Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells
Abstract
1. Introduction
2. Results
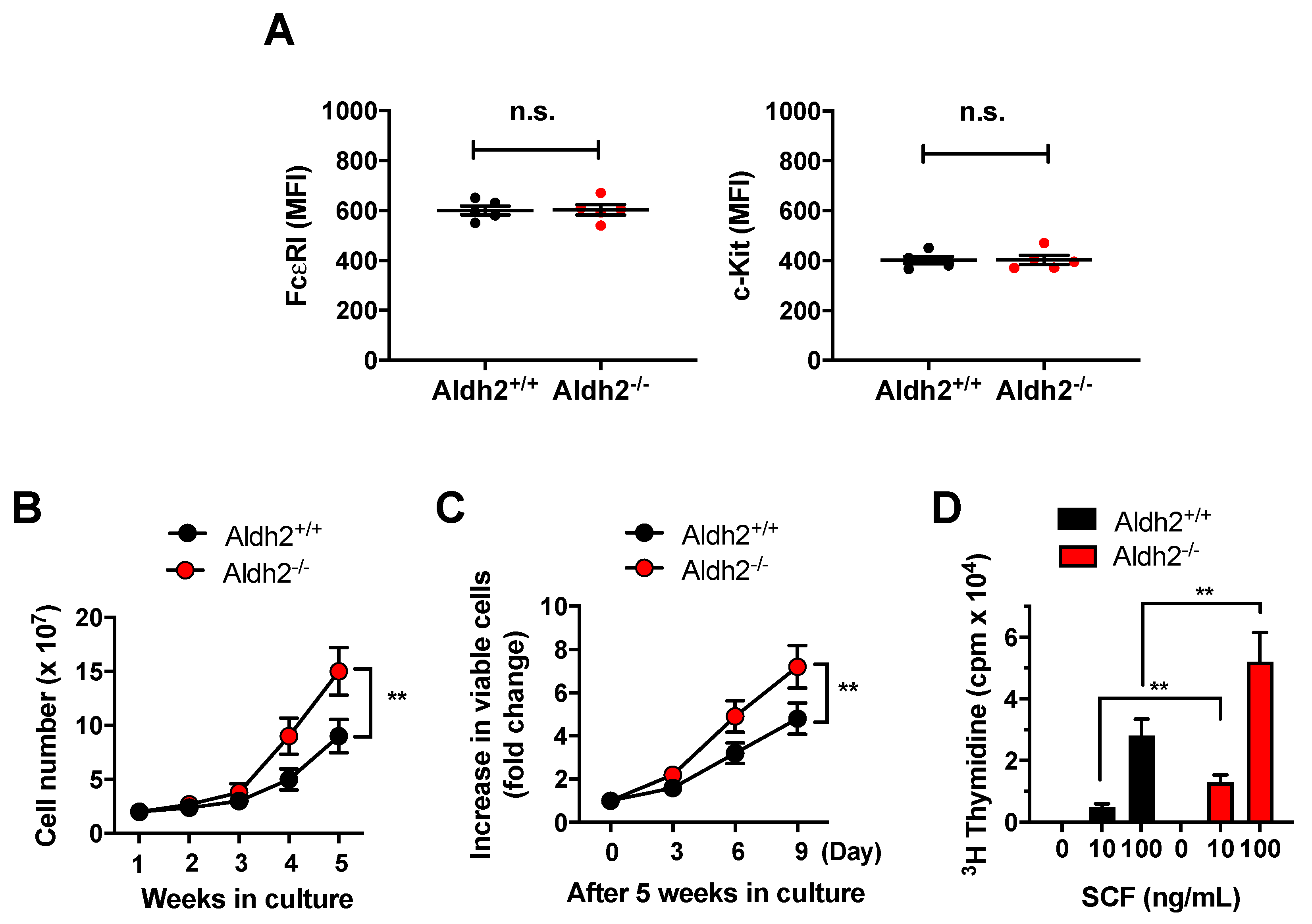
2.1. Aldh2 Deficiency Enhances Mast Cell Proliferation
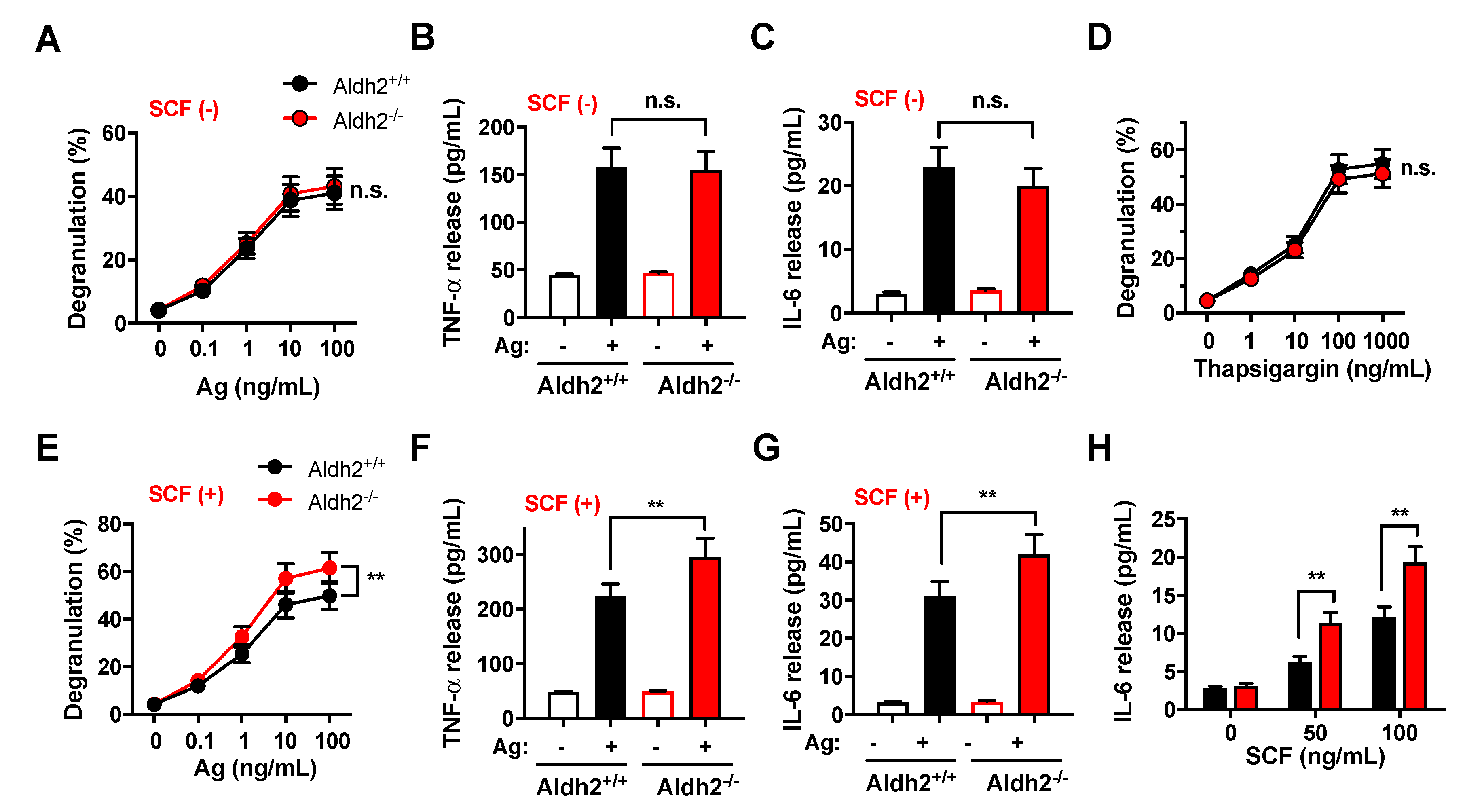
2.2. Responses to SCF Alone or in Combination with IgE/Ag Are Enhanced in Aldh2-Deficient BMMC, While Responses to FcεRI Stimulation Are Unaffected
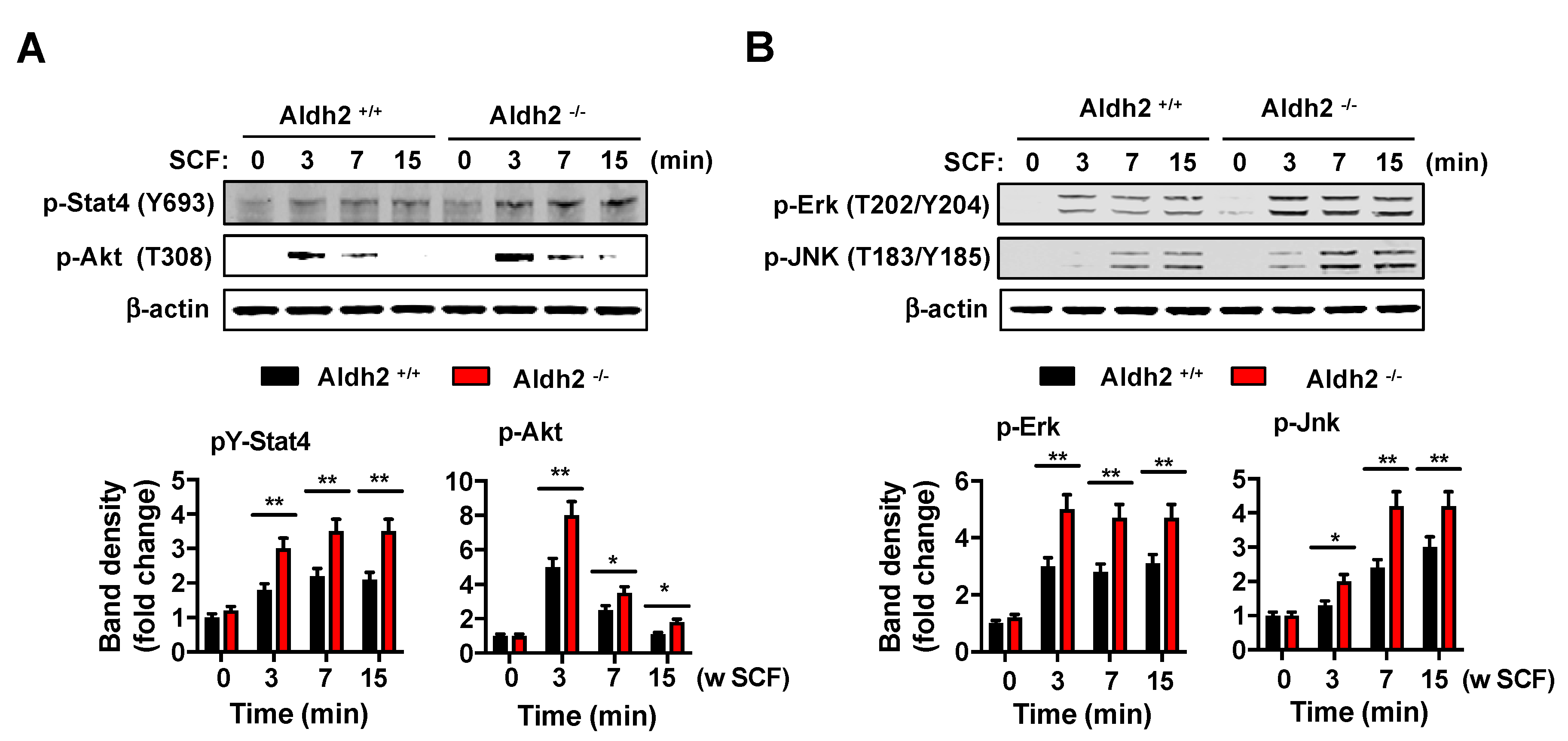
2.3. Kit-Induced Phosphorylation Events Are Upregulated in Aldh2-Deficient BMMCs
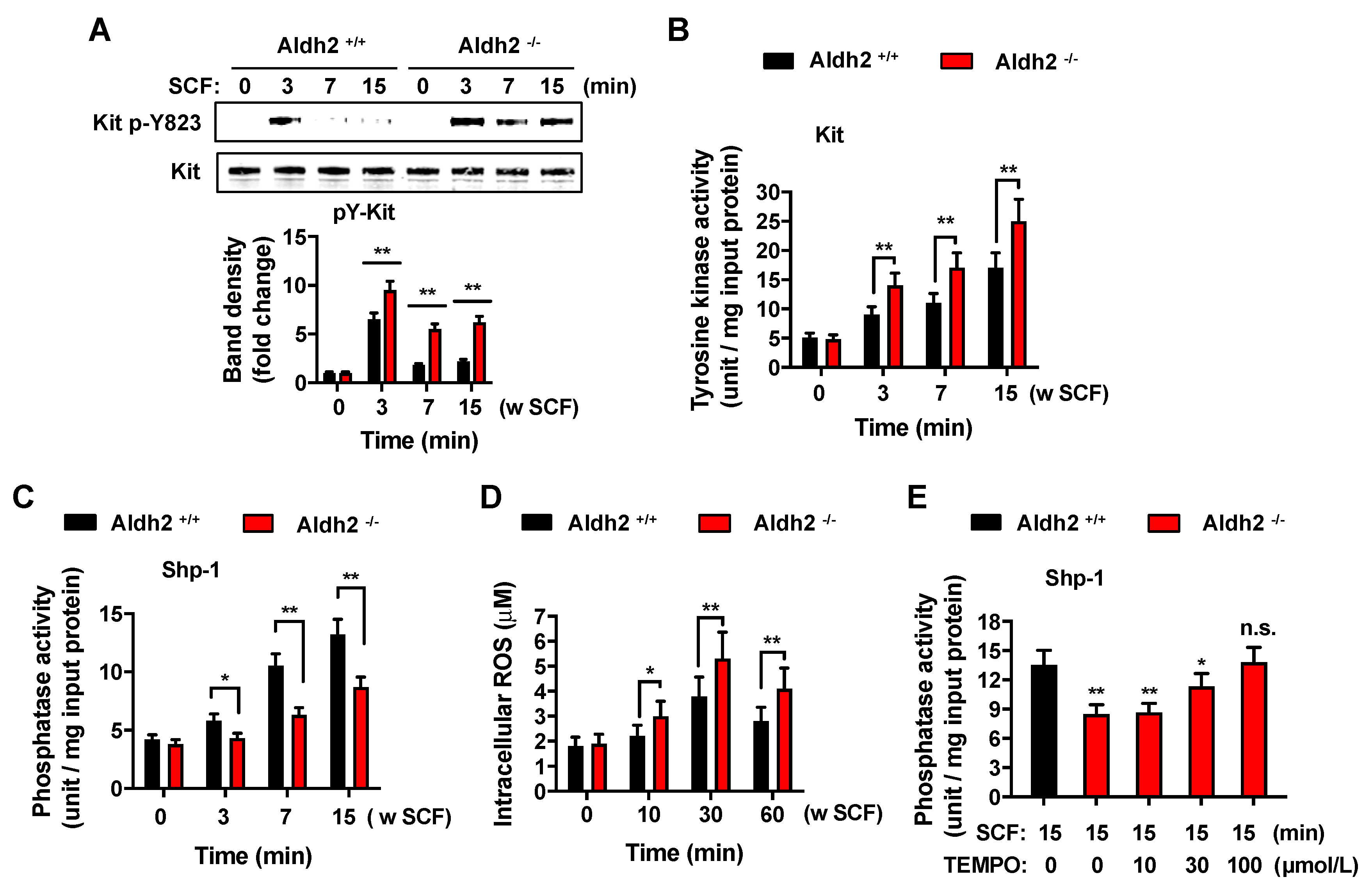
2.4. Deficiency in Aldh2 Results in Increased Kit Activation Concomitant with Reduced Shp-1 Activity and Increased ROS Levels
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mice and BMMC Cultures
4.3. Cell Proliferation Assays
4.4. Measurement of Degranulation and TNF-α and IL-6 Levels
4.5. Immunoprecipitation and Western Blotting Analysis
4.6. In Vitro Kinase Assay
4.7. Measurement of Shp-1 Phosphatase Activity
4.8. Measurement of Intracellular ROS
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ag | Antigen |
| ALDH2 | Aldehyde dehydrogenase |
| Akt | Protein kinase B |
| BMMC | Bone marrow-derived mast cell |
| Kit | Receptor for SCF |
| Erk | Extracellular signal-regulated kinase |
| FcεRI | High affinity receptor for IgE |
| Jnk | Jun N-terminal kinase |
| LAT | Linker for activation of T-cells |
| MAPK | Mitogen-activated protein kinase |
| PLCγ | Phospholipase C gamma |
| ROS | Reactive Oxygen Species |
| SCF | Stem cell factor or Kit ligand |
| Shp-1 | Src homology region 2 domain-containing phosphatase-1 |
| Stat4 | Signal transducer and activator of transcription 4 |
| Syk | Spleen tyrosine kinase |
References
- Chen, C.H.; Ferreira, J.C.; Gross, E.R.; Mochly-Rosen, D. Targeting aldehyde dehydrogenase 2: New therapeutic opportunities. Physiol. Rev. 2014, 94, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.Y.; Fang, Q.; Wang, J.S.; Xie, J.Q.; Chai, B.S.; Li, F.Q.; Cui, X.; Yang, Y. Over-expression of aldehyde dehydrogenase-2 protects against H2O2-induced oxidative damage and apoptosis in peripheral blood mononuclear cells. Acta Pharmacol. Sin. 2011, 32, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Tostes, R.C.; Webb, R.C. Mitochondrial aldehyde dehydrogenase prevents ROS-induced vascular contraction in angiotensin-II hypertensive mice. J. Am. Soc. Hypertens. 2011, 5, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Goedde, H.W.; Agarwal, D.P.; Fritze, G.; Meier-Tackmann, D.; Singh, S.; Beckmann, G.; Bhatia, K.; Chen, L.Z.; Fang, B.; Lisker, R.; et al. Distribution of ADH2 and ALDH2 genotypes in different populations. Hum. Genet. 1992, 88, 344–346. [Google Scholar] [CrossRef]
- Harada, S.; Agarwal, D.P.; Goedde, H.W. Aldehyde dehydrogenase deficiency as cause of facial flushing reaction to alcohol in Japanese. Lancet 1981, 2, 982. [Google Scholar] [CrossRef]
- Rastogi, V.; Singh, D.; Mazza, J.J.; Parajuli, D.; Yale, S.H. Flushing Disorders Associated with Gastrointestinal Symptoms: Part 1, Neuroendocrine Tumors, Mast Cell Disorders and Hyperbasophila. Clin. Med. Res. 2018, 16, 16–28. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef]
- Koivisto, T.; Kaihovaara, P.; Salaspuro, M. Acetaldehyde induces histamine release from purified rat peritoneal mast cells. Life Sci. 1999, 64, 183–190. [Google Scholar] [CrossRef]
- Shimoda, T.; Kohno, S.; Takao, A.; Fujiwara, C.; Matsuse, H.; Sakai, H.; Watanabe, T.; Hara, K.; Asai, S. Investigation of the mechanism of alcohol-induced bronchial asthma. J. Allergy Clin. Immunol. 1996, 97, 74–84. [Google Scholar] [CrossRef]
- Kawano, T.; Matsuse, H.; Kondo, Y.; Machida, I.; Saeki, S.; Tomari, S.; Mitsuta, K.; Obase, Y.; Fukushima, C.; Shimoda, T.; et al. Acetaldehyde induces histamine release from human airway mast cells to cause bronchoconstriction. Int. Arch. Allergy Immunol. 2004, 134, 233–239. [Google Scholar] [CrossRef]
- Miller, N.S.; Goodwin, D.W.; Jones, F.C.; Gabrielli, W.F.; Pardo, M.P.; Anand, M.M.; Hall, T.B. Antihistamine blockade of alcohol-induced flushing in orientals. J. Stud. Alcohol 1988, 49, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.; Olivera, A. A current understanding of Fc epsilon RI-dependent mast cell activation. Curr. Allergy Asthma Rep. 2008, 8, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kalesnikoff, J.; Galli, S.J. New developments in mast cell biology. Nat. Immunol. 2008, 9, 1215–1223. [Google Scholar] [CrossRef] [PubMed]
- Okayama, Y.; Kawakami, T. Development, migration, and survival of mast cells. Immunol. Res. 2006, 34, 97–115. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Rivera, J. The tyrosine kinase network regulating mast cell activation. Immunol. Rev. 2009, 228, 149–169. [Google Scholar] [CrossRef]
- Kim, D.K.; Kim, H.S.; Kim, A.R.; Kim, J.H.; Kim, B.; Noh, G.; Kim, H.S.; Beaven, M.A.; Kim, Y.M.; Choi, W.S. DJ-1 regulates mast cell activation and IgE-mediated allergic responses. J. Allergy Clin. Immunol. 2013, 131, 1653–1662. [Google Scholar] [CrossRef]
- Kim, D.K.; Beaven, M.A.; Kulinski, J.M.; Desai, A.; Bandara, G.; Bai, Y.; Prussin, C.; Schwartz, L.B.; Komarow, H.; Metcalfe, D.D.; et al. Regulation of Reactive Oxygen Species and the Antioxidant Protein DJ-1 in Mastocytosis. PLoS ONE 2016, 11, e0162831. [Google Scholar] [CrossRef][Green Version]
- Kuehn, H.S.; Swindle, E.J.; Kim, M.S.; Beaven, M.A.; Metcalfe, D.D.; Gilfillan, A.M. The phosphoinositide 3-kinase-dependent activation of Btk is required for optimal eicosanoid production and generation of reactive oxygen species in antigen-stimulated mast cells. J. Immunol. 2008, 181, 7706–7712. [Google Scholar] [CrossRef]
- Tagen, M.; Elorza, A.; Kempuraj, D.; Boucher, W.; Kepley, C.L.; Shirihai, O.S.; Theoharides, T.C. Mitochondrial uncoupling protein 2 inhibits mast cell activation and reduces histamine content. J. Immunol. 2009, 183, 6313–6319. [Google Scholar] [CrossRef]
- Cruse, G.; Metcalfe, D.D.; Olivera, A. Functional deregulation of KIT: Link to mast cell proliferative diseases and other neoplasms. Immunol. Allergy Clin. North. Am. 2014, 34, 219–237. [Google Scholar] [CrossRef]
- Desai, A.; Jung, M.Y.; Olivera, A.; Gilfillan, A.M.; Prussin, C.; Kirshenbaum, A.S.; Beaven, M.A.; Metcalfe, D.D. IL-6 promotes an increase in human mast cell numbers and reactivity through suppression of suppressor of cytokine signaling 3. J. Allergy Clin. Immunol. 2016, 137, 1863–1871. [Google Scholar] [CrossRef] [PubMed]
- Hundley, T.R.; Gilfillan, A.M.; Tkaczyk, C.; Andrade, M.V.; Metcalfe, D.D.; Beaven, M.A. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood 2004, 104, 2410–2417. [Google Scholar] [CrossRef] [PubMed]
- Gilfillan, A.M.; Beaven, M.A. Regulation of mast cell responses in health and disease. Crit. Rev. Immunol. 2011, 31, 475–529. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, S.; Arudchandran, R.; Manetz, T.S.; Zhang, W.; Sommers, C.L.; Love, P.E.; Rivera, J.; Samelson, L.E. LAT is essential for Fc(epsilon)RI-mediated mast cell activation. Immunity 2000, 12, 525–535. [Google Scholar] [CrossRef]
- Ishizuka, T.; Chayama, K.; Takeda, K.; Hamelmann, E.; Terada, N.; Keller, G.M.; Johnson, G.L.; Gelfand, E.W. Mitogen-activated protein kinase activation through Fc epsilon receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells. J. Immunol. 1999, 162, 2087–2094. [Google Scholar]
- Kozlowski, M.; Larose, L.; Lee, F.; Le, D.M.; Rottapel, R.; Siminovitch, K.A. SHP-1 binds and negatively modulates the c-Kit receptor by interaction with tyrosine 569 in the c-Kit juxtamembrane domain. Mol. Cell. Biol. 1998, 18, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Paulson, R.; van der Geer, P.; Pawson, T.; Bernstein, A. Oncogenic mutation in the Kit receptor tyrosine kinase alters substrate specificity and induces degradation of the protein tyrosine phosphatase SHP-1. Proc. Natl. Acad. Sci. USA 1996, 93, 14665–14669. [Google Scholar] [CrossRef]
- Zhang, L.; Oh, S.Y.; Wu, X.; Oh, M.H.; Wu, F.; Schroeder, J.T.; Takemoto, C.M.; Zheng, T.; Zhu, Z. SHP-1 deficient mast cells are hyperresponsive to stimulation and critical in initiating allergic inflammation in the lung. J. Immunol. 2010, 184, 1180–1190. [Google Scholar] [CrossRef]
- Nakata, K.; Yoshimaru, T.; Suzuki, Y.; Inoue, T.; Ra, C.; Yakura, H.; Mizuno, K. Positive and negative regulation of high affinity IgE receptor signaling by Src homology region 2 domain-containing phosphatase 1. J. Immunol. 2008, 181, 5414–5424. [Google Scholar] [CrossRef]
- Marino, A.; Sakamoto, T.; Robador, P.A.; Tomita, K.; Levi, R. S1P receptor 1-Mediated Anti-Renin-Angiotensin System Cardioprotection: Pivotal Role of Mast Cell Aldehyde Dehydrogenase Type 2. J. Pharmacol. Exp. Ther. 2017, 362, 230–242. [Google Scholar] [CrossRef]
- Koda, K.; Salazar-Rodriguez, M.; Corti, F.; Chan, N.Y.; Estephan, R.; Silver, R.B.; Mochly-Rosen, D.; Levi, R. Aldehyde dehydrogenase activation prevents reperfusion arrhythmias by inhibiting local renin release from cardiac mast cells. Circulation 2010, 122, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Aldi, S.; Takano, K.; Tomita, K.; Koda, K.; Chan, N.Y.; Marino, A.; Salazar-Rodriguez, M.; Thurmond, R.L.; Levi, R. Histamine H4-receptors inhibit mast cell renin release in ischemia/reperfusion via protein kinase C epsilon-dependent aldehyde dehydrogenase type-2 activation. J. Pharmacol. Exp. Ther. 2014, 349, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Isse, T.; Matsuno, K.; Oyama, T.; Kitagawa, K.; Kawamoto, T. Aldehyde dehydrogenase 2 gene targeting mouse lacking enzyme activity shows high acetaldehyde level in blood, brain, and liver after ethanol gavages. Alcohol Clin. Exp. Res. 2005, 29, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.M.; Swindle, E.J.; Iwaki, S.; Gilfillan, A.M. Generation, isolation, and maintenance of rodent mast cells and mast cell lines. Curr. Protoc. Immunol. 2006, 74, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Kulinski, J.M.; Proia, R.L.; Larson, E.M.; Metcalfe, D.D.; Olivera, A. S1P(4) Regulates Passive Systemic Anaphylaxis in Mice but Is Dispensable for Canonical IgE-Mediated Responses in Mast Cells. Int. J. Mol. Sci. 2018, 19, 1279. [Google Scholar] [CrossRef] [PubMed]
- Kissel, H.; Timokhina, I.; Hardy, M.P.; Rothschild, G.; Tajima, Y.; Soares, V.; Angeles, M.; Whitlow, S.R.; Manova, K.; Besmer, P. Point mutation in kit receptor tyrosine kinase reveals essential roles for kit signaling in spermatogenesis and oogenesis without affecting other kit responses. EMBO J. 2000, 19, 1312–1326. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Radinger, M.; Gilfillan, A.M. Measuring mast cell mediator release. Curr. Protoc. Immunol. 2010, 91, 7–38. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, D.-K.; Cho, Y.-E.; Song, B.-J.; Kawamoto, T.; Metcalfe, D.D.; Olivera, A. Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells. Int. J. Mol. Sci. 2019, 20, 6216. https://doi.org/10.3390/ijms20246216
Kim D-K, Cho Y-E, Song B-J, Kawamoto T, Metcalfe DD, Olivera A. Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells. International Journal of Molecular Sciences. 2019; 20(24):6216. https://doi.org/10.3390/ijms20246216
Chicago/Turabian StyleKim, Do-Kyun, Young-Eun Cho, Byoung-Joon Song, Toshihiro Kawamoto, Dean D. Metcalfe, and Ana Olivera. 2019. "Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells" International Journal of Molecular Sciences 20, no. 24: 6216. https://doi.org/10.3390/ijms20246216
APA StyleKim, D.-K., Cho, Y.-E., Song, B.-J., Kawamoto, T., Metcalfe, D. D., & Olivera, A. (2019). Aldh2 Attenuates Stem Cell Factor/Kit-Dependent Signaling and Activation in Mast Cells. International Journal of Molecular Sciences, 20(24), 6216. https://doi.org/10.3390/ijms20246216





