bernhardt, E.S. Effects of silver nanoparticle exposure on germination and early growth of eleven wetland plants. PLoS ONE 2012, 7, e47674. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, J.; Musante, C.; Sinha, S.K.; White, J.C. Accumulation and phytotoxicity of engineered nanoparticles to Cucurbita Pepo. Int. J. Phytoremediat. 2012, 14, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Musante, C.; White, J.C. Toxicity of silver and copper to Cucurbita pepo: Differential effects of nano and bulk-size particles. Environ. Toxicol. 2012, 27, 510–517. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.K.; Berry, A.; May, L.; Tchounwou, P.B. Genotoxicity of silver nanoparticles in Vicia faba: A pilot study on the environmental monitoring of nanoparticles. Int. J. Environ. Res. Public Health 2012, 9, 1649. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Bhatt, D.; Zaidi, M.G.H.; Saradhi, P.P.; Khanna, P.K.; Arora, S. Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotech. 2012, 167, 2225–2233. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, H.; Ahmed, G. Phytotoxicity effect of silver nanoparticles on Oryza sativa. IJ Chemtech. Res. 2011, 3, 1494–1500. [Google Scholar]
- Panda, K.K.; Achary, V.M.M.; Krishnaveni, R.; Padhi, B.K.; Sarangi, S.N.; Sahu, S.N.; Panda, B.B. In vitro biosynthesis and genotoxicity bioassay of silver nanoparticles using plants. Toxicol. In Vitro 2011, 25, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Mukherjee, A.; Chandrasekaran, N. Genotoxicity of silver nanoparticles in Allium cepa. Sci. Total Environ. 2009, 407, 5243–5246. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhang, M.; Wang, S.; Tang, J. Physical, chemical and microbiological changes in stored green asparagus spears as affected by coating of silver nanoparticles-PVP. LWT Food Sci. Technol. 2008, 41, 1100–1107. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Singh, S.; Pandey, R.; Singh, V.P.; Sharma, N.C.; Prasad, S.M.; Dubey, N.K.; Chauhan, D.K. An overview on manufactured nanoparticles in plants: Uptake, translocation, accumulation and phytotoxicity. Plant Physiol. Biochem. 2017, 110, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Mazumdar, H. Comparative assessment of the adverse effect of silver nanoparticles to Vigna radiata and Brassica campestris crop plants. Int. J. Eng. Res. Appl. 2014, 4, 118–124. [Google Scholar]
- Yan, A.; Chen, Z. Detection methods of nanoparticles in plant tissues. In New Visions in Plant Science; IntechOpen: London, UK, 2018. [Google Scholar]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Mourato, M.; Reis, R.; Martins, L.L. Characterization of plant antioxidative system in response to abiotic stresses: A focus on heavy metal toxicity. In Advances in Selected Plant Physiology Aspects; IntechOpen: London, UK, 2012. [Google Scholar]
- Møller, I.M.; Jensen, P.E.; Hansson, A. Oxidative modifications to cellular components in plants. Annu. Rev. Plant Biol. 2007, 58, 459–481. [Google Scholar] [CrossRef] [PubMed]
- Carocho, M.; Ferreira, I.C.F.R. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Capaldi Arruda, S.C.; Diniz Silva, A.L.; Moretto Galazzi, R.; Antunes Azevedo, R.; Zezzi Arruda, M.A. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Sen Raychaudhuri, S.; Deng, X.W. The role of superoxide dismutase in combating oxidative stress in higher plants. Bot. Rev. 2000, 66, 89–98. [Google Scholar] [CrossRef]
- Yuan, L.; Richardson, C.J.; Ho, M.; Willis, C.W.; Colman, B.P.; Wiesner, M.R. Stress responses of aquatic plants to silver nanoparticles. Environ. Sci. Technol. 2018, 52, 2558–2565. [Google Scholar] [CrossRef] [PubMed]
- Speranza, A.; Crinelli, R.; Scoccianti, V.; Taddei, A.R.; Iacobucci, M.; Bhattacharya, P.; Ke, P.C. In vitro toxicity of silver nanoparticles to kiwifruit pollen exhibits peculiar traits beyond the cause of silver ion release. Environ. Pollut. 2013, 179, 258–267. [Google Scholar] [CrossRef] [PubMed]
- De La Torre-Roche, R.; Hawthorne, J.; Musante, C.; Xing, B.; Newman, L.A.; Ma, X.; White, J.C. Impact of Ag nanoparticle exposure on p,p′-DDE bioaccumulation by Cucurbita pepo (Zucchini) and Glycine max (Soybean). Environ. Sci. Technol. 2013, 47, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Huang, Y.-L.; Huang, S.-F. Lipid peroxidation in liver of rats administrated with methyl mercuric chloride. Biol. Trace Elem. Res. 1996, 54, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Gurunathan, S.; Chung, I.-M. Physiological, metabolic, and transcriptional effects of biologically-synthesized silver nanoparticles in turnip (Brassica rapa ssp. rapa L.). Protoplasma 2015, 252, 1031–1046. [Google Scholar] [CrossRef] [PubMed]
- Dobias, J.; Bernier-Latmani, R. Silver release from silver nanoparticles in natural waters. Environ. Sci. Technol. 2013, 47, 4140–4146. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-J.; Kim, J.Y.; Kim, J.; Lee, J.-H.; Hahn, J.-S.; Gu, M.B.; Yoon, J. Silver-ion-mediated reactive oxygen species generation affecting bactericidal activity. Water Res. 2009, 43, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Liu, S.; Singh, P.K.; Kumar, N.; Pandey, A.C.; Tripathi, D.K.; Chauhan, D.K.; Sahi, S. Differential phytotoxic responses of silver nitrate (AgNO3) and silver nanoparticle (AgNPs) in Cucumis sativus L. Plant Gene 2017, 11, 255–264. [Google Scholar] [CrossRef]
- Montes, A.; Bisson, M.A.; Gardella, J.A.; Aga, D.S. Uptake and transformations of engineered nanomaterials: Critical responses observed in terrestrial plants and the model plant Arabidopsis thaliana. Sci. Total Environ. 2017, 607–608, 1497–1516. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Greppi, G.; Irudayaraj, J. Latest developments of nanotoxicology in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 125–151. [Google Scholar]
- Chen, X.; Schluesener, H.J. Nanosilver: A nanoproduct in medical application. Toxicol. Lett. 2008, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wijnhoven, S.W.P.; Peijnenburg, W.J.G.M.; Herberts, C.A.; Hagens, W.I.; Oomen, A.G.; Heugens, E.H.W.; Roszek, B.; Bisschops, J.; Gosens, I.; Van De Meent, D.; et al. Nano-silver—A review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology 2009, 3, 109–138. [Google Scholar] [CrossRef]
- Anjum, N.A.; Gill, S.S.; Duarte, A.C.; Pereira, E.; Ahmad, I. Silver nanoparticles in soil-plant systems. J. Nanopart. Res. 2013, 15, 1896. [Google Scholar] [CrossRef]
- Gross, E.L. Plastocyanin: Structure and function. Photosynth. Res. 1993, 37, 103–116. [Google Scholar] [CrossRef] [PubMed]
- Sigfridsson, K. Plastocyanin, an electron-transfer protein. Photosynth. Res. 1998, 57, 1–28. [Google Scholar] [CrossRef]
- Sas, K.N.; Haldrup, A.; Hemmingsen, L.; Danielsen, E.; Øgendal, L.H. pH-dependent structural change of reduced spinach plastocyanin studied by perturbed angular correlation of γ-rays and dynamic light scattering. JBIC J. Biol. Inorg. Chem. 2006, 11, 409. [Google Scholar] [CrossRef] [PubMed]
- Sujak, A. Interaction between cadmium, zinc and silver-substituted plastocyanin and cytochrome b6f complex—Heavy metals toxicity towards photosynthetic apparatus. Acta Physiol. Plant 2005, 27, 61–69. [Google Scholar] [CrossRef]
- Jansson, H.; Hansson, Ö. Competitive inhibition of electron donation to photosystem 1 by metal-substituted plastocyanin. Biochim. Biophys. Acta 2008, 1777, 1116–1121. [Google Scholar] [CrossRef] [PubMed]
- Navarro, E.; Piccapietra, F.; Wagner, B.; Marconi, F.; Kaegi, R.; Odzak, N.; Sigg, L.; Behra, R. Toxicity of silver nanoparticles to Chlamydomonas reinhardtii. Environ. Sci. Technol. 2008, 42, 8959–8964. [Google Scholar] [CrossRef] [PubMed]
- Ruotolo, R.; Maestri, E.; Pagano, L.; Marmiroli, M.; White, J.C.; Marmiroli, N. Plant response to metal-containing engineered nanomaterials: An omics-based perspective. Environ. Sci. Technol. 2018, 52, 2451–2467. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, A.; Zivcak, M.; Sytar, O.; Kalaji, H.M.; He, X.; Mbarki, S.; Brestic, M. Impact of metal and metal oxide nanoparticles on plant: A critical review. Front. Chem. 2017, 5, 78. [Google Scholar] [CrossRef] [PubMed]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Azeem, E.A.; Elsayed, B.A. Phytotoxicity of silver nanoparticles on Vicia faba seedlings. N. Y. Sci. J. 2013, 6, 148–156. [Google Scholar]
- Oukarroum, A.; Barhoumi, L.; Pirastru, L.; Dewez, D. Silver nanoparticle toxicity effect on growth and cellular viability of the aquatic plant Lemna gibba. Environ. Toxicol. Chem. 2013, 32, 902–907. [Google Scholar] [CrossRef] [PubMed]
- Levard, C.; Hotze, E.M.; Lowry, G.V.; Brown, G.E. Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environ. Sci. Technol. 2012, 46, 6900–6914. [Google Scholar] [CrossRef] [PubMed]
- Tejamaya, M.; Römer, I.; Merrifield, R.C.; Lead, J.R. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ. Sci. Technol. 2012, 46, 7011–7017. [Google Scholar] [CrossRef] [PubMed]
- Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Chemistry, biochemistry of nanoparticles, and their role in antioxidant defense system in plants. In Nanotechnology and Plant Sciences: Nanoparticles and Their Impact on Plants; Siddiqui, M.H., Al-Whaibi, M.H., Mohammad, F., Eds.; Springer: Cham, Switzerland, 2015; pp. 1–17. [Google Scholar]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.G.; Ramachandran, R.; Abirami, S.M.; Mohan, N.; Kalaichelvan, P.T. Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem. 2012, 47, 651–658. [Google Scholar] [CrossRef]
- Gould, K.S. Nature’s Swiss army knife: The diverse protective roles of anthocyanins in leaves. Biomed Res. Int. 2004, 2004, 314–320. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Jones, A.M.; Garg, S.; Pham, A.N.; Waite, T.D. Silver nanoparticle–reactive oxygen species interactions: Application of a charging-discharging model. J. Phys. Chem. C 2011, 115, 5461–5468. [Google Scholar] [CrossRef]
- Chew, B.P.; Park, J.S. Carotenoid action on the immune response. J. Nutr. 2004, 134, 257S–261S. [Google Scholar] [CrossRef] [PubMed]
- Zechmann, B.; Müller, M.; Zellnig, G. Modified levels of cysteine affect glutathione metabolism in plant cells. In Sulfur Assimilation and Abiotic Stress in Plants; Khan, N.A., Singh, S., Umar, S., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 193–206. [Google Scholar]
- Tangaa, S.R.; Selck, H.; Winther-Nielsen, M.; Khan, F.R. Trophic transfer of metal-based nanoparticles in aquatic environments: A review and recommendations for future research focus. Environ. Sci. Nano 2016, 3, 966–981. [Google Scholar] [CrossRef]
- Kalman, J.; Paul, K.B.; Khan, F.R.; Stone, V.; Fernandes, T.F. Characterisation of bioaccumulation dynamics of three differently coated silver nanoparticles and aqueous silver in a simple freshwater food chain. Environ. Chem. 2015, 12, 662–672. [Google Scholar] [CrossRef]
- McTeer, J.; Dean, A.P.; White, K.N.; Pittman, J.K. Bioaccumulation of silver nanoparticles into Daphnia magna from a freshwater algal diet and the impact of phosphate availability. Nanotoxicology 2014, 8, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-G.; Kim, J.I.; Chang, K.-H.; Lee, B.-C.; Eom, I.-C.; Kim, P.; Nam, D.-H.; Yeo, M.-K. Trophic transfer of citrate, PVP coated silver nanomaterials, and silver ions in a paddy microcosm. Environ. Pollut. 2018, 235, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Unrine, J.M.; Rao, W.; Bertsch, P.M. Bioaccumulation of gold nanomaterials by Manduca sexta through dietary uptake of surface contaminated plant tissue. Environ. Sci. Technol. 2012, 46, 12672–12678. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, J.; De la Torre Roche, R.; Xing, B.; Newman, L.A.; Ma, X.; Majumdar, S.; Gardea-Torresdey, J.; White, J.C. Particle-size dependent accumulation and trophic transfer of cerium oxide through a terrestrial food chain. Environ. Sci. Technol. 2014, 48, 13102–13109. [Google Scholar] [CrossRef] [PubMed]
- De la Torre Roche, R.; Servin, A.; Hawthorne, J.; Xing, B.; Newman, L.A.; Ma, X.; Chen, G.; White, J.C. Terrestrial trophic transfer of bulk and nanoparticle La2O3 does not depend on particle size. Environ. Sci. Technol. 2015, 49, 11866–11874. [Google Scholar] [CrossRef] [PubMed]
- Sung, J.H.; Ji, J.H.; Yoon, J.U.; Kim, D.S.; Song, M.Y.; Jeong, J.; Han, B.S.; Han, J.H.; Chung, Y.H.; Kim, J.; et al. Lung function changes in Sprague-Dawley rats after prolonged inhalation exposure to silver nanoparticles. Inhal. Toxicol. 2008, 20, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Kim, J.S.; Cho, H.S.; Rha, D.S.; Kim, J.M.; Park, J.D.; Choi, B.S.; Lim, R.; Chang, H.K.; Chung, Y.H.; et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal. Toxicol. 2008, 20, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-H.; Chang, L.W.; Lin, P. Metal-based nanoparticles and the immune system: Activation, inflammation, and potential applications. Biomed Res. Int. 2015, 2015, 143720. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lim, D.-H.; Choi, I.-H. The impact of nanomaterials in immune system. Immune Netw. 2010, 10, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular responses induced by silver nanoparticles: In vitro studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef] [PubMed]
| Size (Diameter in nm) | Concentration | Species | Impacts | References |
|---|---|---|---|---|
| 25–70; 7.5–25.0 | 10, 20, 40, 50 ppm | Wheat (Triticumaestivum L.) | Caused various types of chromosomal aberrations | [63] |
| 5–10 | 0, 0.1, 0.3, 0.5 mg/L | Lupinus termis L. | Reduction in shoot and root elongation, shoot and root fresh weights, total chlorophyll, and total protein contents; Decreased sugar contents and caused significant foliar proline accumulation; Caused metabolic disorders | [62] |
| 37.4 ± 13.4 (AgNP-B a); 29.0 ± 6.0 (AgNP-PVP b); 21.5 ± 4.2 (AgNP-Citrate) | 5, 10 μg/mL | Bryophyte (Physcomitrella patens) | Inhibited the growth of the protonema; Changed the thylakoid and chlorophyll contents | [64] |
| 79.0 ± 8.0 | 0.05–2 mg/L | Lemna minor | Caused decays on growth rate and fronds per colony; Induced oxidative stress | [65] |
| 3.1–8.7 | 20, 200, 2000 mg/kg | Wheat (Triticum aestivum L.) | Caused lower biomass, shorter plant height, and lower grain weight; Decreases in the contents of micronutrients (Fe, Cu, and Zn); Decreased the contents of arginine and histidine | [61] |
| 17.2 ± 0.3 | 0, 1, 10 and 30 mg/L (soybean); 0, 0.1, 0.5, 1 mg/L (rice) | Soybean; Rice | Significantly reduced plant biomass; Increased the malondialdehyde and H2O2 contents of leaves | [49] |
| 12.9 ± 9.1 | 0.01, 0.05, 0.1, 0.5, 1 mg/L | Capsicum annuum | Decreased plant height and biomass; Causued a significant increase in total cytokinins in the leaves | [12] |
| 20 | 1000, 3000 μM | Pisum sativum | Declined growth, photosynthetic pigments, and chlorophyll fluorescence; Inhibited activities of glutathione reductase (GR) and dehydroascorbate reductase (DHAR). | [66] |
| 61.2 ± 33.9 (AgNP-Citrate); 9.4 ± 1.3 (AgNP-PVP); 5.6 ± 2.1 (AgNP-CTABc) | 25, 50, 75, 100 μM | Allium cepa | Caused oxidative stress; Led to strong reduction of the root growth | [67] |
| 20 | 5, 10, 20 mg/L | Allium cepa | Induced various chromosomal aberrations in both mitotic and meiotic cells | [68] |
| 200–800 | 1 mg/L | Trigonella foenum-graecum L. | Enhancement in plant growth and diosgenin synthesis | [69] |
| 20 | 10–150 mg/L | Arabidopsis thaliana | Inhibited root gravitropism; Reduced auxin accumulation in root tips; Downregulated expression of auxin receptor-related genes | [70] |
| 47 | 1, 3 mM | Mustard (Brassica sp.) | Declined growth of Brassica seedlings; Induced oxidative stress | [71] |
| 35, 73 | 10 mg/L | Cucumber (Cucumis sativus); Wheat (Triticum aestivum L.) | Teduced growth; Upregulation of genes involved in the ethylene signalling pathway | [72] |
| 5–50 | 800 µg/kg | Vicia faba L. | Declined germination; Decreased shoot and root length; Retarded the process of nodulation; Caused early senescence of root nodules | [73] |
| 20 | 0, 2, 10, 20 mg/L | Potato (Solanum tuberosum L.) | Total reactive oxygen species (ROS) and superoxide anions were increased; Significant increases in the activities ofsuperoxide dismutase (SOD), catalase, ascorbate peroxidase, and glutathione reductase (GR); Higher ion leakage and cell death | [74] |
| 35–40 | 50, 75 mg/L | Triticum aestivum; Vigna sinensis; Brassica juncea | 50 ppm treatment promoted growth and increased root nodulation in cowpea; Improved shoot parameters at 75 ppm in Brassica | [75] |
| 2 | 0, 125, 250, 500 mg/L | Raphanus sativus | Water content was reduced; Root and shoot lengths were reduced at 500 mg/L treatment; Significantly less Ca, Mg, B, Cu, Mn, and Zn | [76] |
| 41 | 100–5000 mg/L | Arabidopsis thaliana | Reduced root length, leaf expansion and photosynthetic efficiency; Induced ROS accumulation; Induced Ca2+ in cytoplasm, inhibited plasma membrane K+ efflux and Ca2+ influx currents | [77] |
| 100 | 50–100 μM | Arabidopsis thaliana | Accumulated more amino acids | [78] |
| 10 | 1, 2, 5, 8, 10 mg/L | Wolffia globosa | Caused oxidative damage, higher malondialdehyde (MDA) content and an upregulation of SOD activity; Decreased contents of chlorophyll a, carotenoids and soluble protein | [79] |
| 20 | 5 mg/L | Arabidopsis thaliana | 111 genes were unique in AgNPs and enriched in three biological functions: response to fungal infection, anion transport, and cell wall/plasma membrane related. | [80] |
| 10, 20, 40, 80 | 0.2 μg/L | Arabidopsis thaliana | Inhibition of root hair development; Repressed transcriptional responses to microbial pathogens, resulting in increased bacterial colonization | [81] |
| 60–100 (Ag2S-NPs); 15–20 (AgNPs) | 0–20mg/L (Ag2S-NPs); 0–1.6 mg/L (Ag-NPs) | Cowpea (Vigna unguiculata L. Walp.); Wheat (Triticum aestivum L.) | Ag2S-NPs reduced growth by up to 52%; Ag accumulated as Ag2S in the root and shoot tissues after exposed to Ag2S-NPs | [82] |
| 20 | 75–300 μg/L | Arabidopsis thaliana | Prolonged vegetative and shortened reproductive growth; Decreased germination rates of offspring | [40] |
| 6, 20 | 0.5, 5, 10 mg/L | Spirodela polyrhiza | Dose dependent increase in levels of ROS, SOD, peroxidase, and the antioxidant glutathione content; Chloroplasts accumulated starch grains and had reduced intergranal thylakoids. | [83] |
| 20 | 0, 0.2, 0.5, 1 mg/L | Arabidopsis thaliana | Significantly reduced total chlorophyll and increased anthocyanin content; Increased lipid peroxidation; a dose-dependent increase in ROS production; Significant upregulated the expression of sulfur assimilation, glutathione biosynthesis, glutathione S-transferase, and glutathione reductase genes | [84] |
| 20 | 0, 0.2, 0.5, 1 mg/L | Oryza sativa L. | Significant reduction in root elongation, shoot and root fresh weights, total chlorophyll, and carotenoids contents; Caused significant increase in H2O2 formation and lipid peroxidation in shoots and roots, increased foliar proline accumulation, and decreased sugar contents; Caused a dose dependent increase in ROS generation; Changes in mitochondrial membrane potential in the roots of seedlings | [53] |
| 8, 45, 47 | 2–100 μM | Arabidopsis thaliana | Induced root growth promotion (RGP) and Cu/Zn superoxide dismutase (CSD2) accumulation; Inhibited ethylene (ET) perception and could interfere with ET biosynthesis | [85] |
| 20,30–60, 70–120, 150 | 0.1, 1, 10, 100, 1000 mg/L | Oryza sativa L. | Seed germination and seedling growth were decreased | [86] |
| 20, 40, 80 | 67–535 μg/L | Arabidopsis thaliana | Inhibited seedling root elongation; AgNPs were apoplastically transported in the cell wall and aggregated at plasmodesmata | [39] |
| 20 | 5–25 mg/L | Arabidopsis thaliana | Upregulation of stress related genes, downregulation of pathogen and hormonal stimuli genes; Oxidative stress | [51] |
| 10 | 0.2, 0.5, 3 mg/L | Arabidopsis thaliana | Root growth inhibition; Disrupted the thylakoid membrane structure and decreased chlorophyll content; Caused alteration of transcription of antioxidant and aquaporin related genes | [55] |
| 11 ± 0.7 (Citrate) | 0.05, 0.1, 1, 18.3, 36.7, 73.4 mg/L | Zea mays Brassica oleracea | Cell erosion in maize root apical meristem | [87] |
| 18.34 | 0.30–60 mg/L | Oryza sativa L. | Damage the cell morphology and its structural features; Total soluble carbohydrates significantly declined; Caused production of the ROS and local root tissue death | [88] |
| 10 | 0.5, 1.5, 2.5, 3.5, 5 mg/kg | Triticum aestivum | Teduced the length of shoots and roots; Caused oxidative stress in roots; Induced expression of a metallothionein gene involved in detoxification | [52] |
| 10–15 | 0, 100, 1000 mg/L | Tomatoes (Lycopersicon esculentum) | Significant decreases in root growth; Decreased chlorophyll contents and Higher SOD activity; Less fruit productivity, | [89] |
| 5, 10, 25 | 0.01–100 mg/L | Arabidopsis thaliana; poplars | Stimulatory effect on root elongation, fresh weight, and evapotranspiration at sublethal concentrations; Toxicity increased with decreasing AgNPs size | [90] |
| 20 (AgNP-PVP) 6 (AgNP-GAd) | 1, 10, 40 mg/L | Eleven species of wetland plants | 40 mg/L AgNPs-GA exposure significantly reduced the germination rate of three species and enhanced the germination rate of one species. | [91] |
| <100 | 250, 750 mg/L | Cucurbita pepo | Reduction in plant biomass and transpiration | [92] |
| 5–25 | 0, 5, 10, 20, 40 mg/L | Phaseolus radiates; Sorghum bicolor | Inhibition of plant growth | [58] |
| <100 | 0, 100, 500 mg/L | Cucurbita pepo | Decreased rate of transpiration | [93] |
| 60 | 12.5, 25, 50, 100 mg/L | Vicia faba | Increased the number of chromosomal aberrations and micronuclei, and decreased the mitotic index | [94] |
| 190–1100 | 0, 25, 50, 100, 200 or 400 mg/L | Brassica juncea | Increase in root length and increase in vigor index; Improved photosynthetic quantum efficiency and higher chlorophyll contents; Induced the activities of antioxidant enzymes, resulting in reduced reactive oxygen species levels | [95] |
| 20, 100 | 5 μg/L | Lemna minor L. | Inhibition of plant growth | [57] |
| 25 | 50, 500, 1000 mg/L | Oryza sativa | Broken the cell wall and damaged the vacuoles of root cells | [96] |
| 24–55 | 0–80 mg/L | Allium cepa | Induced cell death and DNA damage through generation of ROS | [97] |
| <100 | 100 ppm | Allium cepa | Disturbed mitosis, reduction in mitotic index, declined metaphase, sticky chromosome, disintegration and breakdown of cell wall | [98] |
| 20 | 100 mg/L | Green asparagus | Higher ascorbic acid and total chlorophyll contents | [99] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, A.; Chen, Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. Int. J. Mol. Sci. 2019, 20, 1003. https://doi.org/10.3390/ijms20051003
Yan A, Chen Z. Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. International Journal of Molecular Sciences. 2019; 20(5):1003. https://doi.org/10.3390/ijms20051003
Chicago/Turabian StyleYan, An, and Zhong Chen. 2019. "Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism" International Journal of Molecular Sciences 20, no. 5: 1003. https://doi.org/10.3390/ijms20051003
APA StyleYan, A., & Chen, Z. (2019). Impacts of Silver Nanoparticles on Plants: A Focus on the Phytotoxicity and Underlying Mechanism. International Journal of Molecular Sciences, 20(5), 1003. https://doi.org/10.3390/ijms20051003





