Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons
Abstract
1. Introduction
2. Results
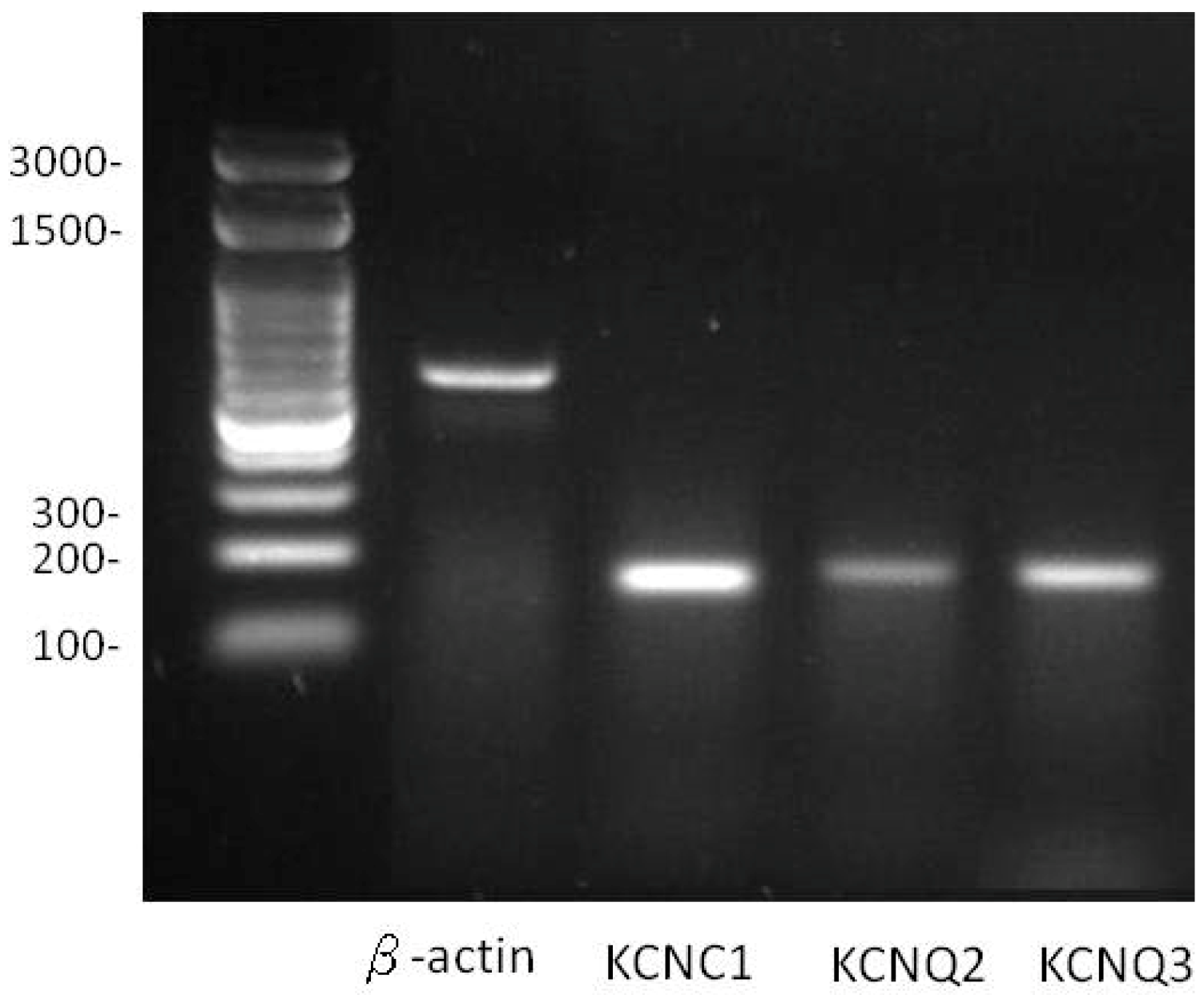
2.1. Expression of KCNC1 (KV3.1), KCNQ2 (KV7.2) and KCNQ3 (KV7.3) in Pituitary GH3 Cells
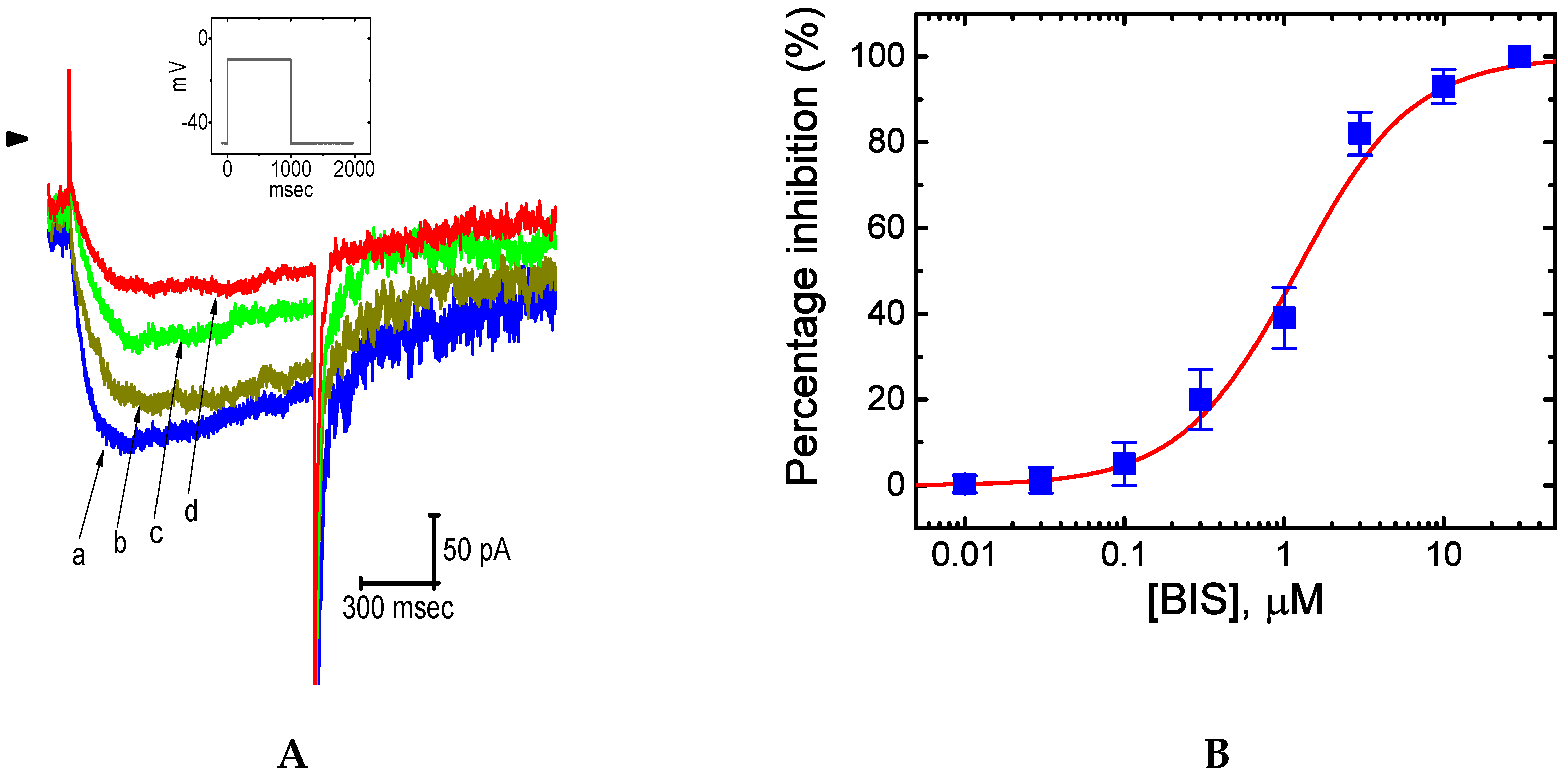
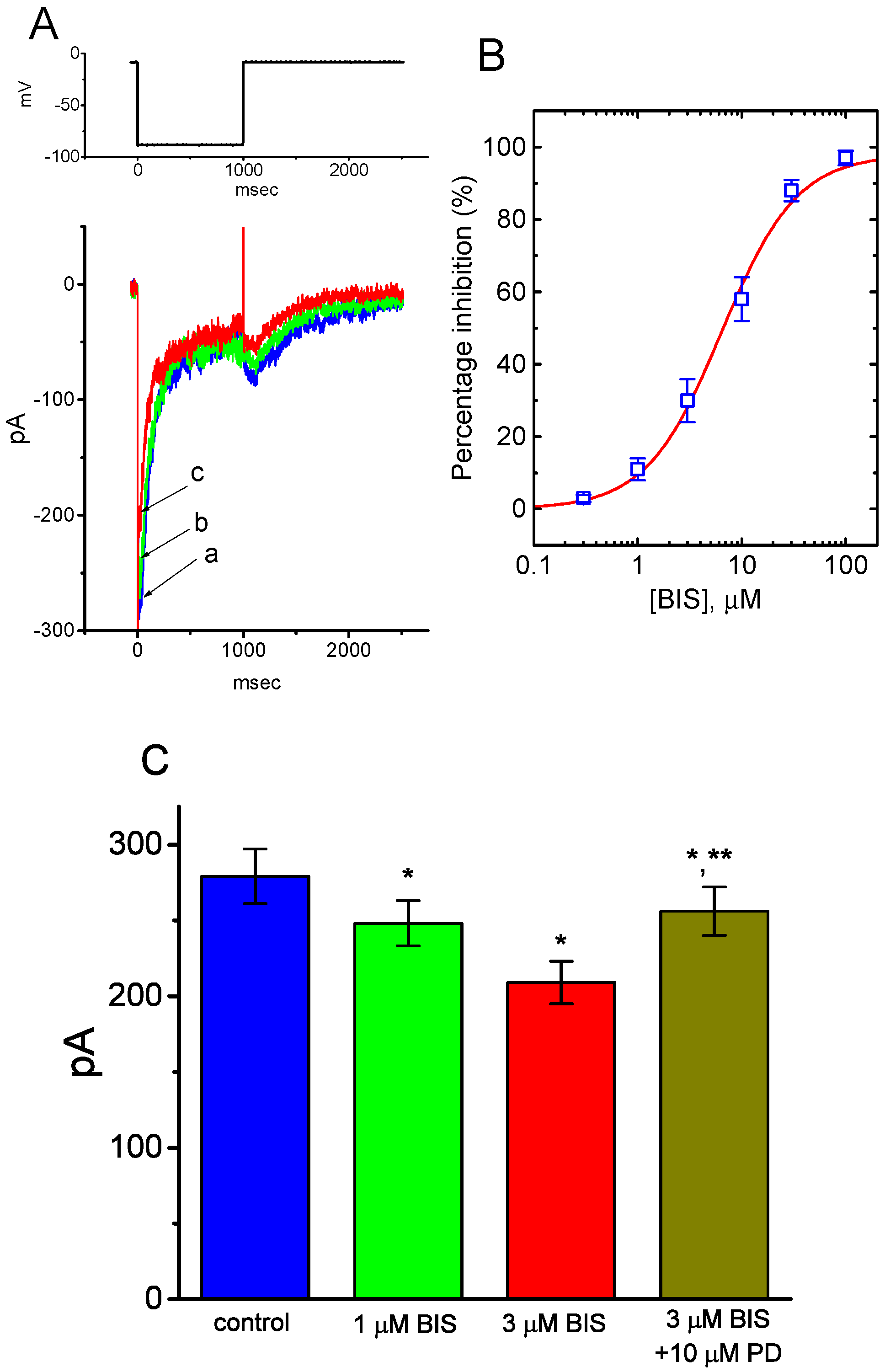
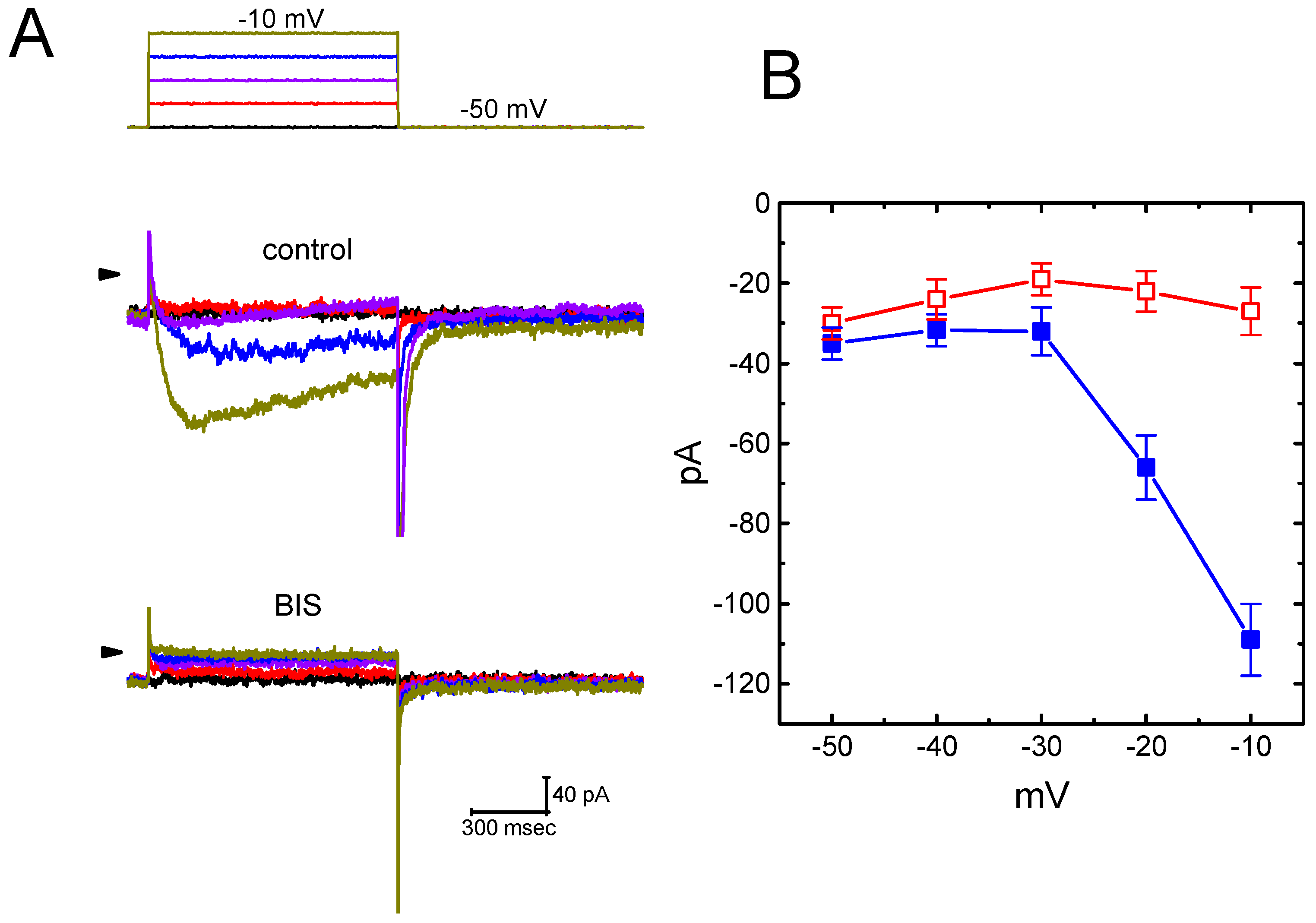
2.2. Effect of Bisoprolol (BIS) on IK(M) in Pituitary GH3 Cells
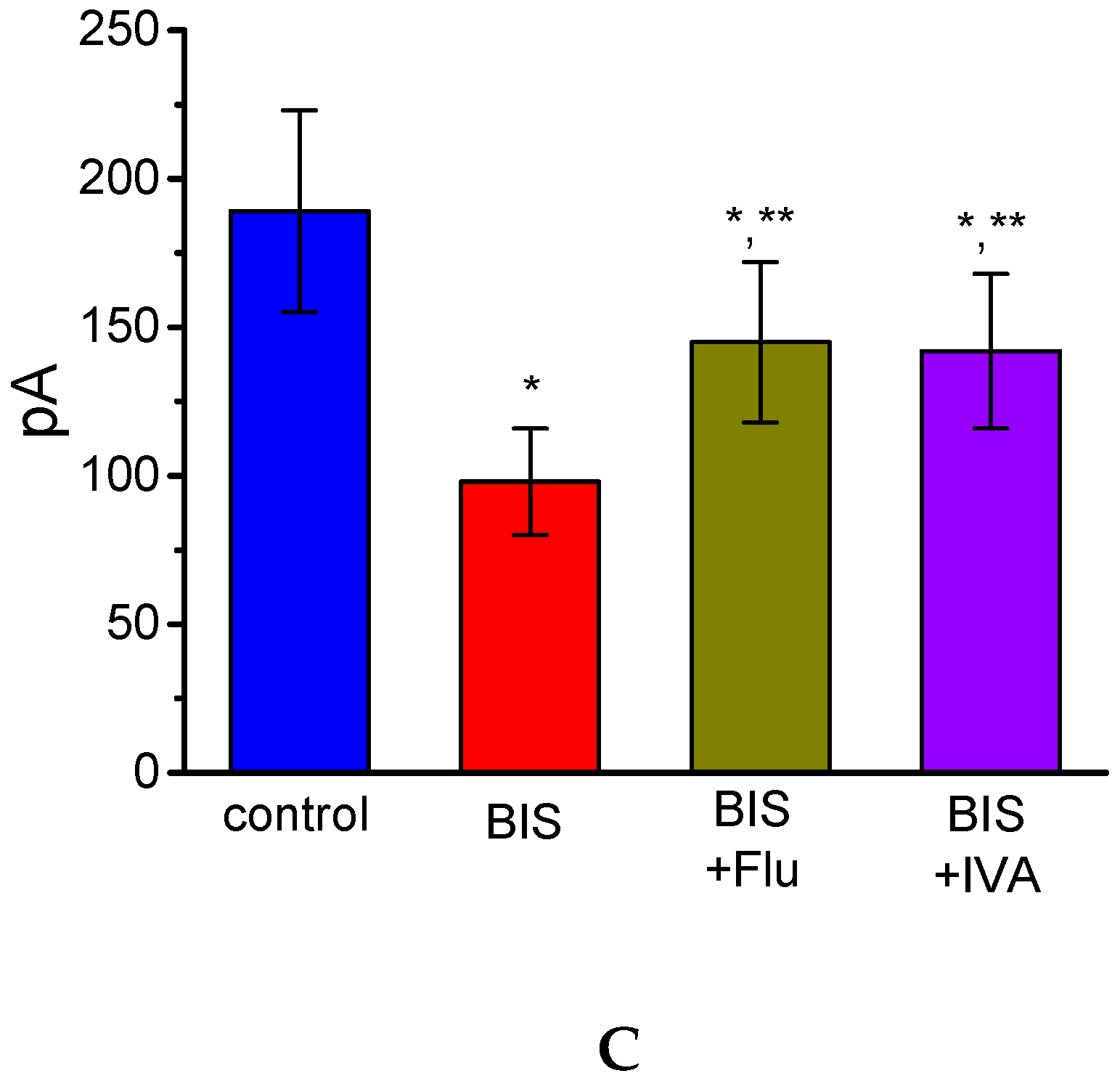
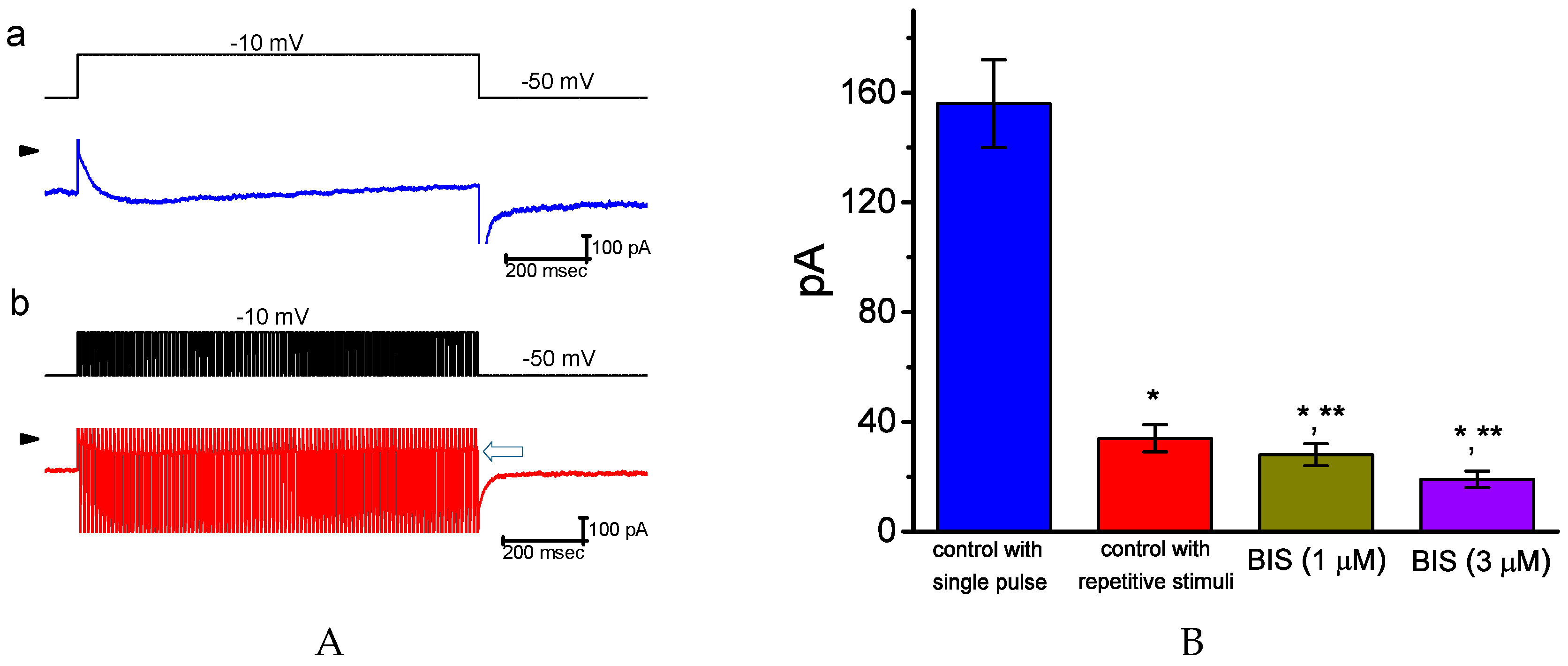
2.3. Inhibitory Effect of BIS on IK(M) Elicited by High Frequency Depolarizing Pulse
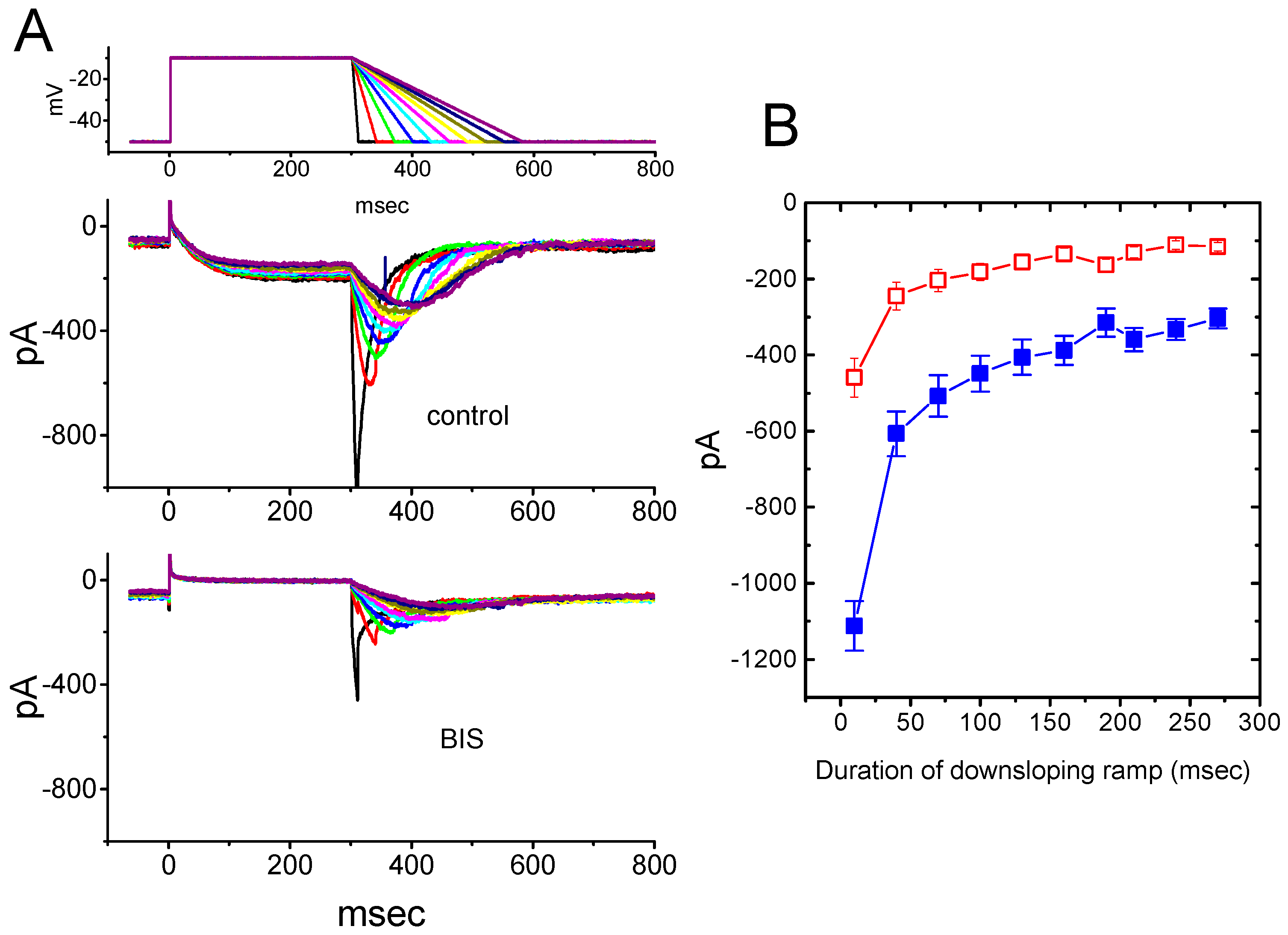
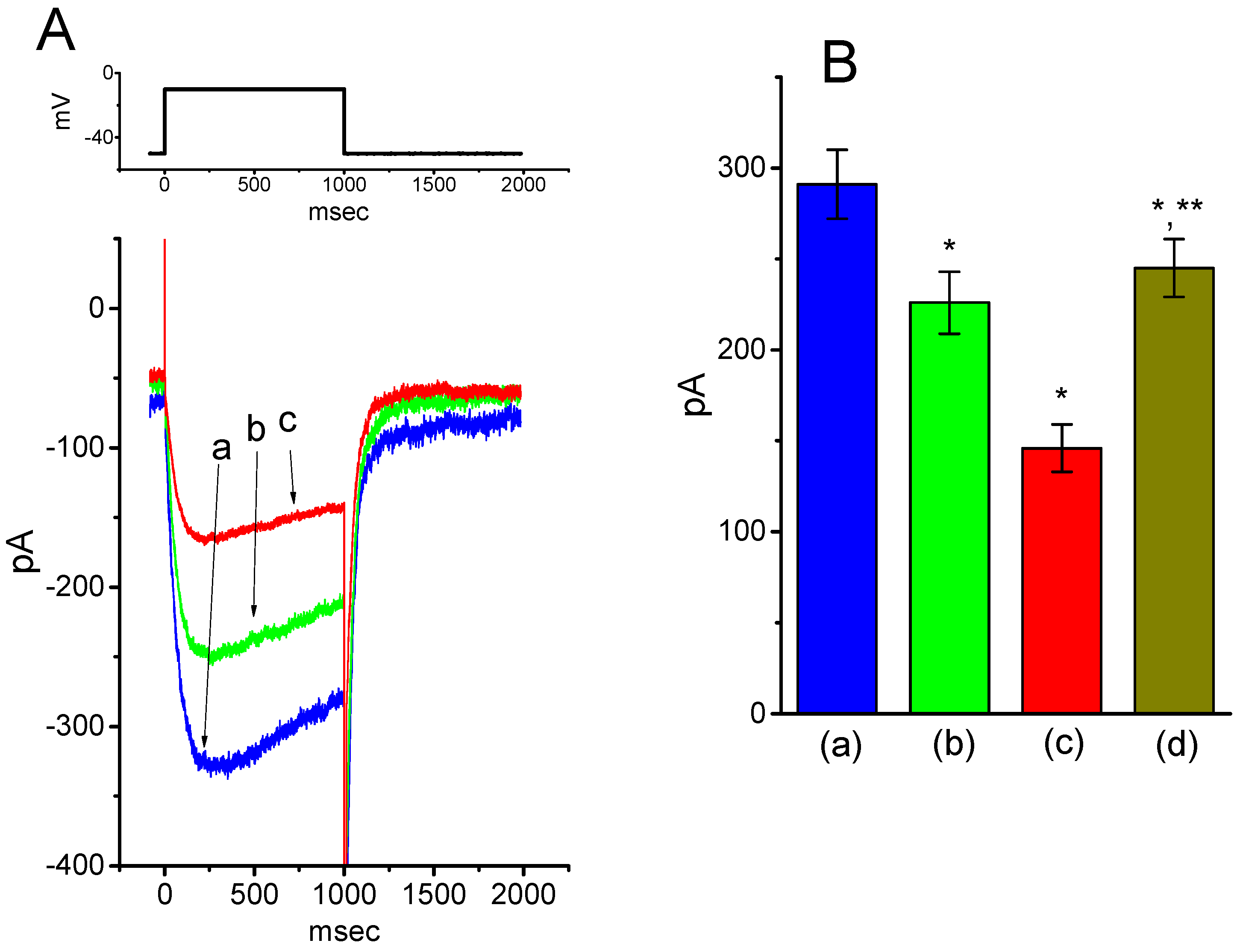
2.4. Effect of BIS on Deactivating IK(M) Elicited Upon Return to Membrane Hyperpolarization with Varying Duration
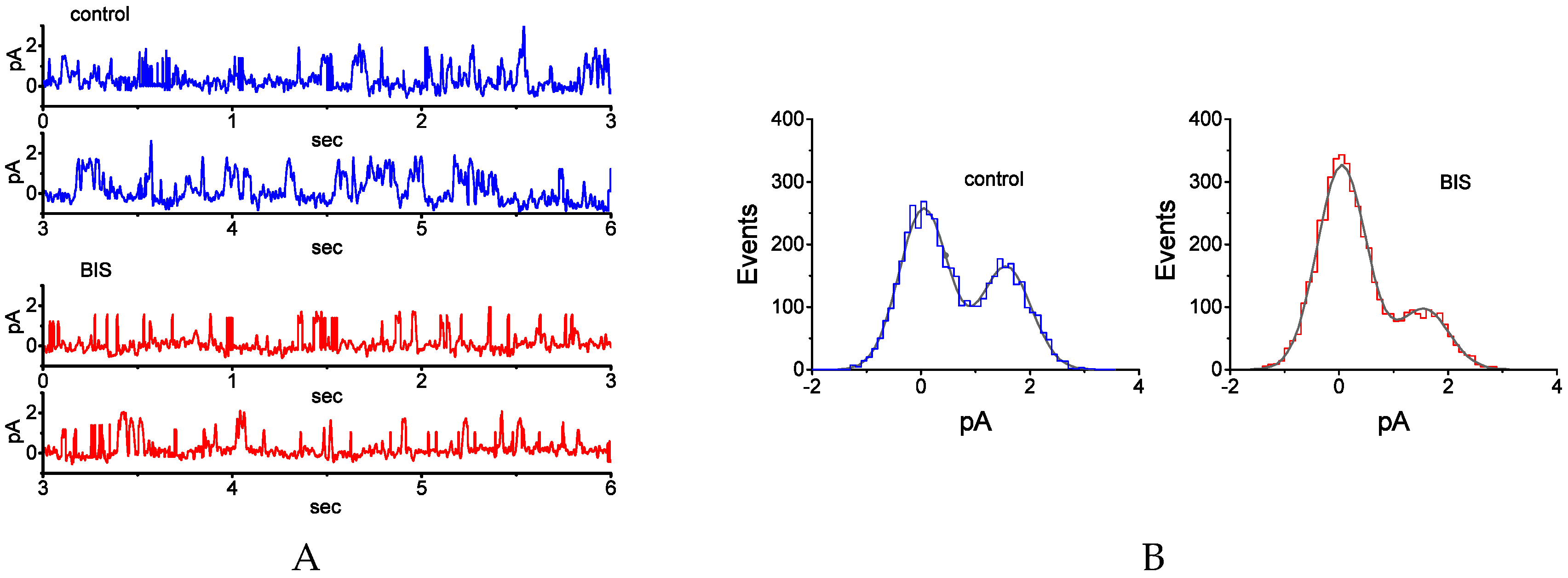
2.5. Inhibitory Effect of BIS on the Activity of Single M-type K+ (KM) Channels in GH3 Cells
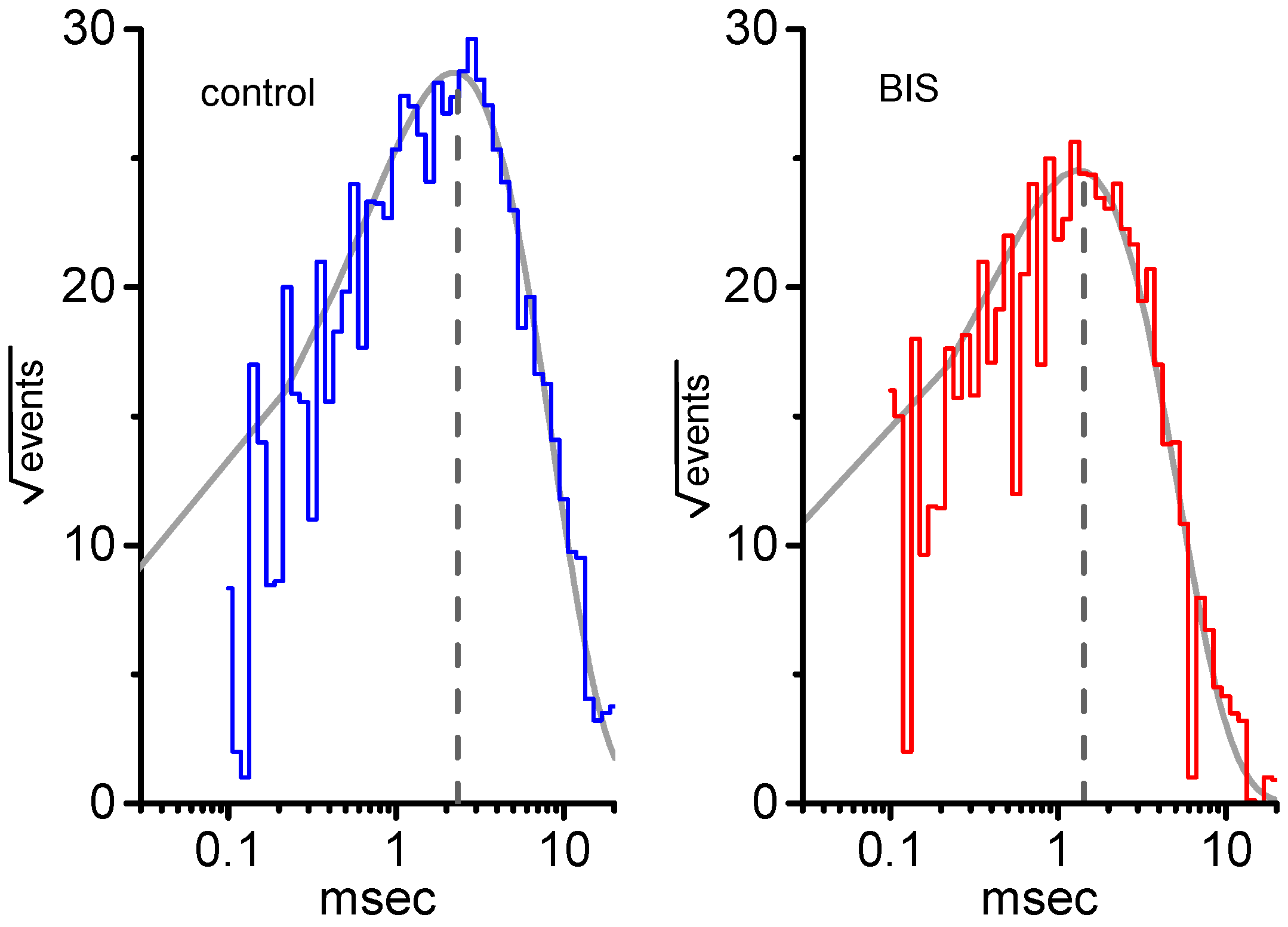
2.6. Effect of BIS on Mean Open Time of KM Channels in GH3 Cells
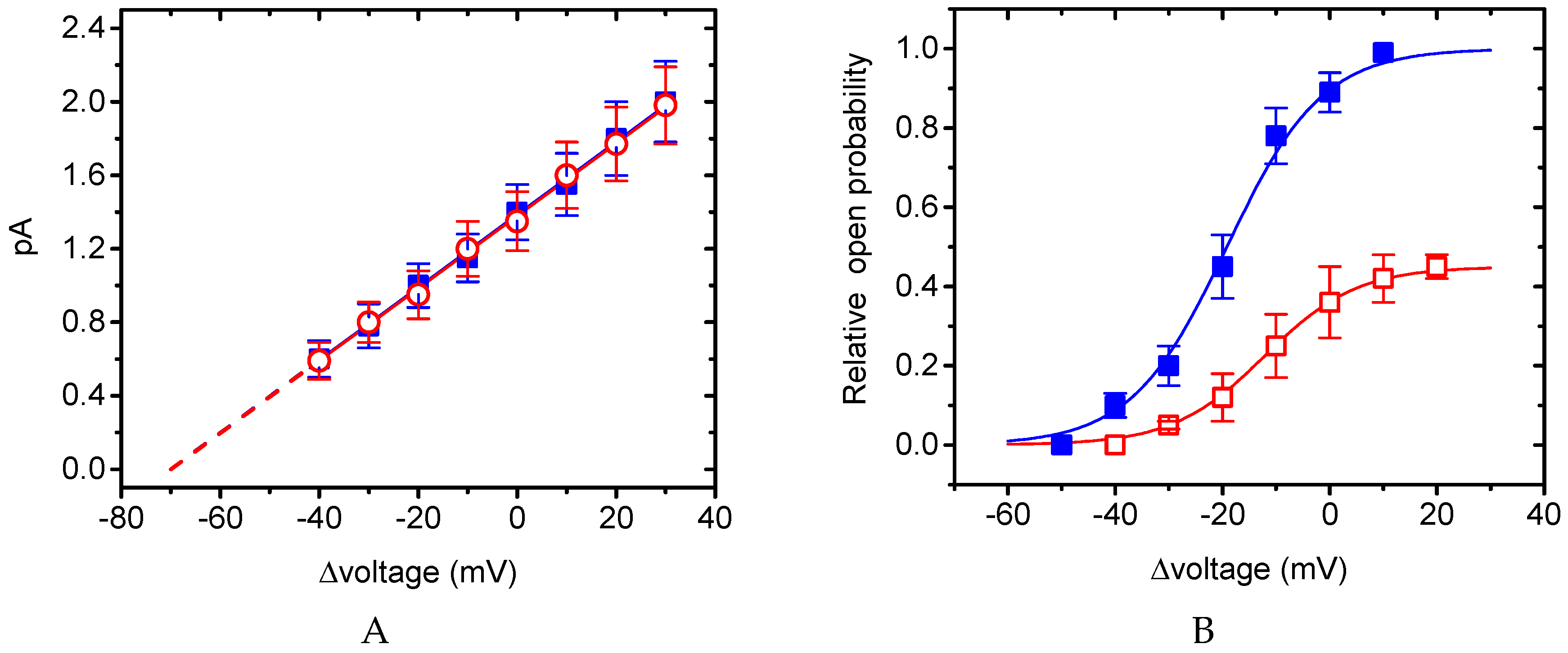
2.7. Lack of BIS Effect on Single-Channel Conductance of KM Channels
2.8. Activation Curve of KM Channels Modified by the Presence of BIS
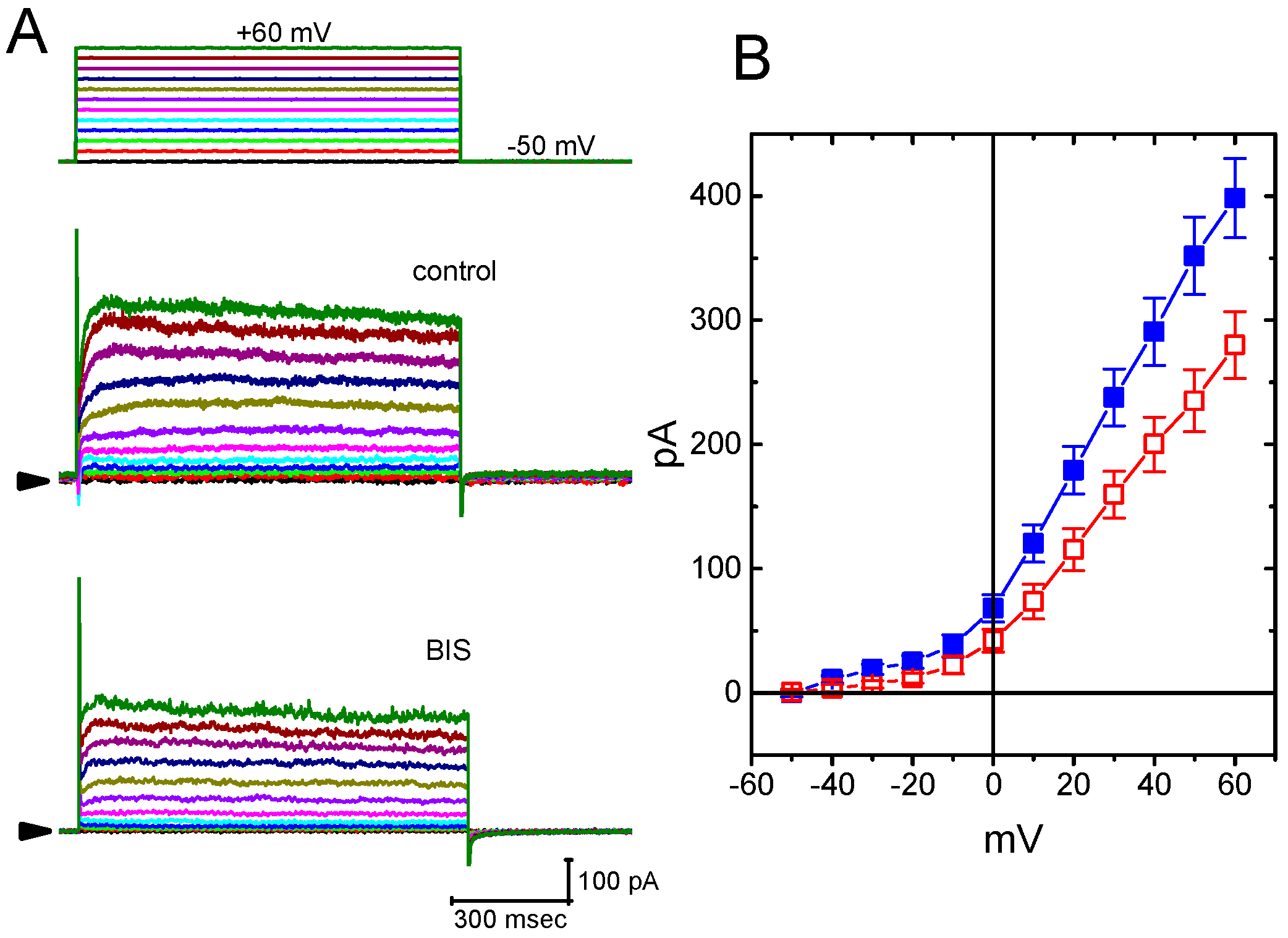
2.9. Effect of BIS on Erg-Mediated K+ Current (IK(erg)) in GH3 Cells
2.10. The Inhibition of Delayed-Rectifier K+ Current (IK(DR)) in GH3 cells Caused by the Presence of BIS
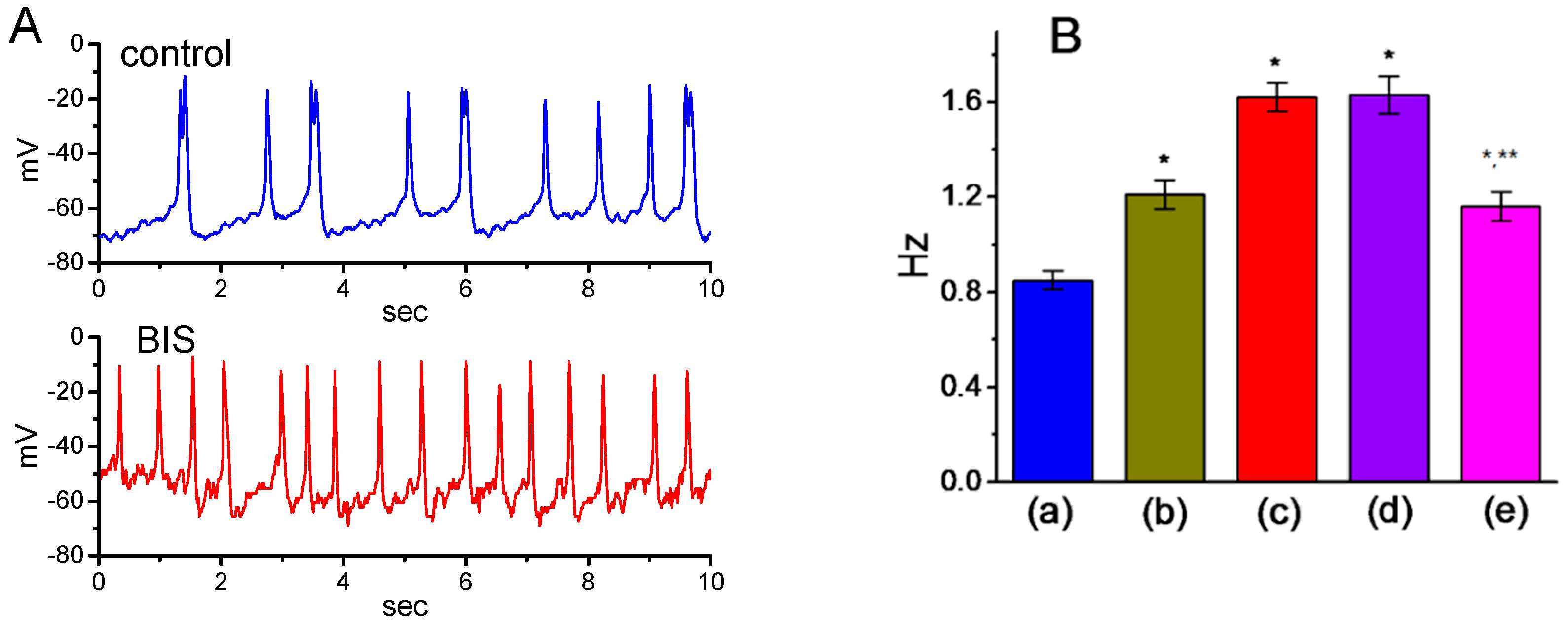
2.11. Effect of BIS on Spontaneous Action Potentials (APs) in GH3 Cells
2.12. Suppressive Effect of BIS on IK(M) in Pituitary R1220 Cells
2.13. Effect of BIS on IK(M) Recorded from Hippocampal mHippoE-14 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solutions
4.2. Cell Preparations
4.3. RNA Isolation and Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
4.4. Electrophysiological Measurements
4.5. Data Recordings
4.6. Data Analyses
4.7. Analyses of Single M-Type K+ (KM) Channels
4.8. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AP | action potential |
| BIS | bisoprolol |
| erg | ether-à-go-go-related gene |
| GMQ | 2-guanidine-4-methylquinazoline |
| I-V | current versus voltage |
| IC50 | the concentration required for 50% inhibition |
| IK(DR) | delayed-rectifier K+ current |
| IK(erg) | erg-mediated K+ current |
| IK(M) | M-type K+ current |
| Kerg | erg-mediated K+ channel |
| KM channel | M-type K+ channel |
| SEM | standard error of mean |
| τact | activation time constant |
| τdeact | deactivation time constant. |
References
- Vasomotor dysfunction in POTS patients. Rev. Port. Cardiol. 2000, 19, 1163–1170.
- Agabiti Rosei, E.; Rizzoni, D. Metabolic profile of nebivolol, a β-adrenoceptor antagonist with unique characteristics. Drugs 2007, 67, 1097–1107. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Murakami, M.; Miura, D.; Yunoki, K.; Enko, K.; Tanaka, M.; Saito, Y.; Nishii, N.; Miyoshi, T.; Yoshida, M.; et al. Beta-Blockers and Oxidative Stress in Patients with Heart Failure. Pharmaceuticals 2011, 4, 1088–1100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; He, S.; Li, Y.; Li, F.; Liu, Z.; Liu, J.; Gong, J. Bisoprolol protects myocardium cells against ischemia/reperfusion injury by attenuating unfolded protein response in rats. Sci. Rep. 2017, 7, 11859. [Google Scholar] [CrossRef] [PubMed]
- Bulpitt, C.J.; Connor, M.; Schulte, M.; Fletcher, A.E. Bisoprolol and nifedipine retard in elderly hypertensive patients: Effect on quality of life. J. Hum. Hypertens. 2000, 14, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Sigaroudi, A.; Kinzig, M.; Wahl, O.; Stelzer, C.; Schroeter, M.; Fuhr, U.; Holzgrabe, U.; Sorgel, F. Quantification of bisoprolol and metoprolol in simultaneous human serum and cerebrospinal fluid samples. Pharmacology 2018, 101, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Soloviev, D.V.; Matarrese, M.; Moresco, R.M.; Todde, S.; Bonasera, T.A.; Sudati, F.; Simonelli, P.; Magni, F.; Colombo, D.; Carpinelli, A.; et al. Asymmetric synthesis and preliminary evaluation of (R)- and (S)-[11C]bisoprolol, a putative β1-selective adrenoceptor radioligand. Neurochem. Int. 2001, 38, 169–180. [Google Scholar] [CrossRef]
- Janssens, K.; Krylyshkina, O.; Hersmus, N.; Vankelecom, H.; Denef, C. β1-Adrenoceptor expression in rat anterior pituitary gonadotrophs and in mouse αT3-1 and LβT2 gonadotrophic cell lines. Endocrinology 2008, 149, 2313–2324. [Google Scholar] [CrossRef]
- Ul Haq, R.; Liotta, A.; Kovacs, R.; Rosler, A.; Jarosch, M.J.; Heinemann, U.; Behrens, C.J. Adrenergic modulation of sharp wave-ripple activity in rat hippocampal slices. Hippocampus 2012, 22, 516–533. [Google Scholar] [CrossRef]
- Ronzoni, G.; Del Arco, A.; Mora, F.; Segovia, G. Enhanced noradrenergic activity in the amygdala contributes to hyperarousal in an animal model of PTSD. Psychoneuroendocrinology 2016, 70, 1–9. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Lin, X.; Tan, H.; Fu, Z. Early Life Exposure to Ractopamine Causes Endocrine-Disrupting Effects in Japanese Medaka (Oryzias latipes). Bull. Environ. Contam. Toxicol. 2016, 96, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Nurmamedova, G.S.; Gumbatov, N.B.; Mustafaev, I.I. Level of hormones of pituitary-gonadal axis, penile blood flow and sexual function in men with arterial hypertension during monotherapy with bisoprolol and nebivolol. Kardiologiia 2007, 47, 50–53. [Google Scholar] [PubMed]
- Liu, C.P.; Chiang, H.T.; Jan, C.R.; Wu, S.N. Mechanism of carvedilol-induced block of delayed rectifier K+ current in the NG108-15 neuronal cell line. Drug Dev. Res. 2003, 58, 196–208. [Google Scholar] [CrossRef]
- Dupuis, D.S.; Klaerke, D.A.; Olesen, S.P. Effect of β-adrenoceptor blockers on human ether-a-go-go-related gene (HERG) potassium channels. Basic Clin. Pharmacol. Toxicol. 2005, 96, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Wang, T.; Zhuo, X.; Song, B.; Zhang, J.; Wei, F.; Bai, H.; Wang, X.; Yang, D.; Gao, L.; et al. Bisoprolol reversed small conductance calcium-activated potassium channel (SK) remodeling in a volume-overload rat model. Mol. Cell. Biochem. 2013, 384, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, J.; Xi, Y.; Wu, G.; Han, K.; Huang, X.; Ma, A.; Wang, T. β1-Adrenergic blocker bisoprolol reverses down-regulated ion channels in sinoatrial node of heart failure rats. J. Physiol. Biochem. 2016, 72, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Selyanko, A.A.; Hadley, J.K.; Wood, I.C.; Abogadie, F.C.; Delmas, P.; Buckley, N.J.; London, B.; Brown, D.A. Two types of K+ channel subunit, Erg1 and KCNQ2/3, contribute to the M-like current in a mammalian neuronal cell. J. Neurosci. 1999, 19, 7742–7756. [Google Scholar] [CrossRef]
- Hsu, H.T.; Tseng, Y.T.; Lo, Y.C.; Wu, S.N. Ability of naringenin, a bioflavonoid, to activate M-type potassium current in motor neuron-like cells and to increase BKCa-channel activity in HEK293T cells transfected with alpha-hSlo subunit. BMC Neurosci. 2014, 15, 135. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, M.C.; Hung, T.Y.; Lin, K.M.; Huang, C.W.; Wu, S.N. Pioglitazone, a PPAR-γ Activator, Stimulates BKCa but Suppresses IKM in Hippocampal Neurons. Front. Pharmacol. 2018, 9, 977. [Google Scholar] [CrossRef]
- Liu, Y.Y.; Hsiao, H.T.; Wang, J.C.; Liu, Y.C.; Wu, S.N. Parecoxib, a selective blocker of cyclooxygenase-2, directly inhibits neuronal delayed-rectifier K+ current, M-type K+ current and Na+ current. Eur. J. Pharmacol. 2018, 844, 95–101. [Google Scholar] [CrossRef]
- Pena, F.; Alavez-Perez, N. Epileptiform activity induced by pharmacologic reduction of M-current in the developing hippocampus in vitro. Epilepsia 2006, 47, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Migliore, R. Know your current Ih: Interaction with a shunting current explains the puzzling effects of its pharmacological or pathological modulations. PLoS ONE 2012, 7, e36867. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Reed, N.; Liu, R.; Aizenman, E.; Wipf, P.; Tzounopoulos, T. Synthesis and evaluation of potent KCNQ2/3-specific channel activators. Mol. Pharmacol. 2016, 89, 667–677. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Gao, H.; Jaffe, D.; Zhang, H.; Gamper, N. M-type K+ channels in peripheral nociceptive pathways. Br. J. Pharmacol. 2018, 175, 2158–2172. [Google Scholar] [PubMed]
- Wu, S.N.; Jan, C.R.; Li, H.F.; Chiang, H.T. Characterization of inhibition by risperidone of the inwardly rectifying K+ current in pituitary GH3 cells. Neuropsychopharmacology 2000, 23, 676–689. [Google Scholar] [CrossRef]
- Furlan, F.; Taccola, G.; Grandolfo, M.; Guasti, L.; Arcangeli, A.; Nistri, A.; Ballerini, L. ERG conductance expression modulates the excitability of ventral horn GABAergic interneurons that control rhythmic oscillations in the developing mouse spinal cord. J. Neurosci. 2007, 27, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.S.; Lo, Y.C.; Peng, H.; Hsu, T.I.; Wu, S.N. Effects of ranolazine, a novel anti-anginal drug, on ion currents and membrane potential in pituitary tumor GH3 cells and NG108-15 neuronal cells. J. Pharmacol. Sci. 2009, 110, 295–305. [Google Scholar] [CrossRef]
- Carretero, L.; Llavona, P.; López-Hernández, A.; Casado, P.; Cutillas, P.R.; de la Peña, P.; Barros, F.; Dominguez, P. ERK and RSK are necessary for TRH-induced inhibition of r-ERG potassium currents in rat pituitary GH3 cells. Cell Signal. 2015, 27, 1720–1730. [Google Scholar] [CrossRef]
- Huang, M.H.; Shen, A.Y.; Wang, T.S.; Wu, H.M.; Kang, Y.F.; Chen, C.T.; Hsu, T.I.; Chen, B.S.; Wu, S.N. Inhibitory action of methadone and its metabolites on erg-mediated K+ current in GH3 pituitary tumor cells. Toxicology 2011, 280, 1–9. [Google Scholar] [CrossRef]
- Wu, S.N.; Hsu, M.C.; Liao, Y.K.; Wu, F.T.; Jong, Y.J.; Lo, Y.C. Evidence for inhibitory effects of flupirtine, a centrally acting analgesic, on delayed rectifier K+ currents in motor neuron-like cells. Evid. Based Complement. Alternat. Med. 2012, 2012, 148403. [Google Scholar] [CrossRef]
- Colbert, W.E.; Williams, P.D.; Williams, G.D. β-Adrenoceptor profile of ractopamine HCl in isolated smooth and cardiac muscle tissues of rat and guinea-pig. J. Pharm. Pharmacol. 1991, 43, 844–847. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.T.; Chen, S.A.; Chiang, C.E.; Lee, S.H.; Wen, Z.C.; Chang, M.S.; Wu, S.N. Influence of beta-adrenergic and vagal activity on the effect of exogenous adenosine on supraventricular tachycardia termination. Am. J. Cardiol. 1997, 79, 1628–1631. [Google Scholar] [CrossRef]
- Vandenberg, G.; Leatherland, J.; Moccia, R.D. The effects of the beta-agonist ractopamine on growth hormone and intermediary metabolite concentrations in rainbow trout, Oncorhynchus mykiss (Walbaum). Aquac. Res. 2008, 29, 9. [Google Scholar] [CrossRef]
- Mills, S.E.; Spurlock, M.E.; Smith, D.J. β-Adrenergic receptor subtypes that mediate ractopamine stimulation of lipolysis. J. Anim. Sci. 2003, 81, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Stephany, R.W. Hormonal growth promoting agents in food producing animals. Handb. Exp. Pharmacol. 2010, 195, 355–367. [Google Scholar]
- Lee, S.H.; Lee, E.H.; Ryu, S.Y.; Rhim, H.; Baek, H.J.; Lim, W.; Ho, W.K. Role of K+ channels in frequency regulation of spontaneous action potentials in rat pituitary GH3 cells. Neuroendocrinology 2003, 78, 260–269. [Google Scholar] [CrossRef] [PubMed]
- Hasebe, M.; Oka, Y. High-frequency firing activity of GnRH1 neurons in female Medaka induces the release of GnRH1 peptide from their nerve terminals in the pituitary. Endocrinology 2017, 158, 2603–2617. [Google Scholar] [CrossRef] [PubMed]
- Caspi, A.; Benninger, F.; Yaari, Y. KV7/M channels mediate osmotic modulation of intrinsic neuronal excitability. J. Neurosci. 2009, 29, 11098–11111. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Ruan, J.S.; Wu, S.N. Evidence of decreased activity in intermediate-conductance calcium-activated potassium channels during retinoic acid-induced differentiation in motor neuron-like NSC-34 cells. Cell. Physiol. Biochem. 2018, 48, 2374–2388. [Google Scholar] [CrossRef]
- So, E.C.; Wang, Y.; Yang, L.Q.; So, K.H.; Lo, Y.C.; Wu, S.N. Multiple regulatory actions of 2-guanidine-4-methylquinazoline (GMQ), an agonist of acid-sensing ion channel type 3, on ionic currents in pituitary GH3 cells and in olfactory sensory (Rolf B1.T) neurons. Biochem. Pharmacol. 2018, 151, 79–88. [Google Scholar] [CrossRef]
- Wu, S.N.; Yang, W.H.; Yeh, C.C.; Huang, H.C. The inhibition by di(2-ethylhexyl)-phthalate of erg-mediated K+ current in pituitary tumor (GH3) cells. Arch. Toxicol. 2012, 86, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.N.; Chern, J.H.; Shen, S.; Chen, H.H.; Hsu, Y.T.; Lee, C.C.; Chan, M.H.; Lai, M.C.; Shie, F.S. Stimulatory actions of a novel thiourea derivative on large-conductance, calcium-activated potassium channels. J. Cell. Physiol. 2017, 232, 3409–3421. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Augelli-Szafran, C.E.; Bradley, J.A.; Chen, X.; Koci, B.J.; Volberg, W.A.; Sun, Z.; Cordes, J.S. Novel potent human ether-a-go-go-related gene (hERG) potassium channel enhancers and their in vitro antiarrhythmic activity. Mol. Pharmacol. 2005, 68, 876–884. [Google Scholar] [PubMed]
- Yu, H.; Wu, M.; Townsend, S.D.; Zou, B.; Long, S.; Daniels, J.S.; McManus, O.B.; Li, M.; Lindsley, C.W.; Hopkins, C.R. Discovery, synthesis, and structure activity relationship of a series of N-aryl- bicyclo[2.2.1]heptane-2-carboxamides: Characterization of ML213 as a novel KCNQ2 and KCNQ4 potassium channel opener. ACS Chem. Neurosci. 2011, 2, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Gatford, K.L.; De Blasio, M.J.; Roberts, C.T.; Nottle, M.B.; Kind, K.L.; van Wettere, W.H.; Smits, R.J.; Owens, J.A. Responses to maternal GH or ractopamine during early-mid pregnancy are similar in primiparous and multiparous pregnant pigs. J. Endocrinol. 2009, 203, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Momčilović, S.; Milovanović, J.R.; Janković, S.M.; Jovanović, A.; Tasić-Otašević, S.; Stanojević, D.; Krstić, M.; Šalinger-Martinović, S.; Radojković, D.D.; Damjanović, M.; et al. Population pharmacokinetic analysis of bisoprolol in patients with acute coronary syndrome. J. Cardiovasc. Pharmacol. 2018. (Epub ahear of print). [Google Scholar]
- Janssens, K.; Boussemaere, M.; Wagner, S.; Kopka, K.; Denef, C. β1-Adrenoceptors in rat anterior pituitary may be constitutively active. inverse agonism of CGP 20712A on basal 3’,5’-cyclic adenosine 5’-monophosphate levels. Endocrinology 2008, 149, 2391–2402. [Google Scholar] [CrossRef]
- Huang, Y.C.; Wu, B.N.; Lin, Y.T.; Chen, S.J.; Chiu, C.C.; Cheng, C.J.; Chen, I.J. Eugenodilol: A third-generation beta-adrenoceptor blocker, derived from eugenol, with alpha-adrenoceptor blocking and beta2-adrenoceptor agonist-associated vasorelaxant activities. J. Cardiovasc. Pharmacol. 1999, 34, 10–20. [Google Scholar] [CrossRef]
- Wu, B.N.; Shen, K.P.; Lin, R.J.; Huang, Y.C.; Chiang, L.C.; Lo, Y.C.; Lin, C.Y.; Chen, I.J. Lipid solubility of vasodilatory vanilloid-type beta-blockers on the functional and binding activities of beta-adrenoceptor subtypes. Gen. Pharmacol. 2000, 34, 321–328. [Google Scholar] [CrossRef]
- Almeman, A.A.; Beshir, Y.A.; Aldosary, A.H. Comparison of the effects of metoprolol and bisoprolol on lipid and glucose profiles in cardiovascular patients. Curr. Drug Saf. 2018. (Epub ahead of print). [Google Scholar] [CrossRef]
- Fowkes, R.C.; Forrest-Owen, W.; McArdle, C.A. C-type natriuretic peptide (CNP) effects in anterior pituitary cell lines: Evidence for homologous desensitisation of CNP-stimulated cGMP accumulation in alpha T3-1 gonadotroph-derived cells. J. Endocrinol. 2000, 166, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.R.; Zhou, H.F.; Liu, B.; Jiang, X.F.; Xu, J.Y.; Jiang, M. The effect of Bushen Zhuyun prescription on gonadotropic hormone secretion and key transcription factor expression in rat pituitary cells. Nanosci. Nanotechnol. Lett. 2017, 9, 1596–1603. [Google Scholar] [CrossRef]
- Gingerich, S.; Kim, G.L.; Chalmers, J.A.; Koletar, M.M.; Wang, X.; Wang, Y.; Belsham, D.D. Estrogen receptor alpha and G-protein coupled receptor 30 mediate the neuroprotective effects of 17beta-estradiol in novel murine hippocampal cell models. Neuroscience 2010, 170, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Chow, J.C.; Tsai, J.J.; Wu, S.N. Characterizing the effects of Eugenol on neuronal ionic currents and hyperexcitability. Psychopharmacology 2012, 221, 575–587. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, E.C.; Foo, N.-P.; Ko, S.Y.; Wu, S.-N. Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons. Int. J. Mol. Sci. 2019, 20, 657. https://doi.org/10.3390/ijms20030657
So EC, Foo N-P, Ko SY, Wu S-N. Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons. International Journal of Molecular Sciences. 2019; 20(3):657. https://doi.org/10.3390/ijms20030657
Chicago/Turabian StyleSo, Edmund Cheung, Ning-Ping Foo, Shun Yao Ko, and Sheng-Nan Wu. 2019. "Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons" International Journal of Molecular Sciences 20, no. 3: 657. https://doi.org/10.3390/ijms20030657
APA StyleSo, E. C., Foo, N.-P., Ko, S. Y., & Wu, S.-N. (2019). Bisoprolol, Known to Be a Selective β1-Receptor Antagonist, Differentially but Directly Suppresses IK(M) and IK(erg) in Pituitary Cells and Hippocampal Neurons. International Journal of Molecular Sciences, 20(3), 657. https://doi.org/10.3390/ijms20030657





