Umbilical Cord SFRP5 Levels of Term Newborns in Relation to Normal and Excessive Gestational Weight Gain
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
- healthy controls—28 pregnant women with normal gestational weight gain;
- patients with EGWG—38 pregnant subjects with excessive gestational weight gain.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ouchi, N.; Higuchi, A.; Ohashi, K.; Oshima, Y.; Gokce, N.; Shibata, R.; Akasaki, Y.; Shimono, A.; Walsh, K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science 2010, 329, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, X.; Liang, G.; Sun, J.; Lin, Z.; Hu, R.; Chen, P.; Zhang, Z.; Zhou, L.; Li, Y. Recombinant SFRP5 protein significantly alleviated intrahepatic inflammation of nonalcoholic steatohepatitis. Nutr. Metab. 2017, 14, 56. [Google Scholar] [CrossRef] [PubMed]
- Oztas, E.; Ozler, S.; Ersoy, E.; Ersoy, A.O.; Tokmak, A.; Ergin, M.; Uygur, D.; Danisman, N. Prediction of gestational diabetes mellitus by first trimester serum secreted frizzle-related protein-5 levels. J. Matern. Fetal Neonatal Med. 2016, 29, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Teliewubai, J.; Bai, B.; Zhou, Y.; Lu, Y.; Yu, S.; Chi, C.; Li, J.; Blacher, J.; Xu, Y.; Zhang, Y. Association of asymptomatic target organ damage with secreted frizzled related protein 5 in the elderly: The Northern Shanghai Study. Clin. Interv. Aging 2018, 13, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.B.; Chen, X.D.; Zhou, X.Y.; Zhu, Q. The Wnt antagonist and secreted frizzled-related protein 5: Implications on lipid metabolism, inflammation, and type 2 diabetes mellitus. Biosci. Rep. 2018, 38, BSR20180011. [Google Scholar] [CrossRef] [PubMed]
- Carstensen-Kirberg, M.; Kannenberg, J.M.; Huth, C.; Meisinger, C.; Koenig, W.; Heier, M.; Peters, A.; Rathmann, W.; Roden, M.; Herder, C.; et al. Inverse associations between serum levels of secreted frizzled-related protein-5 (SFRP5) and multiple cardiometabolic risk factors: KORA F4 study. Cardiovasc. Diabetol. 2017, 16, 109. [Google Scholar] [CrossRef] [PubMed]
- Carstensen-Kirberg, M.; Hatziagelaki, E.; Tsiavou, A.; Chounta, A.; Nowotny, P.; Pacini, G.; Dimitriadis, G.; Roden, M.; Herder, C. Sfrp5 associates with beta-cell function in humans. Eur. J. Clin. Investig. 2016, 46, 535–543. [Google Scholar] [CrossRef]
- Hu, Z.; Deng, H.; Qu, H. Plasma SFRP5 levels are decreased in Chinese subjects with obesity and type 2 diabetes and negatively correlated with parameters of insulin resistance. Diabetes Res. Clin. Pract. 2013, 99, 391–395. [Google Scholar] [CrossRef]
- Hu, W.; Li, L.; Yang, M.; Luo, X.; Ran, W.; Liu, D.; Xiong, Z.; Liu, H.; Yang, G. Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J. Clin. Endocrinol. Metab. 2013, 98, 290–298. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, H.; Li, Y.; Wang, J.; Lai, Y.; Gao, L.; Lei, L.; Yang, G.; Liao, X.; Fang, X.; et al. Plasma Sfrp5 levels correlate with determinants of the metabolic syndrome in Chinese adults. Diabetes Metab. Res. Rev. 2017, 33. [Google Scholar] [CrossRef]
- Yokota, T.; Oritani, K.; Sudo, T.; Ishibashi, T.; Doi, Y.; Habuchi, Y.; Ichii, M.; Fukushima, K.; Okuzaki, D.; Tomizuka, K.; et al. Estrogen-inducible sFRP5 inhibits early B-lymphopoiesis in vivo, but not during pregnancy. Eur. J. Immunol. 2015, 45, 1390–1401. [Google Scholar] [CrossRef] [PubMed]
- Dahlhoff, M.; Pfister, S.; Blutke, A.; Rozman, J.; Klingenspor, M.; Deutsch, M.J.; Rathkolb, B.; Fink, B.; Gimpfl, M.; Hrabě de Angelis, M.; et al. Peri-conceptional obesogenic exposure induces sex-specific programming of disease susceptibilities in adult mouse offspring. Biochim. Biophys. Acta 2014, 1842, 304–317. [Google Scholar] [CrossRef]
- Gao, H.; Tanchico, D.; Yallampalli, U.; Yallampalli, C. Sfrp5 expression is reduced in placental junctional zone in pregnant rats fed a low protein diet. Placenta 2014, 35, 77. [Google Scholar] [CrossRef]
- Atli, M.O.; Guzeloglu, A.; Dinc, D.A. Expression of wingless type (WNT) genes and their antagonists at mRNA levels in equine endometrium during the estrous cycle and early pregnancy. Anim. Reprod. Sci. 2011, 125, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines; Rasmussen, K.M.; Yaktine, A.L. Weight Gain during Pregnancy: Reexamining the Guidelines; National Academies Press: Washington, DC, USA, 2009.
- Goldstein, R.F.; Abell, S.K.; Ranasinha, S.; Misso, M.; Boyle, J.A.; Black, M.H.; Li, N.; Hu, G.; Corrado, F.; Rode, L.; et al. Association of Gestational Weight Gain with Maternal and Infant Outcomes: A Systematic Review and Meta-analysis. JAMA 2017, 317, 2207–2225. [Google Scholar] [CrossRef]
- Guo, L.; Liu, J.; Ye, R.; Liu, J.; Zhuang, Z.; Ren, A. Gestational Weight Gain and Overweight in Children Aged 3–6 Years. J. Epidemiol. 2015, 25, 536–543. [Google Scholar] [CrossRef]
- Diesel, J.C.; Eckhardt, C.L.; Day, N.L.; Brooks, M.M.; Arslanian, S.A.; Bodnar, L.M. Gestational Weight Gain and Offspring Longitudinal Growth in Early Life. Ann. Nutr. Metab. 2015, 67, 49–57. [Google Scholar] [CrossRef]
- Estampador, A.C.; Pomeroy, J.; Renström, F.; Nelson, S.M.; Mogren, I.; Persson, M.; Sattar, N.; Domellöf, M.; Franks, P.W. Infant body composition and adipokine concentrations in relation to maternal gestational weight gain. Diabetes Care 2014, 37, 1432–1438. [Google Scholar] [CrossRef]
- Walsh, J.M.; McGowan, C.A.; Mahony, R.M.; Foley, M.E.; McAuliffe, F.M. Obstetric and metabolic implications of excessive gestational weight gain in pregnancy. Obesity 2014, 22, 1594–1600. [Google Scholar] [CrossRef]
- Lu, Y.C.; Wang, C.P.; Hsu, C.C.; Chiu, C.A.; Yu, T.H.; Hung, W.C.; Lu, L.F.; Chung, F.M.; Tsai, I.T.; Lin, H.C.; et al. Circulating secreted frizzled-related protein 5 (Sfrp5) and wingless-type MMTV integration site family member 5a (Wnt5a) levels in patients with type 2 diabetes mellitus. Diabetes Metab. Res. Rev. 2013, 29, 551–556. [Google Scholar] [CrossRef]
- McDowall, M.D.; Scott, M.S.; Barton, G.J. PIPs: Human protein-protein interaction prediction database. Nucleic Acids Res. 2009, 37, D651–D656. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Rankin, S.A.; Sinner, D.; Kenny, A.P.; Krieg, P.A.; Zorn, A.M. Sfrp5 coordinates foregut specification and morphogenesis by antagonizing both canonical and noncanonical Wnt11 signaling. Genes Dev. 2008, 22, 3050–3063. [Google Scholar] [CrossRef] [PubMed]
- Rauch, A.; Mandrup, S. Lighting the fat furnace without SFRP5. J. Clin. Investig. 2012, 122, 2349–2352. [Google Scholar] [CrossRef] [PubMed]
- Marciniak, A.; Patro-Małysza, J.; Kimber-Trojnar, Ż.; Marciniak, B.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Fetal programming of the metabolic syndrome. Taiwan J. Obstet. Gynecol. 2017, 56, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.J.; Kim, Y.J. What is fetal programming?: A lifetime health is under the control of in utero health. Obstet. Gynecol. Sci. 2017, 60, 506–519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Regnault, T.R.; Barker, P.L.; Botting, K.J.; McMillen, I.C.; McMillan, C.M.; Roberts, C.T.; Morrison, J.L. Placental adaptations in growth restriction. Nutrients 2015, 7, 360–389. [Google Scholar] [CrossRef] [PubMed]
- Almario, R.U.; Karakas, S.E. Roles of circulating WNT-signaling proteins and WNT-inhibitors in human adiposity, insulin resistance, insulin secretion, and inflammation. Horm. Metab. Res. 2015, 47, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, H.; Celik, H.T.; Namuslu, M.; Inan, O.; Onaran, Y.; Karakurt, F.; Ayyildiz, A.; Bilgic, M.A.; Bavbek, N.; Akcay, A. Benefits of the neutrophil-to-lymphocyte ratio for the prediction of gestational diabetes mellitus in pregnant women. Exp. Clin. Endocrinol. Diabetes 2014, 122, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Kharb, S.; Bala, J.; Nanda, S. Markers of obesity and growth in preeclamptic and normotensive pregnant women. J. Obstet. Gynaecol. 2017, 37, 610–615. [Google Scholar] [CrossRef] [PubMed]
- Treviño-Garza, C.; Villarreal-Martínez, L.; Estrada-Zúñiga, C.M.; Leal-Treviño, M.; Rodríguez-Balderrama, I.; Nieto-Sanjuanero, A.; Cárdenas-Del Castillo, B.; Montes-Tapia, F.F.; de la O-Cavazos, M. Leptin, IL-6 and TNF-α levels in umbilical cord blood of healthy term newborns in relation to mode of delivery. J. Obstet. Gynaecol. 2016, 36, 719–721. [Google Scholar] [CrossRef] [PubMed]
- Boutsikou, T.; Briana, D.D.; Boutsikou, M.; Kafalidis, G.; Piatopoulou, D.; Baka, S.; Hassiakos, D.; Gourgiotis, D.; Malamitsi-Puchner, A. Cord blood nesfatin-1 in large for gestational age pregnancies. Cytokine 2013, 61, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Martos-Moreno, G.A.; Barrios, V.; Sáenz de Pipaón, M.; Pozo, J.; Dorronsoro, I.; Martínez-Biarge, M.; Quero, J.; Argente, J. Influence of prematurity and growth restriction on the adipokine profile, IGF1, and ghrelin levels in cord blood: Relationship with glucose metabolism. Eur. J. Endocrinol. 2009, 161, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Kimber-Trojnar, Ż.; Patro-Małysza, J.; Skórzyńska-Dziduszko, K.E.; Oleszczuk, J.; Trojnar, M.; Mierzyński, R.; Leszczyńska-Gorzelak, B. Ghrelin in Serum and Urine of Post-Partum Women with Gestational Diabetes Mellitus. Int. J. Mol. Sci. 2018, 19, 3001. [Google Scholar] [CrossRef] [PubMed]
- Huopio, H.; Hakkarainen, H.; Pääkkönen, M.; Kuulasmaa, T.; Voutilainen, R.; Heinonen, S.; Cederberg, H. Long-Term changes in glucose metabolism after gestational diabetes: A double cohort study. BMC Pregnancy Childbirth 2014, 14, 296. [Google Scholar] [CrossRef] [PubMed]
- Bellamy, L.; Casas, J.; Hingorani, A.; Williams, D. Type 2 diabetes mellitus after gestational diabetes: A systematic review and meta-analysis. Lancet 2009, 373, 1773–1779. [Google Scholar] [CrossRef]
- Luo, Z.C.; Bilodeau, J.F.; Nuyt, A.M.; Fraser, W.D.; Julien, P.; Audibert, F.; Xiao, L.; Garofalo, C.; Levy, E. Perinatal Oxidative Stress May Affect Fetal Ghrelin Levels in Humans. Sci. Rep. 2015, 5, 17881. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, P.D.; Hanson, M.A. Living with the past: Evolution, development, and patterns of disease. Science 2004, 305, 1733–1736. [Google Scholar] [CrossRef]
- Lawrence, G.M.; Shulman, S.; Friedlander, Y.; Sitlani, C.M.; Burger, A.; Savitsky, B.; Granot-Hershkovitz, E.; Lumley, T.; Kwok, P.Y.; Hesselson, S.; et al. Associations of maternal pre-pregnancy and gestational body size with offspring longitudinal change in BMI. Obesity 2014, 22, 1165–1171. [Google Scholar] [CrossRef]
- Van Rossem, L.; Wijga, A.H.; Gehring, U.; Koppelman, G.H.; Smit, H.A. Maternal Gestational and Postdelivery Weight Gain and Child Weight. Pediatrics 2015, 136, e1294–e1301. [Google Scholar] [CrossRef]
- Christodoulides, C.; Lagathu, C.; Sethi, J.K.; Vidal-Puig, A. Adipogenesis and WNT signalling. Trends Endocrinol. Metab. 2009, 20, 16–24. [Google Scholar] [CrossRef]
- Schulte, D.M.; Kragelund, D.; Müller, N.; Hagen, I.; Elke, G.; Titz, A.; Schädler, D.; Schumacher, J.; Weiler, N.; Bewig, B.; et al. The wingless-related integration site-5a/secreted frizzled-related protein-5 system is dysregulated in human sepsis. Clin. Exp. Immunol. 2015, 180, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Prats-Puig, A.; Soriano-Rodríguez, P.; Carreras-Badosa, G.; Riera-Pérez, E.; Ros-Miquel, M.; Gomila-Borja, A.; de Zegher, F.; Ibáñez, L.; Bassols, J.; López-Bermejo, A. Balanced duo of anti-inflammatory SFRP5 and proinflammatory WNT5A in children. Pediatr. Res. 2014, 75, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Bilkovski, R.; Schulte, D.M.; Oberhauser, F.; Gomolka, M.; Udelhoven, M.; Hettich, M.M.; Roth, B.; Heidenreich, A.; Gutschow, C.; Krone, W.; et al. Role of WNT-5a in the determination of human mesenchymal stem cells into preadipocytes. J. Biol. Chem. 2010, 285, 6170–6178. [Google Scholar] [CrossRef] [PubMed]
- Almind, K.; Kahn, C.R. Genetic determinants of energy expenditure and insulin resistance in diet-induced obesity in mice. Diabetes 2004, 53, 3274–3285. [Google Scholar] [CrossRef] [PubMed]
- Schulte, D.M.; Müller, N.; Neumann, K.; Oberhäuser, F.; Faust, M.; Güdelhöfer, H.; Brandt, B.; Krone, W.; Laudes, M. Pro-inflammatory wnt5a and anti-inflammatory sFRP5 are differentially regulated by nutritional factors in obese human subjects. PLoS ONE 2012, 7, e32437. [Google Scholar] [CrossRef]
- Wang, R.; Hong, J.; Liu, R.; Chen, M.; Xu, M.; Gu, W.; Zhang, Y.; Ma, Q.; Wang, F.; Shi, J.; et al. SFRP5 acts as a mature adipocyte marker but not as a regulator in adipogenesis. J. Mol. Endocrinol. 2014, 53, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.W.; Lin, T.Y.; Peng, C.H.; Huang, J.L.; Hung, S.C. Factors Associated with Decreased Lean Tissue Index in Patients with Chronic Kidney Disease. Nutrients 2017, 9, 434. [Google Scholar] [CrossRef]
- Mori, H.; Prestwich, T.C.; Reid, M.A.; Longo, K.A.; Gerin, I.; Cawthorn, W.P.; Susulic, V.S.; Krishnan, V.; Greenfield, A.; Macdougald, O.A. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J. Clin. Investig. 2012, 122, 2405–2416. [Google Scholar] [CrossRef]
- Rulifson, I.C.; Majeti, J.Z.; Xiong, Y.; Hamburger, A.; Lee, K.J.; Miao, L.; Lu, M.; Gardner, J.; Gong, Y.; Wu, H.; et al. Inhibition of secreted frizzled-related protein 5 improves glucose metabolism. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E1144–E1152. [Google Scholar] [CrossRef]
- Van Camp, J.K.; Beckers, S.; Zegers, D.; Verrijken, A.; Van Gaal, L.F.; Van Hul, W. Common genetic variation in sFRP5 is associated with fat distribution in men. Endocrine 2014, 46, 477–484. [Google Scholar] [CrossRef]
- Xu, L.; Li, M.; Yin, J.; Cheng, H.; Yu, M.; Zhao, X.; Xiao, X.; Mi, J. Change of Body Composition and Adipokines and Their Relationship with Insulin Resistance across Pubertal Development in Obese and Nonobese Chinese Children: The BCAMS Study. Int. J. Endocrinol. 2012, 2012, 389108. [Google Scholar] [CrossRef] [PubMed]
- Bush, N.C.; Darnell, B.E.; Oster, R.A.; Goran, M.I.; Gower, B.A. Adiponectin is lower among African Americans and is independently related to insulin sensitivity in children and adolescents. Diabetes 2005, 54, 2772–2778. [Google Scholar] [CrossRef] [PubMed]
- Chiesa, C.; Osborn, J.F.; Haass, C.; Natale, F.; Spinelli, M.; Scapillati, E.; Spinelli, A.; Pacifico, L. Ghrelin, leptin, IGF-1, IGFBP-3, and insulin concentrations at birth: Is there a relationship with fetal growth and neonatal anthropometry? Clin. Chem. 2008, 54, 550–558. [Google Scholar] [CrossRef] [PubMed]
- International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010, 33, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Diabetes Poland (Polish Diabetes Association). 2018 Guidelines on the management of diabetic patients. A position of Diabetes Poland. Clin. Diabetol. 2018, 7, 1–90. [Google Scholar] [CrossRef]
| Variables | Control Group (n = 28) | EGWG Group (n = 38) | p |
|---|---|---|---|
| Maternal characteristics | |||
| age, years | 29 (24–38) | 29 (28–32) | NS |
| pre-pregnancy BMI, kg/m2 | 20.3 (19.5–24.4) | 23.2 (21.6–24.09) | NS |
| gestational weight gain, kg | 15 (11.5–15.6) | 23.9 (21–26) | <0.001 |
| gestational BMI gain, kg/m2 | 5.4 (3.0–5.6) | 8.4 (7.07–9.4) | <0.001 |
| BMI at delivery, kg/m2 | 26.3 (24.2–29.1) | 31.3 (29.7–32.05) | <0.001 |
| cesarean delivery, % | 14 | 26 | NS |
| BMI after delivery, kg/m2 | 22 (21–23.9) | 28.6 (26.2–29.7) | <0.001 |
| FTI after delivery, kg/m2 | 10.1 (9.1–13.8) | 14.7 (13.2–17.2) | <0.001 |
| LTI after delivery, kg/m2 | 10.1. (9.4–13.1) | 12.9 (11.2–13.9) | <0.01 |
| Maternal Serum | |||
| albumin, g/dL | 3.68 (3.43–3.73) | 3.55 (3.41–3.81) | NS |
| total cholesterol, mg/dL | 249 (188–287) | 225 (197–249) | NS |
| HDL, mg/dL | 78 (75–82) | 71 (59–79) | <0.05 |
| LDL, mg/dL | 129 (93–152) | 106 (87–128) | NS |
| triglycerides, mg/dL | 177 (150–254) | 204 (178–258) | <0.05 |
| HgbA1c, % | 5.3 (4.6–5.4) | 5.5 (5.0–5.5) | <0.05 |
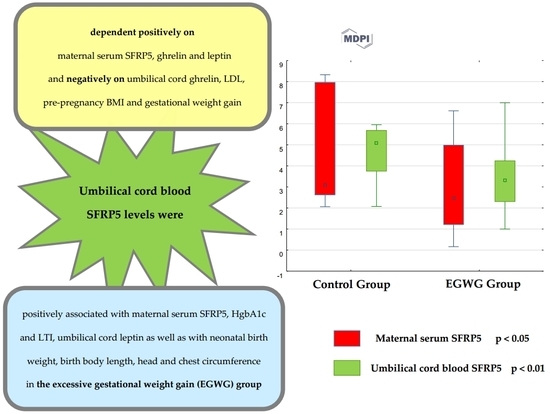
| SFRP5, ng/mL | 3.1 (2.62–8.0) | 2.47 (1.2–5.0) | <0.05 |
| ghrelin, ng/mL | 0.933 (0.646–1.115) | 1.187 (0.343–2.433) | NS |
| leptin, ng/mL | 10.43 (6.04–14.9) | 14.87 (12.6–47.6) | NS |
| Umbilical Cord Blood | |||
| SFRP5, ng/mL | 5.08 (3.74–5.69) | 3.33 (2.3–4.25) | <0.01 |
| ghrelin, ng/mL | 0.0195 (0.187−0.282) | 0.525 (0.265–1.826) | <0.001 |
| leptin, ng/mL | 7.53 (4.9–14.01) | 10.99 (8.5–13.4) | <0.001 |
| Neonatal Anthropometric Measurements | |||
| birth weight, g | 3630 (3200–3920) | 3520 (3400–3650) | NS |
| birth body length, cm | 56 (55–57) | 55 (54–56) | NS |
| head circumference, cm | 34 (33–35) | 34 (33–35) | NS |
| chest circumference, cm | 34 (34–35) | 34 (33–35) | NS |
| Variables | Umbilical Cord SFRP5 | |
|---|---|---|
| Control Group | EGWG Group | |
| Maternal Characteristics | ||
| pre-pregnancy BMI | −0.829 *** | 0.152 |
| gestational weight gain | 0.371 | −0.435 * |
| gestational BMI gain | 0.143 | −0.442 * |
| BMI at delivery | −0.6 ** | −0.105 |
| BMI after delivery | −0.486 * | 0.074 |
| FTI after delivery | 0.086 | −0.342 |
| LTI after delivery | −0.086 | 0.527 ** |
| Maternal Serum | ||
| albumin | −0.143 | −0.603 ** |
| total cholesterol | −0.406 * | −0.436 * |
| HDL | 0.058 | −0.567 ** |
| LDL | −0.371 | −0.087 |
| triglycerides | −0.058 | −0.081 |
| HgbA1c | 0.667 *** | 0.636 *** |
| SFRP5 | 0.429 * | 0.452 * |
| ghrelin | 0.771 *** | −0.394 |
| leptin | 0.6 ** | −0.171 |
| Umbilical Cord Blood | ||
| ghrelin | −0.657 *** | −0.817 *** |
| leptin | −0.086 | 0.495 * |
| Neonatal Anthropometric Measurements | ||
| birth weight | −0.2 | 0.781 *** |
| birth body length | −0.309 | 0.739 *** |
| head circumference | −0.206 | 0.532 ** |
| chest circumference | 0.494 * | 0.516 ** |
| Independent Variable | B | β | 95% CI | p |
|---|---|---|---|---|
| maternal serum SFRP5 | 0.33 | 0.50 | 0.32–0.69 | <0.001 |
| maternal serum ghrelin | 0.12 | 0.39 | 0.19−0.59 | <0.001 |
| umbilical cord ghrelin | −0.26 | −0.79 | −0.99–(−0.59) | <0.001 |
| maternal serum leptin | 0.06 | 0.72 | 0.52–0.92 | <0.001 |
| maternal LDL | −0.01 | −0.23 | −0.38–(−0.07) | <0.01 |
| pre-pregnancy BMI | −0.12 | −0.27 | −0.47–(−0.06) | <0.05 |
| gestational weight gain | −0.08 | −0.29 | −0.48–(−0.11) | <0.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimber-Trojnar, Ż.; Patro-Małysza, J.; Trojnar, M.; Darmochwał-Kolarz, D.; Oleszczuk, J.; Leszczyńska-Gorzelak, B. Umbilical Cord SFRP5 Levels of Term Newborns in Relation to Normal and Excessive Gestational Weight Gain. Int. J. Mol. Sci. 2019, 20, 595. https://doi.org/10.3390/ijms20030595
Kimber-Trojnar Ż, Patro-Małysza J, Trojnar M, Darmochwał-Kolarz D, Oleszczuk J, Leszczyńska-Gorzelak B. Umbilical Cord SFRP5 Levels of Term Newborns in Relation to Normal and Excessive Gestational Weight Gain. International Journal of Molecular Sciences. 2019; 20(3):595. https://doi.org/10.3390/ijms20030595
Chicago/Turabian StyleKimber-Trojnar, Żaneta, Jolanta Patro-Małysza, Marcin Trojnar, Dorota Darmochwał-Kolarz, Jan Oleszczuk, and Bożena Leszczyńska-Gorzelak. 2019. "Umbilical Cord SFRP5 Levels of Term Newborns in Relation to Normal and Excessive Gestational Weight Gain" International Journal of Molecular Sciences 20, no. 3: 595. https://doi.org/10.3390/ijms20030595
APA StyleKimber-Trojnar, Ż., Patro-Małysza, J., Trojnar, M., Darmochwał-Kolarz, D., Oleszczuk, J., & Leszczyńska-Gorzelak, B. (2019). Umbilical Cord SFRP5 Levels of Term Newborns in Relation to Normal and Excessive Gestational Weight Gain. International Journal of Molecular Sciences, 20(3), 595. https://doi.org/10.3390/ijms20030595