A Systematic Review of Neuroprotective Strategies in the Management of Hypoglycemia
Abstract
1. Introduction
2. Results
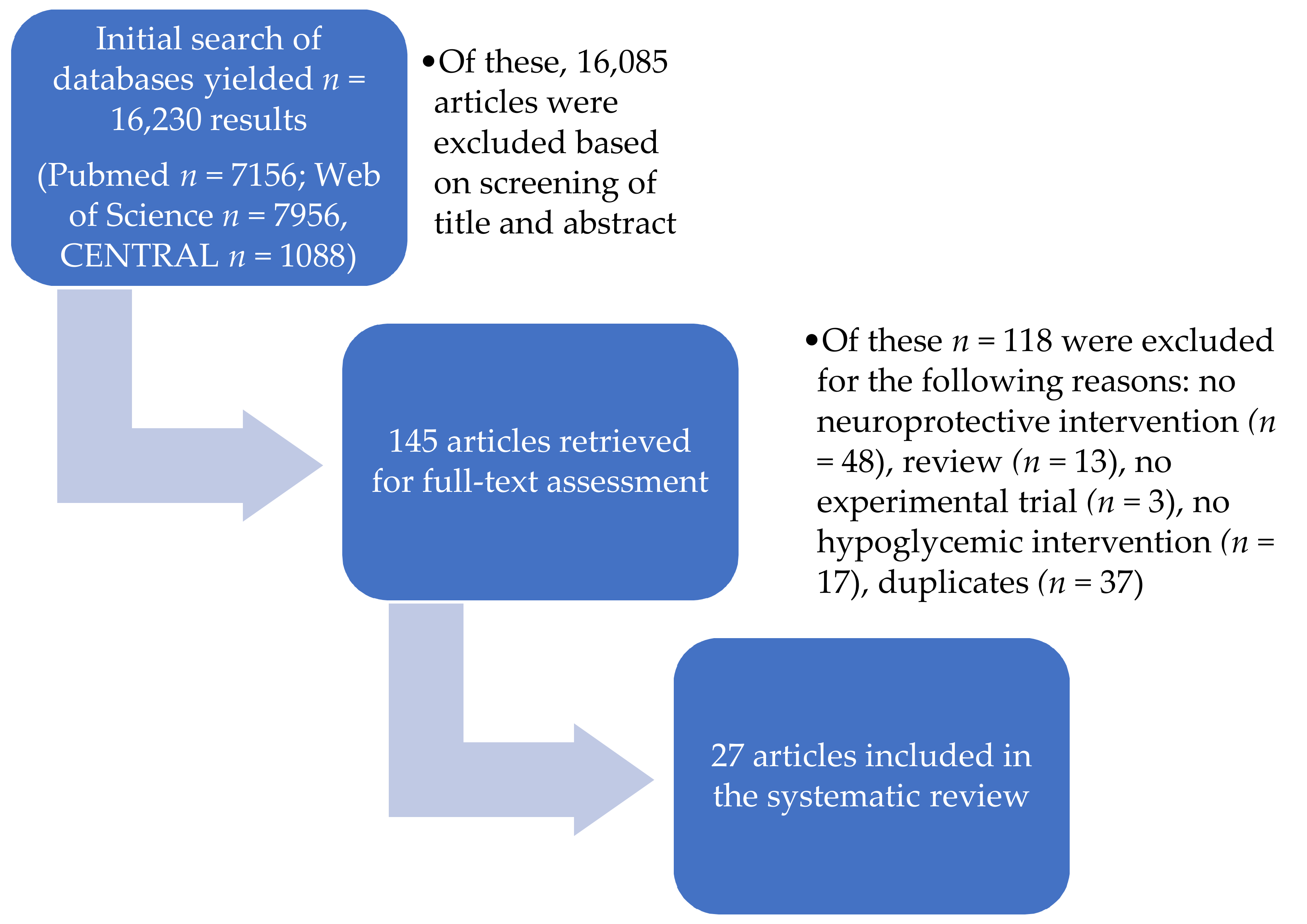
2.1. Study Selection
2.2. Study Characteristics
2.3. Results of Individual Studies
2.3.1. Approaches Regarding Energy Substitution
2.3.2. Amelioration of Impaired Awareness of Hypoglycemia
2.3.3. Other Neuroprotective Interventions
Hypothermia
Glibenclamide and Diazoxide
Target Level of Blood Glucose Post-Hypoglycemia
Mitochondrial Permeability Transition Mitigated by Cyclosporin A
Influence of Sleep Deprivation on Hypoglycemia
Citociline
Erythropoietin
NMDA- and AMPA-Receptor Blockade
Vitamins C and E Pre-Treatment
Antecedent Glycemic Control
Oral Amino Acid Administration in the Context of Glucagon Upregulation
Influence of Chromium on Neuronal Plasticity Markers
Modafinil
2.4. Risk of Bias within Studies
3. Discussion
3.1. Retrieved Studies
3.2. Study Quality and Translational Value
3.3. Limitations
4. Materials and Methods
4.1. Review Protocol
4.2. Eligibility Criteria
- Participants: For humans, participants with hypoglycemia/hypoglycemic episodes that were either not related to an established illness or related to a T1DM or T2DM diagnosis. Both sexes over the age of 18 years were included. For animals, animal experimental models that investigated isolated hypoglycemic episodes and hypoglycemia due to T1DM and T2DM, respectively, and antidiabetic medication, with the inclusion of all species and both sexes;
- Intervention: All neuroprotective interventions;
- Comparators: Usual care/normal practice, no intervention/control group, other neuroprotective interventions (if the respective study contained multiple interventions);
- Outcome measures: Neuroprotective effects;
- Study design: Controlled trials.
4.3. Information Sources and Systematic Search
4.4. Study Selection
4.5. Data Collection Process
4.6. Data Items
- Basic information about the study (author, year of publication);
- General characteristics of the experiment (number of participants/animals, setting) and its participants, including age (mean/SD), gender, and medical history as reported by the authors in human studies and species, gender, age, information regarding housing and keeping, and illness model in animal studies;
- Information regarding the hypoglycemic intervention, including the manner of induction and the depth and duration of hypoglycemia;
- Information regarding the neuroprotective intervention, including the time and duration/number of applications and the dosage;
- Information regarding the employed outcome parameters by the respective study, including all relevant vital parameters, blood values, cerebral and cognitive outcome measures (histopathological, imaging, brain-specific parameters, cognitive tests, etc.), and the length of the observation period subsequent to the intervention.
4.7. Risk of Bias in Individual Studies
4.8. Summary Measures and Analysis
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
- hypoglycemia [MeSH terms]
- hypoglycemic encephalopathy
- hypogly*
- blood sugar
- insulin
- 1 OR 2 OR 3 OR 4 OR 5
- effects, neuroprotective [MeSH terms]
- neuroprotect*
- brain
- cognit*
- cerebral
- 7 OR 8 OR 9 OR 10 OR 11
- rct
- trial
- random*
- experimental
- 13 OR 14 OR 15 OR 16
- 6 AND 12 AND 17
- hypoglyc*mia
- hypoglyc*mic encephalopathy
- hypoglyc*
- blood sugar
- 5. insulin
- diabet*
- antidiabet*
- 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7
- neuroprotect*
- brain
- cerebral
- cognit*
- neuro*
- 9 OR 10 OR 11 OR 12 OR 13
- random*
- rct
- trial
- experimental
- 15 OR 16 OR 17 OR 18
- 8 AND 14 AND 19
- hypoglycemia [MeSH descriptor]
- hypogly*
- blood sugar
- insulin
- #1 or #2 or #3 or #4
- neuroprotective agents [MeSH descriptor]
- neuroprotect*
- brain
- cerebral
- cognit*
- #6 or #7 or #8 or #9 or #10
- #5 and #11
References
- Naghavi, M.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abd-Allah, F.; Abera, S.F.; Aboyans, V.; Adetokunboh, O.; Afshin, A.; Agrawal, A.; et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet (Lond. Engl.) 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef] [PubMed]
- Bommer, C.; Heesemann, E.; Sagalova, V.; Manne-Goehler, J.; Atun, R.; Barnighausen, T.; Vollmer, S. The global economic burden of diabetes in adults aged 20–79 years: A cost-of-illness study. Lancet Diabetes Endocrinol. 2017, 5, 423–430. [Google Scholar] [CrossRef]
- Forbes, J.M.; Cooper, M.E. Mechanisms of diabetic complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef] [PubMed]
- Barendse, S.; Singh, H.; Frier, B.M.; Speight, J. The impact of hypoglycaemia on quality of life and related patient-reported outcomes in Type 2 diabetes: A narrative review. Diabet. Med. A J. Br. Diabet. Assoc. 2012, 29, 293–302. [Google Scholar] [CrossRef]
- Nielsen, H.B.; Ovesen, L.L.; Mortensen, L.H.; Lau, C.J.; Joensen, L.E. Type 1 diabetes, quality of life, occupational status and education level—A comparative population-based study. Diabetes Res. Clin. Pract. 2016, 121, 62–68. [Google Scholar] [CrossRef]
- Cryer, P.E. Glycemic Goals in Diabetes: Trade-off Between Glycemic Control and Iatrogenic Hypoglycemia. Diabetes 2014, 63, 2188–2195. [Google Scholar] [CrossRef]
- Seaquist, E.R.; Anderson, J.; Childs, B.; Cryer, P.; Dagogo-Jack, S.; Fish, L.; Heller, S.R.; Rodriguez, H.; Rosenzweig, J.; Vigersky, R. Hypoglycemia and Diabetes: A Report of a Workgroup of the American Diabetes Association and The Endocrine Society. Diabetes Care 2013, 36, 1384–1395. [Google Scholar] [CrossRef]
- Cryer, P.E. Mechanisms of hypoglycemia-associated autonomic failure and its component syndromes in diabetes. Diabetes 2005, 54, 3592–3601. [Google Scholar] [CrossRef]
- Mohseni, S. Neurologic damage in hypoglycemia. Handb. Clin. Neurol. 2014, 126, 513–532. [Google Scholar] [CrossRef]
- Jensen, V.F.; Bogh, I.B.; Lykkesfeldt, J. Effect of insulin-induced hypoglycaemia on the central nervous system: Evidence from experimental studies. J. Neuroendocrinol. 2014, 26, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.K.; Rawlings, A.M.; Lee, C.J.; Gross, A.L.; Huang, E.S.; Sharrett, A.R.; Coresh, J.; Selvin, E. Severe hypoglycaemia, mild cognitive impairment, dementia and brain volumes in older adults with type 2 diabetes: The Atherosclerosis Risk in Communities (ARIC) cohort study. Diabetologia 2018, 61, 1956–1965. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Sheu, W.H.H. Hypoglycaemic episodes and risk of dementia in diabetes mellitus: 7-year follow-up study. J. Internal Med. 2013, 273, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Mattishent, K.; Loke, Y.K. Bi-directional interaction between hypoglycaemia and cognitive impairment in elderly patients treated with glucose-lowering agents: A systematic review and meta-analysis. Diabetes Obes. Metabol. 2016, 18, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Jensen, V.F.; Molck, A.M.; Bogh, I.B.; Lykkesfeldt, J. Effect of insulin-induced hypoglycaemia on the peripheral nervous system: Focus on adaptive mechanisms, pathogenesis and histopathological changes. J. Neuroendocrinol. 2014, 26, 482–496. [Google Scholar] [CrossRef] [PubMed]
- Harding, J.E.; Harris, D.L.; Hegarty, J.E.; Alsweiler, J.M.; McKinlay, C.J. An emerging evidence base for the management of neonatal hypoglycaemia. Early Hum. Dev. 2017, 104, 51–56. [Google Scholar] [CrossRef]
- Davis, S.; Alonso, M.D. Hypoglycemia as a barrier to glycemic control. J. Diabetes Complicat. 2004, 18, 60–68. [Google Scholar] [CrossRef]
- Maran, A.; Cranston, I.; Lomas, J.; Macdonald, I.; Amiel, S.A. Protection by lactate of cerebral function during hypoglycaemia. Lancet (Lond. Engl.) 1994, 343, 16–20. [Google Scholar] [CrossRef]
- Maran, A.; Crepaldi, C.; Trupiani, S.; Lucca, T.; Jori, E.; Macdonald, I.A.; Tiengo, A.; Avogaro, A.; Del Prato, S. Brain function rescue effect of lactate following hypoglycaemia is not an adaptation process in both normal and Type I diabetic subjects. Diabetologia 2000, 43, 733–741. [Google Scholar] [CrossRef]
- Page, K.A.; Williamson, A.; Yu, N.; McNay, E.C.; Dzuira, J.; McCrimmon, R.J.; Sherwin, R.S. Medium-chain fatty acids improve cognitive function in intensively treated type 1 diabetic patients and support in vitro synaptic transmission during acute hypoglycemia. Diabetes 2009, 58, 1237–1244. [Google Scholar] [CrossRef]
- De Galan, B.E.; Tack, C.J.; Lenders, J.W.; Pasman, J.W.; Elving, L.D.; Russel, F.G.; Lutterman, J.A.; Smits, P. Theophylline improves hypoglycemia unawareness in type 1 diabetes. Diabetes 2002, 51, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Leelarathna, L.; Little, S.A.; Walkinshaw, E.; Tan, H.K.; Lubina-Solomon, A.; Kumareswaran, K.; Lane, A.P.; Chadwick, T.; Marshall, S.M.; Speight, J.; et al. Restoration of self-awareness of hypoglycemia in adultswith long-standing type 1 diabetes: Hyperinsulinemic-hypoglycemic clamp substudy results from the HypoCOMPaSS trial. Diabetes Care 2013, 36, 4063–4070. [Google Scholar] [CrossRef] [PubMed]
- Rooijackers, H.M.; Wiegers, E.C.; van der Graaf, M.; Thijssen, D.H.; Kessels, R.P.C.; Tack, C.J.; de Galan, B.E. A Single Bout of High-Intensity Interval Training Reduces Awareness of Subsequent Hypoglycemia in Patients with Type 1 Diabetes. Diabetes 2017, 66, 1990–1998. [Google Scholar] [CrossRef] [PubMed]
- Tschritter, O.; Schafer, S.A.; Klett, J.; Pfafflin, A.; Haring, H.U.; Hennige, A.M.; Fritsche, A. Insulin detemir causes increased symptom awareness during hypoglycaemia compared to human insulin. Diabetes Obes. Metabol. 2009, 11, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Bingham, E.; Hopkins, D.; Pernet, A.; Reid, H.; Macdonald, I.A.; Amiel, S.A. The effects of KATP channel modulators on counterregulatory responses and cognitive function during acute controlled hypoglycaemia in healthy men: A pilot study. Diabet. Med. A J. Br. Diabet. Assoc. 2003, 20, 231–237. [Google Scholar] [CrossRef]
- Inkster, B.E.; Zammitt, N.N.; Ritchie, S.J.; Deary, I.J.; Morrison, I.; Frier, B.M. Effects of Sleep Deprivation on Hypoglycemia-Induced Cognitive Impairment and Recovery in Adults with Type 1 Diabetes. Diabetes Care 2016, 39, 750–756. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.L.; Hoi-Hansen, T.; Olsen, N.V.; Pedersen-Bjergaard, U.; Thorsteinsson, B. Erythropoietin during hypoglycaemia in type 1 diabetes: Relation to basal renin-angiotensin system activity and cognitive function. Diabetes Res. Clin. Pract. 2009, 85, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.L.; Pedersen-Bjergaard, U.; Kjaer, T.W.; Olsen, N.V.; Dela, F.; Holst, J.J.; Faber, J.; Tarnow, L.; Thorsteinsson, B. Influence of erythropoietin on cognitive performance during experimental hypoglycemia in patients with type 1 diabetes mellitus: A randomized cross-over trial. PLoS ONE 2013, 8, e59672. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, P.; Porcellati, F.; Busciantella Ricci, N.; Candeloro, P.; Cioli, P.; Nair, K.S.; Santeusanio, F.; Bolli, G.B.; Fanelli, C.G. Effect of oral amino acids on counterregulatory responses and cognitive function during insulin-induced hypoglycemia in nondiabetic and type 1 diabetic people. Diabetes 2008, 57, 1905–1917. [Google Scholar] [CrossRef] [PubMed]
- Rossetti, P.; Porcellati, F.; Ricci, N.B.; Candeloro, P.; Cioli, P.; Bolli, G.B.; Fanelli, C.G. Different brain responses to hypoglycemia induced by equipotent doses of the long-acting insulin analog detemir and human regular insulin in humans. Diabetes 2008, 57, 746–756. [Google Scholar] [CrossRef]
- Smith, D.; Pernet, A.; Rosenthal, J.M.; Bingham, E.M.; Reid, H.; Macdonald, I.A.; Amiel, S.A. The effect of modafinil on counter-regulatory and cognitive responses to hypoglycaemia. Diabetologia 2004, 47, 1704–1711. [Google Scholar] [CrossRef] [PubMed]
- Chan, O.; Paranjape, S.A.; Horblitt, A.; Zhu, W.; Sherwin, R.S. Lactate-induced release of GABA in the ventromedial hypothalamus contributes to counterregulatory failure in recurrent hypoglycemia and diabetes. Diabetes 2013, 62, 4239–4246. [Google Scholar] [CrossRef] [PubMed]
- Choi, B.Y.; Kim, J.H.; Kim, H.J.; Yoo, J.H.; Song, H.K.; Sohn, M.; Won, S.J.; Suh, S.W. Pyruvate administration reduces recurrent/moderate hypoglycemia-induced cortical neuron death in diabetic rats. PLoS ONE 2013, 8, e81523. [Google Scholar] [CrossRef] [PubMed]
- Herzog, R.I.; Jiang, L.; Herman, P.; Zhao, C.; Sanganahalli, B.G.; Mason, G.F.; Hyder, F.; Rothman, D.L.; Sherwin, R.S.; Behar, K.L. Lactate preserves neuronal metabolism and function following antecedent recurrent hypoglycemia. J. Clin. Investig. 2013, 123, 1988–1998. [Google Scholar] [CrossRef] [PubMed]
- Agardh, C.D.; Smith, M.L.; Siesjo, B.K. The influence of hypothermia on hypoglycemia-induced brain damage in the rat. Acta Neuropathol. 1992, 83, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Zhao, Y.; Liu, F.; Mi, Y.; Shen, J.; Wang, X.; Liu, J.; Jin, W. Rapidly raise blood sugar will aggravate brain damage after severe hypoglycemia in rats. Cell Biochem. Biophys. 2014, 69, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Ferrand-Drake, M.; Zhu, C.; Gido, G.; Hansen, A.J.; Karlsson, J.O.; Bahr, B.A.; Zamzami, N.; Kroemer, G.; Chan, P.H.; Wieloch, T.; et al. Cyclosporin A prevents calpain activation despite increased intracellular calcium concentrations, as well as translocation of apoptosis-inducing factor, cytochrome c and caspase-3 activation in neurons exposed to transient hypoglycemia. J. Neurochem. 2003, 85, 1431–1442. [Google Scholar] [CrossRef]
- Friberg, H.; Ferrand-Drake, M.; Bengtsson, F.; Halestrap, A.P.; Wieloch, T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J. Neurosci. Off. J. Soc. Neurosci. 1998, 18, 5151–5159. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, B.Y.; Kho, A.R.; Lee, S.H.; Jeong, J.H.; Hong, D.K.; Lee, S.H.; Sohn, M.; Ryu, O.H.; Choi, M.G.; et al. Acetylcholine precursor, citicoline (cytidine 5′-diphosphocholine), reduces hypoglycaemia-induced neuronal death in rats. J. Neuroendocrinol. 2018, 30. [Google Scholar] [CrossRef]
- Nellgard, B.; Wieloch, T. Cerebral protection by AMPA- and NMDA-receptor antagonists administered after severe insulin-induced hypoglycemia. Exp. Brain Res. 1992, 92, 259–266. [Google Scholar] [CrossRef]
- Reno, C.M.; Tanoli, T.; Bree, A.; Daphna-Iken, D.; Cui, C.; Maloney, S.E.; Wozniak, D.F.; Fisher, S.J. Antecedent glycemic control reduces severe hypoglycemia-induced neuronal damage in diabetic rats. Am. J. Physiol. Endocrinol. Metabol. 2013, 304, E1331–E1337. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Tuzcu, M.; Orhan, C.; Ali, S.; Sahin, N.; Gencoglu, H.; Ozkan, Y.; Hayirli, A.; Gozel, N.; Komorowski, J.R. Chromium modulates expressions of neuronal plasticity markers and glial fibrillary acidic proteins in hypoglycemia-induced brain injury. Life Sci. 2013, 93, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, J.M.; Musikantow, D.; Puente, E.C.; Daphna-Iken, D.; Bree, A.J.; Fisher, S.J. Pharmacologic amelioration of severe hypoglycemia-induced neuronal damage. Neurosci. Lett. 2011, 492, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Patockova, J.; Sliva, J.; Marhol, P.; Crkovska, J.; Stipek, S. The influence of vitamin-rich diet on the extent of lipoperoxidation in brain of mice during an acute post-insulin hypoglycaemia. Eur. J. Pharmacol. 2014, 740, 641–644. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ (Clin. Res. ed.) 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed]
- Bouzat, P.; Oddo, M. Lactate and the injured brain: Friend or foe? Curr. Opin. Crit. Care 2014, 20, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Taher, M.; Leen, W.G.; Wevers, R.A.; Willemsen, M.A. Lactate and its many faces. Eur. J. Paediatr. Neurol. EJPN Off. J. Eur. Paediatr. Neurol. Soc. 2016, 20, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bouzat, P.; Sala, N.; Suys, T.; Zerlauth, J.B.; Marques-Vidal, P.; Feihl, F.; Bloch, J.; Messerer, M.; Levivier, M.; Meuli, R.; et al. Cerebral metabolic effects of exogenous lactate supplementation on the injured human brain. Intensive Care Med. 2014, 40, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Patet, C.; Suys, T.; Carteron, L.; Oddo, M. Cerebral Lactate Metabolism After Traumatic Brain Injury. Curr. Neurol. Neurosci. Rep. 2016, 16, 31. [Google Scholar] [CrossRef] [PubMed]
- Vohra, R.; Kolko, M. Neuroprotection of the inner retina: Muller cells and lactate. Neural Regen. Res. 2018, 13, 1741–1742. [Google Scholar] [CrossRef] [PubMed]
- Wiegers, E.C.; Rooijackers, H.M.; Tack, C.J.; Heerschap, A.; de Galan, B.E.; van der Graaf, M. Brain Lactate Concentration Falls in Response to Hypoglycemia in Patients with Type 1 Diabetes and Impaired Awareness of Hypoglycemia. Diabetes 2016, 65, 1601–1605. [Google Scholar] [CrossRef] [PubMed]
- Arbelaez, A.M.; Cryer, P.E. Lactate and the mechanism of hypoglycemia-associated autonomic failure in diabetes. Diabetes 2013, 62, 3999–4001. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.I. Effects of hypoglycaemia on neuronal metabolism in the adult brain: Role of alternative substrates to glucose. J. Inherit. Metab. Dis. 2013, 36, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C. Pyruvate therapy for severe hypoglycemia. Diabetes Technol. Ther. 2005, 7, 583–586. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kim, Y.H.; Koh, J.Y. Protection by pyruvate against transient forebrain ischemia in rats. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, Rc171. [Google Scholar] [CrossRef]
- Mongan, P.D.; Fontana, J.L.; Chen, R.; Bunger, R. Intravenous pyruvate prolongs survival during hemorrhagic shock in swine. Am. J. Physiol. 1999, 277, H2253–H2263. [Google Scholar] [CrossRef]
- Ahren, B. Avoiding hypoglycemia: A key to success for glucose-lowering therapy in type 2 diabetes. Vasc. Health Risk Manag. 2013, 9, 155–163. [Google Scholar] [CrossRef]
- Little, S.; Shaw, J.; Home, P. Hypoglycemia rates with basal insulin analogs. Diabetes Technol. Ther. 2011, 13, S53–S64. [Google Scholar] [CrossRef]
- Strandberg, A.Y.; Khanfir, H.; Makimattila, S.; Saukkonen, T.; Strandberg, T.E.; Hoti, F. Insulins NPH, glargine, and detemir, and risk of severe hypoglycemia among working-age adults. Ann. Med. 2017, 49, 357–364. [Google Scholar] [CrossRef]
- Reno, C.M.; Litvin, M.; Clark, A.L.; Fisher, S.J. Defective counterregulation and hypoglycemia unawareness in diabetes: Mechanisms and emerging treatments. Endocrinol. Metabol. Clin. N. Am. 2013, 42, 15–38. [Google Scholar] [CrossRef] [PubMed]
- Benkhadra, K.; Alahdab, F.; Tamhane, S.U.; McCoy, R.G.; Prokop, L.J.; Murad, M.H. Continuous subcutaneous insulin infusion versus multiple daily injections in individuals with type 1 diabetes: A systematic review and meta-analysis. Endocrine 2017, 55, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Rodbard, D. Continuous Glucose Monitoring: A Review of Successes, Challenges, and Opportunities. Diabetes Technol. Ther. 2016, 18, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Milman, S.; Leu, J.; Shamoon, H.; Vele, S.; Gabriely, I. Magnitude of exercise-induced beta-endorphin response is associated with subsequent development of altered hypoglycemia counterregulation. J. Clin. Endocrinol. Metabol. 2012, 97, 623–631. [Google Scholar] [CrossRef] [PubMed]
- Galassetti, P.; Mann, S.; Tate, D.; Neill, R.A.; Costa, F.; Wasserman, D.H.; Davis, S.N. Effects of antecedent prolonged exercise on subsequent counterregulatory responses to hypoglycemia. Am. J. Physiol. Endocrinol. Metabol. 2001, 280, E908–E917. [Google Scholar] [CrossRef] [PubMed]
- McGregor, V.P.; Greiwe, J.S.; Banarer, S.; Cryer, P.E. Limited impact of vigorous exercise on defenses against hypoglycemia: Relevance to hypoglycemia-associated autonomic failure. Diabetes 2002, 51, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, C.C.; Burgos, C.F.; Gajardo, A.H.; Silva-Grecchi, T.; Gavilan, J.; Toledo, J.R.; Fuentealba, J. Neuroprotective effects of erythropoietin on neurodegenerative and ischemic brain diseases: The role of erythropoietin receptor. Neural Regen. Res. 2017, 12, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Moransard, M.; Bednar, M.; Frei, K.; Gassmann, M.; Ogunshola, O.O. Erythropoietin reduces experimental autoimmune encephalomyelitis severity via neuroprotective mechanisms. J. Neuroinflamm. 2017, 14, 202. [Google Scholar] [CrossRef]
- Fischer, H.S.; Reibel, N.J.; Buhrer, C.; Dame, C. Prophylactic Early Erythropoietin for Neuroprotection in Preterm Infants: A Meta-analysis. Pediatrics 2017, 139. [Google Scholar] [CrossRef]
- Chen, J.; Yang, Z.; Zhang, X. Carbamylated Erythropoietin: A Prospective Drug Candidate for Neuroprotection. Biochem. Insights 2015, 8, 25–29. [Google Scholar] [CrossRef]
- Cariou, A.; Deye, N.; Vivien, B.; Richard, O.; Pichon, N.; Bourg, A.; Huet, L.; Buleon, C.; Frey, J.; Asfar, P.; et al. Early High-Dose Erythropoietin Therapy After Out-of-Hospital Cardiac Arrest: A Multicenter, Randomized Controlled Trial. J. Am. Coll. Cardiol. 2016, 68, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.; Broessner, G. Is there still a role for hypothermia in neurocritical care? Curr. Opin. Crit. Care 2017, 23, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Fan, Y.; Hong, S.M.; Liu, Z.; Matsumori, Y.; Weinstein, P.R.; Swanson, R.A.; Liu, J. Hypoglycemia induces transient neurogenesis and subsequent progenitor cell loss in the rat hippocampus. Diabetes 2005, 54, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Kilbaugh, T.J.; Bhandare, S.; Lorom, D.H.; Saraswati, M.; Robertson, C.L.; Margulies, S.S. Cyclosporin A preserves mitochondrial function after traumatic brain injury in the immature rat and piglet. J. Neurotrauma 2011, 28, 763–774. [Google Scholar] [CrossRef]
- Landstedt-Hallin, L.; Adamson, U.; Lins, P.E. Oral glibenclamide suppresses glucagon secretion during insulin-induced hypoglycemia in patients with type 2 diabetes. J. Clin. Endocrinol. Metabol. 1999, 84, 3140–3145. [Google Scholar] [CrossRef]
- Languren, G.; Montiel, T.; Julio-Amilpas, A.; Massieu, L. Neuronal damage and cognitive impairment associated with hypoglycemia: An integrated view. Neurochem. Int. 2013, 63, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Kahan, B.C.; Rehal, S.; Cro, S. Risk of selection bias in randomised trials. Trials 2015, 16, 405. [Google Scholar] [CrossRef] [PubMed]
- Heller, S.R.; Macdonald, I.A. The measurement of cognitive function during acute hypoglycaemia experimental limitations and their effect on the study of hypoglycaemia unawareness. Diabetic Med. 1996, 13, 607–615. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ (Clin. Res. ed.) 2015, 350, g7647. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Joanna Briggs Institute. JBI Data Extraction form for Experimental/Observational Studies. Joanna Briggs Institute JBI Data Extraction form for Experimental/Observational Studies. Available online: https://joannabriggs.org/assets/docs/jbc/operations/dataExtractionForms/JBC_Form_DataE_ExpObs.pdf (accessed on 2 August 2017).
- Nistor, M.; Behringer, W.; Schmidt, M.; Schiffner, R. A Systematic Review of Neuroprotective Strategies during Hypovolemia and Hemorrhagic Shock. Int. J. Mol. Sci. 2017, 18, 2247. [Google Scholar] [CrossRef] [PubMed]
| Reference | Model | Intervention | Total Number of Participants, n = x | Extent of Hypoglycemia | Observation Period after Intervention |
|---|---|---|---|---|---|
| [32] | Rats | 4CIN vs. aCEF vs. BIC vs. DZ vs. OX, after either lactate or aCEF | 38 | 2.5 ± 0.3 mmol/L | None |
| [33] | Rats | Vehicle vs. pyruvate | 22 | 1–2 mmol/L | 3 days after last recurrent hypoglycemia intervention |
| [34] | Rats | Hypo/EU clamp + lactate in either Ctrl or 3dRH animals | 44 | 2.5 mmol/L | 1 day |
| [19] | Humans | Crossover study: HYPO clamp with lactate vs. without lactate | 14 (7 healthy, 7 DM) | stepwise: 4.8, 3.6, 3.0, 2.8 mmol/L | None |
| [18] | Humans | Crossover study: HYPO clamp with lactate vs. without lactate | 7 (healthy) | stepwise: 5.0, 3.4, 2.8, 2.4 mmol/L | None |
| [20] | Humans | Crossover study: HYPO clamp with medium chain triglycerides vs. placebo | 11 | 2.8 ± 0.16 mmol/L | None |
| [21] | Humans | Crossover study: HYPO clamp with theophylline vs. placebo | 30 (15 DM with HA, 15 healthy) | stepwise: 5.0, 3.5, 2.5 mmol/L | None |
| [22] | Humans | MDI + SMBG vs. MDI + SMBG + RT-CGM vs. CSII + SMBG vs. CSII + SMBG + RT-CGM | 18 | stepwise: 5.0, 3.8, 3.4, 2.8, 2.4 mmol/L | 6 months between two HYPO Clamp procedures with intervention in between |
| [23] | Humans | HIIT vs. rest | 30 | 2.8 mmol/L | None |
| [24] | Humans | Crossover study: Human insulin vs. insulin analogue detemir | 12 | stepwise: 4.4, 3.7, 3.0, 2.7 mmol/L | None |
| [35] | Rats | Hypothermia + halothane vs. hypothermia + isoflurane vs. normothermia + halothane vs. normothermia + isoflurane | 32 | isoelectricity in EEG | 4 hours up to 7 days |
| [25] | Humans | Crossover study: Glibenclamide vs. diazoxide vs. placebo | 10 | stepwise: 5.0, 3.4, 2.8, 2.4 mmol/L | None |
| [36] | Rats | Blood glucose reperfusion to ≤3 mmol/L vs. ≤6 mmol/L vs. ≤9 mmol/L vs. >9 mmol/L | 30 | <1 mmol/L | 7 days |
| [37] | Rats | Cyclosporin A vs. FK506 | 9 | isoelectricity in EEG | 30 min up to 2 days |
| [38] | Rats | Cylosporin A (varying doses) vs. FK506 | 66 | isoelectricity in EEG | 7 days |
| [26] | Humans | Sleep deprivation vs. normal sleep | 14 | 2.5 mmol/L | 85 min |
| [39] | Rats | Citociline vs. vehicle | 42 | isoelectricity in EEG | 7 days |
| [27] | Humans | HYPO clamp in patients with high RAS activity vs. patients with low RAS activity | 18 | 2.5–2.0 mmol/L | 60 min |
| [28] | Humans | Crossover study: Erythropoietin vs. placebo | 11 | 2.2–2.0 mmol/L | 30 min |
| [40] | Rats | NBQX vs. NBQX + dizocilpine vs. dizocilpine vs. CGP 40,116 | 44 | isoelectricity in EEG | 3–4 days |
| [44] | Mice | Vitamin C vs. vitamin E vs. vitamin C + vitamin E | 64 | <1 mmol/L | None |
| [41] | Rats | Insulin-treated DM vs. untreated DM | 55 | 0.8–0.6 mmol/L | 1 week up to 8 weeks |
| [29] | Humans | Crossover study: Human insulin vs. insulin analogue detemir | 10 | stepwise: 5.0, 4.3, 3.6, 3.0 mmol/L | None |
| [30] | Humans | Crossover study: HYPO clamp + oral amino acids vs. HYPO clamp + placebo vs. EU clamp + oral amino acids | 20 | 2.6 mmol/L | None |
| [42] | Rats | CrHis vs. CrPic vs. dextrose | 70 | isoelectricity in EEG | 1 day |
| [43] | Rats | Memantine vs. erythropoietin | 36 | 0.8–0.6 mmol/L | 7 days |
| [31] | Humans | Crossover study: HYPO clamp + modafinil vs. HYPO Clamp + placebo vs. EU clamp + modafinil vs. EU clamp + placebo | 9 | stepwise: 4.4, 3.8, 3.4, 2.8, 2.4 mmol/L | None |
| Reference | Vital Parameters (Hemodynamics, Blood Pressure, Heart Rate, Temperature) Blood Analysis (Blood Gases, Metabolic Products) | Counter-Regulatory Hormones (Catechol-Amines, Glucagon, Growth Hormone, Cortisol) | Brain-Specific Parameters (CBF, EEG, etc.) | Brain Section Staining/ Histopathology) | Neuro-Proteins and Receptors, Apoptosis Markers | Cognitive Function Tests | Symptom Assessment (Autonomic and Neuroglycopenic) |
|---|---|---|---|---|---|---|---|
| [32] | - | + | - | - | GABA | - | - |
| [33] | GSH, Zn | - | - | + | - | - | - |
| [34] | + | - | CBF, EEG | - | GLUT1, GLUT2, GLUT3 | - | - |
| [19] | + | + | - | - | - | 4-CRT | + |
| [18] | - | + | - | - | - | 4-CRT | + |
| [20] | + | + | - | - | - | DS, DSS, WMS | + |
| [21] | + | + | CBF | - | - | - | + |
| [22] | + | + | - | - | - | 4-CRT, Str | + |
| [23] | + | + | - | - | - | DS, VF, PASAT | + |
| [24] | + | + | - | - | - | Str, VRT, VM | + |
| [35] | + | - | - | + | - | - | - |
| [25] | - | + | - | - | - | 4-CRT, Str, FT | + |
| [36] | - | - | - | + | - | - | - |
| [37] | + | - | - | - | Cas3, AIF, Cyt-c, | - | - |
| [38] | - | - | + | MRR | - | - | |
| [26] | - | - | - | - | - | 4-CRT, DSS, NART, WQ, MT | + |
| [39] | + | - | EEG | + | CHAT | - | - |
| [27] | + | - | - | - | - | AQT, CCAP | - |
| [28] | + | + | ÊEG | - | - | CCAP, TM, Str | + |
| [40] | + | - | - | + | - | - | - |
| [44] | + | - | - | - | MAD, SOD, GSHPx | - | - |
| [41] | - | - | - | + | - | LA, SM, MWM | - |
| [29] | + | + | - | - | - | TM, VM, DS, Str, PASAT | + |
| [30] | + | + | - | - | - | TM, VF, VM, DV, DS, Str, PASAT | + |
| [42] | + | - | - | - | MAD, GAP43, NCAM, GLUT1, GLUT3, NF-KB, HNE, Nrf2 | - | - |
| [43] | - | - | - | + | - | - | - |
| [31] | + | + | - | - | - | 4-CRT, FT, Str | + |
| Reference | Model | Intervention | Dosage | Start of Intervention Respective to Hypoglycemia | Length of Subsequent Observation Period |
|---|---|---|---|---|---|
| [32] | Rats | Lactate transporter blockade (4CIN), GABA receptor antagonist (BIC), KATP channel blockade (diazoxide), lactate dehydrogenase inhibitor (OX) | 4CIN = 15 nmol; BIC = 12.5 pmol; diazoxide = 1 nmol; OX = 50 nmol | Immediately before hypoglycemic clamp | None |
| [33] | Rats | Pyruvate | 500 mg/kg | 10 min after termination of daily recurrent hypoglycemia (5 days) | 3 days |
| [34] | Rats | 0.35M [3-13 C] lactate | Initial bolus of 1370 µL/kg body weight, thereafter stepwise reduction from 428 µL/min/kg to 162.8 µL/kg/min over 20 min, thereafter continuous 162.8 µL/kg/min | Immediately after reaching target glucose level | 1 day |
| [18] | Humans | Lactate | Continuous 30 µmoL/kg/min | 40 min before hypoglycemia | None |
| [19] | Humans | Lactate | Continuous 30 µmoL/kg/min | After reporting of first neuroglycopenic response | None |
| [20] | Humans | Medium-chain fatty acids | Total of 40 g (in 25-min intervals: 20 g, 10 g, and 10 g) | First ingestion 5 min before hypoglycemia | None |
| Reference | Model | Intervention | Dosage | Start of Intervention Respective to Hypoglycemia | Length of Subsequent Observation Period |
|---|---|---|---|---|---|
| [21] | Humans | Theophylline | 2.8 mg/kg | Immediately before hypoglycemia | None |
| [22] | Humans | MDI + SMBG vs. MDI + SMBG + RT-CGM vs. CSII + SMBG vs. CSII + SMBG + RT-CGM | Application in daily routine | After the first hypoglycemic procedure | Six months application, thereafter second hypoglycemic experiment |
| [23] | Humans | High-intensity interval training | ~15 mins on cycle ergometer: three 4-min periods at 50 W, interspersed with three 30-s all-out sprints | Before hypoglycemia | None |
| [29] | Humans | Human insulin vs. insulin detemir | Human insulin: Bolus of 10 mU/kg, followed by 240 min of 1 mU/kg/min and 30 min of 2 mU/kg/min; insulin detemir: Bolus of 20 mU/kg, followed by 2 mU/kg/min and 30 min of 4 mU/kg/min | Throughout the entire experiment | None |
| [24] | Humans | Human insulin vs. Insulin detemir | Human insulin: Bolus of 60 mU/kg, followed by continuous infusion of 2 mU/kg/min; insulin detemir: Bolus of 660 mU/kg, followed by a continuous infusion of 5 mU/kg/min | Throughout the entire experiment | None |
| Reference | Humans/Animal Model | Intervention | Dosage | Start of Intervention Respective to Hypoglycemia | Length of Subsequent Observation Period |
|---|---|---|---|---|---|
| [35] | Rats | Hypothermia | 33 °C for 30 min | Immediately before establishment of hypoglycemia | 4 h up to 7 days |
| [25] | Humans | Glibenclamide vs. Diazoxide | Glibenclamide: 10 mg; diazoxide: 5 mg/kg | 45 min before hypoglycemia | None |
| [36] | Rats | Differing blood glucose levels post-hypoglycemia | Infusion of 25% glucose solution until target level was reached | Immediately following the hypoglycemic procedure | 7 days |
| [38] | Rats | CsA vs. FK 506 | CsA: Either 20mg/kg or 50mg/kg; FK506: 2mg/kg | ~30 min before onset of isoelectric EEG | 7 days |
| [37] | Rats | CsA vs. FK 506 | CsA: 50mg/kg; FK506: 2mg/kg | ~30 min before onset of isoelectric EEG | 30 min up to 2 days |
| [26] | Humans | Sleep deprivation | 1 night of sleep deprivation | Night before the hypoglycemic clamp | 85 min |
| [39] | Rats | Citociline | 500 mg/kg | Immediately after hypoglycemia | 7 days |
| [27] | Humans | Measurement of EPO and RAS activity | / | / | 60 min |
| [28] | Humans | EPO | 40,000 IU | 6 days before hypoglycemia | 30 min |
| [43] | Rats | EPO vs. memantine | EPO: 5000 IU/kg on three occasions; memantine: 20 mg/kg | EPO: 24 h before and after hypoglycemia (ip), Immediately after hypoglycemia (iv); memantine: Immediately after hypoglycemia | 7 days |
| [40] | Rats | NBQX vs. NBQX + dizocilpine vs. dizocilpine vs. CGP 40116 | NBQX: 30 mg/kg (ip), followed by 225 µL/kg/min for 6 h i.v.; NBQX + dizolcilpine: 10 mg/kg (ip), followed by 225 µL/kg/min for 6 h i.v. + 2 x 0.33 mg/kg; dizolcilpine: 1 mg/kg (iv); CGP40116: 10 mg/kg (ip) | All immediately after hypoglycemia, except CGP40116 (during EEG isoelectricity) | 3–4 days |
| [44] | Mice | Vitamin C vs. vitamin E | Vitamin C: 1000 mg/kg/day; vitamin E: 100 mg/kg/day | Previous days | None |
| [41] | Rats | Antecedent glycemic control (with insulin) | 2 U/day (target level: 100–250 mg/dL) | Three weeks before hypoglycemia | Either 1 or 8 weeks |
| [30] | Humans | Oral amino acids | At the beginning of hypoglycemia | None | |
| [42] | Rats | CrHis vs. CrPic | 8 µg orally per day for 7 days | 7 days before hypoglycemia | 1 day |
| [31] | Humans | Modafinil | 100 mg orally | Evening before intervention | None |
| Reference | Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Binding (Intervention) | Random Outcome Assessment | Blinding (Outcome) | Incomplete Outcome Data | Selective Outcome Reporting | Other Sources of Bias |
|---|---|---|---|---|---|---|---|---|---|---|
| [35] | - | + | - | - | + | + | + | + | + | + |
| [32] | - | + | - | ? | + | + | ? | + | + | + |
| [33] | - | + | - | ? | - | + | + | + | + | + |
| [36] | ? | + | ? | - | + | ? | + | + | + | - |
| [37] | - | + | - | ? | ? | ? | + | + | + | ? |
| [38] | - | + | - | ? | ? | + | ? | + | + | ? |
| [34] | - | + | - | ? | ? | - | ? | + | + | - |
| [39] | - | + | - | ? | + | - | + | + | + | + |
| [40] | - | + | - | ? | - | + | + | + | + | + |
| [44] | ? | + | ? | N.A. | - | + | + | + | + | + |
| [41] | ? | + | ? | - | ? | ? | ? | + | + | + |
| [42] | ? | + | ? | - | - | + | + | + | + | + |
| [43] | ? | + | ? | ? | - | + | + | + | + | + |
| Reference | Random Sequence Generation | Allocation Concealment | Blinding of Participants and Personnel | Blinding of Outcome Assessment | Incomplete Outcome Data | Selective Reporting | Other Bias |
|---|---|---|---|---|---|---|---|
| [19] | + | ? | + | + | + | + | + |
| [18] | ? | ? | + | + | + | ? | ? |
| [20] | ? | ? | + | + | + | + | + |
| [21] | ? | ? | + | + | + | + | + |
| [22] | + | ? | ? | + | ? | + | + |
| [23] | + | + | ? | + | ? | + | + |
| [24] | ? | ? | + | + | + | + | + |
| [25] | ? | ? | + | + | + | + | + |
| [26] | ? | ? | ? | + | + | + | + |
| [27] | ? | ? | + | + | + | + | + |
| [28] | + | + | + | + | + | + | + |
| [29] | + | ? | + | + | + | + | + |
| [30] | + | ? | + | + | + | + | + |
| [31] | ? | ? | + | + | + | + | ? |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nistor, M.; Schmidt, M.; Graul, I.; Rakers, F.; Schiffner, R. A Systematic Review of Neuroprotective Strategies in the Management of Hypoglycemia. Int. J. Mol. Sci. 2019, 20, 550. https://doi.org/10.3390/ijms20030550
Nistor M, Schmidt M, Graul I, Rakers F, Schiffner R. A Systematic Review of Neuroprotective Strategies in the Management of Hypoglycemia. International Journal of Molecular Sciences. 2019; 20(3):550. https://doi.org/10.3390/ijms20030550
Chicago/Turabian StyleNistor, Marius, Martin Schmidt, Isabel Graul, Florian Rakers, and René Schiffner. 2019. "A Systematic Review of Neuroprotective Strategies in the Management of Hypoglycemia" International Journal of Molecular Sciences 20, no. 3: 550. https://doi.org/10.3390/ijms20030550
APA StyleNistor, M., Schmidt, M., Graul, I., Rakers, F., & Schiffner, R. (2019). A Systematic Review of Neuroprotective Strategies in the Management of Hypoglycemia. International Journal of Molecular Sciences, 20(3), 550. https://doi.org/10.3390/ijms20030550





