Expression and Secretion of an Atrial Natriuretic Peptide in Beige-Like 3T3-L1 Adipocytes
Abstract
1. Introduction
2. Results
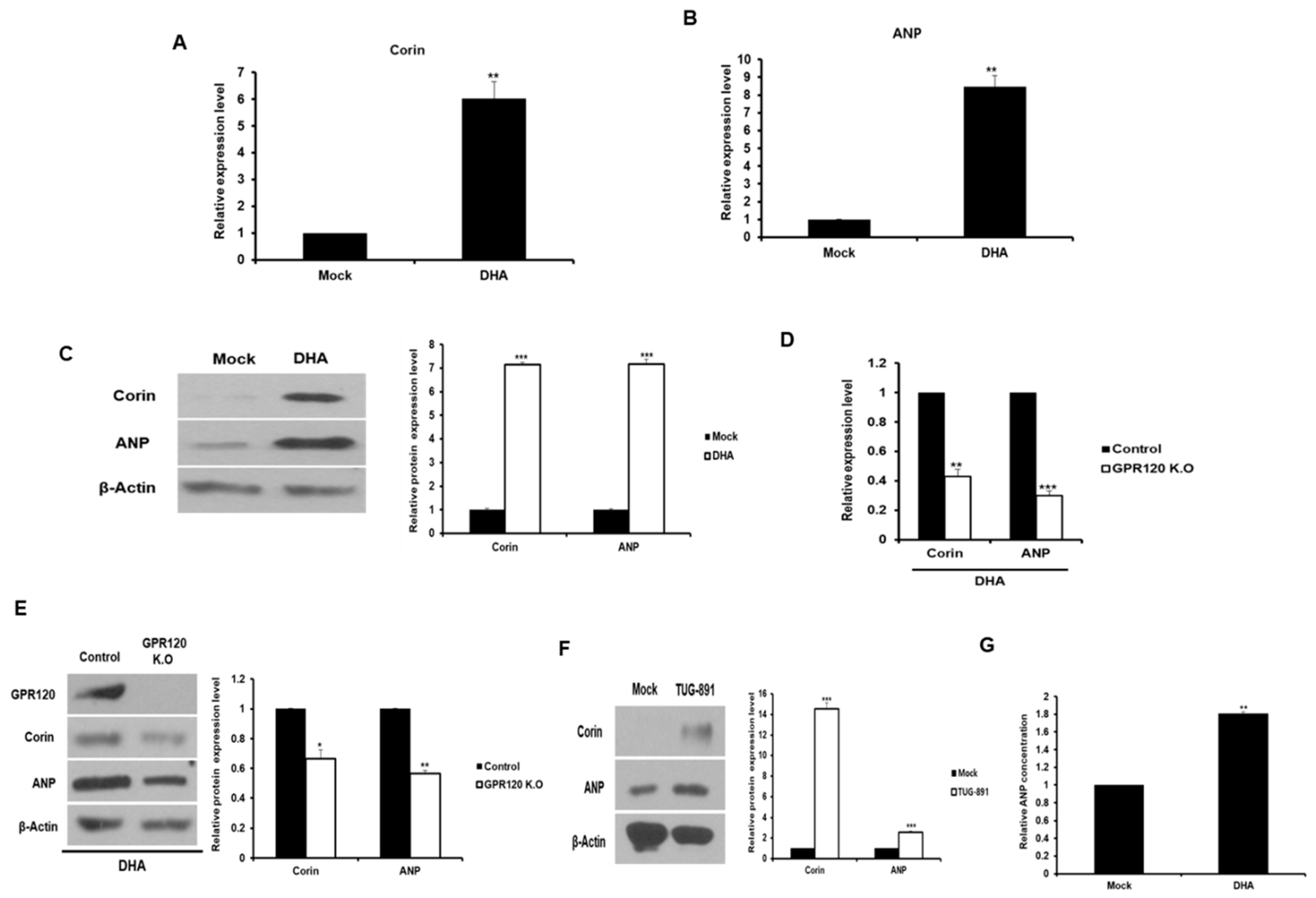
2.1. DHA-Treated Adipocytes Upregulates the Expression of Corin and ANP
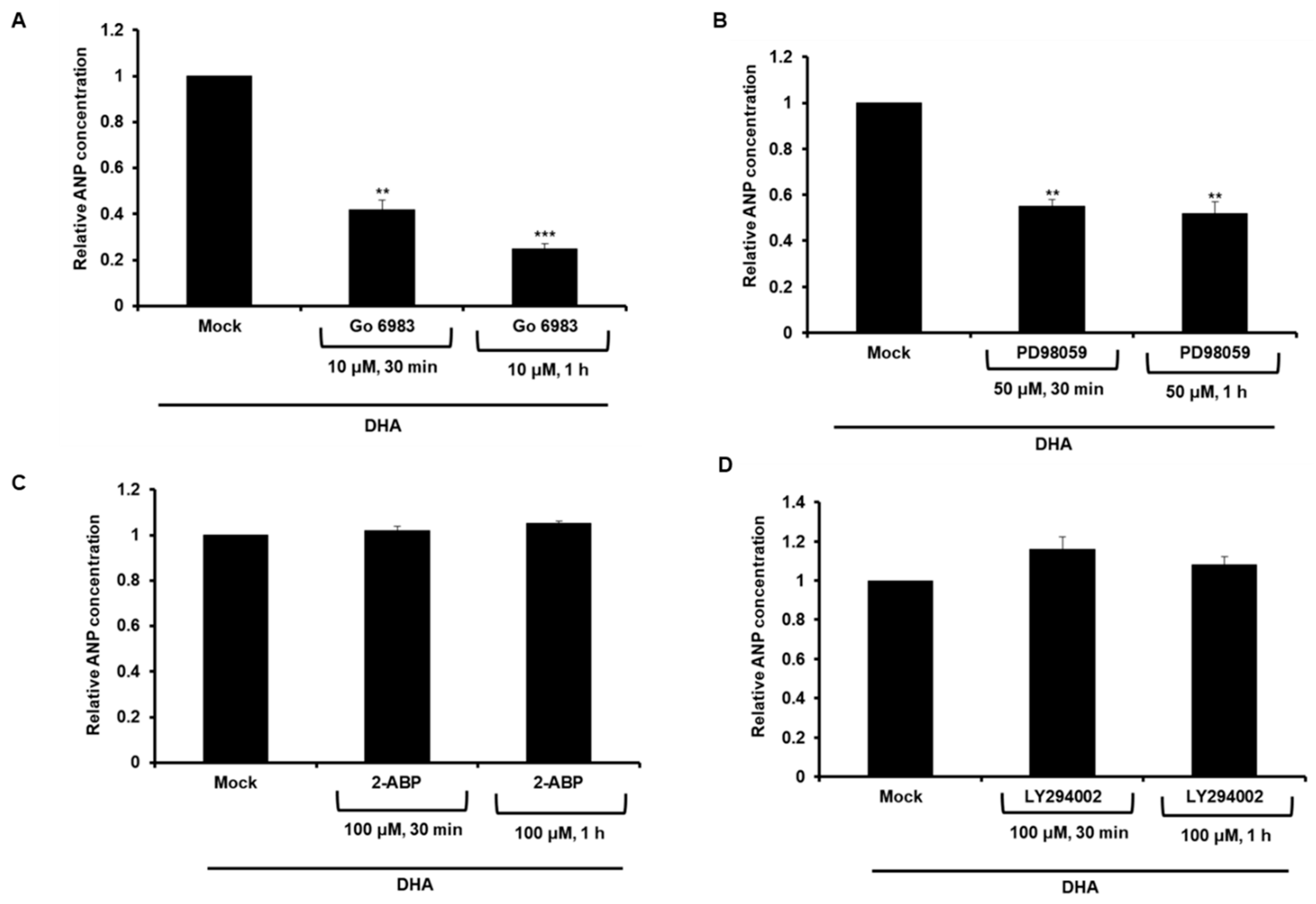
2.2. The Effect of DHA on ANP Secretion is Mediated by PKC/ERK Pathway
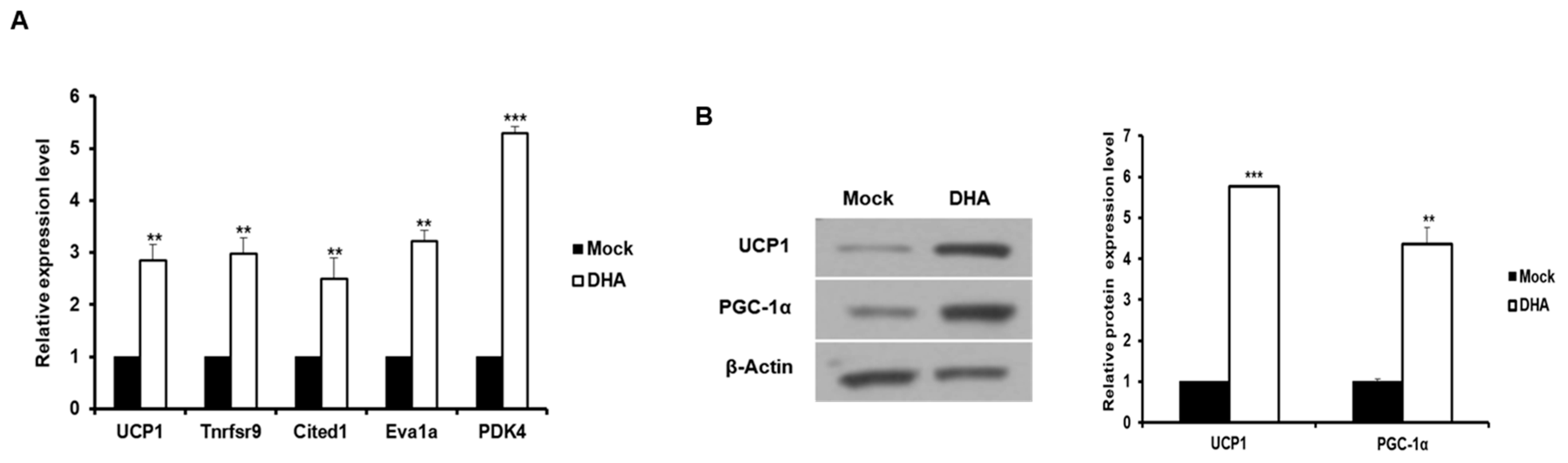
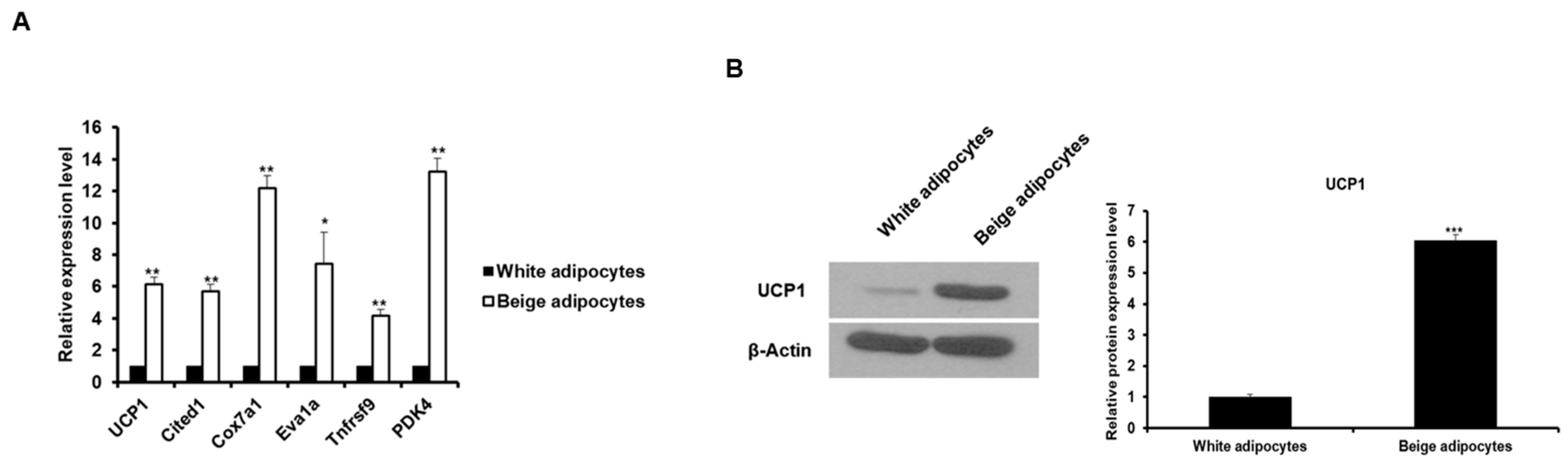
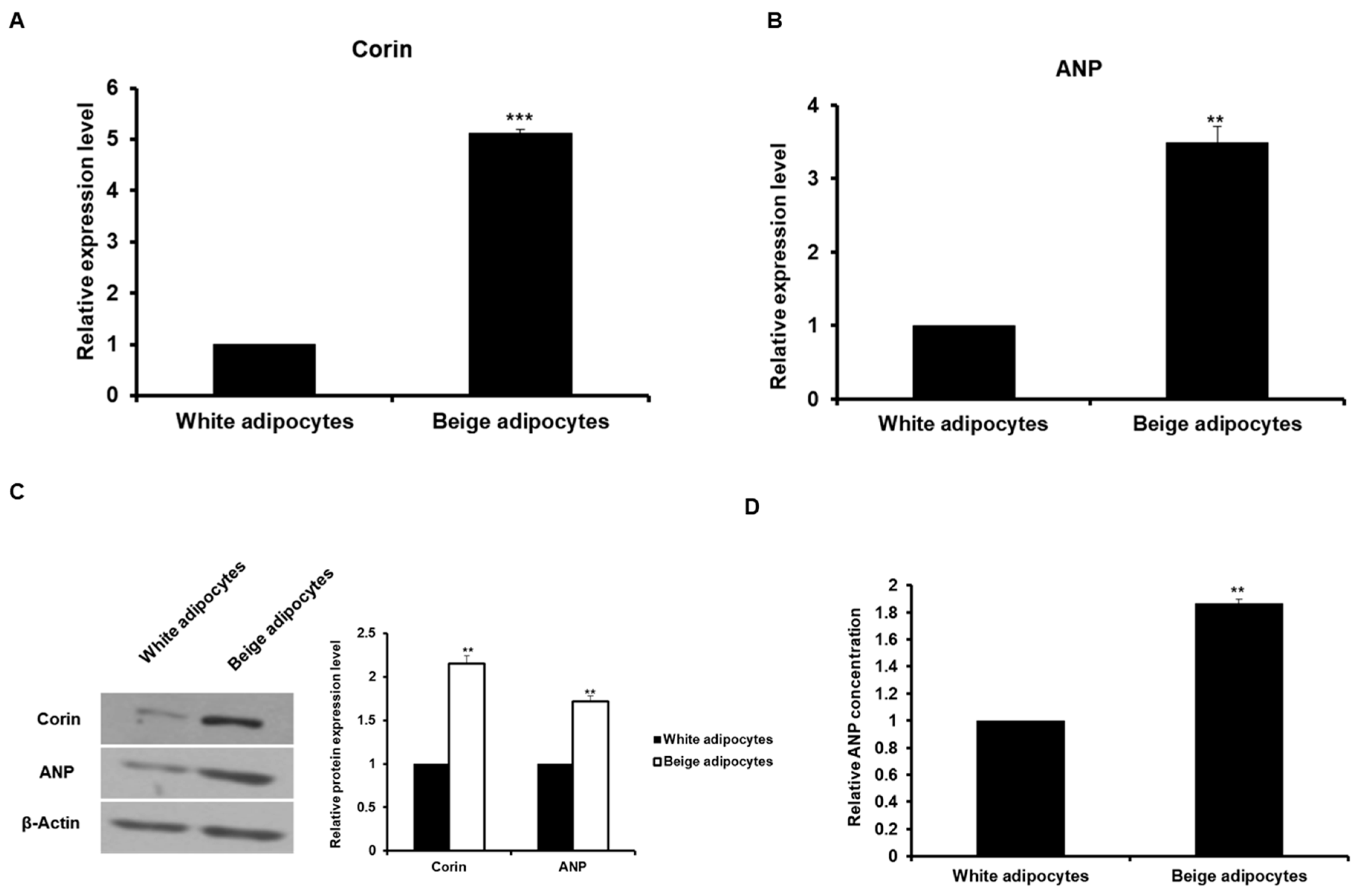
2.3. The Expression of Corin and ANP is Increased in Beige-Like Adipocytes
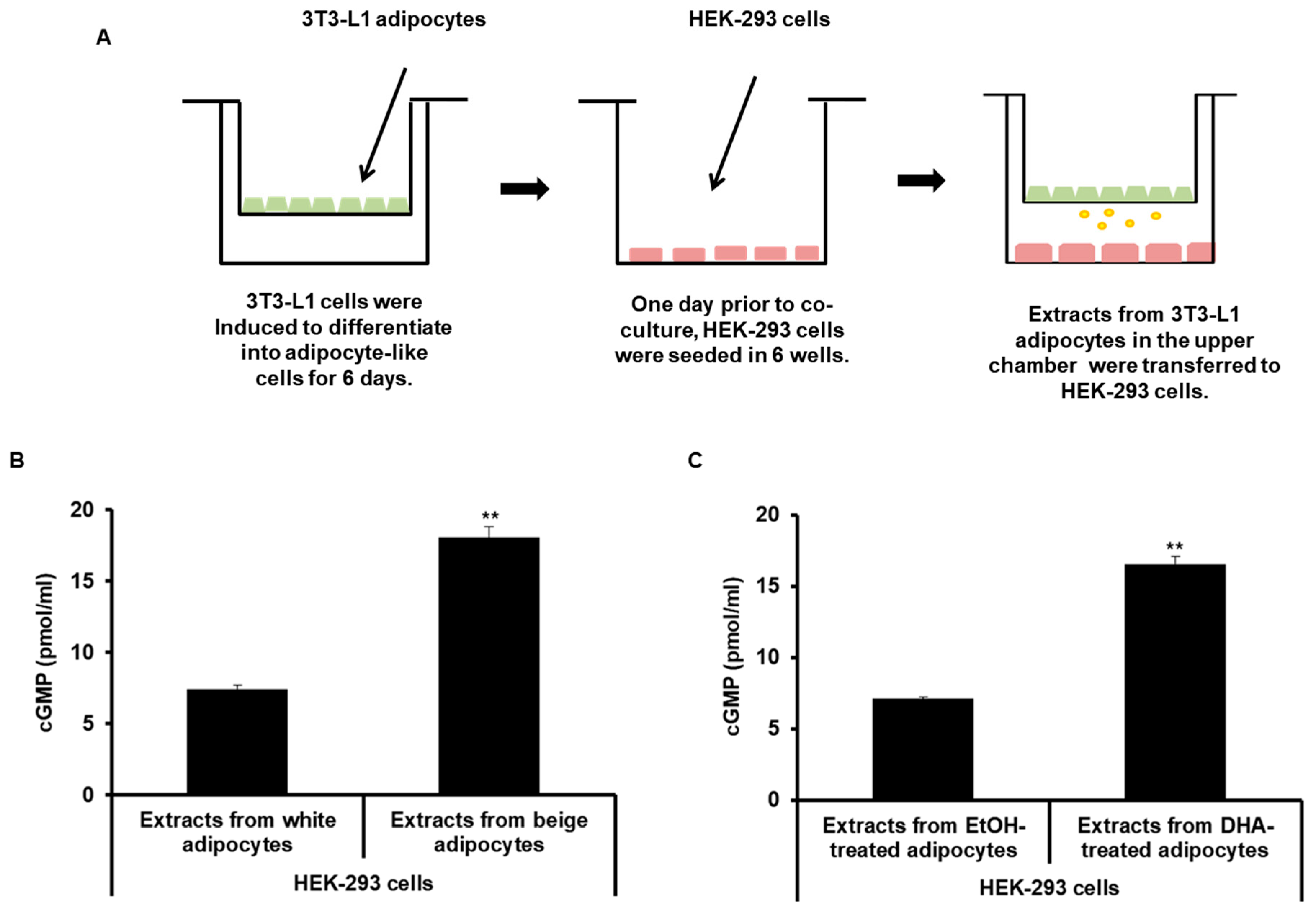
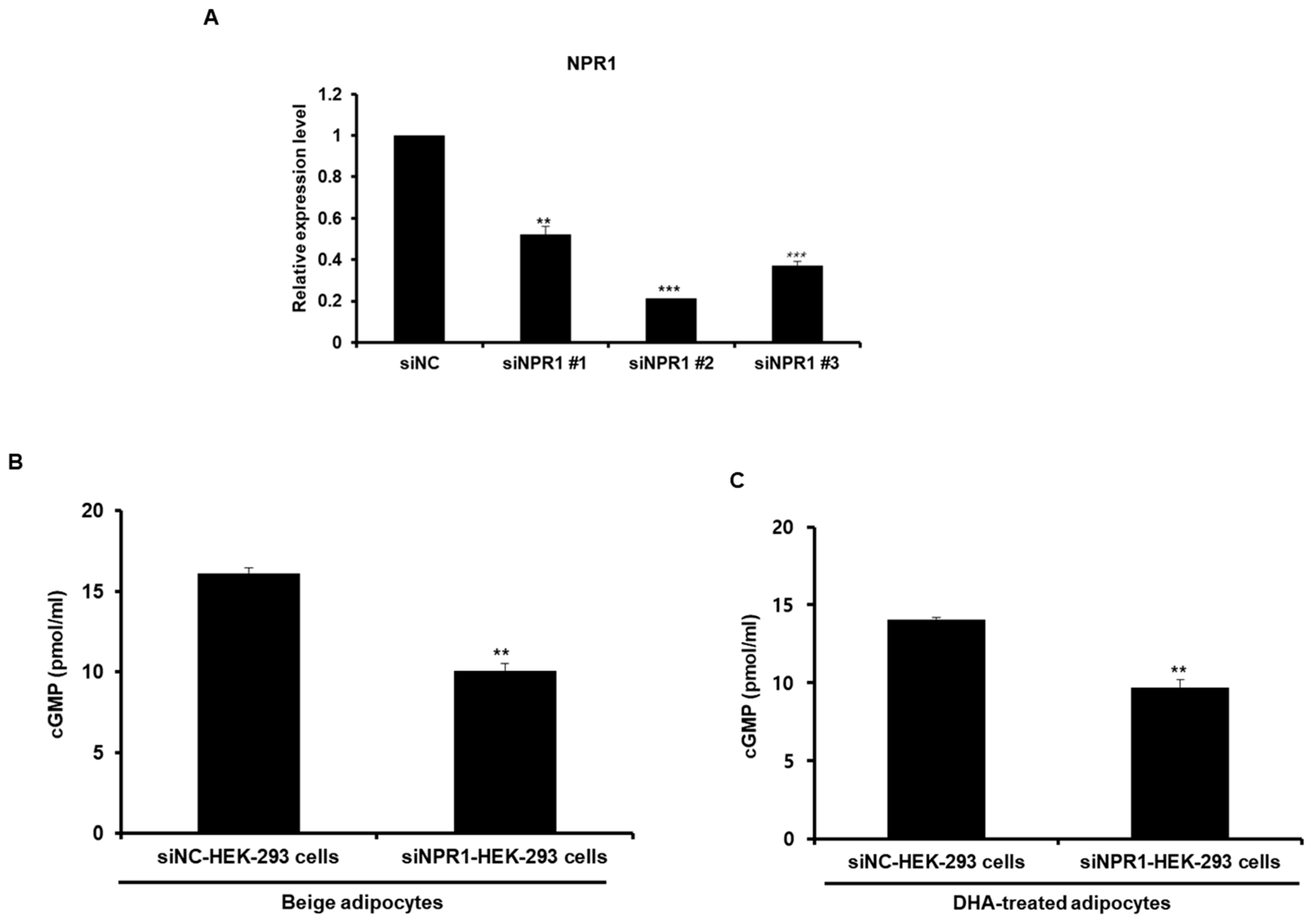
2.4. ANP Derived from Beige-Like Adipocytes Enhances cGMP Levels in HEK-293 Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Adipocyte Differentiation
4.2. RNA-Seq Analysis
4.3. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
4.4. Western Blot Analysis
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Statistical analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Trayhurn, P.; Beattie, J.H. Physiological Role of Adipose Tissue: White Adipose Tissue as an Endocrine and Secretory Organ. Proc. Nutr. Soc. 2001, 60, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Bostrom, P.; Sparks, L.M.; Ye, L.; Choi, J.H.; Giang, A.H.; Khandekar, M.; Virtanen, K.A.; Nuutila, P.; Schaart, G.; et al. Beige Adipocytes Are a Distinct Type of Thermogenic Fat Cell in Mouse and Human. Cell 2012, 150, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Jung, Y.S.; Choi, D. Recent Advance in Brown Adipose Physiology and Its Therapeutic Potential. Exp. Mol. Med. 2014, 46, e78. [Google Scholar] [CrossRef] [PubMed]
- Porter, C. Quantification of UCP1 Function in Human Brown Adipose Tissue. Adipocyte 2017, 6, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Matthias, A.; Jacobsson, A.; Cannon, B.; Nedergaard, J. The Bioenergetics of Brown Fat Mitochondria from UCP1-Ablated Mice. Ucp1 is Not Involved in Fatty Acid-Induced De-Energization (“Uncoupling”). J. Biol. Chem. 1999, 274, 28150–28160. [Google Scholar] [CrossRef]
- Lund, J.; Larsen, L.H.; Lauritzen, L. Fish Oil as a Potential Activator of Brown and Beige Fat Thermogenesis. Adipocyte 2018, 7, 88–95. [Google Scholar] [CrossRef]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.; Li, P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-Inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef]
- Im, D.S. Intercellular Lipid Mediators and GPCR Drug Discovery. Biomol. Ther. 2013, 21, 411–422. [Google Scholar] [CrossRef]
- Blad, C.C.; Tang, C.; Offermanns, S. G Protein-Coupled Receptors for Energy Metabolites as New Therapeutic Targets. Nat. Rev. Drug Discov. 2012, 11, 603–619. [Google Scholar] [CrossRef]
- Schilperoort, M.; van Dam, A.D.; Hoeke, G.; Shabalina, I.G.; Okolo, A.; Hanyaloglu, A.C.; Dib, L.H.; Mol, I.M.; Caengprasath, N.; Chan, Y.W.; et al. The GPR120 Agonist TUG-891 Promotes Metabolic Health by Stimulating Mitochondrial Respiration in Brown Fat. EMBO Mol. Med. 2018, 10. [Google Scholar] [CrossRef]
- Quesada-Lopez, T.; Cereijo, R.; Turatsinze, J.V.; Planavila, A.; Cairo, M.; Gavalda-Navarro, A.; Peyrou, M.; Moure, R.; Iglesias, R.; Giralt, M.; et al. The Lipid Sensor GPR120 Promotes Brown Fat Activation and FGF21 Release from Adipocytes. Nat. Commun. 2016, 7, 13479. [Google Scholar] [CrossRef] [PubMed]
- Ichiki, T.; Huntley, B.K.; Heublein, D.M.; Sandberg, S.M.; McKie, P.M.; Martin, F.L.; Jougasaki, M.; Burnett, J.C., Jr. Corin is Present in the Normal Human Heart, Kidney, and Blood, with Pro-B-Type Natriuretic Peptide Processing in the Circulation. Clin. Chem. 2011, 57, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Sheng, N.; Seto, M.; Morser, J.; Wu, Q. Corin, a Mosaic Transmembrane Serine Protease Encoded by a Novel cDNA from Human Heart. J. Biol. Chem. 1999, 274, 14926–14935. [Google Scholar] [CrossRef]
- Yan, W.; Wu, F.; Morser, J.; Wu, Q. Corin, a Transmembrane Cardiac Serine Protease, Acts as a Pro-Atrial Natriuretic Peptide-Converting Enzyme. Proc. Natl. Acad. Sci. USA 2000, 97, 8525–8529. [Google Scholar] [CrossRef]
- Cameron, V.A.; Aitken, G.D.; Ellmers, L.J.; Kennedy, M.A.; Espiner, E.A. The Sites of Gene Expression of Atrial, Brain, and C-Type Natriuretic Peptides in Mouse Fetal Development: Temporal Changes in Embryos and Placenta. Endocrinology 1996, 137, 817–824. [Google Scholar] [CrossRef]
- Misono, K.S.; Philo, J.S.; Arakawa, T.; Ogata, C.M.; Qiu, Y.; Ogawa, H.; Young, H.S. Structure, Signaling Mechanism and Regulation of the Natriuretic Peptide Receptor Guanylate Cyclase. FEBS J. 2011, 278, 1818–1829. [Google Scholar] [CrossRef]
- Pandey, K.N. Guanylyl cyclase/natriuretic Peptide Receptor-A Signaling Antagonizes Phosphoinositide Hydrolysis, Ca(2+) Release, and Activation of Protein Kinase C. Front. Mol. Neurosci. 2014, 7, 75. [Google Scholar] [CrossRef]
- Schlueter, N.; de Sterke, A.; Willmes, D.M.; Spranger, J.; Jordan, J.; Birkenfeld, A.L. Metabolic Actions of Natriuretic Peptides and Therapeutic Potential in the Metabolic Syndrome. Pharmacol. Ther. 2014, 144, 12–27. [Google Scholar] [CrossRef]
- Kimura, H.; Nagoshi, T.; Yoshii, A.; Kashiwagi, Y.; Tanaka, Y.; Ito, K.; Yoshino, T.; Tanaka, T.D.; Yoshimura, M. The Thermogenic Actions of Natriuretic Peptide in Brown Adipocytes: The Direct Measurement of the Intracellular Temperature using a Fluorescent Thermoprobe. Sci. Rep. 2017, 7, 12978. [Google Scholar] [CrossRef]
- Kessler-Icekson, G.; Barhum, Y.; Schaper, J.; Schaper, W.; Kaganovsky, E.; Brand, T. ANP Expression in the Hypertensive Heart. Exp. Clin. Cardiol. 2002, 7, 80–84. [Google Scholar]
- Nakagawa, Y.; Nishikimi, T.; Kuwahara, K. Atrial and Brain Natriuretic Peptides: Hormones Secreted from the Heart. Peptides 2019, 111, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Garruti, G.; Giusti, V.; Nussberger, J.; Darimont, C.; Verdumo, C.; Amstutz, C.; Puglisi, F.; Giorgino, F.; Giorgino, R.; Cotecchia, S. Expression and Secretion of the Atrial Natriuretic Peptide in Human Adipose Tissue and Preadipocytes. Obesity 2007, 15, 2181–2189. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Cao, P.; Dong, N.; Peng, J.; Zhang, C.; Wang, H.; Zhou, T.; Yang, J.; Zhang, Y.; Martelli, E.E.; et al. PCSK6-mediated corin activation is essential for normal blood pressure. Nat. Med. 2015, 21, 1048. [Google Scholar] [CrossRef] [PubMed]
- Asano, H.; Kanamori, Y.; Higurashi, S.; Nara, T.; Kato, K.; Matsui, T.; Funaba, M. Induction of Beige-Like Adipocytes in 3T3-L1 Cells. J. Vet. Med. Sci. 2014, 76, 57–64. [Google Scholar] [CrossRef]
- Reddy, N.L.; Tan, B.K.; Barber, T.M.; Randeva, H.S. Brown Adipose Tissue: Endocrine Determinants of Function and Therapeutic Manipulation as a Novel Treatment Strategy for Obesity. BMC Obes. 2014, 1, 13. [Google Scholar] [CrossRef]
- Hondares, E.; Iglesias, R.; Giralt, A.; Gonzalez, F.J.; Giralt, M.; Mampel, T.; Villarroya, F. Thermogenic Activation Induces FGF21 Expression and Release in Brown Adipose Tissue. J. Biol. Chem. 2011, 286, 12983–12990. [Google Scholar] [CrossRef]
- Campderros, L.; Moure, R.; Cairo, M.; Gavalda-Navarro, A.; Quesada-Lopez, T.; Cereijo, R.; Giralt, M.; Villarroya, J.; Villarroya, F. Brown Adipocytes Secrete GDF15 in Response to Thermogenic Activation. Obesity 2019, 27, 1606–1616. [Google Scholar] [CrossRef]
- Kristof, E.; Klusoczki, A.; Veress, R.; Shaw, A.; Combi, Z.S.; Varga, K.; Gyory, F.; Balajthy, Z.; Bai, P.; Bacso, Z.; et al. Interleukin-6 Released from Differentiating Human Beige Adipocytes Improves Browning. Exp. Cell Res. 2019, 377, 47–55. [Google Scholar] [CrossRef]
- Bordicchia, M.; Ceresiani, M.; Pavani, M.; Minardi, D.; Polito, M.; Wabitsch, M.; Cannone, V.; Burnett, J.C., Jr.; Dessì-Fulgheri, P.; Sarzani, R. Insulin/glucose induces natriuretic peptide clearance receptor in human adipocytes: A metabolic link with the cardiac natriuretic pathway. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R104–R114. [Google Scholar] [CrossRef]
- Collins, S.; Sarzani, R.; Bordicchia, M. Coordinate control of adipose ‘browning’ and energy expenditure by β-adrenergic and natriuretic peptide signaling. Int. J. Obes. Suppl. 2014, 4, S17–S20. [Google Scholar] [CrossRef]
- Bordicchia, M.; Liu, D.; Amri, E.Z.; Ailhaud, G.; Dessì-Fulgheri, P.; Zhang, C.; Takahashi, N.; Sarzani, R.; Collins, S. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic program in mouse and human adipocytes. J. Clin. Investig. 2012, 122, 1022–1036. [Google Scholar] [CrossRef] [PubMed]
- Kaisanlahti, A.; Glumoff, T. Browning of White Fat: Agents and Implications for Beige Adipose Tissue to Type 2 Diabetes. J. Physiol. Biochem. 2019, 75, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Ferre, P. The Biology of Peroxisome Proliferator-Activated Receptors: Relationship with Lipid Metabolism and Insulin Sensitivity. Diabetes 2004, 53 (Suppl. 1), S43–S50. [Google Scholar] [CrossRef]
- Ohno, H.; Shinoda, K.; Spiegelman, B.M.; Kajimura, S. PPARgamma Agonists Induce a White-to-Brown Fat Conversion through Stabilization of PRDM16 Protein. Cell Metab. 2012, 15, 395–404. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Shearer, G.C.; Chen, Q.; Healy, C.L.; Beyer, A.J.; Nareddy, V.B.; Gerdes, A.M.; Harris, W.S.; O’Connell, T.D.; Wang, D. Omega-3 Fatty Acids Prevent Pressure Overload-Induced Cardiac Fibrosis through Activation of Cyclic GMP/protein Kinase G Signaling in Cardiac Fibroblasts. Circulation 2011, 123, 584–593. [Google Scholar] [CrossRef]
- Duda, M.K.; O’Shea, K.M.; Tintinu, A.; Xu, W.; Khairallah, R.J.; Barrows, B.R.; Chess, D.J.; Azimzadeh, A.M.; Harris, W.S.; Sharov, V.G.; et al. Fish Oil, but Not Flaxseed Oil, Decreases Inflammation and Prevents Pressure Overload-Induced Cardiac Dysfunction. Cardiovasc. Res. 2009, 81, 319–327. [Google Scholar] [CrossRef]
- O’Connell, T.D.; Block, R.C.; Huang, S.P.; Shearer, G.C. Omega3-Polyunsaturated Fatty Acids for Heart Failure: Effects of Dose on Efficacy and Novel Signaling through Free Fatty Acid Receptor 4. J. Mol. Cell. Cardiol. 2017, 103, 74–92. [Google Scholar] [CrossRef]
| Gene | Sequence |
|---|---|
| β-actin | (F)5′-GTGACGTTGACATCCGTAAAGA-3′ |
| (R)5′-GCCGGACTCATCGTACTCC-3′ | |
| UCP1 | (F)5′-AGGCTTCCAGTACCATTAGGT-3′ |
| (R)5′-CTGAGTGAGGCAAAGCTGATTT-3′ | |
| Tnfrsf9 | (F)5′-CGTGCAGAACTCCTGTGATAA C-3′ |
| (R)5′-GTCCACCTATGCTGGAGAAGG-3′ | |
| Cited1 | (F)5′-AACCTTGGAGTGAAGGATCGC-3′ |
| (R)5′-GTAGGAGAGCCTATTGGAGATGT-3′ | |
| Eva1a | (F)5′-GGGGAGACCGAAGGAAATGAGA-3′ |
| (R)5′-CTCCAGCCCTGCACACTCTA-3′ | |
| PDK4 | (F)5′-AGGGAGGTCGAGCTGTTCTC-3′ |
| (R)5′-GGAGTGTTCACTAAGCGGTCA-3′ | |
| Cox7a1 | (F)5′-GCTCTGGTCCGGTCTTTTAGC-3′ |
| (R)5′-GTACTGGGAGGTCATTGTCGG-3′ | |
| Corin | (F)5′-GCTGGTGACTGCTAACTTGCT-3′ |
| (R)5′-CCCATCAGTGACCAAAGGTTC-3′ | |
| ANP | (F)5′-TCGTCTTGGCCTTTTGGCT-3′ |
| (R)5′-TCCAGGTGGTCTAGCAGGTTCT-3′ | |
| NPR1 | (F)5′-GGGATACAGTCAACACAGCCTCAA-3′ |
| (R)5′-CGAAGCTCCAGCTCGAAA C-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, I.-S.; Kim, S.H. Expression and Secretion of an Atrial Natriuretic Peptide in Beige-Like 3T3-L1 Adipocytes. Int. J. Mol. Sci. 2019, 20, 6128. https://doi.org/10.3390/ijms20246128
Bae I-S, Kim SH. Expression and Secretion of an Atrial Natriuretic Peptide in Beige-Like 3T3-L1 Adipocytes. International Journal of Molecular Sciences. 2019; 20(24):6128. https://doi.org/10.3390/ijms20246128
Chicago/Turabian StyleBae, In-Seon, and Sang Hoon Kim. 2019. "Expression and Secretion of an Atrial Natriuretic Peptide in Beige-Like 3T3-L1 Adipocytes" International Journal of Molecular Sciences 20, no. 24: 6128. https://doi.org/10.3390/ijms20246128
APA StyleBae, I.-S., & Kim, S. H. (2019). Expression and Secretion of an Atrial Natriuretic Peptide in Beige-Like 3T3-L1 Adipocytes. International Journal of Molecular Sciences, 20(24), 6128. https://doi.org/10.3390/ijms20246128





