Bacteriotherapy in Breast Cancer
Abstract
1. Introduction
2. Bacteriotherapy and Enhancing of Human Immunity
3. Anticancer Substances Released by Bacteria
3.1. Bacteriocins in Cancer Therapy
3.1.1. Bovicin HC5
3.1.2. Colicins
3.1.3. Laterosporulin 10
3.1.4. Nisin A
3.2. Bacterial Peptides in Cancer Therapy
3.2.1. Ohmyungsamycins A and B
3.2.2. Azurin
3.2.3. Pep27anal2
3.2.4. Entap
3.2.5. Proximicins
3.2.6. Urukthapelstatin A
3.3. Bacterial Toxins in Cancer Therapy
3.3.1. Diphtheria Toxin
3.3.2. Botulinum Neurotoxin Type A
3.3.3. Exotoxin A
3.3.4. Exotoxin T
3.3.5. Hyaluronidase (HylP)
4. Bacteria as a Carrier for Cancer Therapeutic Agents
4.1. Salmonella Typhimurium
4.2. Listeria Monocytogenes
4.3. Bifidobacterium Longum
4.4. Lactobacillus
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Darbre, P.D.; Fernandez, M.F. Environmental oestrogens and breast cancer: Long-term low-dose effects of mixtures of various chemical combinations. J. Epidemiol. Community Health 2013, 67, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.B.; Steindorf, K.; Hein, R.; Flesch-Janys, D.; Chang-Claude, J. Population attributable risk of invasive postmenopausal breast cancer and breast cancer subtypes for modifiable and non-modifiable risk factors. Cancer Epidemiol. 2011, 35, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Song, S.; Vuai, M.S.; Zhong, M. The role of bacteria in cancer therapy–enemies in the past, but allies at present. Infect. Agents Cancer 2018, 13, 9. [Google Scholar] [CrossRef]
- Langie, S.A.; Koppen, G.; Desaulniers, D.; Al-Mulla, F.; Al-Temaimi, R.; Amedei, A.; Azqueta, A.; Bisson, W.H.; Brown, D.; Brunborg, G. Causes of genome instability: The effect of low dose chemical exposures in modern society. Carcinogenesis 2015, 36, S61–S88. [Google Scholar] [CrossRef]
- Kuno, T.; Tsukamoto, T.; Hara, A.; Tanaka, T. Cancer chemoprevention through the induction of apoptosis by natural compounds. J. Biophys. Chem. 2012, 3, 156–173. [Google Scholar] [CrossRef]
- Lax, A.J. Opinion: Bacterial toxins and cancer—A case to answer? Nat. Rev. Microbiol. 2005, 3, 343–349. [Google Scholar] [CrossRef]
- Caygill, C.P.; Braddick, M.; Hill, M.J.; Knowles, R.L.; Sharp, J.C. The association between typhoid carriage, typhoid infection and subsequent cancer at a number of sites. Eur. J. Cancer Prev. 1995, 4, 187–193. [Google Scholar] [CrossRef]
- Lecuit, M.; Abachin, E.; Martin, A.; Poyart, C.; Pochart, P.; Suarez, F.; Bengoufa, D.; Feuillard, J.; Lavergne, A.; Gordon, J.I.; et al. Immunoproliferative small intestinal disease associated with campylobacter jejuni. N. Engl. J. Med. 2004, 350, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.K.; Tripathi, D.; Kulkarni, S.; Rajan, M.G. Mycobacterium tuberculosis H37Rv infected thp-1 cells induce epithelial mesenchymal transition (EMT) in lung adenocarcinoma epithelial cell line (A549). Cell. Immunol. 2016, 300, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Nath, G.; Gulati, A.K.; Shukla, V.K. Role of bacteria in carcinogenesis, with special reference to carcinoma of the gallbladder. World J. Gastroenterol. 2010, 16, 5395. [Google Scholar] [CrossRef] [PubMed]
- Rosadi, F.; Fiorentini, C.; Fabbri, A. Bacterial protein toxins in human cancers. Pathog. Dis. 2016, 74, ftv105. [Google Scholar] [CrossRef]
- De Spiegeleer, B.; Verbeke, F.; D’Hondt, M.; Hendrix, A.; Van De Wiele, C.; Burvenich, C.; Peremans, K.; De Wever, O.; Bracke, M.; Wynendaele, E. The quorum sensing peptides phrg, csp and edf promote angiogenesis and invasion of breast cancer cells in vitro. PLoS ONE 2015, 10, e0119471. [Google Scholar] [CrossRef]
- Roberts, N.J.; Zhang, L.; Janku, F.; Collins, A.; Bai, R.-Y.; Staedtke, V.; Rusk, A.W.; Tung, D.; Miller, M.; Roix, J.; et al. Intratumoral injection of clostridium novyi-nt spores induces antitumor responses. Sci. Transl. Med. 2014, 6, 249ra111. [Google Scholar] [CrossRef]
- Fujimori, M. Genetically engineered bifidobacterium as a drug delivery system for systemic therapy of metastatic breast cancer patients. Breast Cancer 2006, 13, 27–31. [Google Scholar] [CrossRef]
- Zhao, M.; Yang, M.; Ma, H.; Li, X.; Tan, X.; Li, S.; Yang, Z.; Hoffman, R.M. Targeted therapy with a salmonella typhimurium leucine-arginine auxotroph cures orthotopic human breast tumors in nude mice. Cancer Res. 2006, 66, 7647–7652. [Google Scholar] [CrossRef]
- Nauts, H.C.; Swift, W.E.; Coley, B.L. The treatment of malignant tumors by bacterial toxins as developed by the late William B. Coley, M.D., reviewed in the light of modern research. Cancer Res. 1946, 6, 205–216. [Google Scholar]
- Wiemann, B.; Starnes, C.O. Coley’s toxins, tumor necrosis factor and cancer research: A historical perspective. Pharmacol. Ther. 1994, 64, 529–564. [Google Scholar] [CrossRef]
- McCarthy, E.F. The toxins of William B. Coley and the treatment of bone and soft-tissue sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar] [PubMed]
- Gontero, P.; Bohle, A.; Malmstrom, P.-U.; O’Donnell, M.A.; Oderda, M.; Sylvester, R.; Witjes, F. The role of bacillus calmette-guérin in the treatment of non-muscle-invasive bladder cancer. Eur. Urol. 2010, 57, 410–429. [Google Scholar] [CrossRef] [PubMed]
- Zlotta, A.R.; Fleshner, N.E.; Jewett, M.A. The management of BCG failure in non-muscle-invasive bladder cancer: An update. Can. Urol. Assoc. J. 2009, 3, S199–S205. [Google Scholar] [CrossRef] [PubMed]
- Danino, T.; Prindle, A.; Hasty, J.; Bhatia, S. Measuring growth and gene expression dynamics of tumor-targeted S. typhimurium bacteria. J. Vis. Exp. 2013, 77, e50540. [Google Scholar] [CrossRef] [PubMed]
- Alatrash, G.; Jakher, H.; Stafford, P.D.; Mittendorf, E.A. Cancer immunotherapies, their safety and toxicity. Expert Opin. Drug Saf. 2013, 12, 631–645. [Google Scholar] [CrossRef]
- Micallef, I.N.; Stiff, P.J.; Nademanee, A.P.; Maziarz, R.T.; Horwitz, M.E.; Stadtmauer, E.A.; Kaufman, J.L.; McCarty, J.M.; Vargo, R.; Cheverton, P.D. Plerixafor plus granulocyte colony-stimulating factor for patients with non-hodgkin lymphoma and multiple myeloma: Long-term follow-up report. Biol. Blood Marrow Transplant. 2018, 24, 1187–1195. [Google Scholar] [CrossRef]
- Perera, L.P.; Zhang, M.; Nakagawa, M.; Petrus, M.N.; Maeda, M.; Kadin, M.E.; Waldmann, T.A.; Perera, P.Y. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am. J. Hematol. 2017, 92, 892–901. [Google Scholar] [CrossRef]
- Zlotnik, A. Chemokines and cancer. Int. J. Cancer 2006, 119, 2026–2029. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Toldra, J.; Lloris, R.; Carceller, V. American Association for Cancer Research. In Proceedings of the American Association for Cancer Research (AACR)-110th Annual Meeting, Atlanta, GA, USA, 29 March–3 April 2019; pp. 0377–8282. [Google Scholar]
- Chiriva-Internati, M.; Bot, A. A new era in cancer immunotherapy: Discovering novel targets and reprogramming the immune system. Int. Rev. Immunol. 2015, 34, 101–103. [Google Scholar] [CrossRef]
- Zugazagoitia, J.; Guedes, C.; Ponce, S.; Ferrer, I.; Molina-Pinelo, S.; Paz-Ares, L. Current challenges in cancer treatment. Clin. Ther. 2016, 38, 1551–1566. [Google Scholar] [CrossRef] [PubMed]
- Weiner, L.M. Cancer immunology for the clinician. Clin. Adv. Hematol. 2015, 13, 299–306. [Google Scholar]
- Tartari, F.; Santoni, M.; Burattini, L.; Mazzanti, P.; Onofri, A.; Berardi, R. Economic sustainability of anti-pd-1 agents nivolumab and pembrolizumab in cancer patients: Recent insights and future challenges. Cancer Treat. Rev. 2016, 48, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.; Kasnitz, N.; Kocijancic, D.; Trittel, S.; Riese, P.; Guzman, C.A.; Leschner, S.; Weiss, S. Induction of CD4(+) and CD8(+) anti-tumor effector T cell responses by bacteria mediated tumor therapy. Int. J. Cancer 2015, 137, 2019–2028. [Google Scholar] [CrossRef] [PubMed]
- Na, H.S.; Lee, H.C.; Hong, Y.; Rhee, J.H.; Choy, H.E. Immune response induced by salmonella typhimurium defective in ppgpp synthesis. Vaccine 2006, 24, 2027–2034. [Google Scholar] [CrossRef]
- Phan, T.X.; Nguyen, V.H.; Duong, M.T.; Hong, Y.; Choy, H.E.; Min, J.J. Activation of inflammasome by attenuated salmonella typhimurium in bacteria-mediated cancer therapy. Microbiol. Immunol. 2015, 59, 664–675. [Google Scholar] [CrossRef]
- Lee, H.J.; Kim, H.Y. Lantibiotics, class I bacteriocins from the genus bacillus. J. Microbiol. Biotechnol. 2011, 21, 229–235. [Google Scholar]
- Sahl, H.-G.; Bierbaum, G. Lantibiotics: Biosynthesis and biological activities of uniquely modified peptides from gram-positive bacteria. Annu. Rev. Microbiol. 1998, 52, 41–79. [Google Scholar] [CrossRef]
- Kawai, Y.; Kemperman, R.; Kok, J.; Saito, T. The circular bacteriocins gassericin A and circularin A. Curr. Protein Pept. Sci. 2004, 5, 393–398. [Google Scholar] [CrossRef]
- Van Belkum, M.J.; Martin-Visscher, L.A.; Vederas, J.C. Structure and genetics of circular bacteriocins. Trends Microbiol. 2011, 19, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Karpiński, T.; Szkaradkiewicz, A.; Gamian, A. New enterococcal anticancer peptide. In Proceedings of the 23rd European Congress of Clinical Microbiology and Infectious Diseases, Berlin, Germany, 27–30 April 2013; p. 30. [Google Scholar]
- Kaur, S.; Kaur, S. Bacteriocins as potential anticancer agents. Front. Pharmacol. 2015, 6, 272. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.D.; de Oliveira, M.D.; de Paula, S.O.; Baracat-Pereira, M.C.; Breukink, E.; Mantovani, H.C. Toxicity of bovicin HC5 against mammalian cell lines and the role of cholesterol in bacteriocin activity. Microbiology 2012, 158, 2851–2858. [Google Scholar] [CrossRef] [PubMed]
- Chumchalova, J.; Šmarda, J. Human tumor cells are selectively inhibited by colicins. Folia Microbiol. 2003, 48, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Baindara, P.; Gautam, A.; Raghava, G.; Korpole, S. Anticancer properties of a defensin like class IID bacteriocin laterosporulin10. Sci. Rep. 2017, 7, 46541. [Google Scholar] [CrossRef]
- Begde, D.; Bundale, S.; Mashitha, P.; Rudra, J.; Nashikkar, N.; Upadhyay, A. Immunomodulatory efficacy of nisin—A bacterial lantibiotic peptide. J. Pept. Sci. 2011, 17, 438–444. [Google Scholar] [CrossRef]
- Joo, N.E.; Ritchie, K.; Kamarajan, P.; Miao, D.; Kapila, Y.L. Nisin, an apoptogenic bacteriocin and food preservative, attenuates HNSCC tumorigenesis via CHAC 1. Cancer Med. 2012, 1, 295–305. [Google Scholar] [CrossRef]
- Um, S.; Choi, T.J.; Kim, H.; Kim, B.Y.; Kim, S.-H.; Lee, S.K.; Oh, K.-B.; Shin, J.; Oh, D.-C. Ohmyungsamycins A and B: Cytotoxic and antimicrobial cyclic peptides produced by streptomyces sp. from a volcanic island. J. Org. Chem. 2013, 78, 12321–12329. [Google Scholar] [CrossRef]
- Mohamed, M.S.; Fattah, S.A.; Mostafa, H.M. Azurin as antitumor protein and its effect on the cancer cell lines. Curr. Res. J. Biol. Sci. 2010, 2, 396–401. [Google Scholar]
- Gao, M.; Zhou, J.; Su, Z.; Huang, Y. Bacterial cupredoxin azurin hijacks cellular signaling networks: Protein-protein interactions and cancer therapy. Protein Sci. 2017, 26, 2334–2341. [Google Scholar] [CrossRef]
- Sung, W.S.; Park, Y.; Choi, C.-H.; Hahm, K.-S.; Lee, D.G. Mode of antibacterial action of a signal peptide, Pep27 from streptococcus pneumoniae. Biochem. Biophys. Res. Commun. 2007, 363, 806–810. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Hahm, K.-S.; Park, Y.; Kim, H.-Y.; Lee, W.; Lim, S.-C.; Seo, Y.-K.; Choi, C.-H. Functional and structural characteristics of anticancer peptide Pep27 analogues. Cancer Cell Int. 2005, 5, 21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Karpinski, T.M.; Szkaradkiewicz, A.K. Anti-cancer peptides from bacteria. Bangladesh J. Pharmacol. 2013, 8, 343–348. [Google Scholar] [CrossRef]
- Karpiński, T. New Peptide (Entap) with Anti-proliferative Activity Produced by Bacteria of Enterococcus Genus. Post-Doctoral Thesis, Habilitation Thesis, Poznań University of Medical Sciences, Poznań, Poland, 2012. [Google Scholar]
- Fiedler, H.P.; Bruntner, C.; Riedlinger, J.; Bull, A.T.; Knutsen, G.; Goodfellow, M.; Jones, A.; Maldonado, L.; Pathom-aree, W.; Beil, W.; et al. Proximicin A, B and C, novel aminofuran antibiotic and anticancer compounds isolated from marine strains of the actinomycete verrucosispora. J. Antibiot. (Tokyo) 2008, 61, 158–163. [Google Scholar] [CrossRef]
- Matsuo, Y.; Kanoh, K.; Yamori, T.; Kasai, H.; Katsuta, A.; Adachi, K.; Shin-Ya, K.; Shizuri, Y. Urukthapelstatin A, a novel cytotoxic substance from marine-derived mechercharimyces asporophorigenens YM11-542. J. Antibiot. (Tokyo) 2007, 60, 256–260. [Google Scholar] [CrossRef]
- Yamada, T.; Goto, M.; Punj, V.; Zaborina, O.; Chen, M.L.; Kimbara, K.; Majumdar, D.; Cunningham, E.; Das Gupta, T.K.; Chakrabarty, A.M. Bacterial redox protein azurin, tumor suppressor protein p53, and regression of cancer. Proc. Natl. Acad. Sci. USA 2002, 99, 14098–14103. [Google Scholar] [CrossRef]
- Jia, L.; Gorman, G.S.; Coward, L.U.; Noker, P.E.; McCormick, D.; Horn, T.L.; Harder, J.B.; Muzzio, M.; Prabhakar, B.; Ganesh, B.; et al. Preclinical pharmacokinetics, metabolism, and toxicity of azurin-p28 (nsc745104) a peptide inhibitor of p53 ubiquitination. Cancer Chemother. Pharmacol. 2011, 68, 513–524. [Google Scholar] [CrossRef]
- Mehta, R.R.; Yamada, T.; Taylor, B.N.; Christov, K.; King, M.L.; Majumdar, D.; Lekmine, F.; Tiruppathi, C.; Shilkaitis, A.; Bratescu, L.; et al. A cell penetrating peptide derived from azurin inhibits angiogenesis and tumor growth by inhibiting phosphorylation of VEGFR-2, FAK and AKT. Angiogenesis 2011, 14, 355–369. [Google Scholar] [CrossRef]
- Bernardes, N.; Ribeiro, A.S.; Abreu, S.; Mota, B.; Matos, R.G.; Arraiano, C.M.; Seruca, R.; Paredes, J.; Fialho, A.M. The bacterial protein azurin impairs invasion and FAK/ Src signaling in P-cadherin-overexpressing breast cancer cell models. PLoS ONE 2013, 8, e69023. [Google Scholar] [CrossRef]
- Lulla, R.R.; Goldman, S.; Yamada, T.; Beattie, C.W.; Bressler, L.; Pacini, M.; Pollack, I.F.; Fisher, P.G.; Packer, R.J.; Dunkel, I.J.; et al. Phase 1 trial of p28 (NSC745104), a non-HDM2-mediated peptide inhibitor of p53 ubiquitination in pediatric patients with recurrent or progressive central nervous system tumors: A Pediatric Brain Tumor Consortium Study. Neuro-Oncol. 2016, 18, 1319–1325. [Google Scholar] [CrossRef]
- Yamada, T.; Mehta, R.R.; Lekmine, F.; Christov, K.; King, M.L.; Majumdar, D.; Shilkaitis, A.; Green, A.; Bratescu, L.; Beattie, C.W. A peptide fragment of azurin induces a p53-mediated cell cycle arrest in human breast cancer cells. Mol. Cancer Ther. 2009, 8, 2947–2958. [Google Scholar] [CrossRef] [PubMed]
- Yamada, T.; Gupta, T.K.D.; Beattie, C.W. P28-Mediated Activation of p53 in G2-M Phase of the Cell Cycle Enhances the Efficacy of DNA Damaging and Antimitotic Chemotherapy. Cancer Res. 2016, 76, 2354–2365. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.J.; Dao, H., Jr.; Nagarajan, P.; Duvic, M. Primary cutaneous anaplastic large-cell lymphoma: Complete remission for 13 years after denileukin diftitox. JAAD Case Rep. 2017, 3, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Baur, A.S.; Schuler-Thurner, B.; Schuler, G. Immunogenic and tolerogenic effects of the chimeric IL-2-diphtheria toxin cytocidal agent Ontak on CD25+ cells. Oncoimmunology 2014, 3, e28223. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.K. Biology and molecular epidemiology of diphtheria toxin and the tox gene. J. Infect. Dis. 2000, 181, 156–167. [Google Scholar] [CrossRef]
- Bandala, C.; Cortés-Algara, A.; Mejía-Barradas, C.; Ilizaliturri-Flores, I.; Dominguez-Rubio, R.; Bazán-Méndez, C.; Floriano-Sánchez, E.; Luna-Arias, J.; Anaya-Ruiz, M.; Lara-Padilla, E. Botulinum neurotoxin type a inhibits synaptic vesicle 2 expression in breast cancer cell lines. Int. J. Clin. Exp. Pathol. 2015, 8, 8411–8418. [Google Scholar]
- Baudelet, C.; Cron, G.O.; Dessy, C.; Martinive, P.; De Wever, J.; Verrax, J.; Beghein, N.; Gr, V.; Calderon, P.B.; Feron, O. Botulinum toxin potentiates cancer radiotherapy and chemotherapy. Clin. Cancer Res. 2006, 12, 1276–1283. [Google Scholar]
- Hemmati, M.; Tarighi, P.; Amoozadeh, S.; Farajollahi, M.M. Expression and purification of the recombinant pseudomonas exotoxin a conjugated to herceptin and its anti-proliferation effects on sk-br-3. In Proceedings of the International Tehran Breast Cancer Congress (TBCC9), Tehran, Iran, 18–20 October 2017. [Google Scholar]
- Bjorn, M.J.; Groetsema, G.; Scalapino, L. Antibody-pseudomonas exotoxin a conjugates cytotoxic to human breast cancer cells in vitro. Cancer Res. 1986, 46, 3262–3267. [Google Scholar]
- Goldufsky, J.; Wood, S.; Hajihossainlou, B.; Rehman, T.; Majdobeh, O.; Kaufman, H.L.; Ruby, C.E.; Shafikhani, S.H. Pseudomonas aeruginosa exotoxin T induces potent cytotoxicity against a variety of murine and human cancer cell lines. J. Med. Microbiol. 2015, 64, 164–173. [Google Scholar] [CrossRef]
- Lee, J.H.; Moore, L.D.; Kumar, S.; Pritchard, D.G.; Ponnazhagan, S.; Deivanayagam, C. Bacteriophage hyaluronidase effectively inhibits growth, migration and invasion by disrupting hyaluronan-mediated Erk1/2 activation and RhoA expression in human breast carcinoma cells. Cancer Lett. 2010, 298, 238–249. [Google Scholar] [CrossRef]
- Murphy, J.R. Mechanism of diphtheria toxin catalytic domain delivery to the eukaryotic cell cytosol and the cellular factors that directly participate in the process. Toxins 2011, 3, 294–308. [Google Scholar] [CrossRef] [PubMed]
- Martarelli, D.; Pompei, P.; Mazzoni, G. Inhibition of adrenocortical carcinoma by diphtheria toxin mutant CRM197. Chemotherapy 2009, 55, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Li, C.; Jin, N.; Panoskaltsis-Mortari, A.; Hall, W.A. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diphtheria toxin fusion protein DTAT. J. Natl. Cancer Inst. 2002, 94, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Tintner, R.; Jankovic, J. Botulinum toxin type a in the management of oromandibular dystonia and bruxism. In Scientific Therapeutic Aspects of Botulinum Toxin Philadelphia PA; Brin Mitchell, F., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2002; pp. 343–350. [Google Scholar]
- de Groot, M.; Toering, S.T.; Boer, K.; Spliet, W.G.; Heimans, J.J.; Aronica, E.; Reijneveld, J.C. Expression of synaptic vesicle protein 2a in epilepsy-associated brain tumors and in the peritumoral cortex. Neuro-Oncol. 2010, 12, 265–273. [Google Scholar] [CrossRef]
- Coelho, A.; Dinis, P.; Pinto, R.; Gorgal, T.; Silva, C.; Silva, A.; Silva, J.; Cruz, C.D.; Cruz, F.; Avelino, A. Distribution of the high-affinity binding site and intracellular target of botulinum toxin type a in the human bladder. Eur. Urol. 2010, 57, 884–890. [Google Scholar] [CrossRef]
- Vogl, C.; Tanifuji, S.; Danis, B.; Daniels, V.; Foerch, P.; Wolff, C.; Whalley, B.J.; Mochida, S.; Stephens, G.J. Synaptic vesicle glycoprotein 2a modulates vesicular release and calcium channel function at peripheral sympathetic synapses. Eur. J. Neurosci. 2015, 41, 398–409. [Google Scholar] [CrossRef]
- Portela-Gomes, G.M.; Lukinius, A.; Grimelius, L. Synaptic vesicle protein 2, a new neuroendocrine cell marker. Am. J. Pathol. 2000, 157, 1299–1309. [Google Scholar] [CrossRef]
- Zhang, Q.; Fan, H.; Shen, J.; Hoffman, R.M.; Xing, H.R. Human breast cancer cell lines co-express neuronal, epithelial, and melanocytic differentiation markers in vitro and in vivo. PLoS ONE 2010, 5, e9712. [Google Scholar] [CrossRef]
- Dong, M.; Yeh, F.; Tepp, W.H.; Dean, C.; Johnson, E.A.; Janz, R.; Chapman, E.R. SV2 is the protein receptor for botulinum neurotoxin A. Science 2006, 312, 592–596. [Google Scholar] [CrossRef]
- Yates, S.P.; Taylor, P.L.; Jørgensen, R.; Ferraris, D.; Zhang, J.; Andersen, G.R.; Merrill, A.R. Structure–function analysis of water-soluble inhibitors of the catalytic domain of exotoxin a from pseudomonas aeruginosa. Biochem. J. 2005, 385, 667–675. [Google Scholar] [CrossRef]
- Wood, S.J.; Goldufsky, J.W.; Bello, D.; Masood, S.; Shafikhani, S.H. Pseudomonas aeruginosa ExoT induces mitochondrial apoptosis in target host cells in a manner that depends on its GTPase-activating protein (GAP) domain activity. J. Biol. Chem. 2015, 290, 29063–29073. [Google Scholar] [CrossRef] [PubMed]
- Garrity-Ryan, L.; Shafikhani, S.; Balachandran, P.; Nguyen, L.; Oza, J.; Jakobsen, T.; Sargent, J.; Fang, X.; Cordwell, S.; Matthay, M. The ADP ribosyltransferase domain of pseudomonas aeruginosa ExoT contributes to its biological activities. Infect. Immun. 2004, 72, 546–558. [Google Scholar] [CrossRef] [PubMed]
- Murga, C.; Zohar, M.; Teramoto, H.; Gutkind, J.S. Rac1 and RhoG promote cell survival by the activation of PI3K and Akt, independently of their ability to stimulate JNK and NF-kappaB. Oncogene 2002, 21, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.X.; Zhao, J.S.; Li, J.J.; Wang, T.; Cheng, S.Q.; Yuan, Y.; Wang, F.; Wang, X.F.; Xie, D. Liver cancer: EphrinA2 promotes tumorigenicity through rac1/Akt/NF-kappaB signaling pathway. Hepatology 2010, 51, 535–544. [Google Scholar] [CrossRef]
- Aaltomaa, S.; Lipponen, P.; Tammi, R.; Tammi, M.; Viitanen, J.; Kankkunen, J.-P.; Kosma, V.-M. Strong stromal hyaluronan expression is associated with psa recurrence in local prostate cancer. Urol. Int. 2002, 69, 266–272. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Rubinowicz, D.; Schroeder, G.L.; Forgacs, E.; Minna, J.D.; Block, N.L.; Nadji, M.; Lokeshwar, B.L. Stromal and epithelial expression of tumor markers hyaluronic acid and HYAL1 hyaluronidase in prostate cancer. J. Biol. Chem. 2001, 276, 11922–11932. [Google Scholar] [CrossRef]
- Udabage, L.; Brownlee, G.R.; Nilsson, S.K.; Brown, T.J. The over-expression of HAS2, HYAL-2 and CD44 is implicated in the invasiveness of breast cancer. Exp. Cell Res. 2005, 310, 205–217. [Google Scholar] [CrossRef]
- Camenisch, T.D.; Spicer, A.P.; Brehm-Gibson, T.; Biesterfeldt, J.; Augustine, M.L.; Calabro, A.; Kubalak, S.; Klewer, S.E.; McDonald, J.A. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J. Clin. Investig. 2000, 106, 349–360. [Google Scholar] [CrossRef]
- Lokeshwar, V.B.; Cerwinka, W.H.; Isoyama, T.; Lokeshwar, B.L. HYAL1 hyaluronidase in prostate cancer: A tumor promoter and suppressor. Cancer Res. 2005, 65, 7782–7789. [Google Scholar] [CrossRef]
- Lee, J.H.; Moore, L.; Kumar, S.; DeLucas, L.; Pritchard, D.; Ponnazhagan, S.; Deivanayagam, C. In vitro studies on anti-cancer effect of Streptococcus pyogenes phage hyaluronidase (HylP) on breast cancer. In Proceedings of the AACR Annual Meeting, San Diego, CA, USA, 12–16 April 2008. [Google Scholar]
- Khan, S.A.; Everest, P.; Servos, S.; Foxwell, N.; Zähringer, U.; Brade, H.; Rietschel, E.T.; Dougan, G.; Charles, I.G.; Maskell, D.J. A lethal role for lipid A in salmonella infections. Mol. Microbiol. 1998, 29, 571–579. [Google Scholar] [CrossRef]
- Pawelek, J.M.; Low, K.B.; Bermudes, D. Tumor-targeted salmonella as a novel anticancer vector. Cancer Res. 1997, 57, 4537–4544. [Google Scholar] [PubMed]
- Clairmont, C.; Lee, K.C.; Pike, J.; Ittensohn, M.; Low, K.B.; Pawelek, J.; Bermudes, D.; Brecher, S.M.; Margitich, D.; Turnier, J.; et al. Biodistribution and genetic stability of the novel antitumor agent VNP20009, a genetically modified strain of Salmonella typhimurium. J. Infect. Dis. 2000, 181, 1996–2002. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.H.; Min, J.-J. Targeted cancer therapy using engineered salmonella typhimurium. Chonnam. Med. J. 2016, 52, 173–184. [Google Scholar] [CrossRef] [PubMed]
- Gunn, G.R.; Zubair, A.; Peters, C.; Pan, Z.K.; Wu, T.C.; Paterson, Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J. Immunol. 2001, 167, 6471–6479. [Google Scholar]
- Shahabi, V.; Seavey, M.M.; Maciag, P.C.; Rivera, S.; Wallecha, A. Development of a live and highly attenuated listeria monocytogenes-based vaccine for the treatment of Her2/neu-overexpressing cancers in human. Cancer Gene Ther. 2011, 18, 53–62. [Google Scholar] [CrossRef]
- Singh, R.; Dominiecki, M.E.; Jaffee, E.M.; Paterson, Y. Fusion to Listeriolysin O and delivery by Listeria monocytogenes enhances the immunogenicity of HER-2/neu and reveals subdominant epitopes in the FVB/N mouse. J. Immunol. 2005, 175, 3663–3673. [Google Scholar] [CrossRef]
- Liu, C.-F.; Pan, T.-M. In vitro effects of lactic acid bacteria on cancer cell viability and antioxidant activity. J. Food Drug Anal. 2010, 18. [Google Scholar]
- Asoudeh-Fard, A.; Barzegari, A.; Dehnad, A.; Bastani, S.; Golchin, A.; Omidi, Y. Lactobacillus plantarum induces apoptosis in oral cancer KB cells through upregulation of PTEN and downregulation of MAPK signalling pathways. Bioimpacts 2017, 7, 193–198. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Malek, S.N.; Zheng, P.; Liu, Y. Targeting HIF1α eliminates cancer stem cells in hematological malignancies. Cell Stem. Cell 2011, 8, 399–411. [Google Scholar] [CrossRef]
- Jesenberger, V.; Procyk, K.J.; Yuan, J.; Reipert, S.; Baccarini, M. Salmonella-induced caspase-2 activation in macrophages: A novel mechanism in pathogenmediated apoptosis. J. Exp. Med. 2000, 192, 1035–1045. [Google Scholar] [CrossRef]
- Gedde, M.M.; Higgins, D.E.; Tilney, L.G.; Portnoy, D.A. Portnoy. Role of listeriolysin o in cell-to-cell spread of listeria monocytogenes. Infect. Immun. 2000, 68, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Castro, F.; Paterson, Y.; Gravekamp, C. High efficacy of a listeria-based vaccine against metastatic breast cancer reveals a dual mode of action. Cancer Res. 2009, 69, 5860–5866. [Google Scholar] [CrossRef] [PubMed]
- Schell, M.A.; Karmirantzou, M.; Snel, B.; Vilanova, D.; Berger, B.; Pessi, G.; Zwahlen, M.-C.; Desiere, F.; Bork, P.; Delley, M. The genome sequence of bifidobacterium longum reflects its adaptation to the human gastrointestinal tract. Proc. Natl. Acad. Sci. USA 2002, 99, 14422–14427. [Google Scholar] [CrossRef] [PubMed]
- Yazawa, K.; Fujimori, M.; Amano, J.; Kano, Y.; Taniguchi, S. Bifidobacterium longum as a delivery system for cancer gene therapy: Selective localization and growth in hypoxic tumors. Cancer Gene Ther. 2000, 7, 269–274. [Google Scholar] [CrossRef]
- Nakamura, T.; Sasaki, T.; Fujimori, M.; Yazawa, K.; Kano, Y.; Amano, J.; Taniguchi, S. Cloned cytosine deaminase gene expression of bifidobacterium longum and application to enzyme/pro-drug therapy of hypoxic solid tumors. Biosci. Biotechnol. Biochem. 2002, 66, 2362–2366. [Google Scholar] [CrossRef]
- Takeuchi, A.; Matsumura, H.; Kano, Y. Cloning and expression in Escherichia coli of a gene, hup, encoding the histone-like protein HU of Bifidobacterium longum. Biosci. Biotechnol. Biochem. 2002, 66, 598–603. [Google Scholar] [CrossRef]
- Landete, J.M.; Rodríguez, H.; Curiel, J.A.; de las Rivas, B.; de Felipe, F.L.; Muñoz, R. Degradation of phenolic compounds found in olive products by lactobacillus plantarum strains. In Olives and Olive Oil in Health and Disease Prevention; Elsevier: Philadelphia, PA, USA, 2010; pp. 387–396. [Google Scholar]
- Kassayova, M.; Bobrov, N.; Strojný, L.; Kiskova, T.; Mikeš, J.; Demečková, V.; Orendáš, P.; Bojkova, B.; Péč, M.; Kubatka, P.; et al. Preventive effects of probiotic bacteria lactobacillus plantarum and dietary fiber in chemically-induced mammary carcinogenesis. Anticancer Res. 2014, 34, 4969–4975. [Google Scholar]
- Hadrup, S.; Donia, M.; Thor Straten, P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2013, 6, 123–133. [Google Scholar] [CrossRef]
- Gu-Trantien, C.; Loi, S.; Garaud, S.; Equeter, C.; Libin, M.; De Wind, A.; Ravoet, M.; Le Buanec, H.; Sibille, C.; Manfouo-Foutsop, G. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Investig. 2013, 123, 2873–2892. [Google Scholar] [CrossRef]
- Chen, C.; Chan, H.M.; Kubow, S. Kefir extracts suppress in vitro proliferation of estrogen-dependent human breast cancer cells but not normal mammary epithelial cells. J. Med. Food 2007, 10, 416–422. [Google Scholar] [CrossRef]
- Van’t Veer, P.; Van Leer, E.M.; Rietdijk, A.; Kok, F.J.; Schouten, E.G.; Hermus, R.J.; Sturmans, F. Combination of dietary factors in relation to breast-cancer occurrence. Int. J. Cancer 1991, 47, 649–653. [Google Scholar] [CrossRef] [PubMed]
- Rachid, M.; Matar, C.; Duarte, J.; Perdigon, G. Effect of milk fermented with a lactobacillus helveticus R389(+) proteolytic strain on the immune system and on the growth of 4T1 breast cancer cells in mice. Fems. Immunol. Med. Microbiol. 2006, 47, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Kassayova, M.; Bobrov, N.; Strojný, L.; Orendáš, P.; Demečková, V.; Jendželovský, R.; Kubatka, P.; Kiskova, T.; Kružliak, P.; Adamkov, M.; et al. Anticancer and immunomodulatory effects of lactobacillus plantarum ls/07, inulin and melatonin in nmu-induced rat model of breast cancer. Anticancer Res. 2016, 36, 2719–2728. [Google Scholar] [PubMed]
- Aragon, F.; Carino, S.; Perdigon, G.; de Moreno de LeBlanc, A. Inhibition of growth and metastasis of breast cancer in mice by milk fermented with lactobacillus casei CRL 431. J. Immunother. 2015, 38, 185–196. [Google Scholar] [CrossRef]
- Tabarestani, S.; Ghafouri-Fard, S. Cancer stem cells and response to therapy. Asian Pac. J. Cancer Prev. 2012, 13, 5947–5954. [Google Scholar] [CrossRef]
- Shiozawa, Y.; Nie, B.; Pienta, K.J.; Morgan, T.M.; Taichman, R.S. Cancer stem cells and their role in metastasis. Pharmacol. Ther. 2013, 138, 285–293. [Google Scholar] [CrossRef]
- Keith, B.; Simon, M.C. Hypoxia-inducible factors, stem cells, and cancer. Cell 2007, 129, 465–472. [Google Scholar] [CrossRef]
- Riedl, S.; Zweytick, D.; Lohner, K. Membrane-active host defense peptides–challenges and perspectives for the development of novel anticancer drugs. Chem. Phys. Lipids 2011, 164, 766–781. [Google Scholar] [CrossRef]
- Tørfoss, V.; Isaksson, J.; Ausbacher, D.; Brandsdal, B.O.; Flaten, G.E.; Anderssen, T.; Cavalcanti-Jacobsen, C.d.A.; Havelkova, M.; Nguyen, L.T.; Vogel, H.J.; et al. Improved anticancer potency by head-to-tail cyclization of short cationic anticancer peptides containing a lipophilic β2, 2-amino acid. J. Pept. Sci. 2012, 18, 609–619. [Google Scholar] [CrossRef]
- Deutscher, S.L. Phage display in molecular imaging and diagnosis of cancer. Chem. Rev. 2010, 110, 3196–3211. [Google Scholar] [CrossRef]
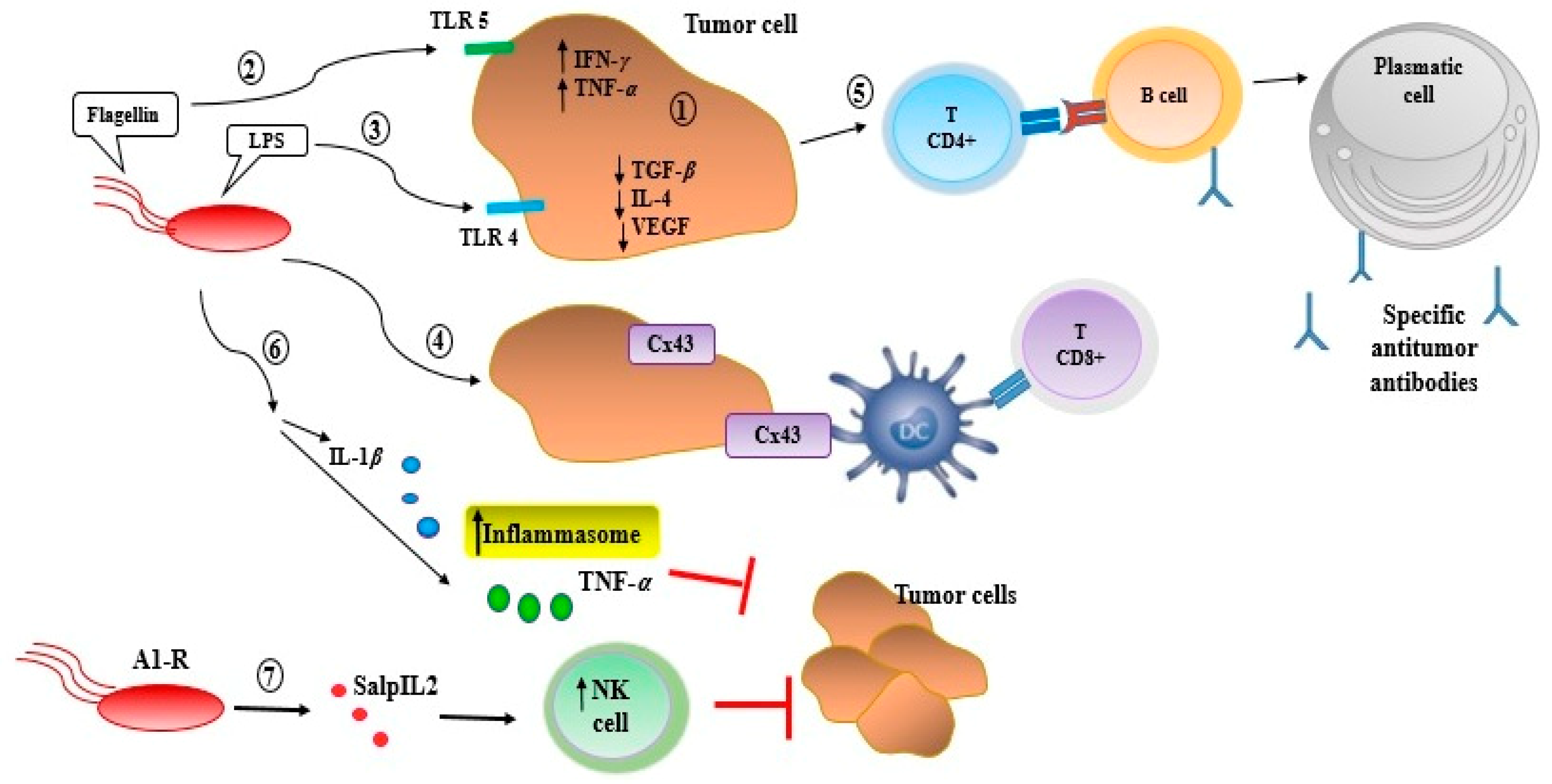
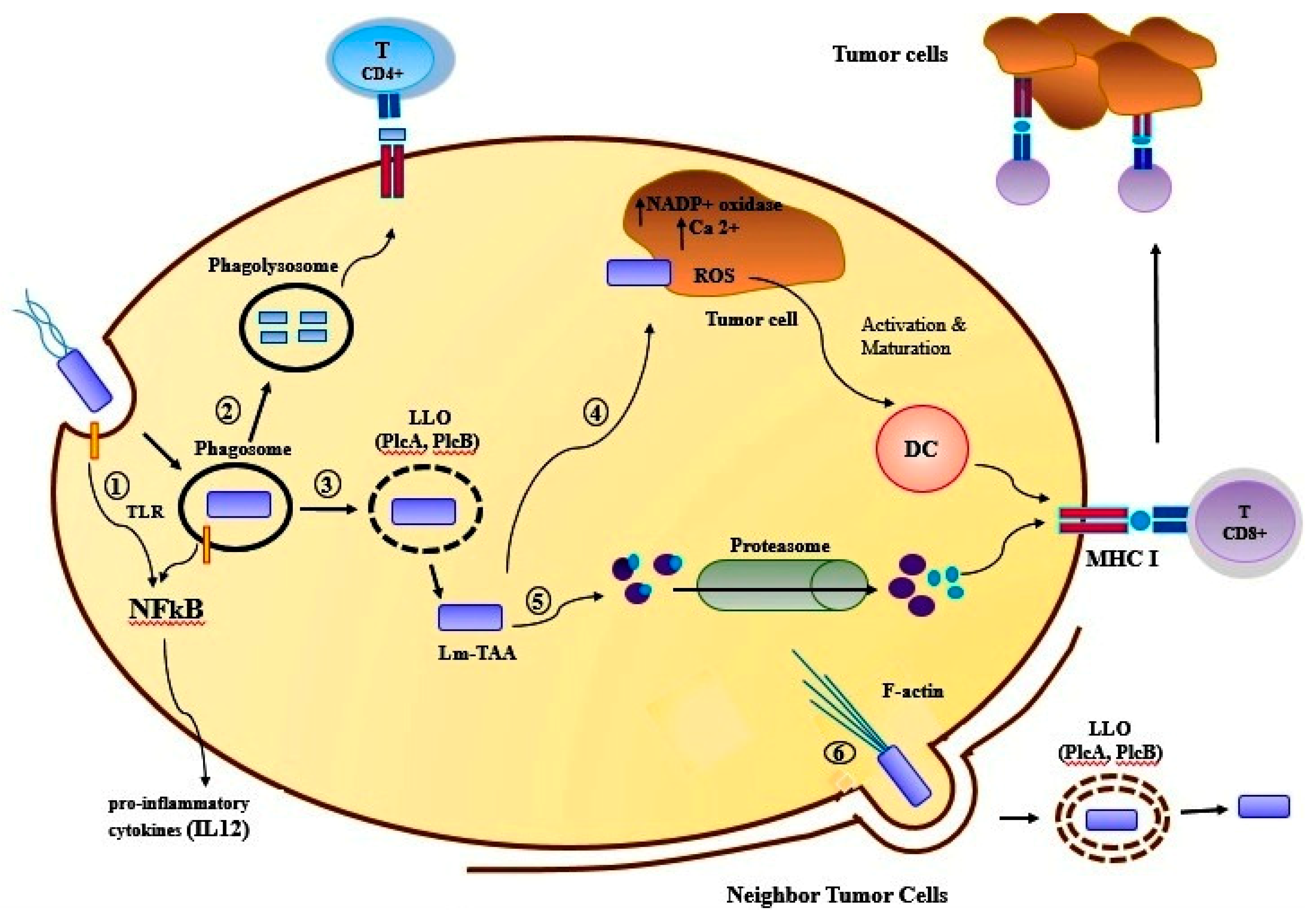
| Bacteriocins | Source | Breast Cancer Cell Line | Other Human Cancer Cells/Cell Lines | Ref. |
|---|---|---|---|---|
| Bovicin HC5 | Streptococcus bovis HC5 | MCF-7 | Liver, hepatocellular carcinoma (HepG2) | [44,45] |
| Colicin E1 and A | Escherichia coli | MCF7, ZR75, BT549, BT474, MDA-MB-231, SKBR3 and T47D | Osteosarcoma (HOS), fibrosarcoma (MRC5, HS913T), leiomyosarcoma (SKUT-1 cells), lung cancer (A-549, PC-14, RERF-LC-AI), ovarian carcinoma, colon cancer (HCT116) | [44,46] |
| Laterosporulin 10 | Brevibacillus sp. strain SKDU10 | MCF-7 | cervical cancer (HeLa), embryonic kidney cancer (HEK293T), fibrosarcoma (HT1080), lung carcinoma (H1299) | [47] |
| Nisin A | Lactococcus lactis | MCF-7 | Head and neck squamous cell carcinoma (UM-SCC-17B, UM-SCC-14A, HSC-3), colon cancer (LS180, SW48, HT29, Caco2), liver hepatocellular carcinoma (HepG2), acute T cell leukaemia (Jurkat) | [48] |
| Protein/Peptide | Source | Breast Cancer Cell Line | Other Human Cancer Cells/Cell Lines | Ref |
|---|---|---|---|---|
| Ohmyungsamycins A and B | Streptomyces sp. | MDAMB231 | diverse cancer cells, colon cancer (HCT116), lung cancer cells (A549), stomach cancer (SNU638), liver cancer (SKHEP1) | [50] |
| Azurin | Pseudomonas aeruginosa strains | MCF7, ZR-75-1, T47D, MDA-MB-157, MDD2, MDA-MB-231 | Normal melanocytes (HFB4), liver cell line (HEPG2), colon cell line (HCT116), progressive pediatric CNS tumors | [51,52] |
| Pep27anal2 | Streptococcus pneumoniae | MCF-7 | leukemia cells (AML-2, HL-60, Jurkat), gastric cancer cells (SNU-601) | [53,54] |
| Entap | Enterococus sp. strains | MDA-MB-231 | Gastric adenocarcinoma cells (AGS), uterine cervix adenocarcinoma cells (HeLa), prostate carcinoma (22Rv1), colorectal adenocarcinoma (HT-29) | [43,55,56] |
| Proximicins | Verrucosispora sp. MG-37 and AB-18-032 | MCF 7 | Human gastric adenocarcinoma (AGS), human hepatocellular carcinoma (HepG2) | [57] |
| Urukthapelstatin A | Mechercharimyces asporophorigenens YM11-542 | MCF-7 | Human lung cancers (A549, DMS114, NCIH460), ovarian cancers (OVCAR-3, OVCAR-4, OVCAR-5, OVCAR-8, SK-OV3), colon cancer (HCT-116) | [58] |
| Toxins | Source | Breast Cancer Cell Line | Other Human Cancer Cells/Cell Lines | Ref |
|---|---|---|---|---|
| Diphtheria toxin | Corynebacterium diphtheria | MCF 7 | Adrenocortical carcinoma (H295R), glioblastomas (U118MG, U373MG, U87MG), cutaneous T cell lymphomas (CTCL), cervical adenocarcinoma (HeLa), colon cancer (SW480, SW620, HCT116, CaCo-2, and HT-29) | [66,67,68] |
| Botulinum neurotoxin type A | Clostridium botulinum | T47D, MDA-MB-231, MDA-MB-453 | prostate cancer (PC-3, LNCaP), neuroblastoma (SH-SY5Y) | [69,70] |
| Exotoxin A | Pseudomonas aeruginosa | MCF-7, BT-20, CAMA-1, SKBR-3 | pancreatic cancer (PaCa-2), melanomas (FEMX, Melmet-1, Melmet-5, Melmet-44, MelRM, MM200), head and neck squamous carcinomas, Burkitt’s lymphoma (Daudi, CA46), leukemias (EHEB, MEC1) | [71,72] |
| Exotoxin T | Pseudomonas aeruginosa | MDA-MB-231, EMT6, 4T1 | murine-derived fibrosarcoma cell line (MCA-205), human melanoma (A375), human lung adenocarcinoma (Calu-3), murine lung carcinoma (LLC1), human ovarian adenocarcinoma (SK-OV-3), human cervical adenocarcinoma (HeLa) | [73] |
| Hyaluronidases (Hyals) | Streptococcus pyogenes | Hs578T, MDA-MB-231, MCF-7 | [74] |
| Bacteria as a Carrier | Source | Breast Cancer Cell Line | Other Human Cancer Cells/Cell Lines | Clinical Phase | Ref |
|---|---|---|---|---|---|
| Salmonella typhimurium (VNP20009) | Salmonella | MDA-MB-435, MDA-MB-361, MDA-MB-231, 4T1, Caco2, RKO, and MCF7 | Metastatic melanoma (MDA-MB-435, B16-F10), human glioblastoma (U87MG), human pancreatic cancer (ASPC-1), colon carcinoma (WiDr, CT26) | Phase I | [97,98,99] |
| Listeriolysin O (Lm-LLO-E7) | Listeria monocytogenes | 4T1, MDA-MB-231 | Cervical cancer, human acute monocytic leukemia cell line THP-1, human ovarian cancer (SKOV3-A2), human prostate cancer (LNCaP), and human colon cancer (Colo205) | Phase I/II | [100] |
| Listeriolysin O (ADXS31-142) | Listeria monocytogenes | EMT6-Luc | Prostate cancer, breast cancer, colorectal cancer, pancreatic cancers(EMT6-Luc, HLA-A2, NT-2 cell line) | Phase I/II | [101,102] |
| Lactobacillus plantarum | Lactobacillus | MCF-7, MDA-MB-231, 4T1 | Colon cancer (Caco-2, BGC-823, HT-29) | [103,104] | |
| Lactobacillus rhamnosus | Lactobacillus | MDA-MB-231 | Colorectal cancer (HT-29), cervical adenocarcinoma (HeLa) | [105] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yaghoubi, A.; Khazaei, M.; Hasanian, S.M.; Avan, A.; C. Cho, W.; Soleimanpour, S. Bacteriotherapy in Breast Cancer. Int. J. Mol. Sci. 2019, 20, 5880. https://doi.org/10.3390/ijms20235880
Yaghoubi A, Khazaei M, Hasanian SM, Avan A, C. Cho W, Soleimanpour S. Bacteriotherapy in Breast Cancer. International Journal of Molecular Sciences. 2019; 20(23):5880. https://doi.org/10.3390/ijms20235880
Chicago/Turabian StyleYaghoubi, Atieh, Majid Khazaei, Seyed Mahdi Hasanian, Amir Avan, William C. Cho, and Saman Soleimanpour. 2019. "Bacteriotherapy in Breast Cancer" International Journal of Molecular Sciences 20, no. 23: 5880. https://doi.org/10.3390/ijms20235880
APA StyleYaghoubi, A., Khazaei, M., Hasanian, S. M., Avan, A., C. Cho, W., & Soleimanpour, S. (2019). Bacteriotherapy in Breast Cancer. International Journal of Molecular Sciences, 20(23), 5880. https://doi.org/10.3390/ijms20235880






