QSAR Models for Predicting Five Levels of Cellular Accumulation of Lysosomotropic Macrocycles
Abstract
:1. Introduction
2. Results and Discussion
2.1. Experimental Determination of Intracellular Accumulation
- Quantification of compound concentration in cells by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS), which is a low throughput method, that is often expensive, requires larger sample, and is rather time consuming to adjust reliable and robust measurements. On the other hand, when validated, the method gives absolute quantification of accumulation (ACC), which can subsequently be used to divide compounds in different levels (classes), according to the intensity of accumulation (here denoted as ACC Class (Table 1)).
- Indirect evaluation of compound accumulation from the measured increase in lysosomal volume induced by the accumulating compound, performed by fluorescent cell microscopy (cell imaging). This method is high throughput, but less precise than the LC-MS/MS method, and can only give an estimation of the accumulation classified in five classes (denoted as LTR ACC Class). Still, this imaging method has an accuracy of 81% in predicting the actual ACC Class (measured by LC-MS/MS) [17].
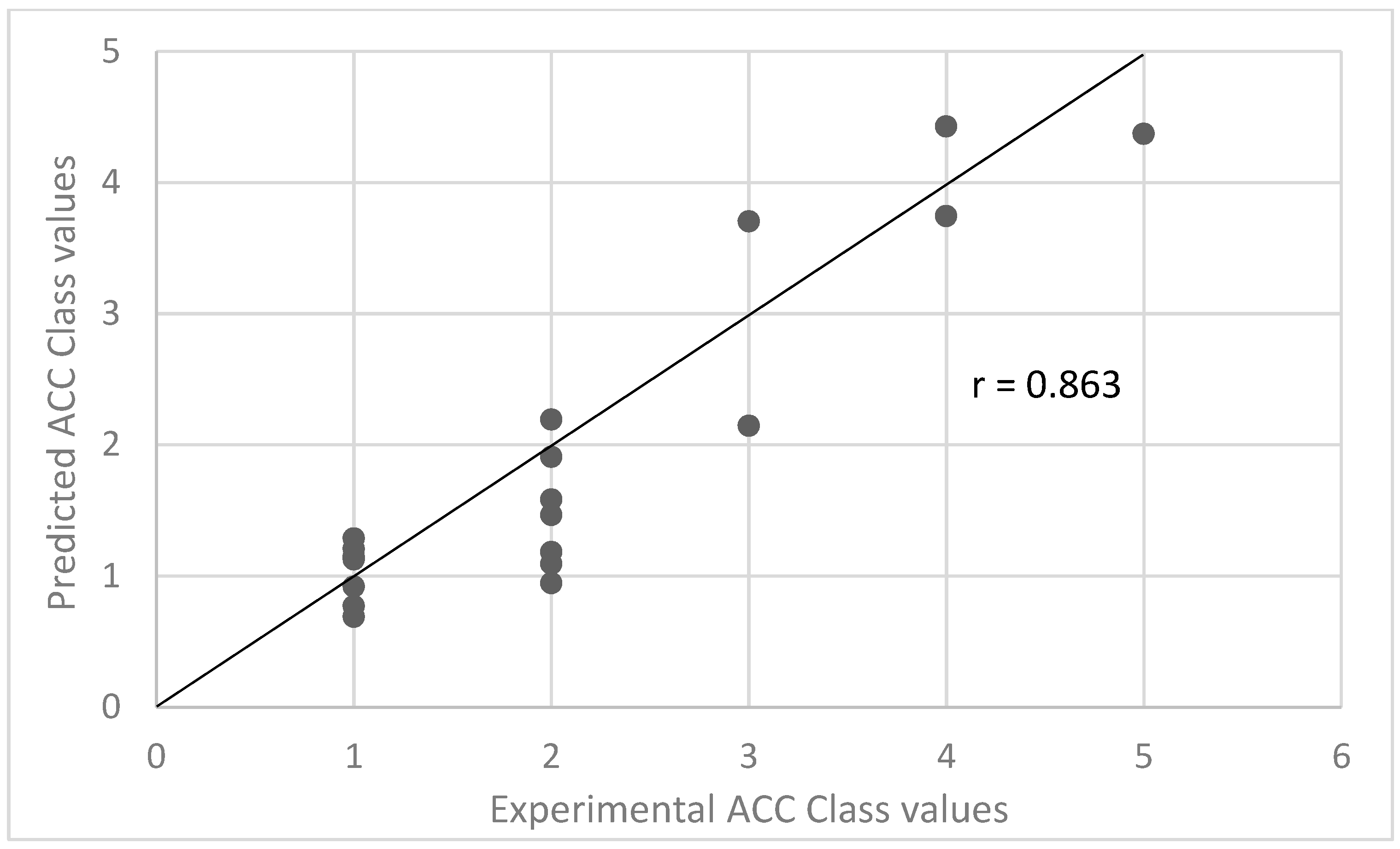
2.2. Prediction of Intracellular Accumulation from Molecular Structure
2.3. Enrichment of the QSAR Model for Intracellular Accumulation with Experimental Imaging Data
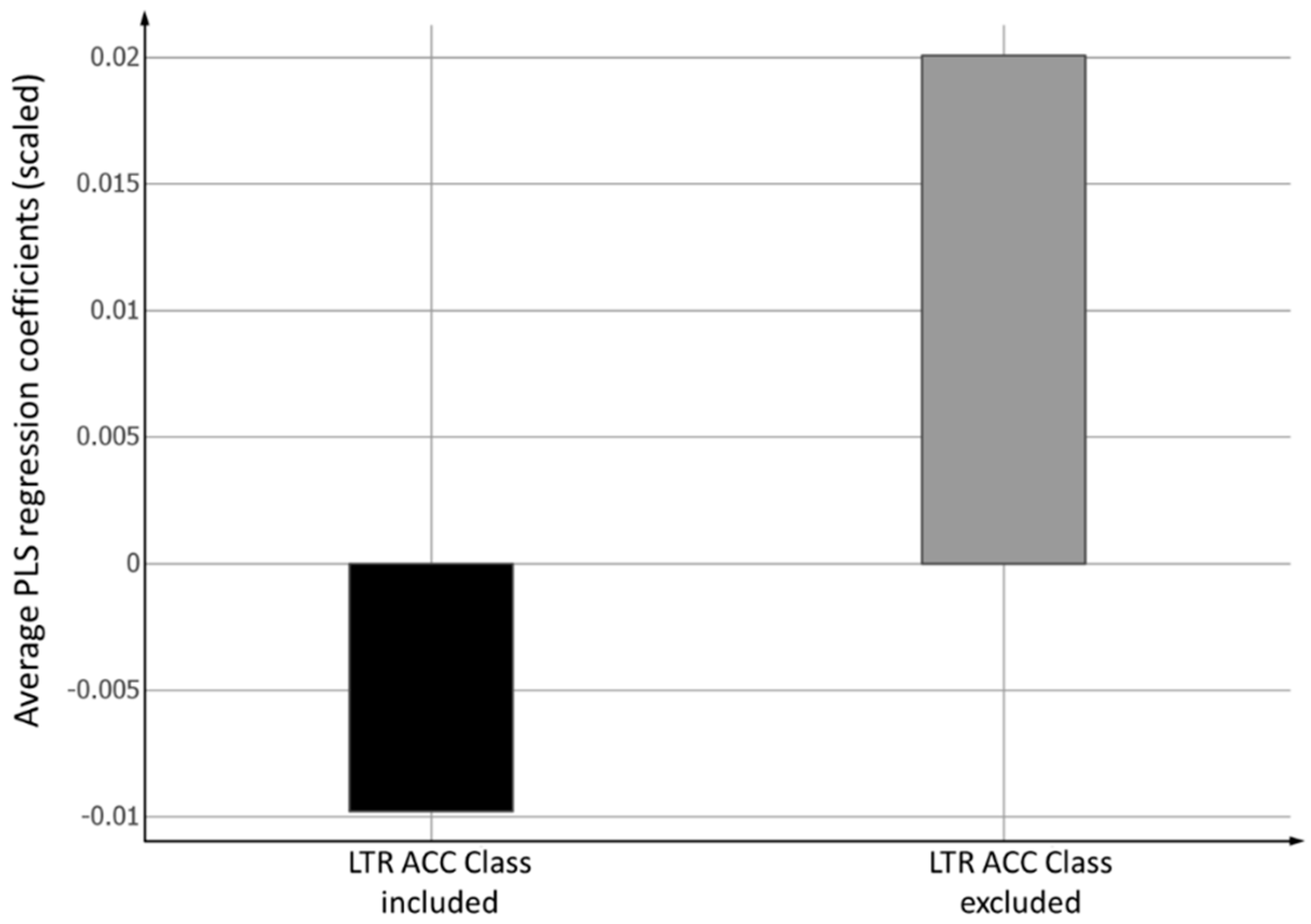
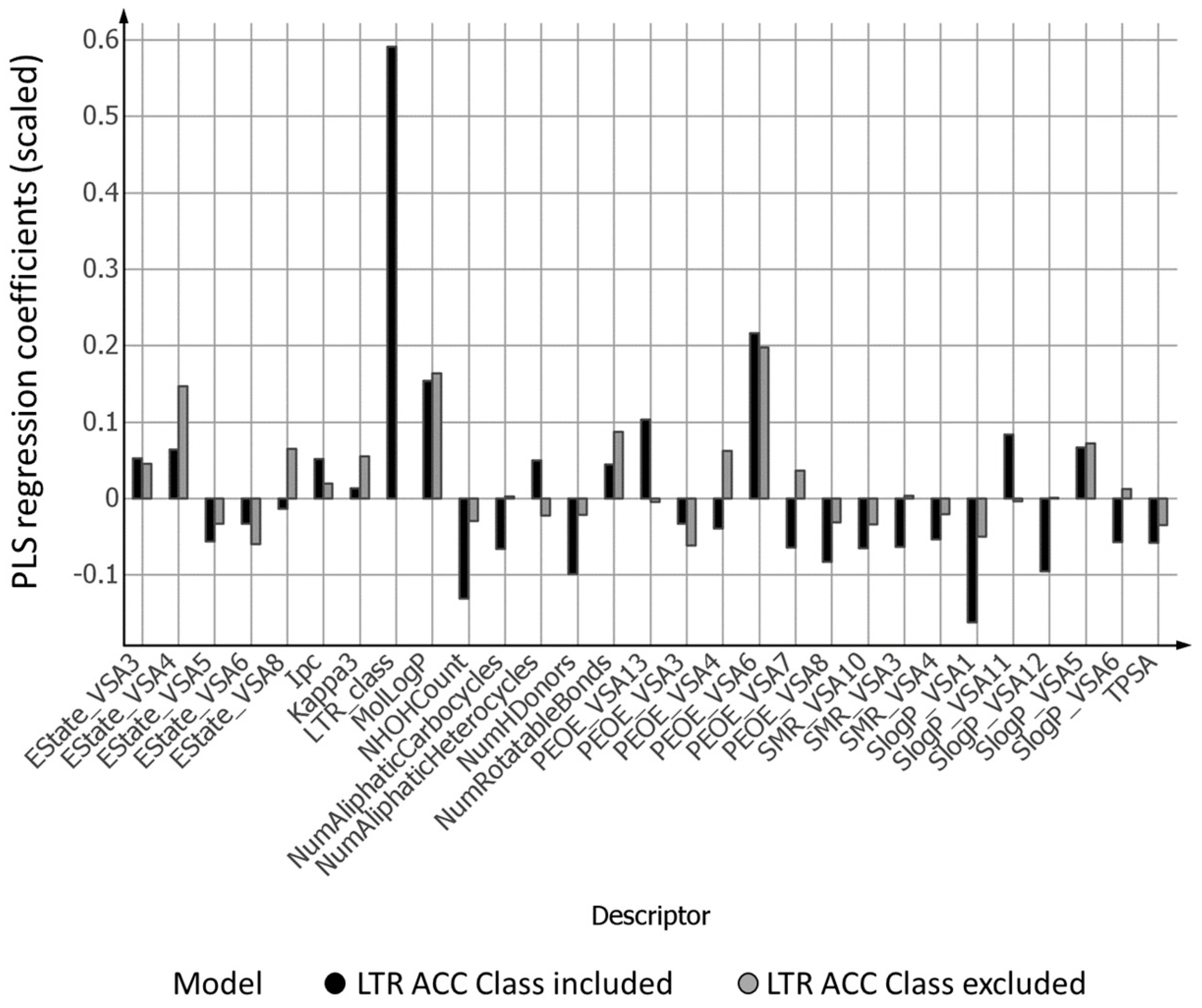
2.4. PLS Coefficients of the Two Models for the Prediction of Intracellular Accumulation
2.5. General Remarks
3. Materials and Methods
3.1. Compounds
3.2. Physicochemical Descriptors
3.3. Experimental Descriptor LTR ACC Class—the Indirect High Throughput Experimental Measure of Cellular Accumulation
3.4. Quantitative Structure-Activity Relationship Model (QSAR) Modeling
- OPLS intracellular accumulation Model 1 using both the 97 physiochemical descriptors and the experimental LTR ACC Class information for 47 compounds with existing experimental LTR ACC Class information. The model was used to predict intracellular accumulation as measured in cells using LC-MS/MS.
- OPLS intracellular accumulation Model 2 using only the 97 physiochemical descriptors for the same 47 compounds in Model 1. The model was used to predict intracellular accumulation as measured in cells using LC-MS/MS.
- OPLS LTR ACC Class Model 3 using only the 97 physiochemical descriptors for 47 compounds with experimental LTR ACC Class information. The model was used to predict the lysosomal volume change as measured in cells using cell imaging (parameter LTR ACC Class), and which was previously found to correlate with intracellular accumulation determined by LC-MS/MS.
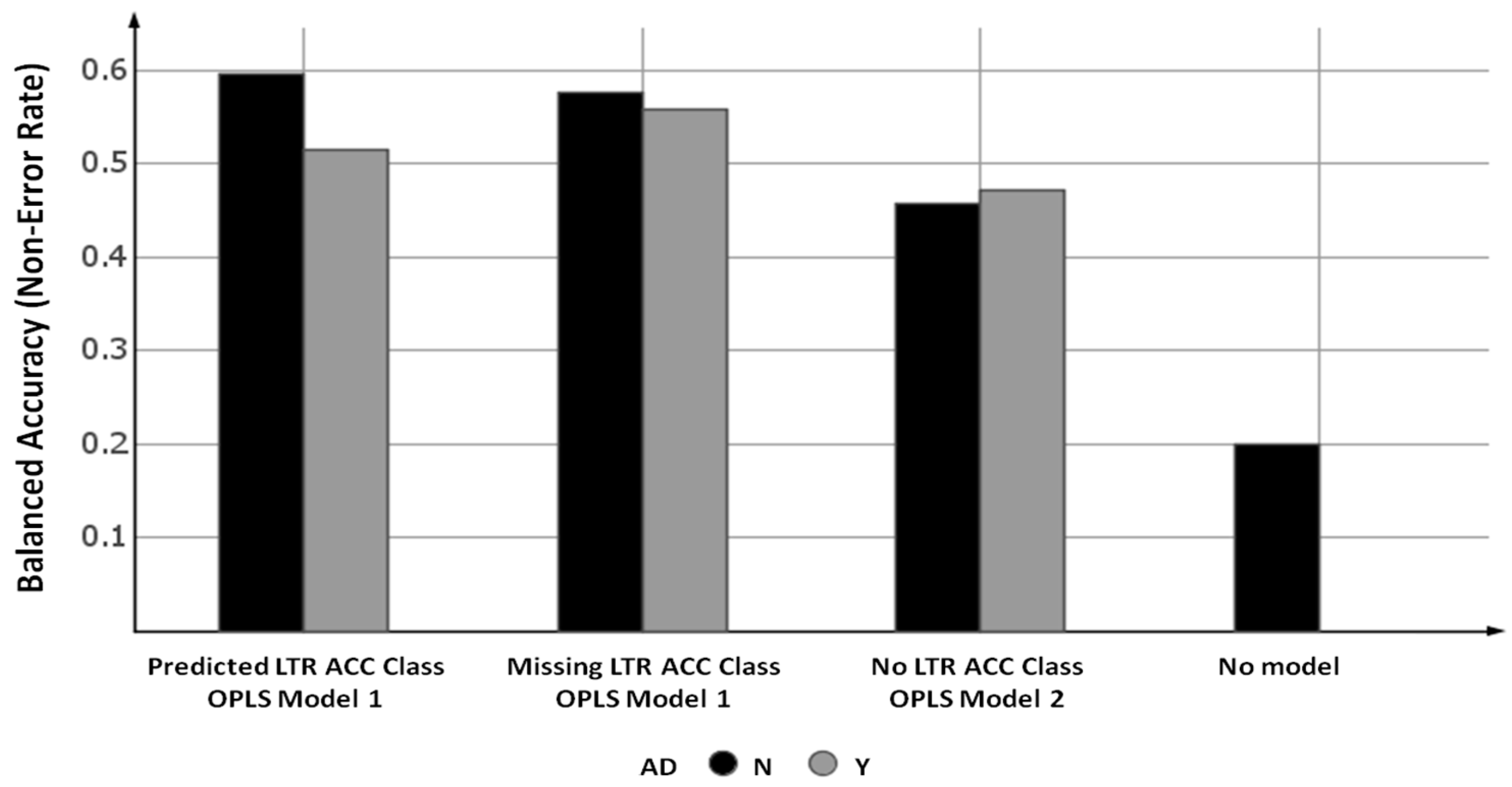
- From OPLS Model 1 where the LTR ACC Class descriptor was treated as ‘missing’ data (“missing LTR ACC class”).
- From OPLS Model 1 where the predicted values from OPLS LTR ACC Class Model 3 were used as the LTR ACC Class descriptor (“predicted LTR ACC class”).
- From OPLS Model 2 (“no LTR ACC class”).
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | Accumulation of compounds in cells expressed as either I/E or % of the accumulation of the reference compound azithromycin |
| ACC Class | Class (level) of accumulation (determined from LC-MS/MS data on ACC) |
| AD | Applicability domain |
| ADME | Absorption distribution metabolism and excretion |
| AZI | Azithromycin, a macrocycle antibiotic |
| CAD | Cationic amphiphilic drug |
| I/E | Intracellular to extracellular concentration ratio; a measure of accumulation |
| LC-MS/MS | Liquid chromatography coupled to tandem mass spectrometry |
| LTR ACC Class | Class (level) of accumulation (determined by an indirect method of cell imaging of lysosome volume change) |
| OPLS | Orthogonal projections to latent structures |
| QSAR | Quantitative structure-activity relationship model |
References
- Yokogawa, K.; Ishizaki, J.; Ohkuma, S.; Miyamoto, K. Influence of lipophilicity and lysosomal accumulation on tissue distribution kinetics of basic drugs: A physiologically based pharmacokinetic model. Methods Find Exp. Clin. Pharm. 2002, 24, 81–93. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C.; de Barsy, T.; Poole, B.; Trouet, A.; Tulkens, P.; Van Hoof, F. Commentary. Lysosomotropic agents. Biochem. Pharm. 1974, 23, 2495–2531. [Google Scholar] [CrossRef]
- Kazmi, F.; Hensley, T.; Pope, C.; Funk, R.S.; Loewen, G.J.; Buckley, D.B.; Parkinson, A. Lysosomal sequestration (trapping) of lipophilic amine (cationic amphiphilic) drugs in immortalized human hepatocytes (Fa2N-4 cells). Drug Metab. Dispos. 2013, 41, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Amsden, G.W. Advanced-generation macrolides: Tissue-directed antibiotics. Int. J. Antimicrob. Agents. 2001, 18, S11–S15. [Google Scholar] [CrossRef]
- Parnham, M.J.; Erakovic Haber, V.; Giamarellos-Bourboulis, E.J.; Perletti, G.; Verleden, G.M.; Vos, R. Azithromycin: Mechanisms of action and their relevance for clinical applications. Pharm. Ther. 2014, 143, 225–245. [Google Scholar] [CrossRef] [PubMed]
- Reasor, M.J.; Kacew, S. Drug-induced phospholipidosis: Are there functional consequences? Exp. Biol. Med. (Maywood). 2001, 226, 825–830. [Google Scholar] [CrossRef]
- Munic, V.; Banjanac, M.; Kostrun, S.; Nujic, K.; Bosnar, M.; Marjanovic, N.; Ralic, J.; Matijasic, M.; Hlevnjak, M.; Erakovic Haber, V. Intensity of macrolide anti-inflammatory activity in J774A.1 cells positively correlates with cellular accumulation and phospholipidosis. Pharm. Res. 2011, 64, 298–307. [Google Scholar] [CrossRef]
- Goracci, L.; Ceccarelli, M.; Bonelli, D.; Cruciani, G. Modeling phospholipidosis induction: Reliability and warnings. J. Chem. Inf. Model. 2013, 53, 1436. [Google Scholar] [CrossRef]
- Rodgers, T.; Rowland, M. Mechanistic Approaches to Volume of Distribution Predictions: Understanding the Processes. Pharm. Res. 2007, 24, 918–933. [Google Scholar] [CrossRef]
- Schmitt, W. General approach for the calculation of tissue to plasma partition coefficients. Toxicol. Vitr. 2008, 22, 457. [Google Scholar] [CrossRef]
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014, 21, 1115. [Google Scholar] [CrossRef]
- Over, B.; Matsson, P.; Tyrchan, C.; Artursson, P.; Doak, B.C.; Foley, M.A.; Hilgendorf, C.; Johnston, S.E.; Lee, M.D., IV; Lewis, R.L.; et al. Structural and conformational determinants of macrocycle cell permeability. Nat. Chem. Biol. 2016, 12, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Giordanetto, F.; Kihlberg, J. Macrocyclic drugs and clinical candidates: What can medicinal chemists learn from their properties? J. Med. Chem. 2014, 57, 278–295. [Google Scholar] [CrossRef] [PubMed]
- Zin, P.P.K.; Williams, G.; Fourches, D. Cheminformatics-based enumeration and analysis of large libraries of macrolide scaffolds. J. Cheminform. 2018, 10, 53. [Google Scholar] [CrossRef] [PubMed]
- Stepanic, V.; Kostrun, S.; Malnar, I.; Hlevnjak, M.; Butkovic, K.; Caleta, I.; Duksi, M.; Kragol, G.; Makaruha-Stegic, O.; Mikac, L.; et al. Modeling cellular pharmacokinetics of 14- and 15-membered macrolides with physicochemical properties. J. Med. Chem. 2011, 54, 719–733. [Google Scholar] [CrossRef]
- Koštrun, S.; Munic Kos, V.; Matanović Škugor, M.; Palej Jakopović, I.; Malnar, I.; Dragojević, S.; Ralić, J.; Alihodžić, S. Around the macrolide—Impact of 3D structure of macrocycles on lipophilicity and cellular accumulation. Eur. J. Med. Chem. 2017, 133, 351–364. [Google Scholar] [CrossRef] [PubMed]
- Easwaranathan, A.; Inci, B.; Ulrich, S.; Brunken, L.; Nikiforova, V.; Norinder, U.; Swanson, S.; Munic Kos, V. Quantification of intracellular accumulation and retention of lysosomotropic macrocyclic compounds by high-throughput imaging of lysosomal changes. J. Pharm. Sci. 2019, 108, 652–660. [Google Scholar] [CrossRef]
- Fay, M.P. Exact McNemar’s Test and Matching Confidence Intervals. Available online: https://cran.r-project.org/web/packages/exact2 × 2/vignettes/exactMcNemar.pdf (accessed on 25 April 2016).
- Labute, P. A widely applicable set of descriptors. J. Mol. Graph. Model. 2000, 18, 464–477. [Google Scholar] [CrossRef]
- RDKit, Open-Source Cheminformatics and Machine Learning. Available online: https://sourceforge.net/p/rdkit/code/2416/tree/trunk/rdkit/Chem/MolSurf.py (accessed on 10 November 2019).
- Sanchez Garcia, D.; Sjödin, M.; Hellstrandh, M.; Norinder, U.; Nikiforova, V.; Lindberg, J.; Wincent, E.; Bergman, Å.; Cotgreave, I.; Munic Kos, V. Cellular accumulation and lipid binding of perfluorinated alkylated substances (PFASs)—A comparison with lysosomotropic drugs. Chem. Biol. Interact. 2018, 281, 1–10. [Google Scholar] [CrossRef]
- Sadowski, J.; Gasteiger, J.; Klebe, G. Comparison of automatic three-dimensional model builders using 639 X-ray structures. J. Chem. Inf. Comput. Sci. 1994, 34, 1000–1008. [Google Scholar] [CrossRef]
- Trygg, J.; Wold, S. Orthogonal projections to latent structures (OPLS). J. Chemom. 2002, 16, 119–128. [Google Scholar] [CrossRef]
- Ballabio, D.; Grisoni, F.; Todeschini, R. Multivariate comparison of classification performance measures. Chemom. Intell. Lab. Syst. 2018, 174, 33–44. [Google Scholar] [CrossRef]
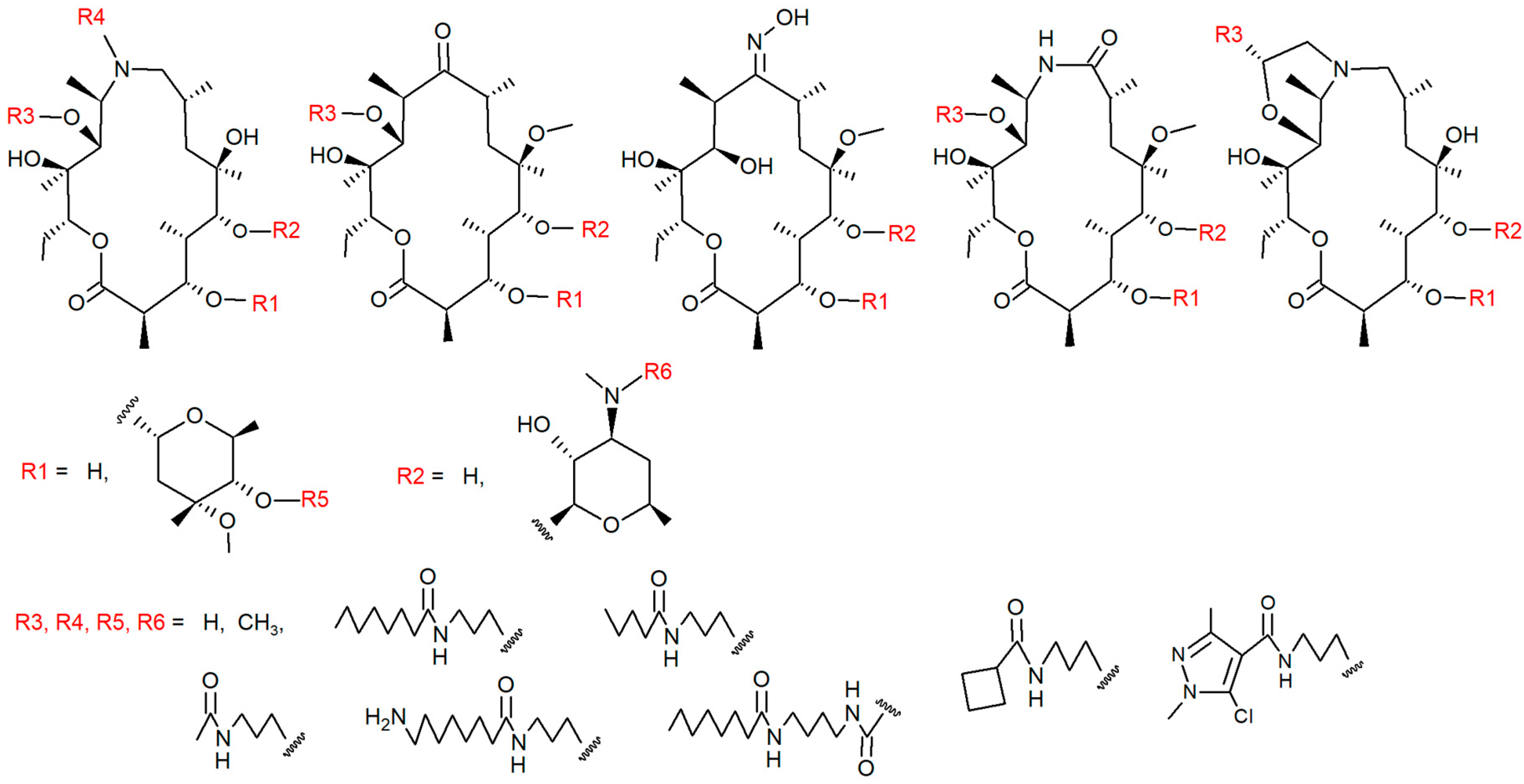

| ACC Class | I/E | % AZI | Accumulation | No of cpds Per class a | No of cpds Per Class b |
|---|---|---|---|---|---|
| 1 | 0–7 | 0–14% | No/low | 22 | 14 |
| 2 | 7.5–33 | 15–66% | Moderate | 23 | 10 |
| 3 | 33.5–92 | 67–184% | High | 14 | 7 |
| 4 | 92.5–220 | 185–440% | Very high | 9 | 11 |
| 5 | >220 | >440% | Extremely high | 7 | 5 |
| OPLS Model | Predicted Endpoint | Name of the Dependent (Predicted) Parameter | No. of Classes of the Dependent (Predicted) Parameter | Descriptors Used in the Model a |
|---|---|---|---|---|
| Model 1 | Intracellular accumulation measured by LC-MS/MS | ACC Class | 5 | ● 97 calculated physicochemical descriptors ● Experimentally measured LTR ACC Class |
| Model 2 | Intracellular accumulation measured by LC-MS/MS | ACC Class | 5 | ● 97 calculated physicochemical descriptors |
| Model 3 | Intracellular accumulation measured by cell imaging | LTR ACC Class | 5 | ● 97 calculated physicochemical descriptors |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Norinder, U.; Munic Kos, V. QSAR Models for Predicting Five Levels of Cellular Accumulation of Lysosomotropic Macrocycles. Int. J. Mol. Sci. 2019, 20, 5938. https://doi.org/10.3390/ijms20235938
Norinder U, Munic Kos V. QSAR Models for Predicting Five Levels of Cellular Accumulation of Lysosomotropic Macrocycles. International Journal of Molecular Sciences. 2019; 20(23):5938. https://doi.org/10.3390/ijms20235938
Chicago/Turabian StyleNorinder, Ulf, and Vesna Munic Kos. 2019. "QSAR Models for Predicting Five Levels of Cellular Accumulation of Lysosomotropic Macrocycles" International Journal of Molecular Sciences 20, no. 23: 5938. https://doi.org/10.3390/ijms20235938
APA StyleNorinder, U., & Munic Kos, V. (2019). QSAR Models for Predicting Five Levels of Cellular Accumulation of Lysosomotropic Macrocycles. International Journal of Molecular Sciences, 20(23), 5938. https://doi.org/10.3390/ijms20235938





