Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine
Abstract
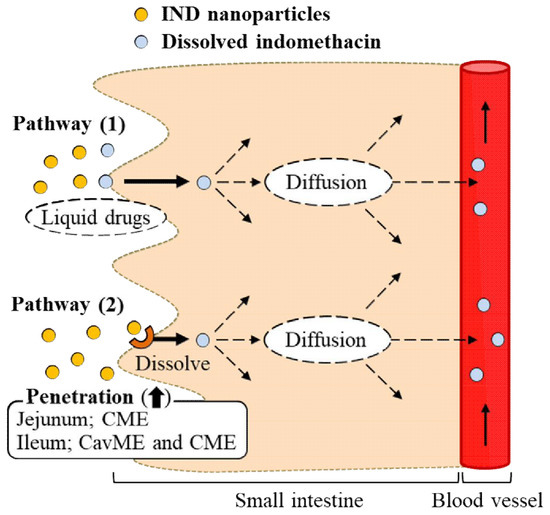
1. Introduction
2. Results
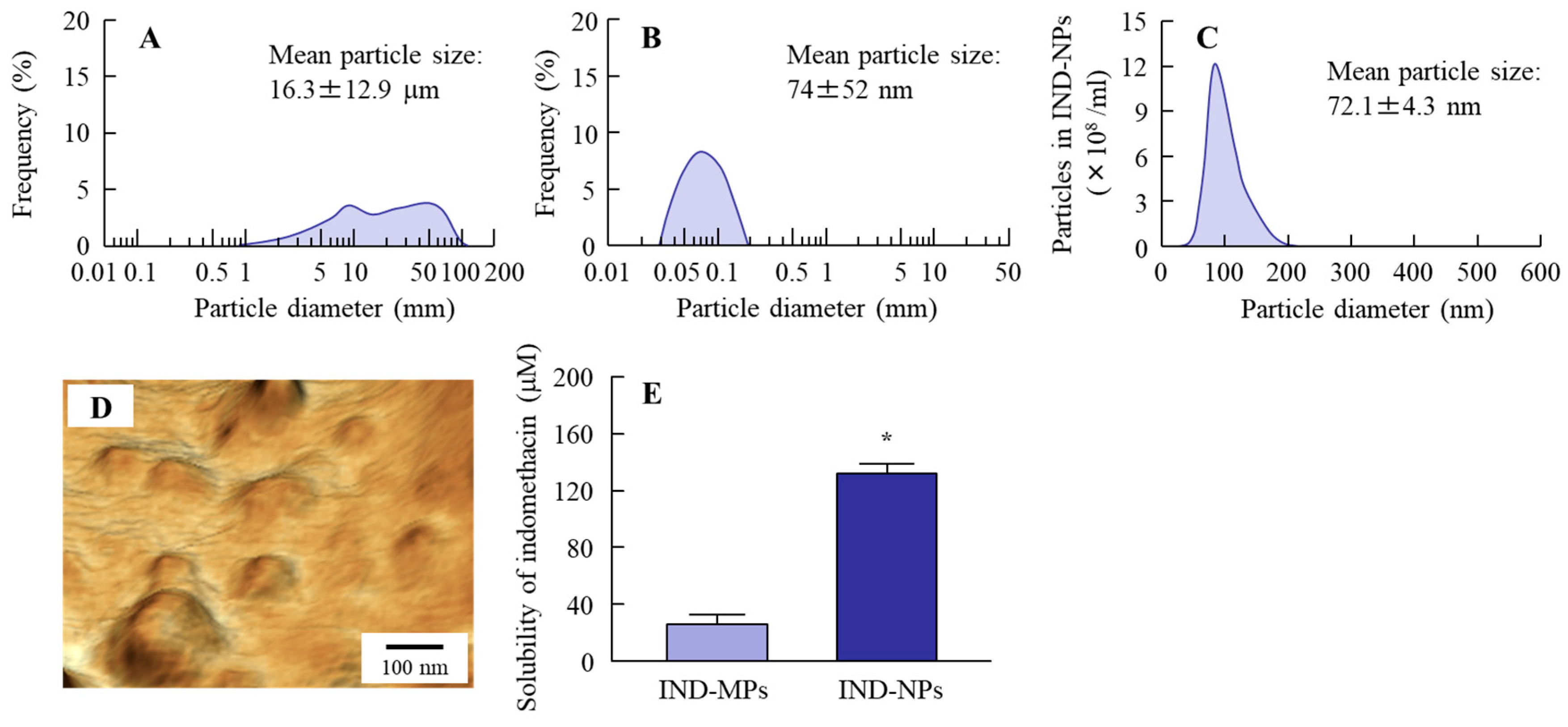
2.1. Design of Oral Formulation Containing Indomethacin Nanoparticles
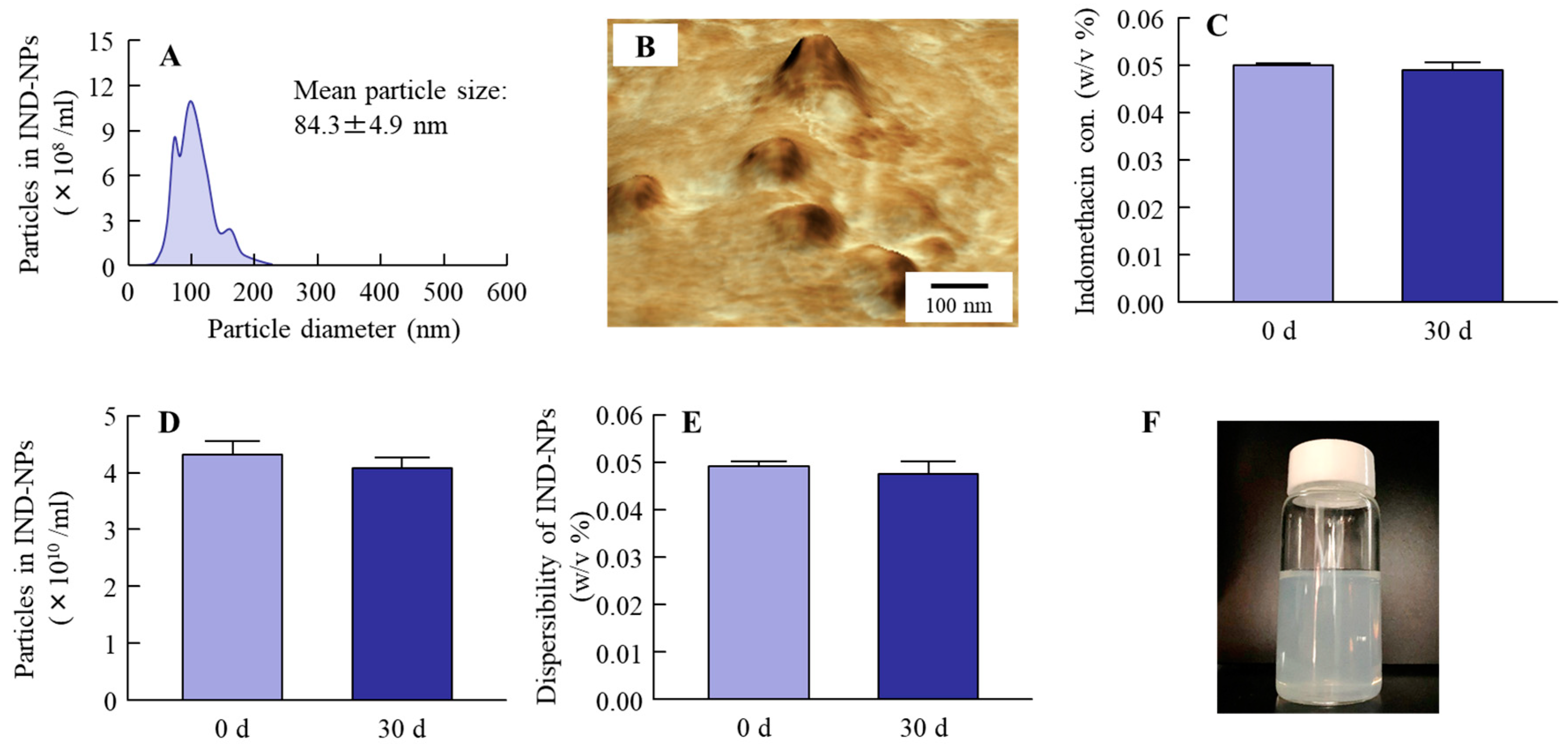
2.2. Stability of the Oral Formulation Containing Indomethacin Nanoparticles
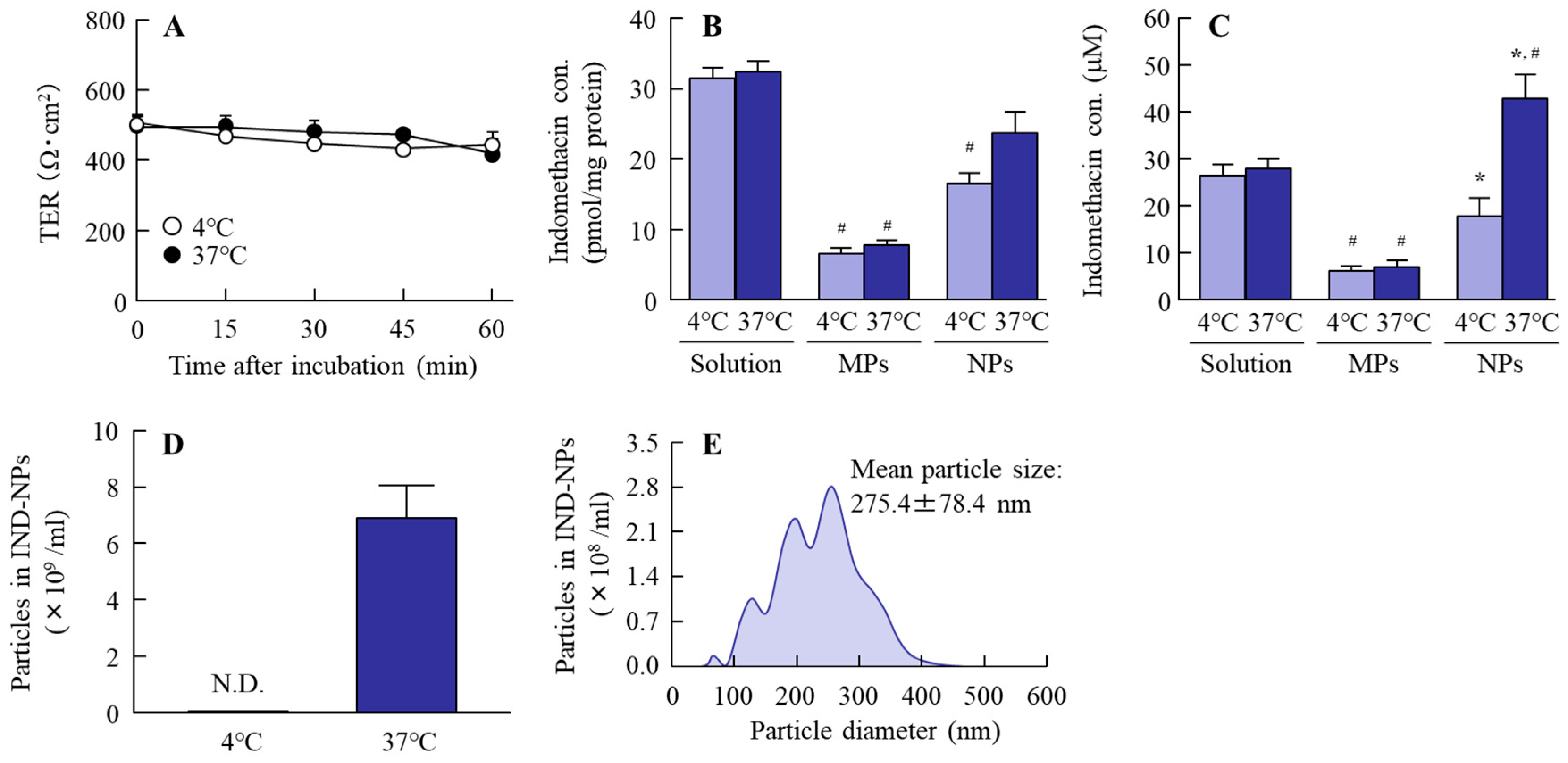
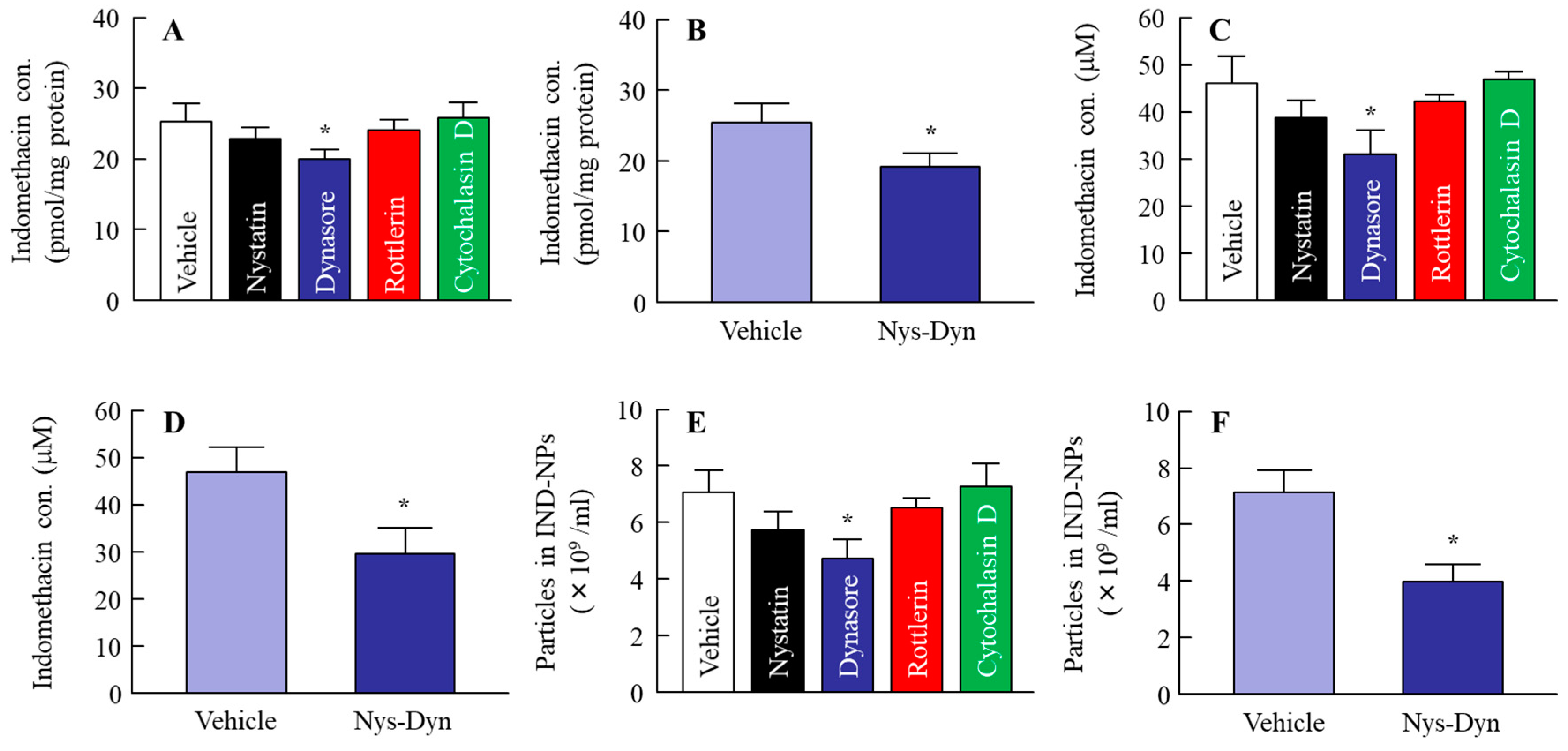
2.3. Effect of the Energy-Dependent Endocytosis on the Transintestinal Penetration of Indomethacin Nanoparticles Using Caco-2 Cell Monolayers
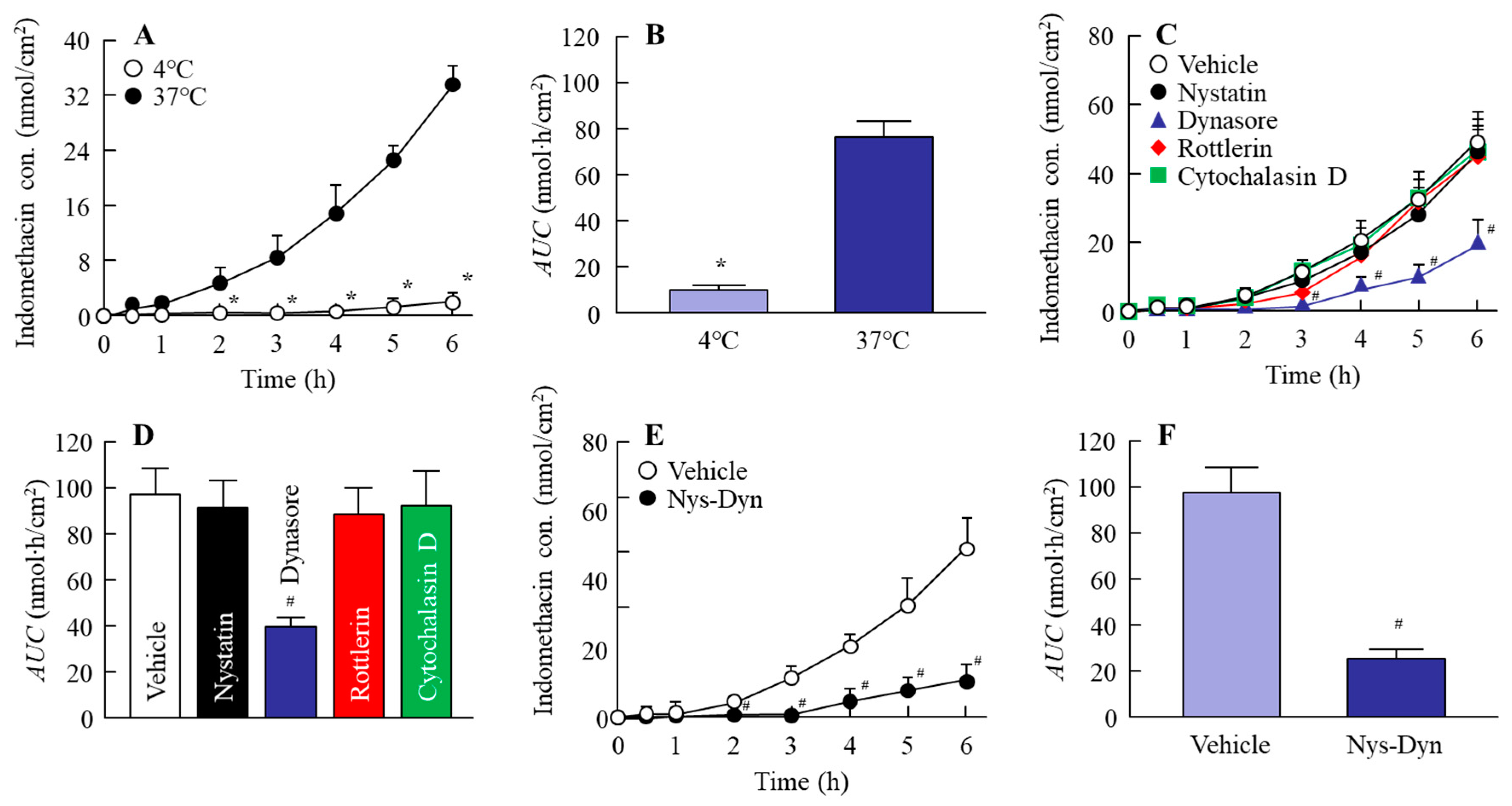
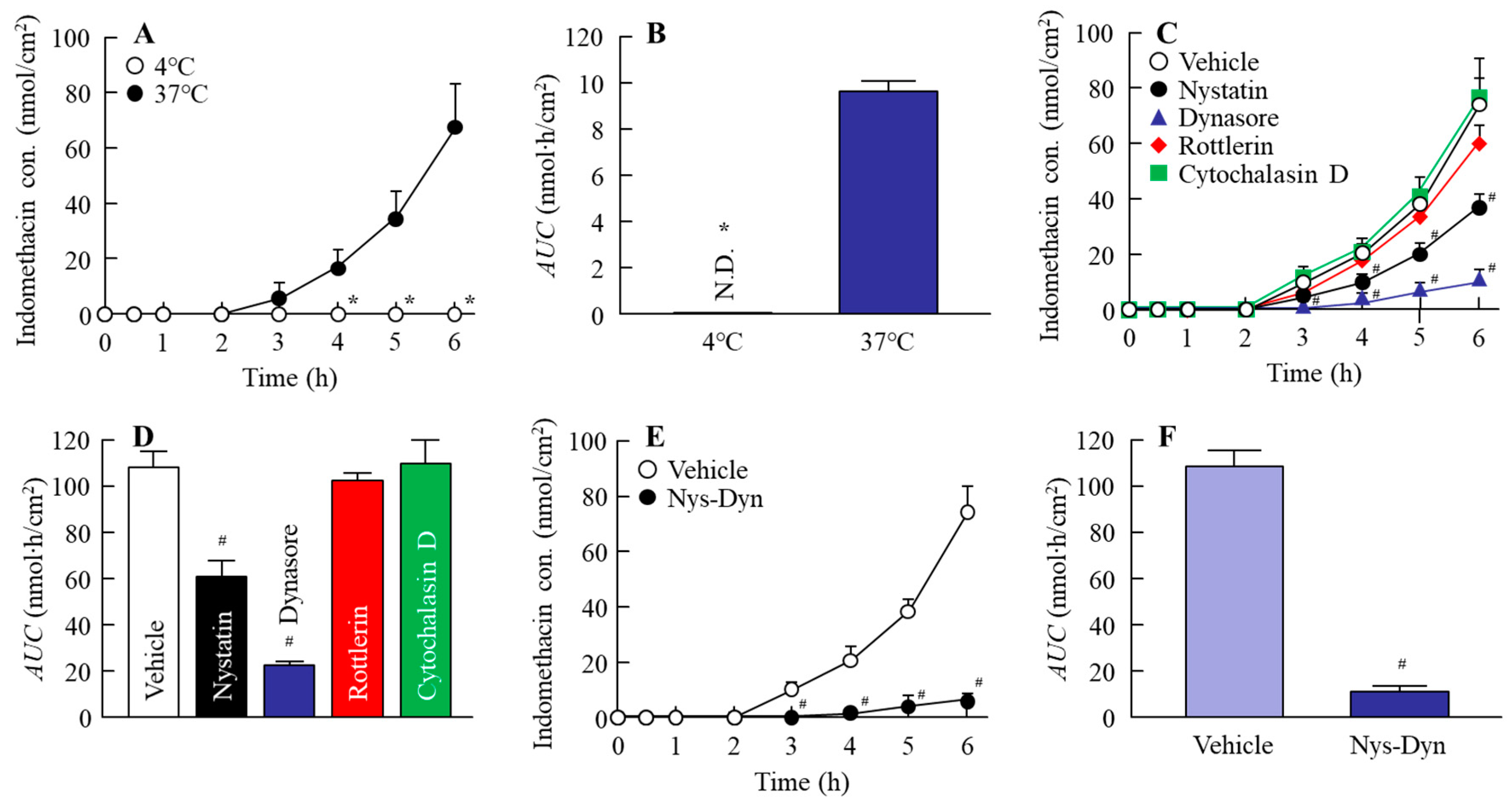
2.4. Effect of Energy-Dependent Endocytosis on the Transintestinal Penetration of Indomethacin Nanoparticles in the Rat Jejunum and Ileum
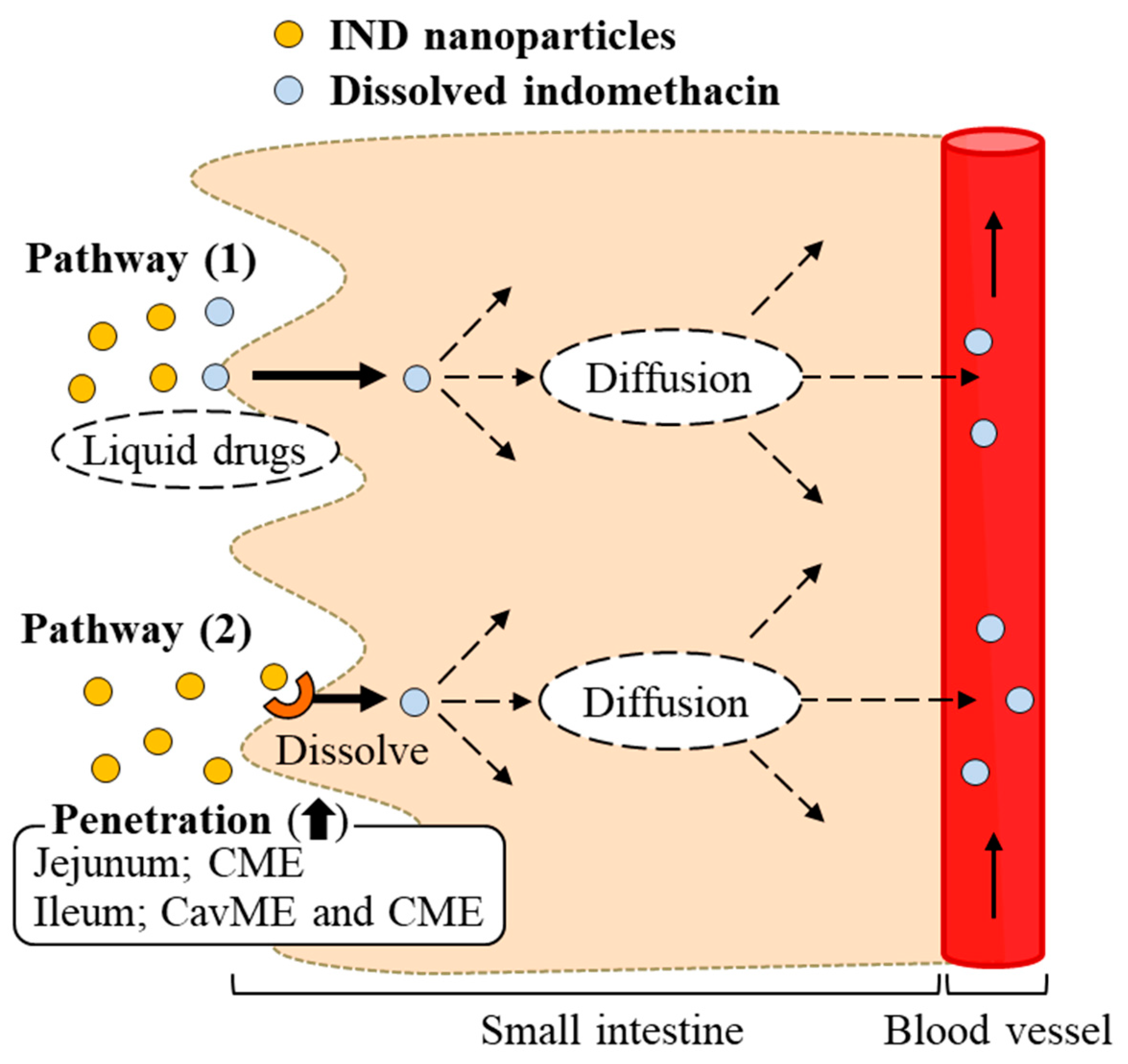
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals
4.3. Preparation of IND-NPs
4.4. Analysis of Particle Size and Number of Indomethacin Nanoparticles
4.5. Evaluation of Dispersibility in IND-NPs
4.6. Measurement of Indomethacin Penetration through Caco-2 Cell Monolayers
4.7. Measurement of Indomethacin Penetration through Removed Small Intestine in Rats
4.8. Inhibition of Energy-Dependent Endocytosis
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| ANOVA | one-way analysis of variance |
| AFM | atomic force microscope |
| AUC | area under the drug concentration-time curve |
| BA | bioavailability |
| Caco-2 | human epithelial colorectal adenocarcinoma cell line |
| CavME | caveolae-dependent endocytosis |
| CME | clathrin-dependent endocytosis |
| COX | cyclooxygenase |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMSO | dimethyl sulfoxide |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| DDS | drug delivery systems |
| IND-MPs | oral formulation containing indomethacin microparticles |
| IND-NPs | oral formulation containing indomethacin nanoparticles |
| IND-solution | liquid indomethacin |
| MP | macropinocytosis |
| MC | methylcellulose |
| NO | nitric oxide |
| NSAID | non-steroidal anti-inflammatory drug |
| PG | prostaglandin |
| S.D. | standard deviation |
| S.E. | standard error |
| TER | transepithelial electrical resistance |
References
- Graham, D.Y.; Opekun, A.R.; Willingham, F.F.; Qureshi, W.A. Visible small-intestinal mucosal injury in chronic NSAID users. Clin. Gastroenterol. Hepatol. 2005, 3, 55–59. [Google Scholar] [CrossRef]
- Higuchi, K.; Umegaki, E.; Watanabe, T.; Yoda, Y.; Morita, E.; Murano, M.; Tokioka, S.; Arakawa, T. Present status and strategy of NSAIDs-induced small bowel injury. J. Gastroenterol. 2009, 44, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Kawahara, R.; Hashimura, H.; Yamanaka, N.; Iimori, M.; Amagase, K.; Kato, S.; Takeuchi, K. Dopamine D(2)-receptor antagonists ameliorate indomethacin-induced small intestinal ulceration in mice by activating alpha7 nicotinic acetylcholine receptors. J. Pharmacol. Sci. 2011, 116, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, R.; Yasuda, M.; Hashimura, H.; Amagase, K.; Kato, S.; Takeuchi, K. Activation of alpha7 nicotinic acetylcholine receptors ameliorates indomethacin-induced small intestinal ulceration in mice. Eur. J. Pharmacol. 2011, 650, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Ikawa, Y.; Fujino, H.; Otake, S.; Murayama, T. Indomethacin antagonizes EP(2) prostanoid receptor activation in LS174T human colon cancer cells. Eur. J. Pharmacol. 2012, 680, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Kunikata, T.; Tanaka, A.; Miyazawa, T.; Kato, S.; Takeuhi, K. 16,16-Dimethyl prostaglandin E2 inhibits indomethacin-induced small intestinal lesions through EP3 and EP4 receptors. Dig. Dis. Sci. 2002, 47, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Hatazawa, R.; Ohno, R.; Tanigami, M.; Takeuchi, K. Roles of endogenous prostaglandins and cyclooxygenase isozymes in healing of indomethacin-induced small intestinal lesions in rats. J. Pharmacol. Exp. Ther. 2006, 318, 691–699. [Google Scholar] [CrossRef]
- Boelsterli, U.A.; Redinbo, M.R.; Saitta, K.S. Multiple NSAID-induced hits injure the small intestine: Underlying mechanisms and novel strategies. Toxicol. Sci. 2013, 131, 654–667. [Google Scholar] [CrossRef]
- Takeuchi, K.; Tanaka, A.; Kato, S.; Amagase, K.; Satoh, H. Roles of COX inhibition in pathogenesis of NSAID-induced small intestinal damage. Clin. Chim. Acta 2010, 411, 459–466. [Google Scholar] [CrossRef]
- Boltze, K.; Brendler, O.; Jacobi, H.; Optiz, W.; Raddatz, S.; Seidel, P.R.; Vollbrecht, D. Chemical structure and anti-inflammatory activity in the group of substituted indole-3 acetic acids. Arzneim-Forsch/Drug Res. 1980, 30, 1314–1325. [Google Scholar]
- Jacobi, H.; Dell, H.D. On the pharmacodynamics of acemetacin. Arzneim-Forsch/Drug Res. 1980, 30, 1348–1362. [Google Scholar]
- Kumakura, S.; Mishima, M.; Kobayashi, S.; Abe, S.; Yamada, K.; Tsurufuji, S. Inhibitory effect of indomethacin farnesyl, a novel anti-inflammatory prodrug, on carrageenan-induced inflammation in rats. Agents Actions 1990, 29, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Singh, S.R.; Yilma, A.N.; Agee, R.D., 2nd; Taha, M.; Dennis, V.A. Poly(lactic acid)-poly(ethylene glycol) nanoparticles provide sustained delivery of a Chlamydia trachomatis recombinant MOMP peptide and potentiate systemic adaptive immune responses in mice. Nanomedicine 2014, 10, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Kalkanidis, M.; Pietersz, G.A.; Xiang, S.D.; Mottram, P.L.; Crimeen-Irwin, B.; Ardipradja, K.; Plebanski, M. Methods for nano-particle based vaccine formulation and evaluation of their immunogenicity. Methods 2006, 40, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y. Effect of solid nanoparticle of indomethacin on therapy for rheumatoid arthritis in adjuvant-induced arthritis rat. Biol. Pharm. Bull. 2014, 37, 1109–1118. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.; Gomez-Orellana, I. Challenges for the Oral Delivery of Macromolecules. Nat. Rev. Drug Discovery 2003, 2, 289–295. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier Properties of Mucus. Adv. Drug Delivery Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Rappoport, J.Z. Focusing on clathrin-mediated endocytosis. Biochem. J. 2008, 412, 415–423. [Google Scholar] [CrossRef]
- Wang, J.; Byrne, J.D.; Napier, M.E.; DeSimone, J.M. More effective nanomedicines through particle design. Small 2011, 7, 1919–1931. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. Impact of particle elasticity on particle-based drug delivery systems. Adv. Drug Delivery Rev. 2017, 108, 51–67. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control. Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y. Topical therapies for rheumatoid arthritis by gel ointments containing indomethacin nanoparticles in adjuvant-induced arthritis rat. J. Oleo Sci. 2015, 64, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Iwamae, A.; Tanimoto, S.; Yoshioka, C.; Ito, Y. Pharmacokinetics and antiinflammatory effect of a novel gel system containing ketoprofen solid nanoparticles. Biol. Pharm. Bull. 2015, 38, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ogata, F.; Otake, H.; Nakazawa, Y.; Kawasaki, N. Design of a transdermal formulation containing raloxifene nanoparticles for osteoporosis treatment. Int. J. Nanomed. 2018, 13, 5215–5229. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Yoshioka, C.; Ito, Y.; Funakami, Y.; Nishikawa, H.; Kawabata, A. Intravenous Administration of Cilostazol Nanoparticles Ameliorates Acute Ischemic Stroke in a Cerebral Ischemia/Reperfusion-Induced Injury Model. Int. J. Mol. Sci. 2015, 16, 29329–29344. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, K.; Manaloto, E.; Casey, A.; Cribaro, G.P.; Byrne, H.J.; Tian, F.; Baracia, C.; Conway, G.E.; Cullen, P.J.; et al. Cold Atmospheric Plasma Induces ATP-Dependent Endocytosis of Nanoparticles and Synergistic U373MG Cancer Cell Death. Sci. Rep. 2018, 8, 5298. [Google Scholar] [CrossRef] [PubMed]
- Mäger, I.; Langel, K.; Lehto, T.; Eiríksdóttir, E.; Langel, U. The role of endocytosis on the uptake kinetics of luciferin-conjugated cell-penetrating peptides. Biochim. Biophys. Acta 2012, 1818, 502–511. [Google Scholar] [CrossRef]
- Malomouzh, A.I.; Mukhitov, A.R.; Proskurina, S.E.; Vyskocil, F.; Nikolsky, E.E. The effect of dynasore, a blocker of dynamin-dependent endocytosis, on spontaneous quantal and non-quantal release of acetylcholine in murine neuromuscular junctions. Dokl. Biol. Sci. 2014, 459, 330–333. [Google Scholar] [CrossRef]
- Hufnagel, H.; Hakim, P.; Lima, A.; Hollfelder, F. Fluid phase endocytosis contributes to transfection of DNA by PEI-25. Mol. Ther. 2009, 17, 1411–1417. [Google Scholar] [CrossRef]
- Dharmani, P.; Srivastava, V.; Kissoon-Singh, V.; Chadee, K. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 2009, 1, 123–135. [Google Scholar] [CrossRef]
- Tlaskalová-Hogenová, H.; Štěpánková, R.; Kozáková, H.; Hudcovic, T.; Vannucci, L.; Tučková, L.; Rossmann, P.; Hrnčĭř, T.; Kverka, M.; Zákostelská, Z.; et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: Contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol. Immunol. 2011, 8, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, J.; Lykotrafitis, G.; Bao, G.; Suresh, S. Size-dependent endocytosis of nanoparticles. Adv. Mater. 2008, 21, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, D.; Qin, M.; Chen, B.; Song, S.; Dai, W.; Zhang, H.; Wang, X.; Wang, Y.; He, B.; et al. Intestinal Mucin Induces More Endocytosis but Less Transcytosis of Nanoparticles across Enterocytes by Triggering Nanoclustering and Strengthening the Retrograde Pathway. ACS. Appl. Mater Interfaces. 2018, 10, 11443–11456. [Google Scholar] [CrossRef] [PubMed]
- Nagai, N.; Ito, Y.; Okamoto, N.; Shimomura, Y. A nanoparticle formulation reduces the corneal toxicity of indomethacin eye drops and enhances its corneal permeability. Toxicology 2014, 319, 53–62. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Jc (pmol/cm2/h) | Kp (×10−5/h) | Km (×10−3) | τ (h) | D (×10−3 cm2/h) |
|---|---|---|---|---|---|
| Normal (37 °C treatment) | 2.6 ± 0.6 | 5.0 ± 1.1 | 1.6 ± 0.4 | 0.69 ± 0.12 | 2.7 ± 0.6 |
| 4 °C treatment | 0.2 ± 0.1*,# | 0.3 ± 0.1*,# | 0.2 ± 0.1*,# | 1.16 ± 0.18*,# | 1.4 ± 0.3*,# |
| Vehicle | 3.9 ± 1.0 | 7.5 ± 1.5 | 2.6 ± 0.3 | 0.72 ± 0.11 | 2.5 ± 0.4 |
| Nystatin | 3.6 ± 0.9 | 6.9 ± 1.3 | 2.4 ± 0.3 | 0.72 ± 0.10 | 2.6 ± 0.4 |
| Dynasore | 1.9 ± 0.1*,# | 3.9 ± 0.4*,# | 1.9 ± 0.6*,# | 0.85 ± 0.15 | 2.0 ± 0.7 |
| Rottlerin | 3.8 ± 1.0 | 7.1 ± 1.1 | 2.5 ± 0.4 | 0.70 ± 0.13 | 2.3 ± 0.4 |
| Cytochalasin D | 3.9 ± 1.2 | 7.3 ± 1.3 | 2.6 ± 0.5 | 0.71 ± 0.11 | 2.3 ± 0.4 |
| Nys-Dyn | 0.7 ± 0.2*,# | 1.2 ± 0.4*,# | 0.5 ± 0.1*,# | 0.71 ± 0.27 | 2.3 ± 0.7 |
| Treatment | Jc (pmol/cm2/h) | Kp (×10−4/h) | Km (×10−3) | τ (h) | D (×10−3 cm2/h) |
|---|---|---|---|---|---|
| Normal (37 °C treatment) | 7.4 ± 1.1 | 1.3 ± 0.2 | 8.9 ± 1.3 | 1.21 ± 0.14 | 1.4 ± 0.1 |
| 4 °C treatment | – | – | – | – | – |
| Vehicle | 7.5 ± 1.0 | 1.4 ± 0.3 | 9.0 ± 0.9 | 1.18 ± 0.11 | 1.5 ± 0.2 |
| Nystatin | 5.0 ± 1.4*,# | 0.9 ± 0.2*,# | 8.1 ± 1.8 | 1.65 ± 0.37 | 1.3 ± 0.4 |
| Dynasore | 2.4 ± 0.1*,# | 0.5 ± 0.1*,# | 2.6 ± 0.7*,# | 0.95 ± 0.21 | 1.9 ± 0.4 |
| Rottlerin | 6.8 ± 0.7 | 1.0 ± 0.2 | 8.7 ± 1.0 | 1.09 ± 0.11 | 1.5 ± 0.4 |
| Cytochalasin D | 7.7 ± 1.4 | 1.4 ± 0.3 | 9.1 ± 0.9 | 1.14 ± 0.13 | 1.5 ± 0.3 |
| Nys-Dyn | 1.4 ± 0.4*,# | 0.3 ± 0.1*,# | 1.9 ± 0.4*,# | 0.92 ± 0.20 | 1.8 ± 0.3 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ishii, M.; Fukuoka, Y.; Deguchi, S.; Otake, H.; Tanino, T.; Nagai, N. Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. Int. J. Mol. Sci. 2019, 20, 476. https://doi.org/10.3390/ijms20030476
Ishii M, Fukuoka Y, Deguchi S, Otake H, Tanino T, Nagai N. Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. International Journal of Molecular Sciences. 2019; 20(3):476. https://doi.org/10.3390/ijms20030476
Chicago/Turabian StyleIshii, Miyu, Yuya Fukuoka, Saori Deguchi, Hiroko Otake, Tadatoshi Tanino, and Noriaki Nagai. 2019. "Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine" International Journal of Molecular Sciences 20, no. 3: 476. https://doi.org/10.3390/ijms20030476
APA StyleIshii, M., Fukuoka, Y., Deguchi, S., Otake, H., Tanino, T., & Nagai, N. (2019). Energy-Dependent Endocytosis Is Involved in the Absorption of Indomethacin Nanoparticles in the Small Intestine. International Journal of Molecular Sciences, 20(3), 476. https://doi.org/10.3390/ijms20030476