Antiviral Activity of Benzoic Acid Derivative NC-5 Against Influenza A Virus and Its Neuraminidase Inhibition
Abstract
1. Introduction
2. Results
2.1. The Antiviral Activities of NC-5 and its Analogs against Influenza Virus A/FM/1/47 (H1N1)
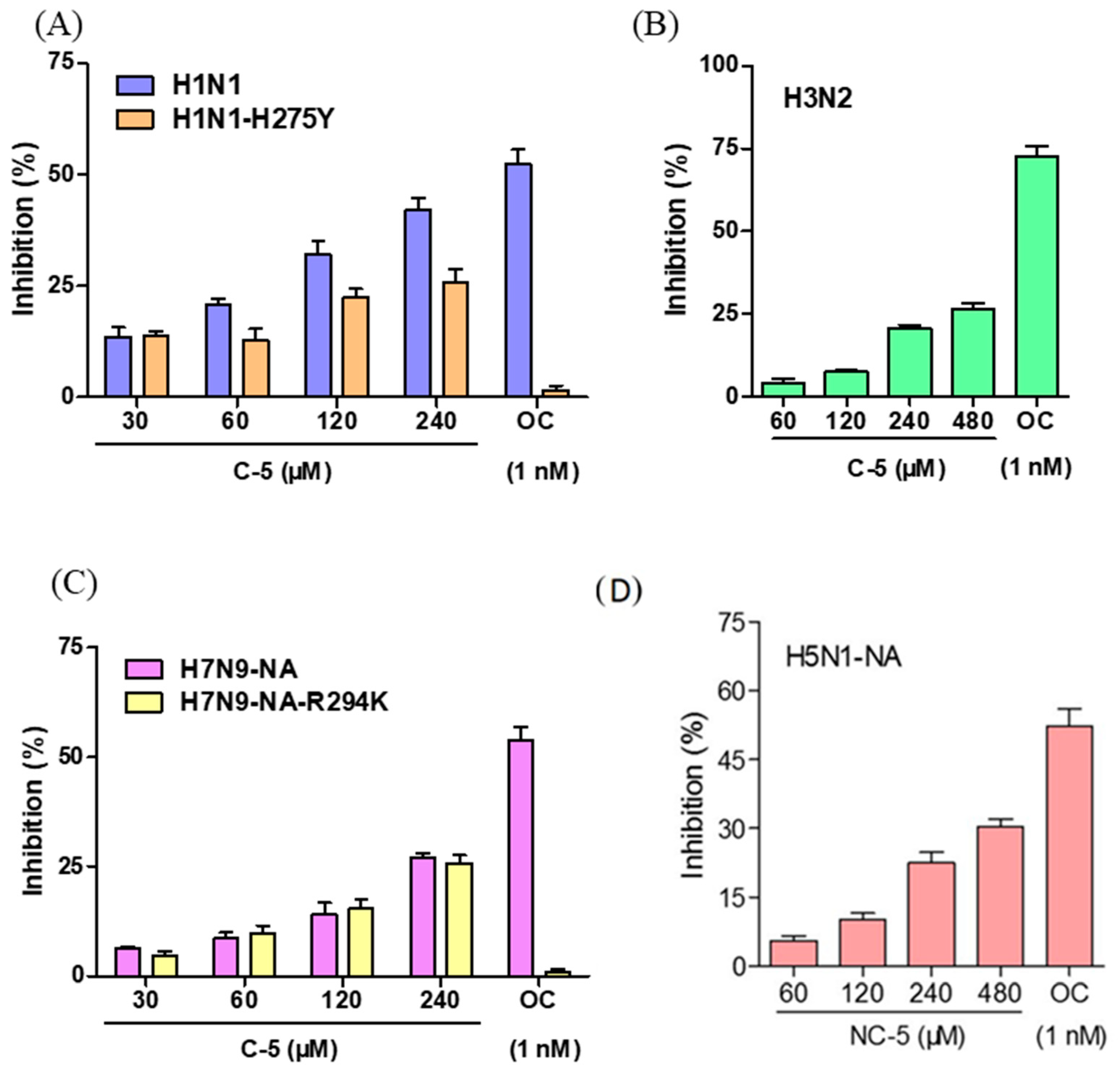
2.2. NC-5 has the Ability to Inhibit Neuraminidase Activity
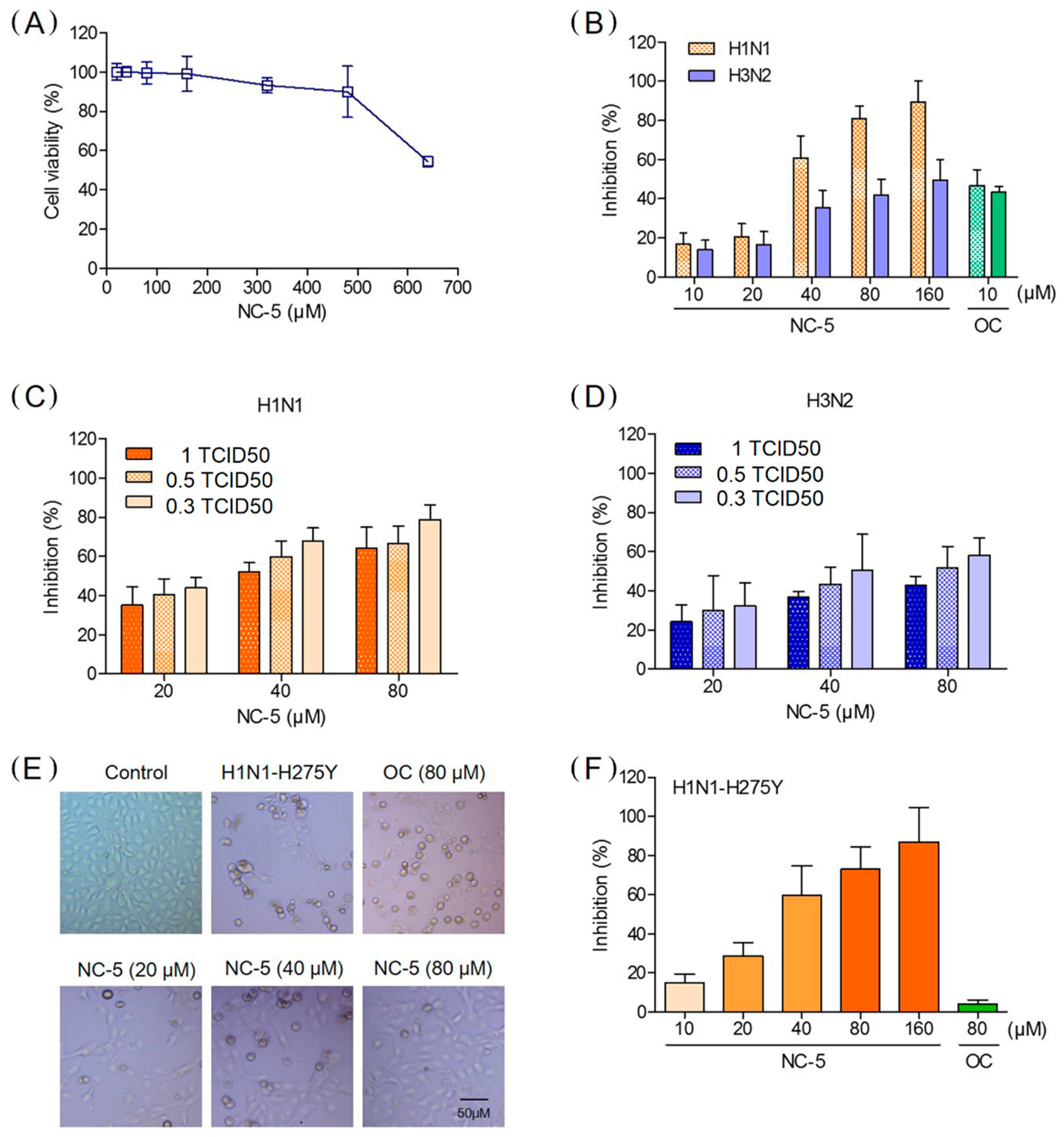
2.3. NC-5 inhibited the Replication of Influenza Viruses A/FM/1/47 (H1N1), A/FM/1/47-H275Y (H1N1-H275Y) and A/Beijing/32/92 (H3N2) in Cell Culture
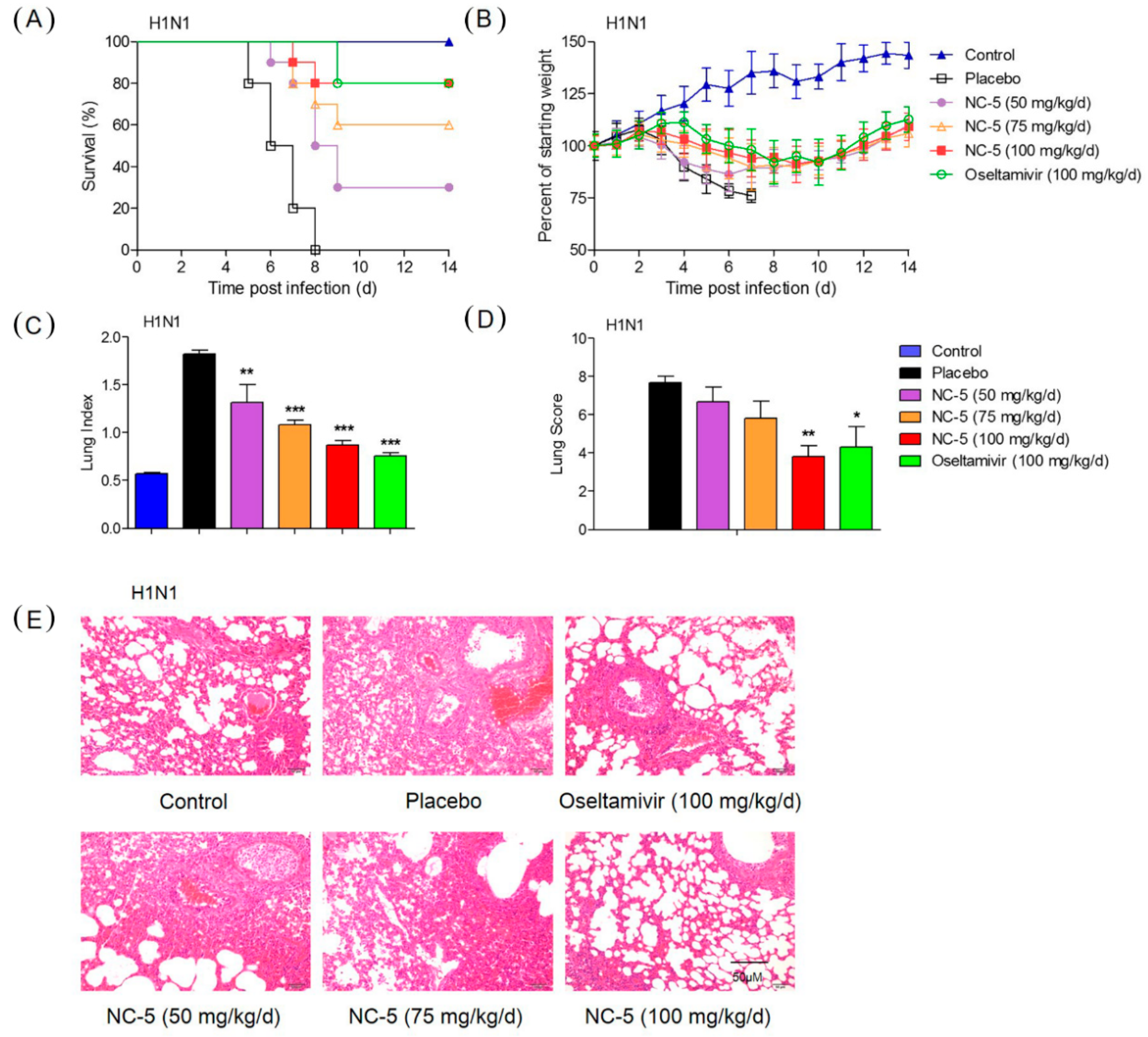
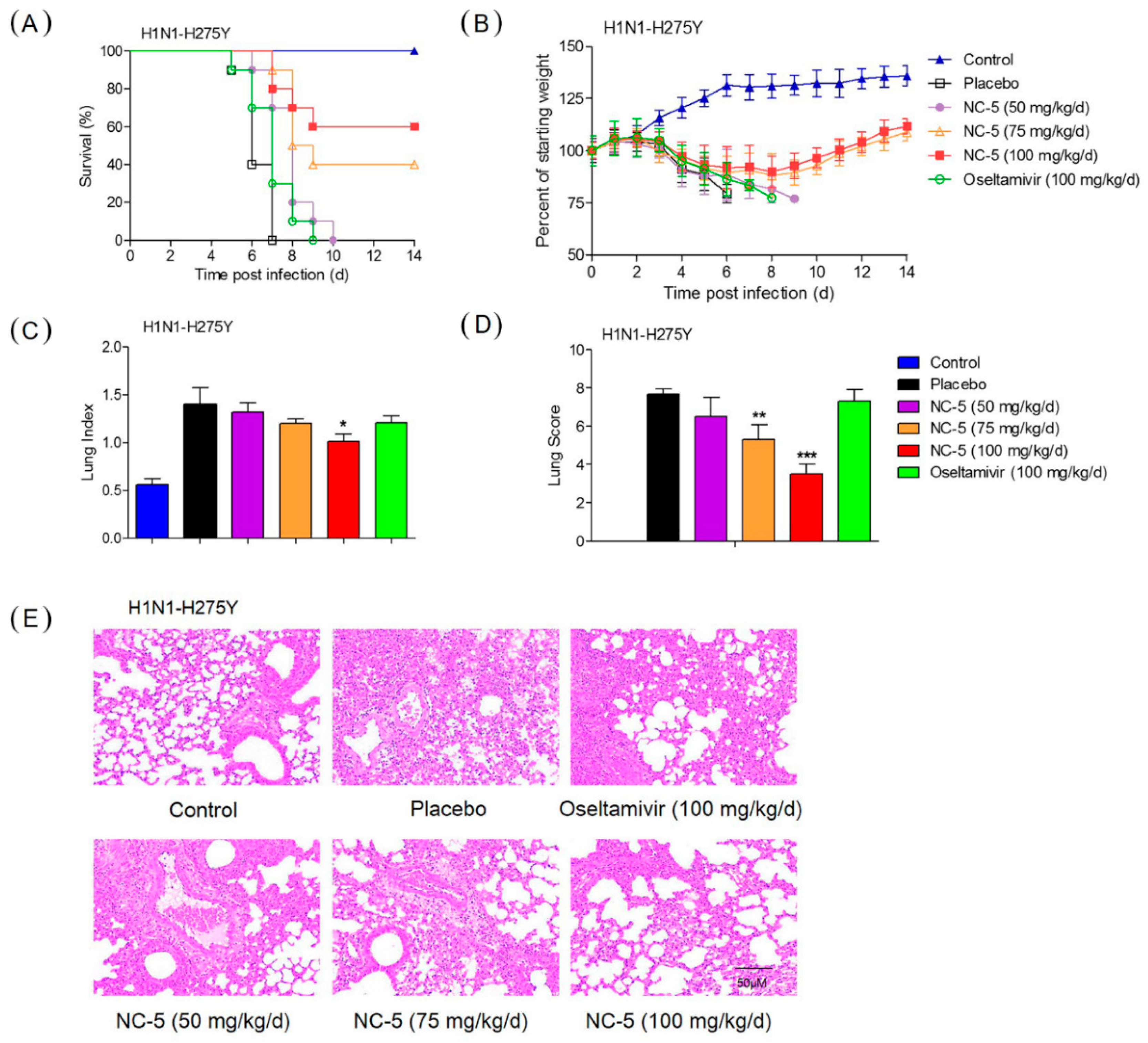
2.4. Effective Protection by NC-5 in Mice Infected with Influenza Virus A/FM/1/47 (H1N1) and Oseltamivir-Resistant Mutant A/FM/1/47 (H1N1-H275Y)
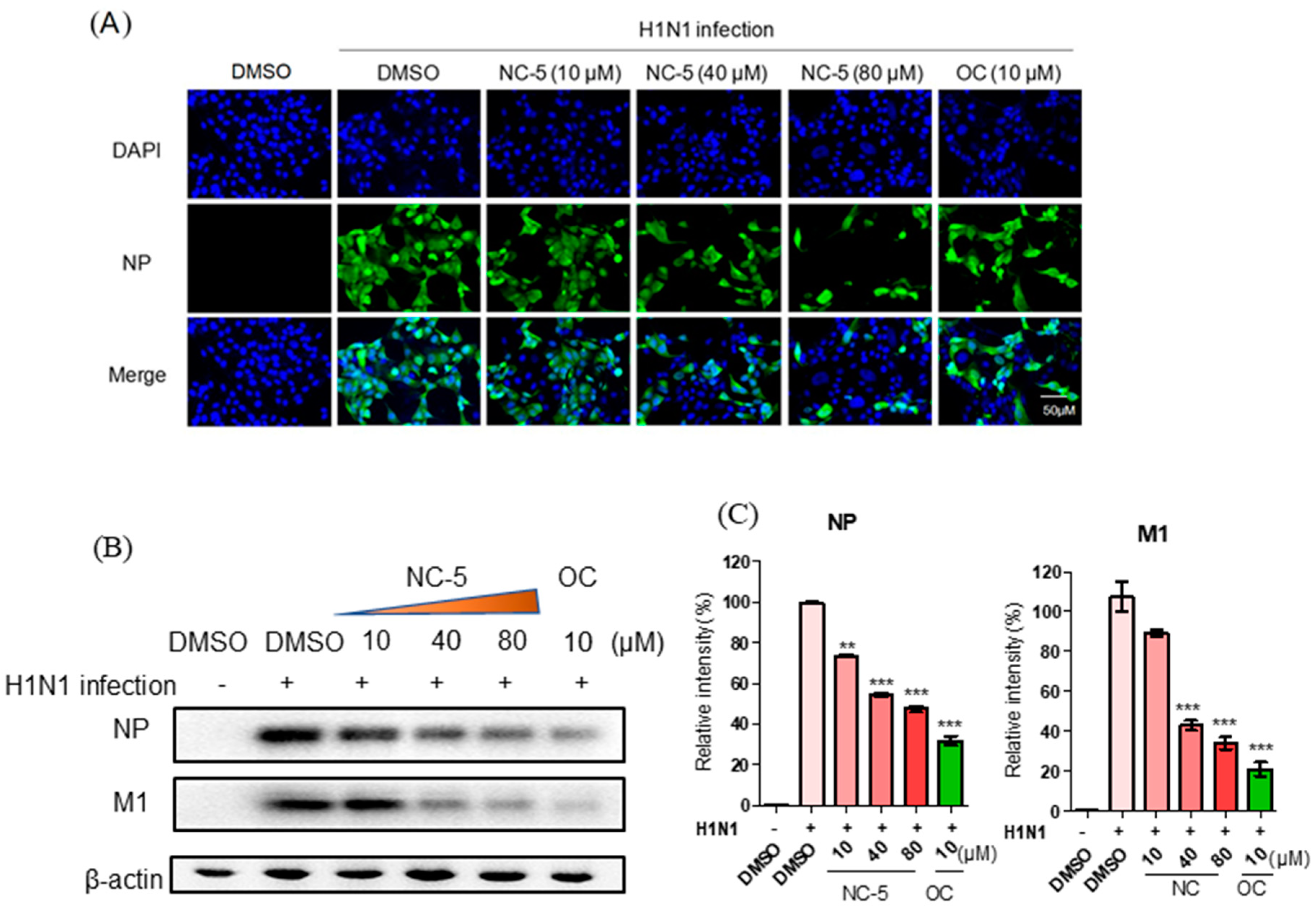
2.5. NC-5 Suppressed Influenza Virus A/FM/1/47 (H1N1) in the Late Stages of its Life Cycle by Interfering NP and M1 Protein Expression but Showed no Effect on RNA Polymerase
3. Discussion
4. Materials and Methods
4.1. Cells, Viruses and Plasmids
4.2. Compounds
4.3. Determination of Influenza a Virus TCID50
4.4. Cytopathic Effect (CPE) Reduction Assay
4.5. Antiviral Activity of NC-5 in vivo
4.6. Western Blot Analysis
4.7. Luciferase Assay
4.8. Real-Time qPCR Analysis
4.9. Fluorescence Microscopy
4.10. Neuraminidase (NA) Inhibition Assay
4.11. Data Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NC-5 | 4-[2,2-bis(hydroxymethyl)-5-oxopyrrolidin-l-yl]-3-(5-cyclohexyl-4H-1, 2,4-triazol-3-yl) amino) benzoic acid |
| NA | neuraminidase |
| H1N1 | influenza A virus A/FM/1/47 (H1N1) |
| H1N1-H275Y | influenza A virus A/FM/1/47-H275Y (H1N1-H275Y) |
| H3N2 | influenza A virus A/Beijing/32/92 (H3N2) |
| H5N1 | influenza A virus A/Vietnam/1203/2004 (H5N1) |
| H7N9 | influenza A virus A/Anhui/1/2013 (H7N9) |
| H7N9-R294K | influenza A virus A/Anhui/1/2013-R294K (H7N9-R294K |
| MDCK | Madin–Darby canine kidney cells |
| DMEM | Dulbecco’s modified Eagle’s medium |
| CHO-K1 | Chinese hamster ovary |
| OS | oseltamivir phosphate |
| OC | oseltamivir carboxylate |
| RBV | ribavirin |
| CPE | cytopathic effect |
| EC50 | 50% effective concentrations |
| CC50 | 50% cytotoxic concentration |
| TCID50 | 50% tissue culture infectious dose |
| H & E stain | haematoxylin and eosin stain |
References
- Yu, M.; Si, L.; Wang, Y.; Wu, Y.; Zhou, D.J.J.o.M.C. Discovery of Pentacyclic Triterpenoids as Potential Entry Inhibitors of Influenza. Viruses 2014, 57, 10058–10071. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y. Sialobiology of influenza molecular mechanism of host range variation of influenza viruses. Biol. Pharm. Bull. 2005, 28, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.M.Y.; Yen, H.L. Targeting the host or the virus: Current and novel concepts for antiviral approaches against influenza virus infection. Antivir. Res. 2012, 96, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Rudrawar, S.; Dyason, J.C.; Rameix-Welti, M.A.; Rose, F.J.; Kerry, P.S.; Russell, R.J.; van der Werf, S.; Thomson, R.J.; Naffakh, N.; von Itzstein, M. Novel sialic acid derivatives lock open the 150-loop of an influenza A virus group-1 sialidase. Nat. Commun. 2010, 1, 113. [Google Scholar] [CrossRef]
- Russell, R.J.; Haire, L.F.; Stevens, D.J.; Collins, P.J.; Lin, Y.P.; Blackburn, G.M.; Hay, A.J.; Gamblin, S.J.; Skehel, J.J.J.N. The structure of H5N1 avian influenza neuraminidase suggests new opportunities for drug design. Nature 2006, 443, 45–49. [Google Scholar] [CrossRef]
- Vonitzstein, M.; Wu, W.Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Phan, T.V.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational Design of Potent Sialidase-Based Inhibitors of Influenza-Virus Replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.T.; Zhang, L.J.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza neuraminidase inhibitors possessing a novel hydrophobic interaction in the enzyme active site: Design, synthesis, and structural analysis of carbocyclic sialic acid analogues with potent anti-influenza activity. J. Am. Chem Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- Bright, R.A.; Medina, M.J.; Xu, X.Y.; Perez-Oronoz, G.; Wallis, T.R.; Davis, X.H.M.; Povinelli, L.; Cox, N.J.; Klimov, A.I. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: A cause for concern. Lancet 2005, 366, 1175–1181. [Google Scholar] [CrossRef]
- Deyde, V.M.; Xu, X.Y.; Bright, R.A.; Shaw, M.; Smith, C.B.; Zhang, Y.; Shu, Y.L.; Gubareva, L.V.; Cox, N.J.; Klimov, A.I. Surveillance of resistance to adamantanes among influenza A(H3N2) and A(H1N1) viruses isolated worldwide. J. Infect. Dis. 2007, 196, 249–257. [Google Scholar] [CrossRef]
- Moscona, A. Global Transmission of Oseltamivir-Resistant Influenza. New Engl. J. Med. 2009, 360, 953–956. [Google Scholar] [CrossRef]
- Sun, C.W.; Huang, H.; Feng, M.Q.; Shi, X.L.; Zhang, X.D.; Zhou, P. A novel class of potent influenza virus inhibitors: Polysubstituted acylthiourea and its fused heterocycle derivatives. Bioorg. Med. Chem. Lett 2006, 16, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Burgeson, J.R.; Moore, A.L.; Boutilier, J.K.; Cerruti, N.R.; Gharaibeh, D.N.; Lovejoy, C.E.; Amberg, S.M.; Hruby, D.E.; Tyavanagimatt, S.R.; Allen, R.D.; et al. SAR analysis of a series of acylthiourea derivatives possessing broad-spectrum antiviral activity. Bioorg. Med. Chem. Lett 2012, 22, 4263–4272. [Google Scholar] [CrossRef] [PubMed]
- Tarbet, E.B.; Hamilton, S.; Vollmer, A.H.; Luttick, A.; Ng, W.C.; Pryor, M.; Hurst, B.L.; Crawford, S.; Smee, D.F.; Tucker, S.P. A zanamivir dimer with prophylactic and enhanced therapeutic activity against influenza viruses. J. Antimicrob. Chemoth. 2014, 69, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.C.; Fang, J.M.; Jan, J.T.; Cheng, T.J.R.; Wang, S.Y.; Yang, S.T.; Cheng, Y.S.E.; Wong, C.H. Enhanced Anti-influenza Agents Conjugated with Anti-inflammatory Activity. J. Med. Chem. 2012, 55, 8493–8501. [Google Scholar] [CrossRef]
- Atigadda, V.R.; Brouillette, W.J.; Duarte, F.; Ali, S.M.; Babu, Y.S.; Bantia, S.; Chand, P.; Chu, N.; Montgomery, J.A.; Walsh, D.A.; et al. Potent inhibition of influenza sialidase by a benzoic acid containing a 2-pyrrolidinone substituent. J. Med. Chem. 1999, 42, 2332–2343. [Google Scholar] [CrossRef]
- Shapiro, G.I.; Gurney, T.; Krug, R.M. Influenza virus gene expression: Control mechanisms at early and late times of infection and nuclear-cytoplasmic transport of virus-specific RNAs. J. Virol. 1987, 61, 764–773. [Google Scholar]
- Atkins, C.; Evans, C.W.; White, E.L.; Noah, J.W. Screening methods for influenza antiviral drug discovery. Expert. Opin. Drug. Dis. 2012, 7, 429–438. [Google Scholar] [CrossRef]
- Amaro, R.E.; Swift, R.V.; Votapka, L.; Li, W.W.; Walker, R.C.; Bush, R.M. Mechanism of 150-cavity formation in influenza neuraminidase. Nat. Commun. 2011, 2. [Google Scholar] [CrossRef]
- Wang, W.L.; Li, R.Q.; Deng, Y.; Lu, N.; Chen, H.; Meng, X.; Wang, W.; Wang, X.P.; Yan, K.X.; Qi, X.R.; et al. Protective Efficacy of the Conserved NP, PB1, and M1 Proteins as Immunogens in DNA- and Vaccinia Virus-Based Universal Influenza A Virus Vaccines in Mice. Clin. Vaccine. Immunol. 2015, 22, 618–630. [Google Scholar] [CrossRef]
- Hurt, A.C. The epidemiology and spread of drug resistant human influenza viruses. Curr. Opin. Virol. 2014, 8, 22–29. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.C.; Jin, J.; Xu, J.; Li, M.W.; Wang, H.; Dou, J.; Zhou, C.L. Antiviral activity of SA-2 against influenza A virus in vitro/vivo and its inhibition of RNA polymerase. Antivir. Res. 2016, 127, 68–78. [Google Scholar] [CrossRef]
- Zhang, J.J.; Liu, T.; Tong, X.M.; Li, G.; Yan, J.H.; Ye, X. Identification of novel virus inhibitors by influenza A virus specific reporter cell based screening. Antivir. Res. 2012, 93, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.L.; Jin, J.; Zhang, H.X.; Dou, J.; Wang, D.C.; Jia, Y.B. Synthesis and Anti-influenza Application for a Series of Benzoic Acid Triazole Compounds. Chinese patent CN106432031A, 25 March 2015. [Google Scholar]
- Takeuchi, A.; Mishina, Y.; Miyaishi, O.; Kojima, E.; Hasegawa, T.; Isobe, K. Heterozygosity with respect to Zfp148 causes complete loss of fetal germ cells during mouse embryogenesis. Nat. Genet. 2003, 33, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Dou, J.; Teng, Z.J.; Yu, J.; Wang, T.T.; Lu, N.; Wang, H.; Zhou, C.L. Antiviral activity of baicalin against influenza A (H1N1/H3N2) virus in cell culture and in mice and its inhibition of neuraminidase. Arch. Virol. 2014, 159, 3269–3278. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Wei, X.L.; Zhang, F.Y.; Hao, J.F.; Huang, F.; Zhang, C.L.; Liang, W. A dual character of flavonoids in influenza A virus replication and spread through modulating cell-autonomous immunity by MAPK signaling pathways. Sci. Rep.-Uk 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.R.; Chu, T.Y.; Reddy, G.R.; Tseng, S.N.; Chen, H.L.; Tang, W.F.; Wu, M.S.; Yeh, J.Y.; Chao, Y.S.; Hsu, J.T.A.; et al. Pyrazole compound BPR1P0034 with potent and selective anti-influenza virus activity. J. Biomed. Sci. 2010, 17. [Google Scholar] [CrossRef]
- Kim, J.H.; Resende, R.; Wennekes, T.; Chen, H.M.; Bance, N.; Buchini, S.; Watts, A.G.; Pilling, P.; Streltsov, V.A.; Petric, M.; et al. Mechanism-Based Covalent Neuraminidase Inhibitors with Broad-Spectrum Influenza Antiviral Activity. Science 2013, 340, 71–75. [Google Scholar] [CrossRef]
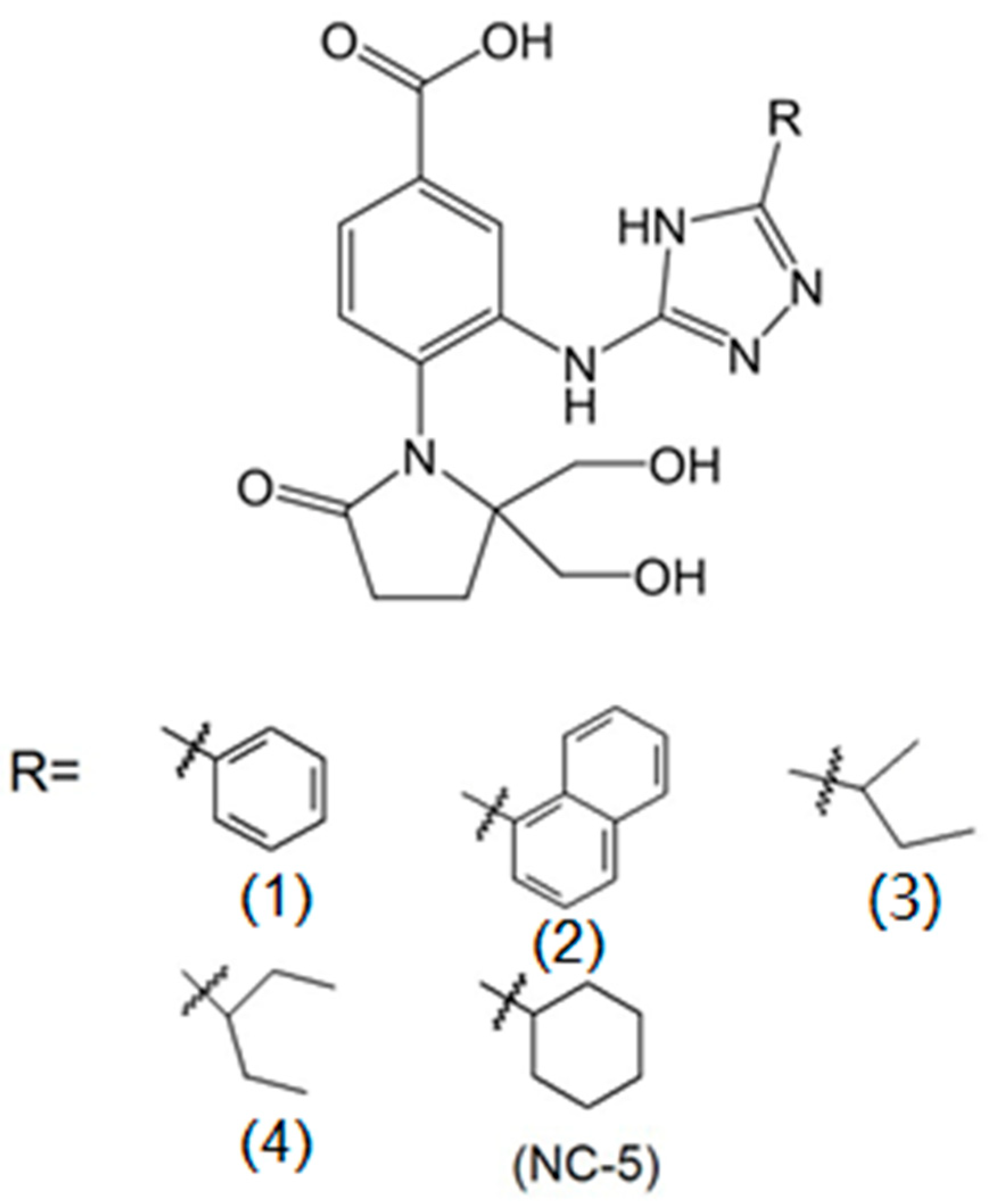

| Compound | Ra | Inhibition Rate (%) | |
|---|---|---|---|
| A/FM/1/47 (H1N1) | |||
| 10 μM | 100 μM | ||
| 1 | 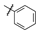 | 7.3 ± 4.66 | 7.8 ± 3.2 |
| 2 |  | 16.0 ± 6.14 | 30.3 ± 6.71 |
| 3 | 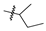 | 8.5 ± 3.50 | 16.8 ± 4.05 |
| 4 | 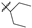 | 12.8 ± 4.88 | 23.6 ± 8.92 |
| 5 (NC-5) | 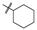 | 16.7 ± 5.76 | 83.7 ± 7.38 |
| OC b | 55.2 ± 6.89 | ND c | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, M.; Ni, J.; Yu, J.; Jin, J.; Ma, L.; Zhang, H.; Wang, D.; Zhang, X.; Dou, J.; Zhou, C. Antiviral Activity of Benzoic Acid Derivative NC-5 Against Influenza A Virus and Its Neuraminidase Inhibition. Int. J. Mol. Sci. 2019, 20, 6261. https://doi.org/10.3390/ijms20246261
Guo M, Ni J, Yu J, Jin J, Ma L, Zhang H, Wang D, Zhang X, Dou J, Zhou C. Antiviral Activity of Benzoic Acid Derivative NC-5 Against Influenza A Virus and Its Neuraminidase Inhibition. International Journal of Molecular Sciences. 2019; 20(24):6261. https://doi.org/10.3390/ijms20246261
Chicago/Turabian StyleGuo, Min, Jiawei Ni, Jie Yu, Jing Jin, Lingman Ma, Huixing Zhang, Dechuan Wang, Xue Zhang, Jie Dou, and Changlin Zhou. 2019. "Antiviral Activity of Benzoic Acid Derivative NC-5 Against Influenza A Virus and Its Neuraminidase Inhibition" International Journal of Molecular Sciences 20, no. 24: 6261. https://doi.org/10.3390/ijms20246261
APA StyleGuo, M., Ni, J., Yu, J., Jin, J., Ma, L., Zhang, H., Wang, D., Zhang, X., Dou, J., & Zhou, C. (2019). Antiviral Activity of Benzoic Acid Derivative NC-5 Against Influenza A Virus and Its Neuraminidase Inhibition. International Journal of Molecular Sciences, 20(24), 6261. https://doi.org/10.3390/ijms20246261




