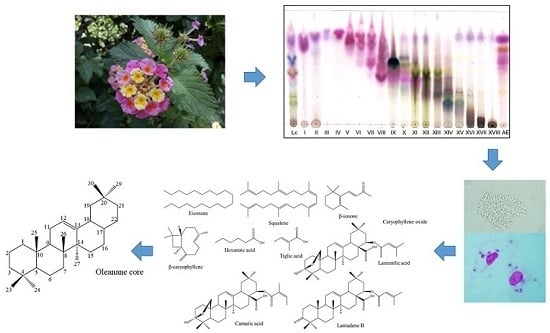
Chemical Constituents with Leishmanicidal Activity from a Pink-Yellow Cultivar of Lantana camara var. aculeata (L.) Collected in Central Mexico
Abstract
1. Introduction
2. Results and Discussion
2.1. Leishmanicidal and Cytotoxic Activity of the Crude Extract and the Chromatographic Fractions Obtained from L. camara
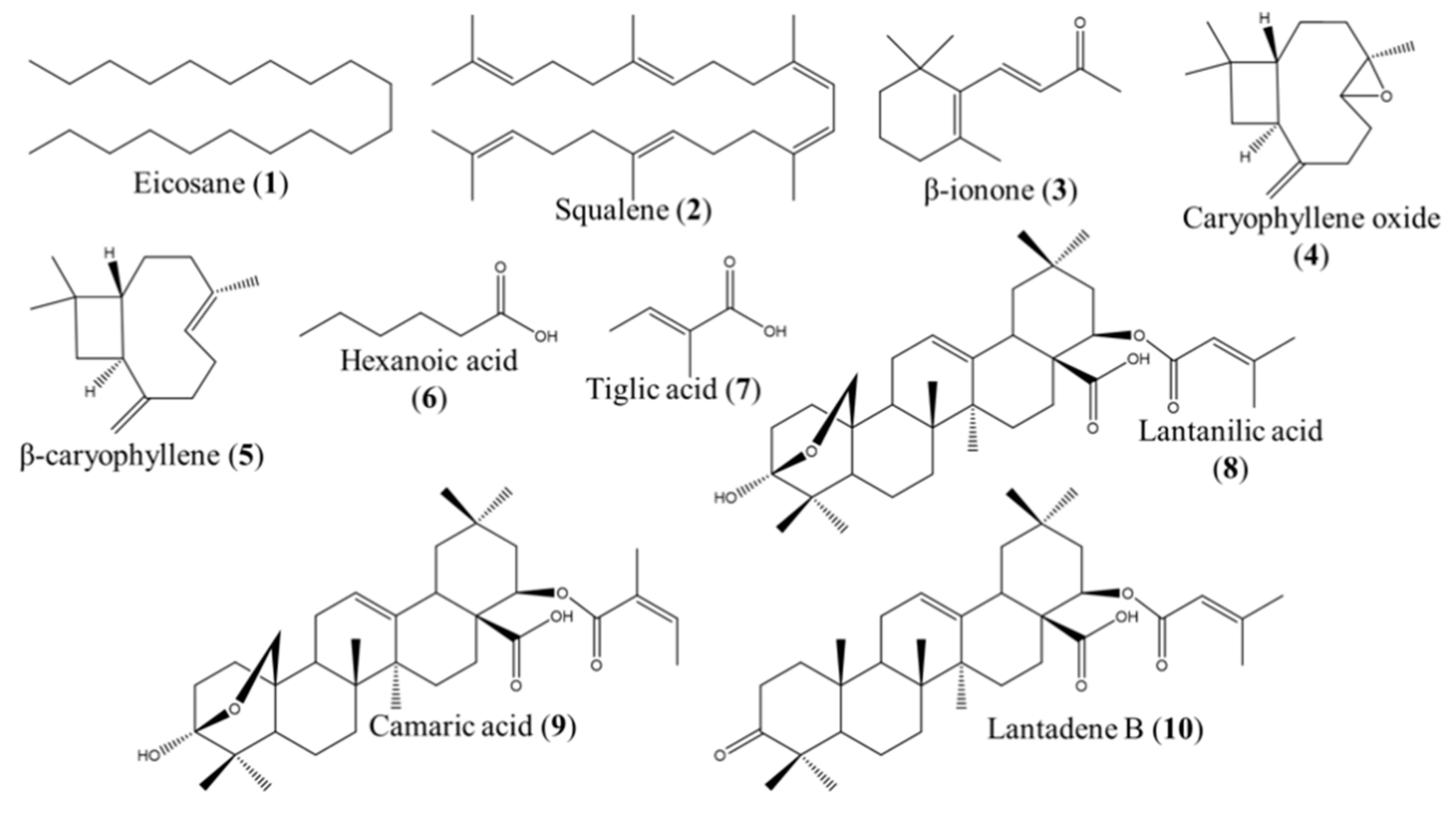
2.2. GC–MS and NMR Analysis for the Identification of the Active Compounds Contained in FII, FX, FXI, and FXV
2.3. Leishmanicidal Activity of the Compounds Identified in the Fractions FII, FX, FXI, and FXV
3. Materials and Methods
3.1. Chemicals
3.2. Biological Material
3.3. Plant Material
3.4. Extraction and Isolation
3.5. GC–MS Analysis of FII
3.6. HPLC-PDA Analysis of Fractions FX, FXI, and FXV
3.7. NMR Analysis for the Identification of the Triterpenes Isolated from Fractions FX, FXI, and FXV
3.8. In Vitro Biological Testing
3.8.1. Cytotoxicity Assay on BALB/c Mice Peritoneal Macrophages
3.8.2. In Vitro Anti-Amastigote Activity on Leishmania amazonensis
3.8.3. In Vitro Anti-Promastigote Activity on Leishmania mexicana
3.8.4. In Vitro Anti-Amastigote Activity on Leishmania mexicana
3.8.5. Selectivity Index
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; de Boer, M. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef] [PubMed]
- Akhoundi, M.; Kuhls, K.; Cannet, A.; Votýpka, J.; Marty, P.; Delaunay, P.; Sereno, D. A historical overview of the classification, evolution, and dispersion of Leishmania parasites and sandflies. PLoS Negl. Trop. Dis. 2016, 10, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, V.; Cordeiro-da-Silva, A.; Laforge, M.; Silvestre, R.; Estaquier, J. Regulation of immunity during visceral Leishmania infection. Parasit. Vectors 2016, 9, 118. [Google Scholar] [CrossRef] [PubMed]
- Scott, P.; Novais, F.O. Cutaneous leishmaniasis: Immune responses in protection and pathogenesis. Nat. Rev. Immunol. 2016, 16, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Séguin, O.; Descoteaux, A. Leishmania, the phagosome, and host responses: The journey of a parasite. Cell. Immunol. 2016, 309, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; Barnes, J.L.; Anstead, G.M.; Jimenez, F.; Travi, B.L.; Peniche, A.G.; Osorio, E.Y.; Ahuja, S.S.; Melby, P.C. The malnutrition-related increase in early visceralization of Leishmania donovani is associated with a reduced number of lymph node phagocytes and altered conduit system flow. PLoS Negl. Trop. Dis. 2013, 7, e2329. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.K.; Barnes, J.L.; Osorio, E.Y.; Anstead, G.M.; Jimenez, F.; Osterholzer, J.J.; Travi, B.L.; Ahuja, S.S.; White, A.C.; Melby, P.C. Deficiency of lymph node-resident dendritic cells (DCs) and dysregulation of DC chemoattractants in a malnourished mouse model of Leishmania donovani infection. Infect. Immun. 2014, 82, 3098–3112. [Google Scholar] [CrossRef]
- Malafaia, G. Protein-energy malnutrition as a risk factor for visceral leishmaniasis: A review. Parasite Immunol. 2009, 31, 587–596. [Google Scholar] [CrossRef]
- Kumar, V.; Bimal, S.; Singh, S.K.; Chaudhary, R.; Das, S.; Lal, C.; Pandey, K.; Das, V.R.; Das, P. Leishmania donovani: Dynamics of L. donovani evasion of innate immune cell attack due to malnutrition in visceral leishmaniasis. Nutrition 2014, 30, 449–458. [Google Scholar] [CrossRef]
- Lestinova, T.; Rohousova, I.; Sima, M.; De Oliveira, C.I.; Volf, P. Insights into the sand fly saliva: Blood-feeding and immune interactions between sand flies, hosts, and Leishmania. PLoS Negl. Trop. Dis. 2017, 13, 1–26. [Google Scholar] [CrossRef]
- Reithinger, R.; Dujardin, J.; Louzir, H.; Pirmez, C.; Alexander, B.; Brooker, S. Cutaneous leishmaniasis. Lancet Infect. Dis. 2007, 7, 581–596. [Google Scholar] [CrossRef]
- Pedrosa, M.S.C. Clinical manifestations of visceral leishmaniasis (American visceral leishmaniasis). In The Epidemiology and Ecology of Leishmaniasis; IntechOpen Limited: London, UK, 2017; pp. 17–30. [Google Scholar]
- Özkeklikçi, A.; Karakuş, M.; Özbel, Y.; Töz, S. The new situation of cutaneous leishmaniasis after Syrian civil war in Gaziantep city, Southeastern region of Turkey. Acta Trop. 2017, 166, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Doganay, M.; Demiraslan, H. Refugees of the Syrian civil war: Impact on reemerging infections, health services, and biosecurity in Turkey. Heal. Secur. 2016, 14, 220–225. [Google Scholar] [CrossRef]
- Desjeux, P. Leishmaniasis: Current situation and new perspectives. Comp. Immunol. Microbiol. Infect. Dis. 2004, 27, 305–318. [Google Scholar] [CrossRef] [PubMed]
- Desjeux, P. Worldwide increasing risk factors for leishmaniasis. Med. Microbiol. Immunol. 2001, 190, 77–79. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Akbari, M. Asian Paci fi c Journal of Tropical Medicine. Asian Pac. J. Trop. Med. 2016, 9, 925–932. [Google Scholar] [CrossRef] [PubMed]
- Alasaad, S. War diseases revealed by the social media: Massive leishmaniasis outbreak in the Syrian spring. Parasit. Vectors 2013, 6, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.H.; Sen, P.; Roy, S. Use of antimony in the treatment of leishmaniasis: Current status and future directions. Mol. Biol. Int. 2011, 1–23. [Google Scholar]
- Sundar, S.; Chakravarty, J. Antimony toxicity. Int. J. Environ. Res. Public Health 2010, 7, 4267–4277. [Google Scholar] [CrossRef] [PubMed]
- Rajasekaran, R.; Chen, Y.-P.P. Potential therapeutic targets and the role of technology in developing novel antileishmanial drugs. Drug Discov. Today 2015, 20, 958–968. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Mishra, B.B.; Bajpai, S.; Singh, R.K.; Tiwari, V.K. Natural product based leads to fight against leishmaniasis. Bioorg. Med. Chem. 2014, 22, 18–45. [Google Scholar] [CrossRef] [PubMed]
- Corpas-López, V.; Morillas-Márquez, F.; Navarro-Moll, M.C.; Merino-Espinosa, G.; Díaz-Sáez, V.; Martín-Sánchez, J. (−)-α-bisabolol, a promising oral compound for the treatment of visceral leishmaniasis. J. Nat. Prod. 2015, 78, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Corpas-López, V.; Merino-Espinosa, G.; López-Viota, M.; Gijón-Robles, P.; Morillas-Mancilla, M.J.; López-Viota, J.; Díaz-Sáez, V.; Morillas-Márquez, F.; Navarro Moll, M.C.; Martín-Sánchez, J. Topical treatment of Leishmania tropica infection using (−)-α-bisabolol ointment in a hamster model: Effectiveness and safety assessment. J. Nat. Prod. 2016, 79, 2403–2407. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, J.D.; Aligiannis, N.; Polychronopoulos, P.; Skaltsounis, A.L.; Dotsika, E. Leishmanicidal activity assessment of olive tree extracts. Phytomedicine 2013, 20, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Kyriazis, I.D.; Koutsoni, O.S.; Aligiannis, N.; Karampetsou, K.; Skaltsounis, A.-L.; Dotsika, E. The leishmanicidal activity of oleuropein is selectively regulated through inflammation- and oxidative stress-related genes. Parasit. Vectors 2016, 9, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Altamirano, R.; Monzote, L.; Piñón-Tápanes, A.; Vibrans, H.; Rivero-Cruz, J.F.; Ibarra-Alvarado, C.; Rojas-Molina, A. In vitro antileishmanial activity of Mexican medicinal plants. Heliyon 2017, 3, e00394. [Google Scholar] [CrossRef] [PubMed]
- Ghisalberti, E.L. Lantana camara L. (Verbenaceae). Fitoterapia 2000, 71, 467–486. [Google Scholar] [CrossRef]
- Goncalves, E.; Herrera, I.; Duarte, M.; Bustamante, R.O.; Lampo, M.; Velásquez, G.; Sharma, G.P.; García-Rangel, S. Global invasion of Lantana camara: Has the climatic niche been conserved across continents? PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Sharma, S.; Pattabhi, V.; Mahato, S.B.; Sharma, P.D. A review of the hepatotoxic plant Lantana camara. Crit. Rev. Toxicol. 2007, 37, 313–352. [Google Scholar] [CrossRef] [PubMed]
- Bhagwat, S.A.; Breman, E.; Thekaekara, T.; Thornton, T.F.; Willis, K.J. A battle lost? Report on two centuries of invasion and management of Lantana camara L. in Australia, India and South Africa. PLoS One 2012, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves Ferreira Macedo, J.; Alencar De Menezes, I.R.; Alves Ribeiro, D.; De Oliveira Santos, M.; Gonçalves De Mâcedo, D.; Ferreira Macêdo, M.J.; Vilar De Almeida, B.; Geraldo Souza De Oliveira, L.; Pereira Leite, C.; De Almeida Souza, M.M. Analysis of the variability of therapeutic indications of medicinal species in the Northeast of Brazil: Comparative study. Evid.-Based Complement. Altern. Med. 2018, 2018, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Bisi-Johnson, M.A.; Obi, C.L.; Hattori, T.; Oshima, Y.; Li, S.; Kambizi, L.; Eloff, J.N.; Vasaikar, S.D. Evaluation of the antibacterial and anticancer activities of some South African medicinal plants. BMC Complement. Altern. Med. 2011, 11, 14. [Google Scholar] [CrossRef] [PubMed]
- Cheruiyot, K.R.; Olila, D.; Kateregga, J. In-vitro antibacterial activity of selected medicinal plants from Longisa region of Bomet district, Kenya. Afr. Health Sci. 2009, 9, S42–S46. [Google Scholar] [PubMed]
- Moyo, B.; Masika, P.J.; Dube, S.; Maphosa, V. An in-vivo study of the efficacy and safety of ethno-veterinary remedies used to control cattle ticks by rural farmers in the Eastern Cape Province of South Africa. Trop. Anim. Health Prod. 2009, 41, 1569–1576. [Google Scholar] [CrossRef] [PubMed]
- Jonville, M.C.; Kodja, H.; Humeau, L.; Fournel, J.; De Mol, P.; Cao, M.; Angenot, L.; Frédérich, M. Screening of medicinal plants from Reunion Island for antimalarial and cytotoxic activity. J. Ethnopharmacol. 2008, 120, 382–386. [Google Scholar] [CrossRef]
- Magassouba, F.B.; Diallo, A.; Kouyaté, M.; Mara, F.; Mara, O.; Bangoura, O.; Camara, A.; Traoré, S.; Diallo, A.K.; Zaoro, M.; et al. Ethnobotanical survey and antibacterial activity of some plants used in Guinean traditional medicine. J. Ethnopharmacol. 2007, 114, 44–53. [Google Scholar] [CrossRef] [PubMed]
- Dabur, R.; Gupta, A.; Mandal, T.K.; Singh, D.D.; Bajpai, V.; Gurav, A.M.; Lavekar, G.S. Antimicrobial activity of some Indian medicinal plants. Afr. J. Tradit. Complement. Altern. Med. 2007, 4, 313–318. [Google Scholar] [CrossRef]
- Basu, S.; Hazra, B. Evaluation of nitric oxide scavenging activity, in vitro and ex vivo, of selected medicinal plants traditionally used in infammatory diseases. Phyther. Res. 2006, 20, 896–900. [Google Scholar] [CrossRef]
- Hernández, T.; Canales, M.; Avila, J.G.; Duran, A.; Caballero, J.; Romo de Vivar, A.; Lira, R. Ethnobotany and antibacterial activity of some plants used in traditional medicine of Zapotitlán de las Salinas, Puebla (México). J. Ethnopharmacol. 2003, 88, 181–188. [Google Scholar] [CrossRef]
- Begum, S.; Ayub, A.; Zehra, S.Q.; Siddiqui, B.S.; Choudhary, M.I.; Sciences, B. Leishmanicidal triterpenes from Lantana camara. Chem. Biodivers. 2014, 11, 709–718. [Google Scholar] [CrossRef]
- Braga, F.G.; Bouzada, M.L.M.; Fabri, R.L.; Matos, M.D.O.; Moreira, F.O.; Scio, E.; Coimbra, E.S. Antileishmanial and antifungal activity of plants used in traditional medicine in Brazil. J. Ethnopharmacol. 2007, 111, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Weyerstahl, P.; Marschall, H.; Eckhardt, A.; Christiansen, C. Constituents of commercial Brazilian lantana oil. Flavour Fragr. J. 1999, 14, 15–28. [Google Scholar] [CrossRef]
- Santos, I.E.M. A taxonomic revision of Lantana sect. Lantana (Verbenaceae) in the Greater Antilles. Willdenowia 2002, 32, 285–301. [Google Scholar] [CrossRef]
- Queensland, D. Lantana—A Weed of National Significance Identification guide: Lantana Flowers. 2003; QNRM03382. Available online: https://www.daf.qld.gov.au/__data/assets/pdf_file/0004/60376/IPA-Lantana-Flowers-Id-Guide.pdf (accessed on 1 February 2019).
- Sharma, O.P.; Vaid, J.; Sharma, P.D. Comparison of lantadenes content and toxicity of different taxa of the Lantana plant. J. Chem. Ecol. 1991, 17, 2283–2291. [Google Scholar] [CrossRef] [PubMed]
- Randrianalijaona, J.-A.; Ramanoelina, P.A.R.; Rasoarahona, J.R.E.; Gaydou, E.M. Seasonal and chemotype influences on the chemical composition of Lantana camara L. Anal. Chim. Acta 2005, 545, 46–52. [Google Scholar] [CrossRef]
- Ghobakhloo, N.; Motazedian, M.H.; Pourmohammadi, B.; Yousefi, Z. Evaluation of correlation between the in vitro susceptibility of field isolates of Leishmania major and clinical outcomes of meglumine antimoniate therapy in Fars Province, Iran. J. Arthropod. Borne. Dis. 2017, 11, 132–138. [Google Scholar] [PubMed]
- Perez-Franco, J.E.; Cruz-Barrera, M.L.; Robayo, M.L.; Lopez, M.C.; Daza, C.D.; Bedoya, A.; Mariño, M.L.; Saavedra, C.H.; Echeverry, M.C. Clinical and parasitological features of patients with American cutaneous leishmaniasis that did not respond to treatment with meglumine antimoniate. PLoS Negl. Trop. Dis. 2016, 10, 1–13. [Google Scholar] [CrossRef]
- Martín-Montes, Á.; Plano, D.; Martín-Escolano, R.; Alcolea, V.; Díaz, M.; Pérez-Silanes, S.; Espuelas, S.; Moreno, E.; Marín, C.; Gutiérrez-Sánchez, R.; et al. Library of seleno-compounds as novel agents against Leishmania species. Antimicrob. Agents Chemother. 2017, 61, 1–13. [Google Scholar] [CrossRef]
- Mahmoudvand, H.; Kheirandish, F.; Mirbadie, S.R.; Kayedi, M.H.; Rezaei Riabi, T.; Ghasemi, A.A.; Bamorovat, M.; Sharifi, I. The potential use of methotrexate in the treatment of cutaneous leishmaniasis: In vitro assays against sensitive and meglumine antimoniate-resistant strains of Leishmania tropica. Iran J. Parasitol. 2017, 12, 339–347. [Google Scholar]
- Ginouvès, M.; Simon, S.; Nacher, M.; Demar, M.; Carme, B.; Couppié, P.; Prévot, G. In vitro sensitivity of cutaneous Leishmania promastigote isolates circulating in French guiana to a set of drugs. Am. J. Trop. Med. Hyg. 2017, 96, 1143–1150. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Vanden, D.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro ‘proof-of-concept’. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- García, M.; Monzote, L.; Scull, R.; Herrera, P. Activity of Cuban plants extracts against Leishmania amazonensis. ISRN Pharmacol. 2012, 2012, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Altamirano, R.; Rojas, A.; Esturau-Escofet, N. 1H and 13C NMR reassignment of some chemical shifts of lantanilic acid and camaric acid. Magn. Reson. Chem. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; García, M.; Pastor, J.; Gil, L.; Scull, R.; Maes, L.; Cos, P.; Gille, L. Essential oil from Chenopodium ambrosioides and main components: Activity against Leishmania, their mitochondria and other microorganisms. Exp. Parasitol. 2014, 136, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Monzote, L.; Geroldinger, G.; Tonner, M.; Scull, R.; De Sarkar, S.; Bergmann, S.; Bacher, M.; Staniek, K.; Chatterjee, M.; Rosenau, T.; et al. Interaction of ascaridole, carvacrol, and caryophyllene oxide from essential oil of Chenopodium ambrosioides L. with mitochondria in Leishmania and other eukaryotes. Phyther. Res. 2018, 1–12. [Google Scholar] [CrossRef]
- Monzote, L.; Pastor, J.; Scull, R.; Gille, L. Antileishmanial activity of essential oil from Chenopodium ambrosioides and its main components against experimental cutaneous leishmaniasis in BALB/c mice. Phytomedicine 2014, 21, 1048–1052. [Google Scholar] [CrossRef]
- DNDi Drugs for Neglected Diseases iniciative: Leishmaniasis. Available online: https://www.dndi.org/diseases-projects/leishmaniasis/ (accessed on 1 February 2019).
- Soares, D.C.; Portella, N.A.; Ramos, M.F.D.S.; Siani, A.C.; Saraiva, E.M. Trans-β-Caryophyllene: An effective antileishmanial compound found in commercial copaiba Oil (Copaifera spp.). Evid.-Based Complement. Altern. Med. 2013, 2013, 1–13. [Google Scholar] [CrossRef]
- Ridoux, O.; Di Giorgio, C.; Delmas, F.; Elias, R.; Mshvildadze, V.; Dekanosidze, G.; Kemertelidze, E.; Balansard, G.; Timon-David, P. In vitro antileishmanial activity of three saponins isolated from ivy, α-hederin, β-hederin and hederacolchiside A1, in association with pentamidine and amphotericin B. Phyther. Res. 2001, 15, 298–301. [Google Scholar] [CrossRef]
- Torres-Santos, E.C.; Lopes, D.; Rodrigues Oliveira, R.; Carauta, J.P.P.; Bandeira Falcao, C.A.; Kaplan, M.A.C.; Rossi-Bergmann, B. Antileishmanial activity of isolated triterpenoids from Pourouma guianensis. Phytomedicine 2004, 11, 114–120. [Google Scholar] [CrossRef]
- Sifaoui, I.; López-Arencibia, A.; Martín-Navarro, C.M.; Reyes-Batlle, M.; Mejri, M.; Lorenzo-Morales, J.; Abderabba, M.; Piñero, J.E. Selective activity of oleanolic and maslinic acids on the amastigote form of Leishmania spp. Iran. J. Pharm. Res. 2017, 16, 1190–1193. [Google Scholar]
- Gnoatto, S.C.B.; Vechia Dalla, L.; Lencina, C.L.; Dassonville-Klimpt, A.; Da Nascimento, S.; Mossalayi, D.; Guillon, J.; Gosmann, G.; Sonnet, P. Synthesis and preliminary evaluation of new ursolic and oleanolic acids derivatives as antileishmanial agents. J. Enzyme Inhib. Med. Chem. 2008, 23, 604–610. [Google Scholar] [CrossRef]
- Camacho, M.D.R.; Mata, R.; Castaneda, P.; Kirby, G.C.; Warhurst, D.C.; Croft, S.L.; Phillipson, J.D. Bioactive compounds from Celaenodendron mexicanum. Planta Med. 2000, 66, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Tan, N.; Kaloga, M.; Radtke, O.A.; Kiderlen, A.F.; Öksüz, S.; Ulubelen, A.; Kolodziej, H. Abietane diterpenoids and triterpenoic acids from Salvia cilicica and their antileishmanial activities. Phytochemistry 2002, 61, 881–884. [Google Scholar] [CrossRef]
- Tiwari, N.; Gedda, M.R.; Tiwari, V.K.; Singh, S.P.; Singh, R.K. Limitations of current therapeutic options, possible drug targets and scope of natural products in control of leishmaniasis. Mini-Rev. Med. Chem. 2017, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- De Souza, W.; Rodrigues, F.C.J. Sterol biosynthesis pathway as target for anti-trypanosomatid drugs. Interdiscip. Perspect. Infect. Dis. 2009, 1–19. [Google Scholar] [CrossRef]
- McCall, L.-I.; El-Aroussi, A.; Yong Choi, J.; Vieira, D.F.; De Muylder, G.; Johnston, J.B.; Chen, S.; Kellar, D.; Siqueira-Neto, J.L.; Roush, W.R.; et al. Targeting ergosterol biosynthesis in Leishmania donovani: Essentiality of sterol 14α-demethylase. PLoS Negl. Trop. Dis. 2015, 9, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.T.; Rocha, G.C.; Costa, S.D.; Rodrigues, C.M.; Ferreira, C.; Tavares, M.T.; Saraiva, E.; Parise-filho, R.; Braden, H.; Delorenzi, J.C. Oleanolic acid (OA) as an antileishmanial agent: Biological evaluation and in silico mechanistic insights. Parasitol. Int. 2016, 65, 227–237. [Google Scholar]
- Warfield, J.; Setzer, W.N.; Ogungbe, I.V. Interactions of antiparasitic sterols with sterol 14α-demethylase (CYP51) of human pathogens. Springer Plus 2014, 3, 1–11. [Google Scholar] [CrossRef]
- Goad, L.J.; Holz, G.G.; Beach, D.H. Sterols of Leishmania species, implications for biosynthesis. Mol. Biochem. Parasitol. 1984, 10, 161–170. [Google Scholar] [CrossRef]
- Yao, C.; Wilson, M.E. Dynamics of sterol synthesis during development of Leishmania spp. parasites to their virulent form. Parasites Vectors 2016, 9, 1–12. [Google Scholar] [CrossRef]
- Begum, S.; Zehra, S.Q.; Siddiqui, B.S.; Fayyaz, S.; Ramzan, M. Pentacyclic triterpenoids from the aerial parts of Lantana camara and their nematicidal activity. Chem. Biodivers. 2008, 5, 1856–1866. [Google Scholar] [CrossRef] [PubMed]
- Qamar, F.; Begum, S.; Raza, S.M.; Wahab, A.; Siddiqui, B.S.; Begum, S.; Raza, S.M. Nematicidal natural products from the aerial parts of Lantana camara Linn. Nat. Prod. Res. 2005, 19, 609–613. [Google Scholar] [CrossRef]
- Saleh, M.; Kamel, A.; Li, X.; Swaray, J. Antibacterial triterpenoids isolated from Lantana camara. Pharm. Biol. 1999, 37, 63–66. [Google Scholar] [CrossRef]
- Ishibashi, M.; Oda, H.; Mitamura, M.; Okuyama, E.; Komiyama, K.; Kawaguchi, K.; Watanabe, T.; de Mello Alves, S.; Maekawa, T.; Ohtsuki, K. Casein kinase II inhibitors isolated from two Brazilian plants Hymenaea parvifolia and Wulffia baccata. Bioorganic Med. Chem. Lett. 1999, 9, 2157–2160. [Google Scholar] [CrossRef]
- Innocent, E.; Joseph, C.C.; Gikonyo, N.K.; Moshi, M.J.; Nkunya, M.H.H.; Hassanali, A. Mosquito larvicidal constituents from Lantana viburnoides sp. viburnoides var kisi (A. rich) Verdc (Verbenaceae). J. Vector Borne Dis. 2008, 45, 240–244. [Google Scholar] [PubMed]
- Mohamed, N.M.; Makboul, M.A.; Farag, S.F.; Jain, S.; Jacob, M.R.; Tekwani, B.L.; Ross, S.A. Triterpenes from the roots of Lantana montevidensis with antiprotozoal activity. Phytochem. Lett. 2016, 15, 30–36. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information Lantadene B Toxicity. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/15560077#section=Non-Human-Toxicity-Excerpts (accessed on 2 February 2019).
- Sharma, S.; Sharma, O.P.; Singh, B.; Bhat, T.K. Biotransformation of lantadenes, the pentacyclic triterpenoid hepatotoxins of lantana plant, in guinea pig. Toxicon 2000, 38, 1191–1202. [Google Scholar] [CrossRef]
| Extract/Fraction | CC50 ± SD (μg/mL) BALB/c Peritoneal Macrophages | IC50 ± SD (μg/mL) Leishmania amazonensis Amastigotes | SI on L. amazonensis | IC50 ± SD (μg/mL) L. mexicana Promastigotes | SI on L. mexicana |
|---|---|---|---|---|---|
| Crude extract | >100 | 21.8 ± 2.4 | >5 | 42.6 ± 1.9 | >2 |
| FI | 65.2 ± 2.3 | 12.2 ± 1.8 | 5 | >200 | <0.5 |
| FII | >100 | 9.1 ± 3.4 | >11 | 132.1 ± 7.9 | 0.49 |
| FIII | >100 | 18.3 ± 1.5 | >5 | >200 | N.C. |
| FIV | >100 | 30.2 ± 3.5 | >3 | >200 | <0.5 |
| FV | >100 | 17.9 ± 3.8 | >6 | >200 | <0.5 |
| FVI | >100 | 21.5 ± 4.3 | >5 | >200 | <0.5 |
| FVII | 96.8 ± 2.2 | 17.9 ± 2.5 | >5 | >200 | <0.5 |
| FVIII | 92.3 ± 3.1 | 24.7 ± 2.9 | >4 | >200 | <0.5 |
| FIX | >100 | 18.6 ± 1.5 | >5 | 96.0 ± 1.6 | >1 |
| FX | >100 | 7.9 ± 0.3 | >13 | 11.2 ± 2.2 | >9 |
| FXI | >100 | 8.0 ± 1.1 | >13 | 22.0 ± 9.3 | >4 |
| FXII | >100 | 16.7 ± 3.7 | >6 | 87.3 ± 2.1 | >1 |
| FXIII | 82.2 ± 2.6 | 12.1 ± 0.7 | >7 | 83.3 ± 6.9 | 1 |
| FXIV | 60.8 ± 1.4 | 15.1 ± 1.0 | >4 | 89.4 ± 2.4 | N.C. |
| FXV | >100 | 23.6 ± 2.6 | >4 | 2.8 ± 0.5 | >35 |
| FXVI | >100 | 8.5 ± 1.7 | >12 | 16.5 ± 1.6 | >6 |
| FXVII | >100 | 19.8 ± 3.9 | >5 | 157.8 ± 15.5 | <0.6 |
| FXVIII | >100 | 15.6 ± 4.7 | >6 | >200 | <0.5 |
| Pentamidine | 11.7 ± 1.7 | 1.3 ± 0.1 | 9 | N.D. | N.D. |
| Glucantime® | 60.0 ± 3.0 | N.D. | N.D. | 4019.7 ± 140.4 | 0.015 |
| Fraction | Compound | IC50 ± SD (μM) L. mexicana promastigotes |
|---|---|---|
| FII | Eicosane (1) | >500 a |
| Squalene (2) | >500 a | |
| β-ionone (3) | 80.80 ± 7.09 b | |
| Caryophyllene oxide (4) | 81.62 ± 2.16 b | |
| β-caryophyllene (5) | 74.43 ± 4.38 b | |
| Hexanoic acid (6) | 42.96 ± 6.02 c | |
| Tiglic acid (7) | >500 a | |
| FX, FXV | Lantanilic acid (8)/Camaric acid (9) (79%/21%) | 12.02 ± 0.36 d (9.50 ± 0.28 lantanilic acid, 8) (2.52 ± 0.08 camaric acid, 9) |
| FX, FXI | Lantadene B (10) | 23.45 ± 2.15 d |
| Glucantime® | 11,000.00 ± 2.15 e |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delgado-Altamirano, R.; López-Palma, R.I.; Monzote, L.; Delgado-Domínguez, J.; Becker, I.; Rivero-Cruz, J.F.; Esturau-Escofet, N.; Vázquez-Landaverde, P.A.; Rojas-Molina, A. Chemical Constituents with Leishmanicidal Activity from a Pink-Yellow Cultivar of Lantana camara var. aculeata (L.) Collected in Central Mexico. Int. J. Mol. Sci. 2019, 20, 872. https://doi.org/10.3390/ijms20040872
Delgado-Altamirano R, López-Palma RI, Monzote L, Delgado-Domínguez J, Becker I, Rivero-Cruz JF, Esturau-Escofet N, Vázquez-Landaverde PA, Rojas-Molina A. Chemical Constituents with Leishmanicidal Activity from a Pink-Yellow Cultivar of Lantana camara var. aculeata (L.) Collected in Central Mexico. International Journal of Molecular Sciences. 2019; 20(4):872. https://doi.org/10.3390/ijms20040872
Chicago/Turabian StyleDelgado-Altamirano, Ronna, Rosa Isela López-Palma, Lianet Monzote, José Delgado-Domínguez, Ingeborg Becker, José Fausto Rivero-Cruz, Nuria Esturau-Escofet, Pedro A. Vázquez-Landaverde, and Alejandra Rojas-Molina. 2019. "Chemical Constituents with Leishmanicidal Activity from a Pink-Yellow Cultivar of Lantana camara var. aculeata (L.) Collected in Central Mexico" International Journal of Molecular Sciences 20, no. 4: 872. https://doi.org/10.3390/ijms20040872
APA StyleDelgado-Altamirano, R., López-Palma, R. I., Monzote, L., Delgado-Domínguez, J., Becker, I., Rivero-Cruz, J. F., Esturau-Escofet, N., Vázquez-Landaverde, P. A., & Rojas-Molina, A. (2019). Chemical Constituents with Leishmanicidal Activity from a Pink-Yellow Cultivar of Lantana camara var. aculeata (L.) Collected in Central Mexico. International Journal of Molecular Sciences, 20(4), 872. https://doi.org/10.3390/ijms20040872