Abstract
Duchenne muscular dystrophy (DMD) is one of the most severe forms of inherited muscular dystrophies. The disease is caused by the lack of dystrophin, a structurally essential protein; hence, a definitive cure would necessarily have to pass through some form of gene and/or cell therapy. Cell- and genetic-based therapeutics for DMD have been explored since the 1990s; recently, two of the latter have been approved for clinical use, but their efficacy is still very low. In parallel, there have been great ongoing efforts aimed at targeting the downstream pathogenic effects of dystrophin deficiency using classical pharmacological approaches, with synthetic or biological molecules. However, as it is always the case with rare diseases, R&D costs for new drugs can represent a major hurdle for researchers and patients alike. This problem can be greatly alleviated by experimenting the use of molecules that had originally been developed for different conditions, a process known as drug repurposing or drug repositioning. In this review, we will describe the state of the art of such an approach for DMD, both in the context of clinical trials and pre-clinical studies.
1. Introduction
Duchenne muscular dystrophy (DMD [MIM: 310200]) is an X-linked, progressive form of inherited muscular dystrophies, with an incidence of about 1:5000 [1,2]. The disease is caused by lack of the dystrophin protein, due to mutations of the DMD gene. Dystrophin is a cytoskeletal component that plays an essential role in maintaining muscle fiber membrane integrity, by connecting the fiber’s contractile apparatus to the sarcolemma and ultimately to the external extracellular matrix. Its deficiency results in dramatic muscle deterioration, with repeated cycles of fibers’ degeneration/regeneration that eventually result in fibro-adipose substitution of the muscle tissue. Patients present a clear histopathological phenotype already at birth, but clinical symptoms, in the form of delayed motor milestones, usually appear at age 3–5. The disease is invariably progressive and DMD boys are usually wheelchair-bound by age 10–13. Thanks to a steady improvement in palliative care, chiefly nocturnal assisted ventilation, life quality and expectancy have increased, but most patients eventually succumb due to respiratory/cardiac failure by the third decade [3].
Given that DMD is caused by the lack of a structurally essential protein, a definitive cure would necessarily have to pass through some form of gene and/or cell therapy. During the past two decades, many promising therapeutic approaches have been developed and tested in DMD animal models, but so far, clinical trials in patients have led to much less impressive results. Two novel ‘genetic-based’ drugs have recently been approved for clinical use by either Food and Drug Administration (FDA) or European Medicines Agency (EMA), but they are aimed at specific subsets of patients and their efficacy is still subjected to debate. For instance, at the time of this writing, exon 51 skipping drug Eteplirsen has been approved for commercialization by FDA but not by EMA, and it is potentially applicable to less than 15% of the DMD population [4]. DMD is a complex disease, whose management requires a multi-disciplinary approach [5]. When it comes to pharmacological management, the only available options capable of delaying the progression of the disease in terms of skeletal muscle function are two glucocorticoids, deflazacort or prednisone, which to this day represent the gold standard for DMD in terms of pharmacological therapy [6]. Both drugs can significantly prolong ambulation and preserve muscle force in most DMD patients, albeit at the cost of heavy side effects. In this situation, the search for ‘druggable’ targets is of paramount importance in DMD research and is indeed the subject of intense investigation, with almost 50 active clinical trials with various molecules at the time of writing (https://clinicaltrials.gov/).
DMD belongs to the category of the rare diseases (a.k.a. “orphan disease”), defined by a prevalence below 1:2000 in Europe and of less than 200,000 patients in the USA [7]. The quest for a cure for rare diseases is hampered by the fact that the high costs of basic research and subsequent clinical trials have to be faced in the front of a relatively small revenue potential for the pharmaceutical companies. Part of the burden, especially for basic research, is often sustained by patients’ Associations, but these can rarely afford the costs of development and clinical testing for new drugs. For these reasons, when it comes to rare diseases, a cost- and time-effective strategy relies on drug repurposing, i.e., the finding of new indications for a drug that has already been approved for a different condition [8]. This allows for skipping most, if not all, pharmacokinetics and safety studies, greatly speeding up the process of assessing actual efficacy in patients. Such an approach is based on the consideration that rare and common diseases often share several clinical features, and that the similarities in many cases are also reflected at the level of pathogenetic mechanisms [9]. Indeed, even in the case of classic Mendelian diseases, the pathological phenotype is rarely linked solely to the (mal)function of a single gene product, but rather derives from the alteration of multiple molecular networks [10]. This consideration, compounded with the fact that in most cases drugs interact with multiple targets besides the intended ones [11], explains why a molecule originally designed for a specific disease could also prove useful in a different one.
In this review, we will summarize the state of the art of drug repurposing for DMD, both at the level of pre-clinical studies in dystrophic (mdx) mice and of clinical trials, taking into account only molecules that have been already approved for commercialization. The drugs are summarized in Table 1 and their structural formulas in Figure 1.

Table 1.
Examples of repurposed drugs that are being investigated for Duchenne muscular dystrophy (DMD) treatment.
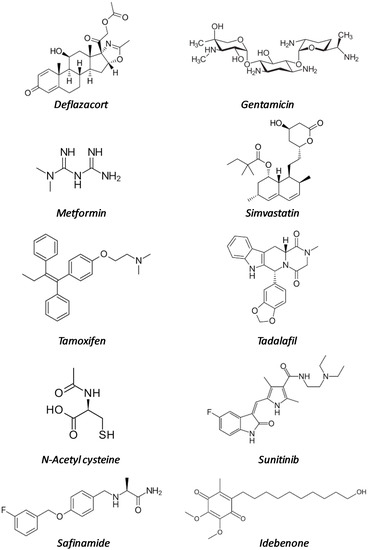
Figure 1.
Structural formulas of the repurposed drugs cited in Table 1.
2. The Gold Standard: Glucocorticoid Corticosteroids
Glucocorticoid corticosteroids (GCs) are potent anti-inflammatory agents first isolated from adrenal cortex in the 1940s, and initially adopted with exceptional results in rheumatoid arthritis. This discovery resulted in the Nobel Prize in Physiology and Medicine being awarded in 1950 to Kendall, Reichstein, and Hench—one of the shortest periods of time occurring between any discovery and the attribution of a Nobel Prize [12].
One of the main oral forms of GCs, prednisolone or its pro-drug prednisone, was first used to treat DMD in the early 1970s, with clear evidence of efficacy even in a small seminal study of 14 patients by Drachmann and Colleagues [13]. This may be considered the original, and so far, the most successful example of drug repurposing for DMD (Table 1).
Since then, several other studies have been conducted, as effectively summarized in a recent Cochrane review [14]. It is demonstrated beyond doubt that glucocorticoids are effective in improving and stabilizing skeletal muscle strength in DMD over a time period of one to two years. The benefit in preserving motor function in the long term is also well established (e.g., prolonged ambulation by approximately 2–3 years), but the evidence is weaker as it is mostly based on observational studies [15,16] rather than randomized controlled trials.
Side effects of glucocorticoids, especially occurring with long-term treatment, are well known across pediatric and adult populations. Different from adults, in which hypertension and glucose intolerance are frequent, in young boys the main issues are weight gain, behavioral disturbances, growth stunting, and osteoporosis [6]. Gastrointestinal symptoms and cataracts are also quite common. Virtually all DMD patients who undergo prolonged GC treatment according to standards of care [17] experience some degree of GC-related adverse effects. However, these are usually manageable and must be proactively screened for, prevented, and counteracted, tailoring dose on the individual benefit/risk profile. Nevertheless, there is clearly a dire need for safer treatment options in DMD.
Several treatment regimens (daily, alternate day, 10 days on and 10 days off, etc.) have been experimented and applied in the clinic, using both prednisone/prednisolone and deflazacort, and with few randomized studies comparing the relative efficacy and safety of different regimens [18]. A global, National Institutes of Health-sponsored, randomized clinical trial comparing daily prednisone, daily deflazacort, and 10-days-on-10-days-off prednisone named finding the optimum corticosteroid regimen for DMD (FOR-DMD) is currently reaching its completion in the U.S. and several European countries.
Despite lingering uncertainties about the optimum regimen, GC treatment is unanimously considered useful and recommended as a standard of care in DMD [6,17]. Treatment is usually started around the age of 4 to 7 years, at the dose of 0.75 mg/kg/day prednisone or 0.9 mg/kg/day deflazacort, and is adjusted according to individual response and adverse effects (osteoporosis, weight gain, cataracts, etc.). Increasingly, treatment is protracted beyond loss of independent ambulation into the early and late non-ambulatory stages of DMD, because of evidence from observational studies of beneficial long-term effects on upper limb function, lung function, and efficacy in preventing severe scoliosis [16,19].
The mechanisms of action of glucocorticoids in dystrophinopathy are complex and manifold. The potent inhibition of nuclear actor kappa-B (NF-κB) and its downstream pro-inflammatory effects are surely crucial mechanisms (Figure 2), but other relevant pathways also include fiber type transition from glycolytic to oxidative, widespread regulation of gene expression, membrane stabilization, stimulation of regeneration and repair, regulation of calcium metabolism, and possibly regulation of utrophin expression [20,21,22,23].
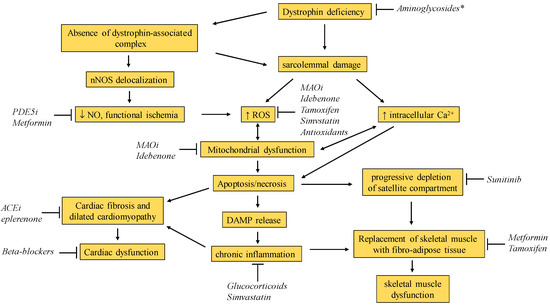
Figure 2.
Schematic representation of pathogenic mechanisms in DMD. Blunted arrows indicate the hypothesized point of actions of repurposed drugs currently under investigation. NO, nitric oxide; nNOS, neuronal nitric oxide synthase; PDE5i, phosphodiesterase 5 inhibitors; ROS, reactive oxygen species; MAOi, Monoamine oxidase inhibitors; ACEi, angiotensin converting enzyme inhibitors; DAMP, damage-associated molecular patterns; * initially considered for stop codon readthrough, but now replaced by a specifically designed molecule.
3. Simvastatin
Simvastatin is a lipid-lowering agent widely used in clinics for treating high blood LDL cholesterol levels and associated cardiovascular diseases. It derives synthetically from a fermentation product of the fungus Aspergillus terreus. Hydrolyzed in vivo to an active metabolite, simvastatin competitively inhibits hepatic hydroxymethyl-glutaryl coenzyme A (HMG-CoA) reductase, the enzyme that catalyzes the conversion of HMG-CoA to mevalonate, a key step in cholesterol synthesis. Besides these cholesterol lowering effects, statins also reduce inflammation, oxidative stress, and fibrosis through a cholesterol-independent mechanism [36,37,38].
Recently, a preclinical study in mdx mice showed that simvastatin reduced muscle damage and enhanced muscle function, by reducing inflammation, oxidative stress, and fibrosis [39]. Further analyses also showed positive effects on cardiac function in the same murine model [39,40]. More preclinical experimentations are underway to help better characterize the risks and benefits of statins in DMD and inform the optimal molecule to move into clinical studies.
4. N-acetylcysteine and Antioxidants
N-acetyl cysteine has been approved by FDA as the mainstay of therapy for acetaminophen toxicity, as it is highly effective in the treatment of potentially hepatotoxic overdoses. It is also approved for diseases associated with excessive, viscous mucous secretions such as pneumonia, bronchitis, and cystic fibrosis. The main molecular mechanism is due to its ability to replete glutathione reserves by providing cysteine, which is an essential precursor in glutathione synthesis. Glutathione, in its reduced form, is a crucial antioxidant by itself and also a substrate for different antioxidant enzymes [50]. In case of significant depletion of glutathione, N-acetyl cysteine also acts as a direct antioxidant, as a thiol compound. The use of N-acetylcysteine in mdx mice has been found to alleviate skeletal muscle dysfunction and pathologic histology [51]. Similar results were observed by treating mdx mice with another antioxidant, (−)-epigallocatechin gallate, the major polyphenolic component of green tea extract, [52]; this molecule has also been used in a recently completed DMD clinical trial (NCT01183767), for which no results have yet been published. However, the use of non-selective antioxidants is quite controversial, as recently discussed [64,65,66].
5. Safinamide and MAO Inhibitors
Oxidative stress and mitochondrial dysfunction are known to play a key role in DMD [55,67,68,69,70,71,72]. A crucial source of reactive oxygen species (ROS) in dystrophic muscles is monoamine oxidase (MAO) [55,56,72], a mitochondrial enzyme widely studied for its role in the central nervous system [57]. The two isoforms of MAO, A and B, are located in the outer mitochondrial membrane and catalyze the oxidative deamination of different biogenic amines to generate aldehydes and H2O2. Pathologic excess of H2O2 have been shown to be involved in the oxidation of contractile proteins both in ischemic heart and dystrophic skeletal muscle [56,72,73,74,75]. Consistently, treatment with pargyline, an inhibitor of both MAO-A and MAO-B, reduced tropomyosin oxidation and led to improvement of the dystrophic phenotype in mdx and col6a1−/− mice [72]. MAO has also been shown to be overactivated in myoblasts from patients with collagen VI myopathies and DMD [55,56]. More recently, novel and better tolerated inhibitors of the B isoform (MAO-Bi) have been introduced in the clinic for neurological disorders [76]. The advantage of inhibiting MAO-B is to avoid the risk of hypertensive crises, which is associated with inhibition of the MAO-A isoform. In addition, the molecular structure of MAO-B has been identified at high resolution [77,78,79], thus allowing the design of highly specific inhibitors. Among them, safinamide is a selective and reversible MAO-Bi, with an improved profile of efficacy and safety, that has been introduced in the market for Parkinson’s disease. In a recent report, Safinamide has been shown to markedly improve muscle function in mdx mice, as well as to reduce oxidative stress and mitochondrial dysfunction in muscle cells from DMD patients [56].
6. Sunitinib
Recently, Fontelonga and Colleagues have shown that sunitinib (SU11248), a multi receptor tyrosine kinase (RTK) inhibitor approved for the treatment of renal cell carcinoma [53] and gastrointestinal stromal tumors, provided benefits in mdx mice [54]. Treatment with this drug promoted satellite cell (SC) activation and myogenic regeneration, leading to significantly improved muscle disease pathology and functional skeletal muscle force production. Such effects have been linked to Sunitinib’s capability to act as a potent α7ß1 integrin enhancer, thereby stimulating satellite cell activation and increasing myofiber fusion via the STAT3 pathway [54].
7. Idebenone
Idebenone is a synthetic short-chain coenzyme Q10 analogue, which was initially developed for the treatment of degenerative neurocognitive disorders, but in this indication it never reached significant success. However, it has been approved by Europe Medicines Agency (EMA) for the treatment of Leber’s hereditary optic neuropathy. It transfers electrons directly to the mitochondrial complex III of the respiratory chain, thereby restoring ATP intracellular levels, and also acts as an antioxidant and a free radical scavenger. As discussed above, there is a strong rationale for treatment with respiratory chain co-factors and antioxidants in a disease, such as DMD, whose pathophysiology is characterized by a cellular energetic failure and chronic oxidative stress [80].
As idebenone had been applied in the treatment of the cardiological complications of a different neurogenetic disease, Friedreich’s Ataxia [81,82], it does not come as a surprise that the first clinical trials of idebenone in the DMD field were targeted at treating dilated cardiomyopathy. An early, phase IIa “pilot” study compared 13 DMD patients aged 8–16 years, treated with 450 mg/day idebenone, with eight matched controls [58] with a 12-month follow-up (DELPHI trial). Echocardiographic measures of left ventricular contractility showed a positive trend but no significant treatment-related differences; however, a significant difference between treated and control groups was observed in peak expiratory flow (PEF) values. Subsequent trials then focused on respiratory endpoints, and primarily on GC-naïve patients who had shown maximum idebenone-related effects on PEF, according to post-hoc analyses of DELPHI data [59]. The phase 3 DELOS trial [60,61] therefore recruited 64 DMD patients aged 10 to 18 years and not on concomitant GCs, who were randomized 1:1 to receive a higher idebenone dose (900 mg/day divided into three doses) or placebo. As in previous phase II trials, idebenone was well tolerated, and it attenuated the decrease of PEF over 12 months by about 6% of age/height predicted values (p < 0.05). This positive trial established idebenone as a promising treatment option for DMD at several disease stages, including later non-ambulatory phases for which the availability of treatments is even scanter than for ambulatory patients. Some limitations included the relatively small sample size for a phase 3 trial and some age discrepancy between treated and placebo groups. Furthermore, at the time when these results were published in 2015, it was somewhat difficult to gauge the clinical significance of the 6% difference in PEF reduction, because of the lack of longitudinal “natural history” studies of pulmonary function tests (PFTs). This knowledge gap has recently been filled and the average decrease of PEF in DMD patients aged 10–18 seems to be around 5%/year [16]. Post-hoc analyses of DELOS data showed a reduced rate of respiratory complications [62] (primarily chest infections) and longer retainment of PFT values above thresholds indicating the need for non-invasive ventilation [63]. Currently, another global phase 3 study (SIDEROS) is ongoing, aiming to demonstrate idebenone efficacy also in GC-treated DMD patients.
8. Tamoxifen
Tamoxifen, a selective estrogen receptor modulator (SERM), is a one of the most commonly used drugs in the treatment of estrogen receptor-positive breast cancer. However, it is also known to possess numerous other therapeutically relevant effects, as it has been shown to act a ROS scavenger, an anti-apoptotic agent, and an inhibitor of fibroblast proliferation [41]. Starting from these considerations, Dorchies and Colleagues tested its use in the 5Cv strain of mdx mice, subjecting the animals to long-term treatment with the drug. This resulted in remarkable improvements of muscle force and reduction of fibrosis of the diaphragm and the heart [41]. Interestingly, the efficacy of tamoxifen was observed also in mice with another severe congenital muscular disorder, the fatal X-linked myotubular myopathy [42].
Shifting from murine to human dystrophinopathy, in DMD patients the influence of sexual hormones has been demonstrated to be patho-physiologically relevant. Because of a combination of causes related to the disease itself and GC treatment, DMD patients often exhibit some degree of hypogonadism leading to pubertal delay [17], and their muscles overexpress estrogen receptors (ERs). Recently, tamoxifen has been granted an orphan drug designation for the treatment of DMD by the EMA, and a randomized, placebo-controlled phase 3 clinical trial with a multi-center design is has started to assess the efficacy of tamoxifen in DMD [43].
9. Metformin
Metformin belongs to the class of biguanides, a family of compounds with complex mechanisms of action, including mainly a regulation of respiratory chain activity and the AMP-activated protein kinase (AMPK) cascade, which ultimately inhibits gluconeogenesis from the liver [28]. Initially approved and widely used for its blood glucose lowering effects, metformin is a drug that has a multifaceted potential for repurposing because of its pleiotropic effects. For instance, it is also approved for the treatment of polycystic ovary syndrome, and its regulatory action on the mammalian target of rapamycin (mTOR) signaling pathway implies an interesting potential as an anti-cancer agent [29]. Preclinical studies of metformin in the mdx model of dystrophinopathy have suggested that its long-term beneficial effects on muscle fibrosis and strength, as assessed by muscle histology and functional tests, respectively, can be independent from metabolic and AMPK-related effects, suggesting the existence of alternative mechanisms [30]. Other researchers have identified, in treated mdx mice, the activation of well-characterized phenotype-modifying pathways, such as peroxisome proliferator-activated receptor (PPAR)γ coactivator-1α (PGC-1α) and the upregulation of utrophin [31].
The first application of metformin to human DMD in the literature is described in a trial that recruited a heterogeneous population of pediatric patients affected with conditions characterized by motor dysfunction [32] (also including neural tube defects and other neuromuscular conditions), and successfully showed a reduction of visceral adiposity and insulin resistance. However, later studies have focused on more disease-specific mechanisms of action of metformin in muscle dystropathology. For example, the ability of metformin to upregulate neuronal nitric oxide synthase (nNOS), which is defective in dystrophin-deficient muscle (see also the following paragraph), has provided a strong rationale for its association with nitric oxide (NO) precursors such as l-citrulline. After initial, small proof-of-concept studies of the metformin/l-citrulline association in Becker muscular dystrophy (BMD) and DMD [33,34], which showed promising results on muscle metabolism biomarkers and preliminary clinical evaluations, metformin/l-citrulline experimentation is being moved on to a larger, randomized, double-blind, placebo-controlled trial with a duration of six months and the clinically oriented motor function measure (MFM) as a primary outcome [35].
10. PDE5 Inhibitors and Nitrate Drugs
Phosphodiesterase type 5 inhibitors (PDE5i) are a class of molecules approved for the treatment of erectile disfunction and pulmonary hypertension, as blocking PDE5 leads to increased levels of cGMP and hence to the relaxation of smooth muscle cells within blood vessels. Starting from the observation that one of the pathological features of dystrophic muscle is the presence of functional ischemia (i.e., the incapability of achieving sufficient blood flow upon muscle activity), Asai and Colleagues treated mdx mice with tadalafil, reporting decreased fiber necrosis upon repeated tetanic contractions [44]. Shortly after, another group reported that the heightened fatigue response to mild exercise present in mdx mice was indeed due to impaired vascular adaptation upon muscle contraction, due to the loss of sarcolemma-localized nNOS and consequent impairment of cGMP-mediated vaso-modulation [45]. In the same report, Kobayashi et al. showed that treatment with a PDE5i could greatly improve blood flow and reduce edema in the muscles of mdx mice after exercise [45]. Subsequently, the group led by RG Victor tested the efficacy of administering the PDE5i tadalafil in improving functional muscle ischemia pilot trials in BMD and DMD patients [46]. Both studies yielded positive results, although the outcome parameter was limited to the measurement of blood flow in the forearm upon handgrip exercise. More recently, other preclinical studies reported that the use of tadalafil led to functional and histological improvements in skeletal muscle in mdx mice [47] and could delay the onset of cardiomyopathy both in mdx mice and in the golden retriever canine model of DMD [48]. The clinical development program of Tadalafil culminated in a large phase 3, double blind, placebo-controlled, randomized clinical trial, with a three-arm design: Placebo, 0.3 mg/kg/day tadalafil, and 0.6 mg/kg/day tadalafil [49]. Notably, this trial represented one of the largest recruitment efforts in the history of DMD trials, with 331 randomized participants. While the known safety profile of tadalafil was confirmed, with no additional safety concerns in the Duchenne population than in the general population, unfortunately both tadalafil doses failed to slow down the decline in walking ability of the patients, as measured by the primary outcome, the 6-min walk test (6MWT). Secondary measures including other motor function scales, measurements of respiratory and cardiac function, and patient-centered assessments of quality of life, showed no significant drug-related effect.
11. Food Supplements
Flavocoxid is a mixture of bioflavonoid of plant origin, that was originally approved in 2004 by the FDA as a “medical food” with anti-inflammatory properties. It was commercialized as an integration to the treatment of osteoarthritis. Flavocoxid has an antioxidant/anti-inflammatory effect, and an ability to reduce levels of NF-κB signaling. After promising preclinical studies in mice [83], flavocoxid entered human experimentation in the form of phase I study, whose results, reported only as a conference abstract, suggested good tolerability and decreased levels of circulating inflammatory cytokines [84]. However, in 2017 FDA revoked the marketing authorization after several cases of acute liver injuries were reported and no further experimentations were reported after that.
12. Aminoglycosides (for Stop Codon Readthrough)
About 10% to 15% of DMD cases are due to nonsense mutations, i.e., point mutation [85] (most usually a single nucleotide substitution) leading to a single premature termination codon to be inserted into the open reading frame (ORF). Different from more frequent frameshifting mutations, in which the ORF downstream of the mutation becomes “meaningless” to the advancing ribosome, and fraught with tens of stop codons, with nonsense mutations the downstream genetic information is preserved, and could be translated by the ribosome if the mutation itself could be surpassed. Usually, the presence of a nonsense mutation causes the ribosomal complex to halt and activate the nonsense mediated decay pathway [86], resulting in the degradation of the transcript and absence of protein expression. However, the ribosomal complex does have the ability, in the right molecular context, to “read through” the nonsense mutation by inserting a random amino acid and continuing downstream translation [87]; an ability that can be enhanced by specific drugs. Aminoglycosides, a class of antibiotics approved for the treatment of several bacterial infections, have been known to be able to suppress nonsense mutations in animal models. Starting from this consideration, Barton-Davis and Colleagues tested the efficacy of gentamicin in mdx mice, demonstrating partial dystrophin restoration and increased resistance to force loss upon eccentric contraction, a typical hallmark of dystrophic muscle [24]. Gentamicin treatment led to positive results also in exercised mdx mice, a model that better reproduces the pathologic features found in DMD patients [25]. Therefore, an attempt was made to use gentamicin to obtain full-length dystrophin re-expression in a small study of four DMD patients [83]. Unfortunately, this could not be demonstrated in the short duration of the two-week study. On the other hand, a different small-scale study on four individuals who were either ambulatory or had lost ambulation for no more than four months, showed dystrophin re-expression in muscle biopsies performed after gentamicin infusions, as shown by immunohistochemistry and immunoblot [26]. Interestingly, the effect was apparent only in those patients with the more permissive UGA stop codon, and not in one patient with the UAA codon. A few years later, the largest study on aminoglycosides in DMD was published [27]. In this multi-center trial, different cohorts were treated with gentamicin infusions: A cohort of 10 nonsense DMD patients and a “control” cohort of eight patients with frameshift mutations (in order to control for hypothetical “non-genetic” effects of gentamicin) received a 14-day course of treatment, while two additional cohorts of 12 and 4 nonsense DMD patients were treated weekly and twice weekly, respectively. Dystrophin increase evaluated by immunofluorescence was statistically significant in the six-month treatment group, and the data did not support a preferential response with the UGA codon as observed by Politano et al.; rather, those patients who had higher levels of dystrophin at baseline appeared to respond better. Subsequent to the publication of these data, concerns about oto- and nephrotoxicity hindered a larger clinical development of gentamicin in DMD. Interestingly, a recent study in mdx mice suggested that aminoglycosides could also be beneficial for DMD treatment in a different capacity, namely as facilitators of the delivery of morpholino antisense oligonucleotides to skeletal muscle [88]. As for the stop-codon readthrough strategy, in DMD this is now being pursued with the use of novel, non-aminoglycoside, synthetic molecules [89,90]. Of these, Ataluren [84,85] has progressed through all the phases of clinical trials and has recently been approved by EMA.
13. Cardiological Drugs (ACE Inhibitors, Beta-Blockers, Eplerenone)
Dilated cardiomyopathy (DCM) is one of the major complications of DMD, and one of the most relevant determinants of life expectancy [91], especially after the implementation of mechanical ventilation has significantly reduced and delayed death due to respiratory insufficiency. Several drugs are used in the clinical management of dystrophin-related DCM, and it could be debated whether this can be regarded as “repurposing”. In fact, several physio-pathological mechanisms are shared between dystrophin-related and more common (e.g., ischemic) cardiomyopathies: Fibrosis, dilated remodeling, increased oxygen consumption, and adrenergic hyperactivation being some of the most relevant. Therefore, cardiac drugs target these mechanisms in similar ways in DMD as in “common” cardiopathic patients. Here, we will only briefly review the main pharmacological categories employed in the treatment of dystrophin-related DCM, and the most important evidence supporting their use.
One of the main challenges in studying cardiological endpoints in DMD is that several years of observation are usually needed to observe a meaningful change in available measures of cardiac function, effectively summarized in a recent review of the literature [92], to which our readers are pointed for more in-depth discussion. The most widely used outcomes are echographic measures of left ventricular systolic function, such as fractional shortening and ejection fraction, as well as of dilation, e.g., left ventricular telediastolic volume normalized by body surface. Electrocardiographic alterations such as pseudo-necrotic Q waves are also relevant and often precede echographic changes. The recent advances in magnetic resonance imaging of the heart, which allow a very accurate characterization of myocardial fibrosis—revealed by late gadolinium enhancement—and provide very sensitive contractility measures such as myocardial strain estimations, have increased its role both in clinical management and research.
Angiotensin-converting enzyme inhibitors (ACEi) are probably the most frequently prescribed cardiac medications in DMD [93]. By inhibiting the renin-angiotensin pathway, they reduce peripheral circulatory resistance and blood volume (through a reduction of mineralocorticoid secretion). The hypothesis behind their application in DMD was that the subsequent reduction of afterload would release the myocardium from the mechanical stress that is known to cause cellular damage when dystrophin is lacking [94]. In fact, the ACEi perindopril did show an ability to delay the progression to a reduced systolic function of the left ventricle, in a randomized, blinded trial [95]. The effect of ACEi may also be due to their secondary anti-fibrotic properties, a consequence of the reduction of mineralocorticoids. Longitudinal follow-up studies collecting data up to 10 years of observation proved that perindopril is also able to prevent and delay the onset of DCM [96], and not just to slow down its progression once it has started. Currently, therefore, prophylactic treatment with ACEi is increasingly being implemented, starting from the age of around 10, even in DMD children with no evidence of DCM, although international standard-of-care guidelines leave this clinical choice at the discretion of cardiologists [93].
The second mainstay of DMD cardiac therapy are ß-blockers (BBs), which reduce hyperadrenergic activation, oxygen consumption, and the risk of dangerous arrhythmias started by foci of myocardial fibrosis [97]. Their use is recommended by current standards of care [43], especially in patients with marked tachycardia. The association with ACEi has shown good results both as treatment and prophylaxis of DCM [98], although randomized, controlled trials with significant primary endpoint results are lacking.
As mentioned above while discussing the effects of ACEi, the secretion of mineralocorticoids has detrimental consequences for the DMD heart, because of both afterload increase (i.e., salt retention) and the activation of pro-fibrotic pathways. Mineralocorticoid antagonists, also known as potassium-sparing diuretics, are effective in contrasting these mechanisms and have a place in the treatment of DMD DCM [93]. While the most common agent of this class was spironolactone, a recent study of a newer molecule, eplerenone [99], showed significantly improved left ventricular function in 22 treated DMD patients vs. 20 controls, on the background of treatment with either ACEi or BBs. A strength of this study was the use of sensitive, heart-MRI derived outcome measures such as left ventricular circumferential strain, which allowed to overcome the relatively small sample size and short observation time of 12 months.
Other agents (diuretics, inotropic drugs, etc.) may sporadically be prescribed; a newer drug combination, sacubitril/valsartan, has recently been shown to exhibit superior therapeutic efficiency compared to standard ACEi in heart failure patients, both adult and pediatric [100,101]. For this reason, its use has been suggested also in DMD patients, but at present, no specific studies have been reported [102]. Currently, though, the combination of ACEi, BBs, and mineralocorticoid antagonists, under the guidance of a cardiologist with experience in dystrophinopathies, represents the core of cardiac care for DMD, and clearly—despite the relative lack of high-class evidence, in the context of evidence-based cardiology—has the potential to significantly enhance life quality and expectancy in DMD [102].
14. Conclusions
Duchenne muscular dystrophy was the second genetic disease whose underlying molecular defect was discovered by the then novel “reverse genetics” approach, more than 30 years ago [103]. Since then, the intrinsic features of dystrophin (e.g., its large size and complex structural role, the multiple type of mutations found in patients) have hampered the many attempts at developing resolutive therapies. The fact that in the recent past two genetic-based therapies have been approved for commercialization offers hope for further positive developments, as do the indications that gene replacement therapy with specifically crafted micro-dystrophins appears to be providing positive results in clinical trials [104]. However, molecular therapies with mutation-specific drugs, such as exon skipping oligonucleotides or small molecules promoting stop codon readthrough, are intrinsically limited to restricted DMD sub-populations [92]. Furthermore, some patients may not be eligible for gene therapy because of pre-existing immunity to viral vectors and other reasons [92]. Last but not least, both exon skipping and micro-dystrophin expression would be of relatively little help for all those patients in whom the amount of actual muscle tissue has already been drastically reduced. In these latter cases, a cell transplantation approach would be the ideal solution, but attempts in this direction have so far failed and no clinical trials are presently ongoing. For all of these reasons, there is a definite and pressing need to develop pharmacological therapies capable of addressing the numerous downstream consequences of dystrophin deficiency, in order to improve life expectancy and quality of life in patients. This is why drug repurposing is playing an increasingly important role in the quest for new, efficacious and affordable therapies for DMD, and will increasingly do so in the foreseeable future.
Author Contributions
L.V., L.B., and M.C., writing—original draft and review and editing; L.T. and E.P., review and editing.
Funding
This research was funded by: University of Padova, Department of Biology (PRID-Seed Project 2018 to L.V.); Cariparo Foundation (Progetto di Eccellenza “GenMod” 2017 to E.P.); Telethon Genetic BioBank (GTB12001D to E.P.); the Eurobiobank network (to E.P.); IRP-PENTA Grant 18/07-1 to M.C., University of Padova (DOR1907358/19 to M.C.).
Conflicts of Interest
L.B. reports speaker honoraria from PTC Therapeutics, participation in Advisory Boards for Sarepta Therapeutics, Santhera Pharmaceuticals, and PTC Therapeutics, and participation in research sponsored by Santhera Pharmaceuticals. The other authors declare no conflict of interest.
References
- Mah, J.K.; Korngut, L.; Dykeman, J.; Day, L.; Pringsheim, T.; Jette, N. A systematic review and meta-analysis on the epidemiology of Duchenne and Becker muscular dystrophy. Neuromuscul. Disord. 2014, 24, 482–491. [Google Scholar] [CrossRef]
- Ryder, S.; Leadley, R.M.; Armstrong, N.; Westwood, M.; De Kock, S.; Butt, T.; Jain, M.; Kleijnen, J. The burden, epidemiology, costs and treatment for Duchenne muscular dystrophy: An evidence review. Orphanet J. Rare Dis. 2017, 12, 79. [Google Scholar] [CrossRef]
- Bourke, J.P.; Bueser, T.; Quinlivan, R. Interventions for preventing and treating cardiac complications in Duchenne and Becker muscular dystrophy and X-linked dilated cardiomyopathy. Cochrane Database Syst. Rev. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Aartsma-Rus, A.; Goemans, N. A Sequel to the Eteplirsen Saga: Eteplirsen Is Approved in the United States but Was Not Approved in Europe. Nucleic Acid Ther. 2019, 29, 13–15. [Google Scholar] [CrossRef] [PubMed]
- Bushby, K.; Finkel, R.; Birnkrant, D.J.; Case, L.E.; Clemens, P.R.; Cripe, L.; Kaul, A.; Kinnett, K.; McDonald, C.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and pharmacological and psychosocial management. Lancet Neurol. 2010, 9, 77–93. [Google Scholar] [CrossRef]
- Gloss, D.; Moxley, R.T.; Ashwal, S.; Oskoui, M. Practice guideline update summary: Corticosteroid treatment of Duchenne muscular dystrophy—Report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2016, 86, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Aronson, J. Rare diseases, orphan drugs, and orphan diseases. BMJ 2006, 333, 127. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2018, 18, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.Y.; Loscalzo, J. The emerging paradigm of network medicine in the study of human disease. Circ. Res. 2012, 111, 359–374. [Google Scholar] [CrossRef]
- Piro, R.M. Network medicine: Linking disorders. Hum. Genet. 2012, 131, 1811–1820. [Google Scholar] [CrossRef]
- Guengerich, F.P. Mechanisms of drug toxicity and relevance to pharmaceutical development. Drug Metab. Pharmacokinet. 2011, 26, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.M. The History of Cortisone Discovery and Development. Rheum. Dis. Clin. N. Am. 2016, 42, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Drachman, D.B.; Toyka, K.V.; Myer, E. Predisone in Duchenne muscular dystrophy. Lancet 1974, 2, 1409–1412. [Google Scholar] [CrossRef]
- Matthews, E.; Brassington, R.; Kuntzer, T.; Jichi, F.; Manzur, A.Y. Corticosteroids for the treatment of Duchenne muscular dystrophy. Cochrane Database Syst. Rev. 2016, 5. [Google Scholar] [CrossRef]
- Bello, L.; Gordish-Dressman, H.; Morgenroth, L.P.; Henricson, E.K.; Duong, T.; Hoffman, E.P.; Cnaan, A.; McDonald, C.M. Prednisone/prednisolone and deflazacort regimens in the CINRG Duchenne Natural History Study. Neurology 2015, 85, 1048–1055. [Google Scholar] [CrossRef]
- McDonald, C.M.; Henricson, E.K.; Abresch, R.T.; Duong, T.; Joyce, N.C.; Hu, F.; Clemens, P.R.; Hoffman, E.P.; Cnaan, A.; Gordish-Dressman, H.; et al. Long-term effects of glucocorticoids on function, quality of life, and survival in patients with Duchenne muscular dystrophy: A prospective cohort study. Lancet 2018, 391, 451–461. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Apkon, S.D.; Blackwell, A.; Brumbaugh, D.; Case, L.E.; Clemens, P.R.; Hadjiyannakis, S.; Pandya, S.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: Diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018, 17, 251–267. [Google Scholar] [CrossRef]
- Griggs, R.C.; Herr, B.E.; Reha, A.; Elfring, G.; Atkinson, L.; Cwik, V.; Mccoll, E.; Tawil, R.; Pandya, S.; Mcdermott, M.P.; et al. Corticosteroids in Duchenne muscular dystrophy: Major variations in practice. Muscle Nerve 2013, 48, 27–31. [Google Scholar] [CrossRef]
- Mc Donald, C.M.; Gordish-Dressman, H.; Henricson, E.K.; Duong, T.; Joyce, N.C.; Jhawar, S.; Leinonen, M.; Hsu, F.; Connolly, A.M.; Cnaan, A.; et al. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: Long-term natural history with and without glucocorticoids. Neuromuscul. Disord. 2018, 28, 897–909. [Google Scholar] [CrossRef]
- Khan, M.A. Corticosteroid therapy in Duchenne muscular dystrophy. J. Neurol. Sci. 1993, 120, 8–14. [Google Scholar] [CrossRef]
- Anderson, J.E.; Mcintosh, L.M.; Poettcker, R. Deflazacort but not prednisone improves both muscle repair and fiber growth in diaphragm and limb muscle in vivo in the mdx dystrophic mouse. Muscle Nerve 1996, 19, 1576–1585. [Google Scholar] [CrossRef]
- Anderson, J.E.; Weber, M.; Vargas, C. Deflazacort increases laminin expression and myogenic repair, and induces early persistent functional gain in mdx mouse muscular dystrophy. Proc. Cell Transp. 2000, 9, 551–564. [Google Scholar] [CrossRef] [PubMed]
- St-Pierre, S.J.G.; Chakkalakal, J.V.; Kolodziejczyk, S.M.; Knudson, J.C.; Jasmin, B.J.; Megeney, L. A Glucocorticoid treatment alleviates dystrophic myofiber pathology by activation of the calcineurin/NF-AT pathway. FASEB J. 2004, 18, 1937–1939. [Google Scholar] [CrossRef] [PubMed]
- Barton-Davis, E.R.; Cordier, L.; Shoturma, D.I.; Leland, S.E.; Sweeney, H.L. Aminoglycoside antibiotics restore dystrophin function to skeletal muscles of mdx mice. J. Clin. Investig. 1999, 104, 375–381. [Google Scholar] [CrossRef] [PubMed]
- De Luca, A.; Nico, B.; Rolland, J.F.; Cozzoli, A.; Burdi, R.; Mangieri, D.; Giannuzzi, V.; Liantonio, A.; Cippone, V.; De Bellis, M.; et al. Gentamicin treatment in exercised mdx mice: Identification of dystrophin-sensitive pathways and evaluation of efficacy in work-loaded dystrophic muscle. Neurobiol. Dis. 2008, 32, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Politano, L.; Nigro, G.; Nigro, V.; Pilus, G.; Papparella, S.; Paciello, O.; Comi, L.I. Gentamicin administration in Duchenne patients with premature stop codon. Preliminary results. Acta Myol. 2003, 22, 15–21. [Google Scholar]
- Malik, V.; Rodino-Klapac, L.R.; Viollet, L.; Wall, C.; King, W.; Al-Dahhak, R.; Lewis, S.; Shilling, C.J.; Kota, J.; Serrano-Munuera, C.; et al. Gentamicin-induced readthrough of stop codons in Duchenne muscular dystrophy. Ann. Neurol. 2010, 67, 771–780. [Google Scholar] [CrossRef]
- Wróbel, M.P.; Marek, B.; Kajdaniuk, D.; Rokicka, D.; Szymborska-Kajanek, A.; Strojek, K. Metformin—A new old drug. Endokrynol. Pol. 2017, 68, 482–496. [Google Scholar] [CrossRef]
- Amin, S.; Lux, A.; O’Callaghan, F. The journey of metformin from glycaemic control to mTOR inhibition and the suppression of tumour growth. Br. J. Clin. Pharmacol. 2019, 85, 37–46. [Google Scholar] [CrossRef]
- Mantuano, P.; Sanarica, F.; Conte, E.; Morgese, M.G.; Capogrosso, R.F.; Cozzoli, A.; Fonzino, A.; Quaranta, A.; Rolland, J.F.; De Bellis, M.; et al. Effect of a long-term treatment with metformin in dystrophic mdx mice: A reconsideration of its potential clinical interest in Duchenne muscular dystrophy. Biochem. Pharmacol. 2018, 154, 89–103. [Google Scholar] [CrossRef]
- Ljubicic, V.; Jasmin, B.J. Metformin increases peroxisome proliferator-activated receptor γ Co-activator-1α and utrophin a expression in dystrophic skeletal muscle. Muscle Nerve 2015, 52, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Casteels, K.; Fieuws, S.; van Helvoirt, M.; Verpoorten, C.; Goemans, N.; Coudyzer, W.; Loeckx, D.; de Zegher, F. Metformin therapy to reduce weight gain and visceral adiposity in children and adolescents with neurogenic or myogenic motor deficit. Pediatr. Diabetes 2010, 11, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Hafner, P.; Bonati, U.; Erne, B.; Schmid, M.; Rubino, D.; Pohlman, U.; Peters, T.; Rutz, E.; Frank, S.; Neuhaus, C.; et al. Improved muscle function in duchenne muscular dystrophy through l-arginine and metformin: An investigator-initiated, open-label, single-center, proof-of-concept-study. PLoS ONE 2016, 11, e0147634. [Google Scholar] [CrossRef] [PubMed]
- Hanff, E.; Hafner, P.; Bollenbach, A.; Bonati, U.; Kayacelebi, A.A.; Fischer, D.; Tsikas, D. Effects of single and combined metformin and l-citrulline supplementation on l-arginine-related pathways in Becker muscular dystrophy patients: Possible biochemical and clinical implications. Amino Acids 2018, 50, 1391–1406. [Google Scholar] [CrossRef] [PubMed]
- Hafner, P.; Bonati, U.; Rubino, D.; Gocheva, V.; Zumbrunn, T.; Gueven, N.; Fischer, D. Treatment with l-citrulline and metformin in Duchenne muscular dystrophy: Study protocol for a single-centre, randomised, placebo-controlled trial. Trials 2016, 17, 389. [Google Scholar] [CrossRef]
- Schönbeck, U.; Libby, P. Inflammation, immunity, and HMG-CoA reductase inhibitors: Statins as antiinflammatory agents? Circulation 2004, 109. [Google Scholar] [CrossRef]
- Pignatelli, P.; Carnevale, R.; Pastori, D.; Cangemi, R.; Napoleone, L.; Bartimoccia, S.; Nocella, C.; Basili, S.; Violi, F. Immediate antioxidant and antiplatelet effect of atorvastatin via inhibition of Nox2. Circulation 2012, 126, 92–103. [Google Scholar] [CrossRef]
- Violi, F.; Pignatelli, P. Statins as Regulators of Redox Signaling in Platelets. Antioxid. Redox Signal. 2014, 20, 1300–1312. [Google Scholar] [CrossRef]
- Whitehead, N.P.; Kim, M.J.; Bible, K.L.; Adams, M.E.; Froehner, S.C. A new therapeutic effect of simvastatin revealed by functional improvement in muscular dystrophy. Proc. Natl. Acad. Sci. USA 2015, 112, 12864–12869. [Google Scholar] [CrossRef]
- Kim, M.J.; Bible, K.L.; Regnier, M.; Adams, M.E.; Froehner, S.C.; Whitehead, N.P. Simvastatin provides long-term improvement of left ventricular function and prevents cardiac fibrosis in muscular dystrophy. Physiol. Rep. 2019, 7, e14018. [Google Scholar] [CrossRef]
- Dorchies, O.M.; Reutenauer-Patte, J.; Dahmane, E.; Ismail, H.M.; Petermann, O.; Patthey-Vuadens, O.; Comyn, S.A.; Gayi, E.; Piacenza, T.; Handa, R.J.; et al. The anticancer drug tamoxifen counteracts the pathology in a mouse model of duchenne muscular dystrophy. Am. J. Pathol. 2013, 182, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Gayi, E.; Neff, L.A.; Massana Muñoz, X.; Ismail, H.M.; Sierra, M.; Mercier, T.; Décosterd, L.A.; Laporte, J.; Cowling, B.S.; Dorchies, O.M.; et al. Tamoxifen prolongs survival and alleviates symptoms in mice with fatal X-linked myotubular myopathy. Nat. Commun. 2018, 9, 4848. [Google Scholar] [CrossRef] [PubMed]
- Gayi, E.; Neff, L.A.; Ismail, H.M.; Ruegg, U.T.; Scapozza, L.; Dorchies, O.M. Repurposing the selective oestrogen receptor modulator tamoxifen for the treatment of Duchenne muscular dystrophy. Proc. Chim. 2018, 182, 485–504. [Google Scholar] [CrossRef] [PubMed]
- Asai, A.; Sahani, N.; Kaneki, M.; Ouchi, Y.; Jeevendra Martyn, J.A.; Yasuhara, S.E. Primary role of functional ischemia, quantitative evidence for the two-hit mechanism, and phosphodiesterase-5 inhibitor therapy in mouse muscular dystrophy. PLoS ONE 2007, 2, e806. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.M.; Rader, E.P.; Crawford, R.W.; Iyengar, N.K.; Thedens, D.R.; Faulkner, J.A.; Parikh, S.V.; Weiss, R.M.; Chamberlain, J.S.; Moore, S.A.; et al. Sarcolemma-localized nNOS is required to maintain activity after mild exercise. Nature 2008, 456, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.D.; Rader, F.; Tang, X.; Tavyev, J.; Nelson, S.F.; Miceli, M.C.; Elashoff, R.M.; Sweeney, H.L.; Victor, R.G. PDE5 inhibition alleviates functional muscle ischemia in boys with Duchenne muscular dystrophy. Neurology 2014, 82, 2085–2091. [Google Scholar] [CrossRef]
- De Arcangelis, V.; Strimpakos, G.; Gabanella, F.; Corbi, N.; Luvisetto, S.; Magrelli, A.; Onori, A.; Passananti, C.; Pisani, C.; Rome, S.; et al. Pathways Implicated in Tadalafil Amelioration of Duchenne Muscular Dystrophy. J. Cell. Physiol. 2016, 231, 224–232. [Google Scholar] [CrossRef]
- Hammers, D.W.; Sleeper, M.M.; Forbes, S.C.; Shima, A.; Walter, G.A.; Sweeney, H.L. Tadalafil Treatment Delays the Onset of Cardiomyopathy in Dystrophin-Deficient Hearts. J. Am. Heart Assoc. 2016, 5, e003911. [Google Scholar] [CrossRef]
- Victor, R.G.; Sweeney, H.L.; Finkel, R.; McDonald, C.M.; Byrne, B.; Eagle, M.; Goemans, N.; Vandenborne, K.; Dubrovsky, A.L.; Topaloglu, H.; et al. A phase 3 randomized placebo-controlled trial of tadalafil for Duchenne muscular dystrophy. Neurology 2017, 89, 1811–1820. [Google Scholar] [CrossRef]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Whitehead, N.P.; Pham, C.; Gervasio, O.L.; Allen, D.G. N-Acetylcysteine ameliorates skeletal muscle pathophysiology in mdx mice. J. Physiol. 2008, 586, 2003–2014. [Google Scholar] [CrossRef] [PubMed]
- Dorchies, O.M.; Wagner, S.; Vuadens, O.; Waldhauser, K.; Buetler, T.M.; Kucera, P.; Ruegg, U.T. Green tea extract and its major polyphenol (−)-epigallocatechin gallate improve muscle function in a mouse model for Duchenne muscular dystrophy. Am. J. Physiol. Cell Physiol. 2006, 290, C616–C625. [Google Scholar] [CrossRef] [PubMed]
- Faivre, S.; Delbaldo, C.; Vera, K.; Robert, C.; Lozahic, S.; Lassau, N.; Bello, C.; Deprimo, S.; Brega, N.; Massimini, G.; et al. Safety, pharmacokinetic, and antitumor activity of SU11248, a novel oral multitarget tyrosine kinase inhibitor, in patients with cancer. J. Clin. Oncol. 2006, 24, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Fontelonga, T.M.; Jordan, B.; Nunes, A.M.; Barraza-Flores, P.; Bolden, N.; Wuebbles, R.D.; Griner, L.M.; Hu, X.; Ferrer, M.; Marugan, J.; et al. Sunitinib promotes myogenic regeneration and mitigates disease progression in the mdx mouse model of Duchenne muscular dystrophy. Hum. Mol. Genet. 2019, 28, 2120–2132. [Google Scholar] [CrossRef]
- Sorato, E.; Menazza, S.; Zulian, A.; Sabatelli, P.; Gualandi, F.; Merlini, L.; Bonaldo, P.; Canton, M.; Bernardi, P.; Di Lisa, F. Monoamine oxidase inhibition prevents mitochondrial dysfunction and apoptosis in myoblasts from patients with collagen VI myopathies. Free Radic. Biol. Med. 2014, 75, 40–47. [Google Scholar] [CrossRef]
- Vitiello, L.; Marabita, M.; Sorato, E.; Nogara, L.; Forestan, G.; Mouly, V.; Salviati, L.; Acosta, M.; Blaauw, B.; Canton, M. Drug repurposing for Duchenne muscular dystrophy: The monoamine oxidase B inhibitor safinamide Ameliorates the pathological phenotype in mdx mice and in myogenic cultures From DMD patients. Front. Physiol. 2018, 9, 1087–1096. [Google Scholar] [CrossRef]
- Youdim, M.B.H.; Edmondson, D.; Tipton, K.F. The therapeutic potential of monoamine oxidase inhibitors. Nat. Rev. Neurosci. 2006, 7, 295–309. [Google Scholar] [CrossRef]
- Buyse, G.M.; Goemans, N.; van den Hauwe, M.; Thijs, D.; de Groot, I.J.M.; Schara, U.; Ceulemans, B.; Meier, T.; Mertens, L. Idebenone as a novel, therapeutic approach for Duchenne muscular dystrophy: Results from a 12 month, double-blind, randomized placebo-controlled trial. Neuromuscul. Disord. 2011, 21, 396–405. [Google Scholar] [CrossRef]
- Buyse, G.M.; Goemans, N.; Van Den Hauwe, M.; Meier, T. Effects of glucocorticoids and idebenone on respiratory function in patients with duchenne muscular dystrophy. Pediatr. Pulmonol. 2013, 48, 912–920. [Google Scholar] [CrossRef]
- Buyse, G.M.; Van der Mieren, G.; Erb, M.; D’hooge, J.; Herijgers, P.; Verbeken, E.; Jara, A.; Van Den Bergh, A.; Mertens, L.; Courdier-Fruh, I.; et al. Long-term blinded placebo-controlled study of SNT-MC17/idebenone in the dystrophin deficient mdx mouse: Cardiac protection and improved exercise performance. Eur. Heart J. 2009, 30, 116–124. [Google Scholar] [CrossRef]
- Buyse, G.M.; Voit, T.; Schara, U.; Straathof, C.S.M.; D’Angelo, M.G.; Bernert, G.; Cuisset, J.M.; Finkel, R.S.; Goemans, N.; McDonald, C.M.; et al. Efficacy of idebenone on respiratory function in patients with Duchenne muscular dystrophy not using glucocorticoids (DELOS): A double-blind randomised placebo-controlled phase 3 trial. Lancet 2015, 385, 1748–1757. [Google Scholar] [CrossRef]
- McDonald, C.; Meier, T.; Voit, T.; Schara, U.; Straathof, C.; D’Angelo, M.; Bernert, G.; Cuisset, J.; Finkel, R.; Goemans, N.; et al. Idebenone reduces respiratory complications in patients with Duchenne muscular dystrophy. Neuromuscul. Disord. 2016, 26, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Mayer, O.H.; Leinonen, M.; Rummey, C.; Meier, T.; Buyse, G.M. Efficacy of Idebenone to Preserve Respiratory Function above Clinically Meaningful Thresholds for Forced Vital Capacity (FVC) in Patients with Duchenne Muscular Dystrophy. J. Neuromuscul. Dis. 2017, 4, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Head, S.I. Antioxidant therapy in a mouse model of Duchenne muscular dystrophy: Some promising results but with a weighty caveat. J. Physiol. 2017, 595, 7015. [Google Scholar] [CrossRef]
- Pinniger, G.J.; Terrill, J.R.; Assan, E.B.; Grounds, M.D.; Arthur, P.G. Pre-clinical evaluation of N-acetylcysteine reveals side effects in the mdx mouse model of Duchenne muscular dystrophy. J. Physiol. 2017, 595, 7093–7107. [Google Scholar] [CrossRef]
- O’Halloran, K.D.; Murphy, K.H.; Burns, D.P. Antioxidant therapy for muscular dystrophy: Caveat lector! J. Physiol. 2018, 596, 737–738. [Google Scholar] [CrossRef]
- Rando, T.A.; Disatnik, M.H.; Yu, Y.; Franco, A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul. Disord. 1998, 8, 14–21. [Google Scholar] [CrossRef]
- Disatnik, M.H.; Dhawan, J.; Yu, Y.; Beal, M.F.; Whirl, M.M.; Franco, A.; Rando, T.A. Evidence of oxidative stress in mdx mouse muscle: Studies of the pre-necrotic state. J. Neurol. Sci. 1998, 161, 77–84. [Google Scholar] [CrossRef]
- Irwin, W.A.; Bergamin, N.; Sabatelli, P.; Reggiani, C.; Megighian, A.; Merlini, L.; Braghetta, P.; Columbaro, M.; Volpin, D.; Bressan, G.M.; et al. Mitochondrial dysfunction and apoptosis in myopathic mice with collagen VI deficiency. Nat. Genet. 2003, 35, 367–371. [Google Scholar] [CrossRef]
- Palma, E.; Tiepolo, T.; Angelin, A.; Sabatelli, P.; Maraldi, N.M.; Basso, E.; Forte, M.A; Bernardi, P.; Bonaldo, P. Genetic ablation of cyclophilin D rescues mitochondrial defects and prevents muscle apoptosis in collagen VI myopathic mice. Hum. Mol. Genet. 2009, 18, 2024–2031. [Google Scholar] [CrossRef]
- Millay, D.P.; Sargent, M.A.; Osinska, H.; Baines, C.P.; Barton, E.R.; Vuagniaux, G.; Sweeney, H.L.; Robbins, J.; Molkentin, J.D. Genetic and pharmacologic inhibition of mitochondrial-dependent necrosis attenuates muscular dystrophy. Nat. Med. 2008, 14, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Menazza, S.; Blaauw, B.; Tiepolo, T.; Toniolo, L.; Braghetta, P.; Spolaore, B.; Reggiani, C.; Di Lisa, F.; Bonaldo, P.; Canton, M. Oxidative stress by monoamine oxidases is causally involved in myofiber damage in muscular dystrophy. Hum. Mol. Genet. 2010, 19, 4207–4215. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Neverova, I.; Menabò, R.; Van Eyk, J.; Di Lisa, F. Evidence of myofibrillar protein oxidation induced by postischemic reperfusion in isolated rat hearts. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H870–H877. [Google Scholar] [CrossRef] [PubMed]
- Canton, M.; Skyschally, A.; Menabò, R.; Boengler, K.; Gres, P.; Schulz, R.; Haude, M.; Erbel, R.; Di Lisa, F.; Heusch, G. Oxidative modification of tropomyosin and myocardial dysfunction following coronary microembolization. Eur. Heart J. 2006, 27, 875–881. [Google Scholar] [CrossRef]
- Canton, M.; Menazza, S.; Sheeran, F.L.; Polverino De Laureto, P.; Di Lisa, F.; Pepe, S. Oxidation of myofibrillar proteins in human heart failure. J. Am. Coll. Cardiol. 2011, 57, 300–309. [Google Scholar] [CrossRef]
- Finberg, J.P.M.; Rabey, J.M. Inhibitors of MAO-A and MAO-B in psychiatry and neurology. Front. Pharmacol. 2016, 7, 340–348. [Google Scholar] [CrossRef]
- Binda, C.; Newton-Vinson, P.; Hubálek, F.; Edmondson, D.E.; Mattevi, A. Structure of human monoamine oxidase B, a drug target for the treatment of neurological disorders. Nat. Struct. Biol. 2002, 9, 22–26. [Google Scholar] [CrossRef]
- Binda, C.; Li, M.; Hubalek, F.; Restelli, N.; Edmondson, D.E.; Mattevi, A. Insights into the mode of inhibition of human mitochondrial monoamine oxidase B from high-resolution crystal structures. Proc. Natl. Acad. Sci. USA 2003, 100, 9750–9755. [Google Scholar] [CrossRef]
- De Colibus, L.; Li, M.; Binda, C.; Lustig, A.; Edmondson, D.E.; Mattevi, A. Three-dimensional structure of human monoamine oxidase A (MAO A): Relation to the structures of rat MAO A and human MAO B. Proc. Natl. Acad. Sci. USA 2005, 102, 12684–12689. [Google Scholar] [CrossRef]
- Bello, L.; Hoffman, E.P.; Pegoraro, E. Dystrophinopathies. In Muscular Dystrophy Causes and Management; Angelini, C., Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 69–95. ISBN 978-1-62618-460-2. [Google Scholar]
- Kearney, M.; Orrell, R.W.; Fahey, M.; Pandolfo, M. Antioxidants and other pharmacological treatments for Friedreich ataxia. Cochrane Database Syst. Rev. 2009, 4. [Google Scholar] [CrossRef]
- Schiff, M.; Rustin, P. Idebenone in Friedreich ataxia and Leber’s hereditary optic neuropathy: Close mechanisms, similar therapy? Brain 2016, 139, e39. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Bitto, A.; Aguennouz, M.; Mazzeo, A.; Migliorato, A.; Polito, F.; Irrera, N.; Altavilla, D.; Vita, G.L.; Russo, M.; et al. Flavocoxid counteracts muscle necrosis and improves functional properties in mdx mice: A comparison study with methylprednisolone. Exp. Neurol. 2009, 220, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Messina, S.; Vita, G.L.; Licata, N.; Sframeli, M.; Bitto, A.; Distefano, M.G.; Barcellona, C.; La Rosa, M.; Romeo, S.; Ciranni, A.; et al. Pilot study of flavocoxid in ambulant DMD patients. Neuromuscul. Disord. 2014, 24, 825. [Google Scholar] [CrossRef]
- Aartsma-Rus, A.; Van Deutekom, J.C.T.; Fokkema, I.F.; Van Ommen, G.J.B.; Den Dunnen, J.T. Entries in the Leiden Duchenne muscular dystrophy mutation database: An overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve 2006, 34, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Kishor, A.; Fritz, S.E.; Hogg, J.R. Nonsense-mediated mRNA decay: The challenge of telling right from wrong in a complex transcriptome. Wiley Interdiscip. Rev. RNA 2019, 10, e1548. [Google Scholar] [CrossRef] [PubMed]
- Dabrowski, M.; Bukowy-Bieryllo, Z.; Zietkiewicz, E. Advances in therapeutic use of a drug-stimulated translational readthrough of premature termination codons. Mol. Med. 2018, 24, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wu, B.; Shah, S.N.; Lu, P.; Lu, Q. Aminoglycoside Enhances the Delivery of Antisense Morpholino Oligonucleotides In Vitro and in mdx Mice. Mol. Ther. Nucleic Acids 2019, 16, 663–674. [Google Scholar] [CrossRef]
- Baradaran-Heravi, A.; Balgi, A.D.; Zimmerman, C.; Choi, K.; Shidmoossavee, F.S.; Tan, J.S.; Bergeaud, C.; Krause, A.; Flibotte, S.; Shimizu, Y.; et al. Novel small molecules potentiate premature termination codon readthrough by aminoglycosides. Nucleic Acids Res. 2016, 44, 6583–6598. [Google Scholar] [CrossRef]
- Kayali, R.; Ku, J.M.; Khitrov, G.; Jung, M.E.; Prikhodko, O.; Bertoni, C. Read-through compound 13 restores dystrophin expression and improves muscle function in the mdx mouse model for Duchenne muscular dystrophy. Hum. Mol. Genet. 2012, 21, 4007–4020. [Google Scholar] [CrossRef]
- Melacini, P. Cardiac problems in DMD. In Muscular Dystrophy: Causes and Management; Angelini, C, Ed.; Nova Science Publishers, Inc.: Hauppauge, NY, USA, 2013; pp. 367–380. ISBN 978-1-62618-460-2. [Google Scholar]
- Hor, K.N.; Mah, M.L.; Johnston, P.; Cripe, T.P.; Cripe, L.H. Advances in the diagnosis and management of cardiomyopathy in Duchenne muscular dystrophy. Neuromuscul. Disord. 2018, 28, 711–716. [Google Scholar] [CrossRef]
- Birnkrant, D.J.; Bushby, K.; Bann, C.M.; Alman, B.A.; Apkon, S.D.; Blackwell, A.; Case, L.E.; Cripe, L.; Hadjiyannakis, S.; Olson, A.K.; et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: Respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018, 17, 347–361. [Google Scholar] [CrossRef]
- Sanders, S.P. Did they lower stress in the trial?: Or was it just wasted energy? J. Am. Coll. Cardiol. 2005, 45, 858–859. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Duboc, D.; Meune, C.; Lerebours, G.; Devaux, J.Y.; Vaksmann, G.; Bécane, H.M. Effect of perindopril on the onset and progression of left ventricular dysfunction in Duchenne muscular dystrophy. J. Am. Coll. Cardiol. 2005, 45, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Duboc, D.; Meune, C.; Pierre, B.; Wahbi, K.; Eymard, B.; Toutain, A.; Berard, C.; Vaksmann, G.; Bécane, H.M. Perindopril preserves left ventricular function in X-linked Duchenne muscular dystrophy. Eur. Hear. J. Suppl. 2007, 9, E20–E24. [Google Scholar] [CrossRef][Green Version]
- Matsumura, T. Beta-blockers in Children with Duchenne Cardiomyopathy. Rev. Recent Clin. Trials 2014, 9, 76–81. [Google Scholar] [CrossRef]
- Viollet, L.; Thrush, P.T.; Flanigan, K.M.; Mendell, J.R.; Allen, H.D. Effects of angiotensin-converting enzyme inhibitors and/or beta blockers on the cardiomyopathy in duchenne muscular dystrophy. Am. J. Cardiol. 2012, 110, 98–102. [Google Scholar] [CrossRef]
- Raman, S.V.; Hor, K.N.; Mazur, W.; Halnon, N.J.; Kissel, J.T.; He, X.; Tran, T.; Smart, S.; McCarthy, B.; Taylor, M.D.; et al. Eplerenone for early cardiomyopathy in Duchenne muscular dystrophy: A randomised, double-blind, placebo-controlled trial. Lancet Neurol. 2015, 14, 153–161. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- Shaddy, R.; Canter, C.; Halnon, N.; Kochilas, L.; Rossano, J.; Bonnet, D.; Bush, C.; Zhao, Z.; Kantor, P.; Burch, M.; et al. Design for the sacubitril/valsartan (LCZ696) compared with enalapril study of pediatric patients with heart failure due to systemic left ventricle systolic dysfunction (PANORAMA-HF study). Am. Heart J. 2017, 193, 23–34. [Google Scholar] [CrossRef]
- Buddhe, S.; Cripe, L.; Friedland-Little, J.; Kertesz, N.; Eghtesady, P.; Finder, J.; Hor, K.; Judge, D.P.; Kinnett, K.; McNally, E.M.; et al. Cardiac management of the patient with Duchenne muscular dystrophy. Pediatrics 2018, 142, S72–S81. [Google Scholar] [CrossRef]
- Koenig, M.; Hoffman, E.P.; Bertelson, C.J.; Monaco, A.P.; Feener, C.; Kunkel, L.M. Complete cloning of the duchenne muscular dystrophy (DMD) cDNA and preliminary genomic organization of the DMD gene in normal and affected individuals. Cell 1987, 50, 509–517. [Google Scholar] [CrossRef]
- Verhaart, I.E.C.; Aartsma-Rus, A. Therapeutic developments for Duchenne muscular dystrophy. Nat. Rev. Neurol. 2019, 15, 373–386. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).