Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications
Abstract
1. Introduction
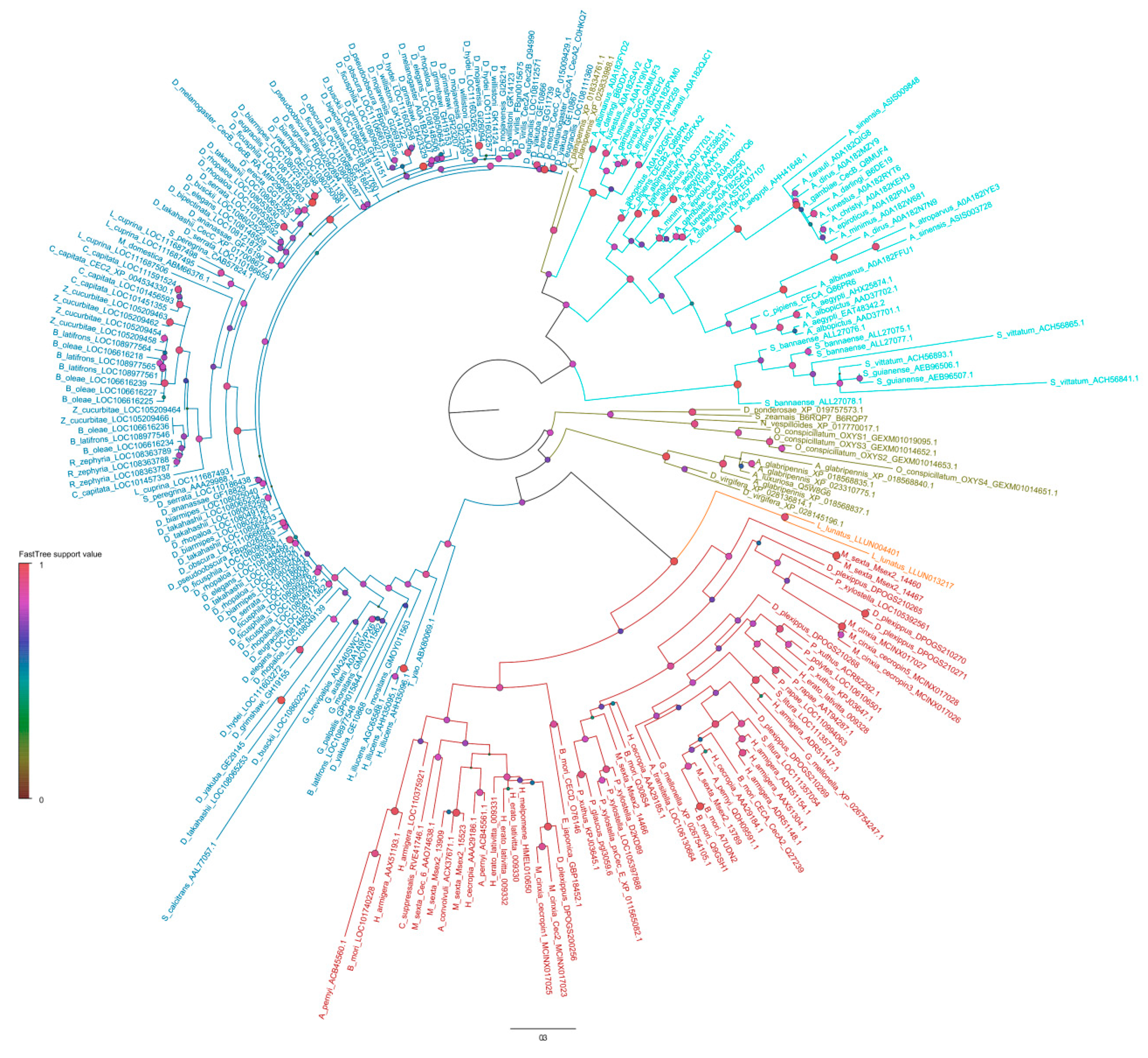
2. The Family of Cecropins in Insects
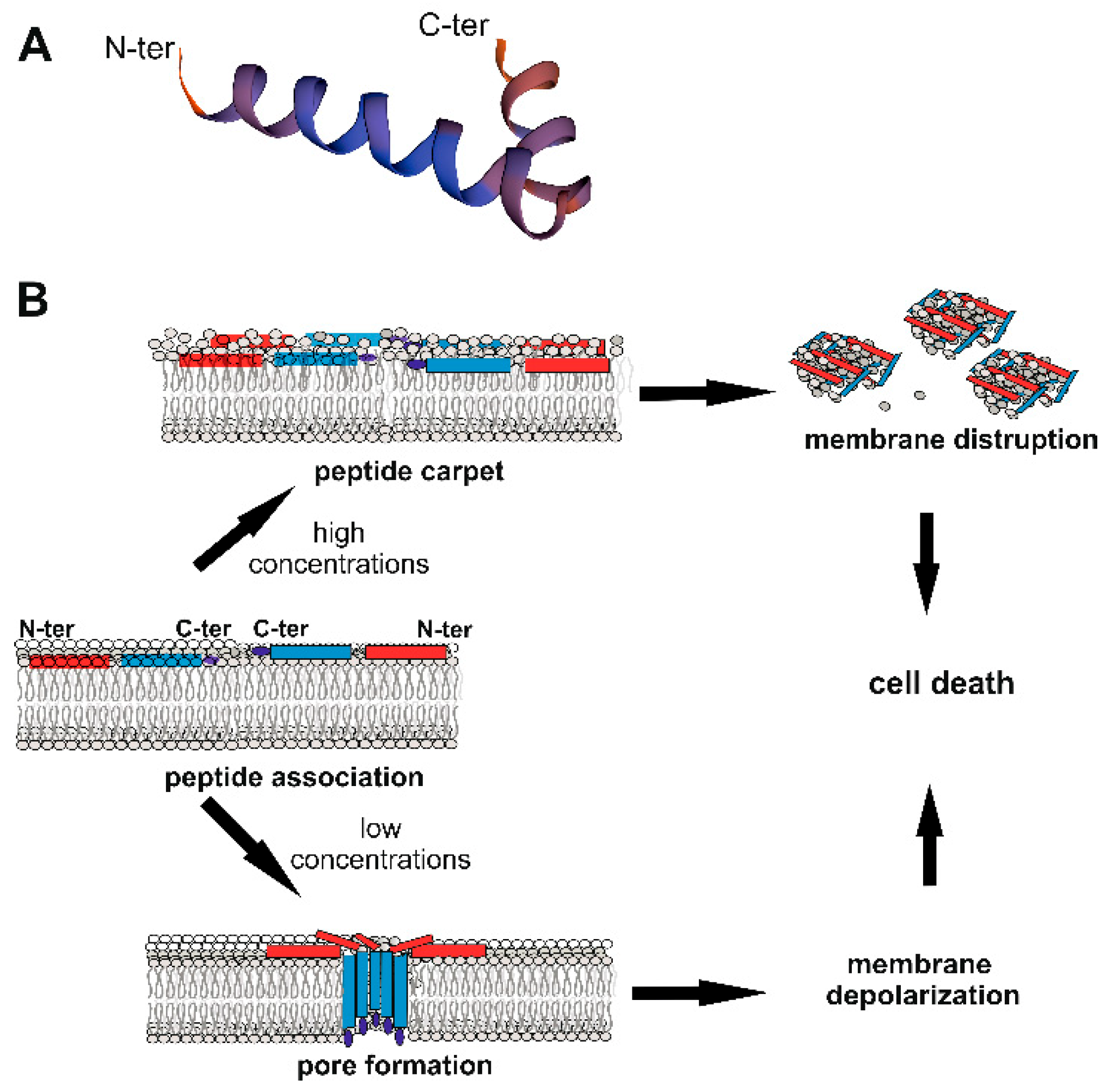
3. Cec Gene Expression and Mechanism of Action Against Microorganisms
4. In Vitro Antimicrobial Activity of Natural Cecs and Synthetic Cec-Analogs
5. Anti-Inflammatory Properties of Natural Cecs and Synthetic Cec-Analogs
6. Antitumor Activity of Natural Cecs and Synthetic Cec-Analogs
7. Health Benefits of Natural Cecs and Synthetic Cec-analogs: Future Potential and Limitations
7.1. Potential of Natural Cecs and Cec-analogs as Antibacterial Drugs
7.2. Natural Cecs and Cec-Analogs as Anti-Biofilm Compounds
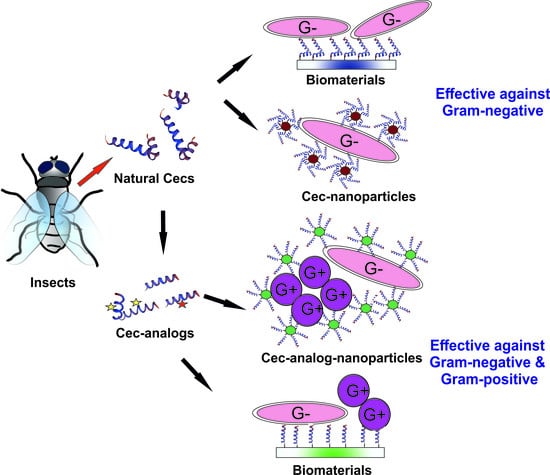
7.3. Biomedical Applications of Natural Cecs and Cec-Analogs: Limitations and Potential Solutions
8. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Report to the Secretary General of the Nations: No Time to Wait–Securing the Future from Drug-Resistant Infections; Interagency Coordination Group on Antimicrobial Resistance (IACG): New York, NY, USA, 2019.
- Zhang, L.-J.; Gallo, R.L. Antimicrobial peptides. Curr. Biol. 2016, 26, R14-9. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial peptides: Diversity, mechanism of action and strategies to improve the activity and biocompatibility in vivo. Biomolecules 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, X.; Wang, Z. APD3: The antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 2015, 44, D1087–D1093. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Patočka, J.; Kuča, K. Insect antimicrobial peptides, a mini review. Toxins 2018, 10, 461. [Google Scholar] [CrossRef]
- Tonk, M.; Vilcinskas, A. The medical potential of antimicrobial peptides from insects. Curr. Top. Med. Chem. 2017, 17, 554–575. [Google Scholar] [CrossRef]
- Yi, H.-Y.; Chowdhury, M.; Huang, Y.-D.; Yu, X.-Q. Insect antimicrobial peptides and their applications. Appl. Microbiol. Biotechnol. 2014, 98, 5807–5822. [Google Scholar] [CrossRef]
- Hultmark, D.; Steiner, H.; Rasmuson, T.; Boman, H.G. Insect immunity. Purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980, 106, 7–16. [Google Scholar] [CrossRef]
- Hultmark, D.; Engström, Å.; Bennich, H.; Kapur, R.; Boman, H.G. Insect immunity: Isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur. J. Biochem. 1982, 127, 207–217. [Google Scholar] [CrossRef]
- Steiner, H.; Hultmark, D.; Engström, Å.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246. [Google Scholar] [CrossRef]
- Mylonakis, E.; Podsiadlowski, L.; Muhammed, M.; Vilcinskas, A. Diversity, evolution and medical applications of insect antimicrobial peptides. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2016, 371, 20150290. [Google Scholar] [CrossRef]
- Misof, B.; Liu, S.; Meusemann, K.; Peters, R.S.; Donath, A.; Mayer, C.; Frandsen, P.B.; Ware, J.; Flouri, T.; Beutel, R.G.; et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 2014, 346, 763–767. [Google Scholar] [CrossRef]
- Zhao, C.; Liaw, L.; Lee, I.H.; Lehrer, R.I. cDNA cloning of three cecropin-like antimicrobial peptides (Styelins) from the tunicate, Styela clava. FEBS Lett. 1997, 412, 144–148. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Boman, A.; Sun, C.X.; Andersson, M.; Jörnvall, H.; Mutt, V.; Boman, H.G. Antibacterial peptides from pig intestine: Isolation of a mammalian cecropin. Proc. Natl. Acad. Sci. USA 1989, 86, 9159–9162. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M.; Boman, A.; Boman, H.G. Ascaris nematodes from pig and human make three anti-bacterial peptides: Isolation of cecropin P1 and two ASABF peptides. Cell. Mol. Life Sci. 2003, 60, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Pütsep, K.; Normark, S.; Boman, H.G. The origin of cecropins; implications from synthetic peptides derived from ribosomal protein L1. FEBS Lett. 1999, 451, 249–252. [Google Scholar] [CrossRef]
- Tamang, D.G.; Saier, M.H., Jr. The cecropin superfamily of toxic peptides. J. Mol. Microbiol. Biotechnol. 2006, 11, 94–103. [Google Scholar] [CrossRef]
- Tarr, D.E.K. Distribution and characteristics of ABFs, cecropins, nemapores, and lysozymes in nematodes. Dev. Comp. Immunol. 2012, 36, 502–520. [Google Scholar] [CrossRef]
- Segovia, L.J.T.; Ramirez, G.A.T.; Arias, D.C.H.; Duran, J.D.R.; Bedoya, J.P.; Osorio, J.C.C. Identification and characterization of novel cecropins from the Oxysternon conspicillatum neotropic dung beetle. PLoS ONE 2017, 12, e0187914. [Google Scholar] [CrossRef]
- Saito, A.; Ueda, K.; Imamura, M.; Atsumi, S.; Tabunoki, H.; Miura, N.; Watanabe, A.; Kitami, M.; Sato, R. Purification and cDNA cloning of a cecropin from the longicorn beetle, Acalolepta luxuriosa. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 142, 317–323. [Google Scholar] [CrossRef]
- Memarpoor-Yazdi, M.; Zare-Zardini, H.; Asoodeh, A. A novel antimicrobial peptide derived from the insect Paederus dermatitis. Int. J. Pept. Res. Ther. 2013, 19, 99–108. [Google Scholar] [CrossRef]
- Wu, J.; Mu, L.; Zhuang, L.; Han, Y.; Liu, T.; Li, J.; Yang, Y.; Yang, H.; Wei, L. A cecropin-like antimicrobial peptide with anti-inflammatory activity from the black fly salivary glands. Parasites Vectors 2015, 8, 561. [Google Scholar] [CrossRef] [PubMed]
- Vizioli, J.; Bulet, P.; Charlet, M.; Lowenberger, C.; Blass, C.; Müller, H.M.; Dimopoulos, G.; Hoffmann, J.; Kafatos, F.C.; Richman, A. Cloning and analysis of a cecropin gene from the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 2000, 9, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lowenberger, C.; Charlet, M.; Vizioli, J.; Kamal, S.; Richman, A.; Christensen, B.M.; Bulet, P. Antimicrobial activity spectrum, cDNA cloning, and mRNA expression of a newly isolated member of the cecropin family from the mosquito vector Aedes aegypti. J. Biol. Chem. 1999, 274, 20092–20097. [Google Scholar] [CrossRef] [PubMed]
- Jayamani, E.; Rajamuthiah, R.; Larkins-Ford, J.; Fuchs, B.B.; Conery, A.L.; Vilcinskas, A.; Ausubel, F.M.; Mylonakis, E. Insect-derived cecropins display activity against Acinetobacter baumannii in a whole-animal high-throughput Caenorhabditis elegans model. Antimicrob. Agents Chemother. 2015, 59, 1728–1737. [Google Scholar] [CrossRef]
- Wei, L.; Yang, Y.; Zhou, Y.; Li, M.; Yang, H.; Mu, L.; Qian, Q.; Wu, J.; Xu, W. Anti-inflammatory activities of Aedes aegypti cecropins and their protection against murine endotoxin shock. Parasites Vectors 2018, 11, 470. [Google Scholar] [CrossRef]
- Sun, D.; Eccleston, E.D.; Fallon, A.M. Peptide sequence of an antibiotic cecropin from the vector mosquito, Aedes albopictus. Biochem. Biophys. Res. Commun. 1998, 249, 410–415. [Google Scholar] [CrossRef]
- Kaushal, A.; Gupta, K.; Shah, R.; van Hoek, M.L. Antimicrobial activity of mosquito cecropin peptides against Francisella. Dev. Comp. Immunol. 2016, 63, 171–180. [Google Scholar] [CrossRef]
- Xu, X.; Yang, H.; Ma, D.; Wu, J.; Wang, Y.; Song, Y.; Wang, X.; Lu, Y.; Yang, J.; Lai, R. Toward an understanding of the molecular mechanism for successful blood feeding by coupling proteomics analysis with pharmacological testing of horsefly salivary glands. Mol. Cell. Proteomics 2008, 7, 582–590. [Google Scholar] [CrossRef]
- Park, S.I.; Yoe, S.M. A novel cecropin-like peptide from black soldier fly, Hermetia illucens: Isolation, structural and functional characterization. Entomol. Res. 2017, 47, 115–124. [Google Scholar] [CrossRef]
- Ekengren, S.; Hultmark, D. Drosophila cecropin as an antifungal agent. Insect Biochem. Mol. Biol. 1999, 29, 965–972. [Google Scholar] [CrossRef]
- Samakovlis, C.; Kimbrell, D.A.; Kylsten, P.; Engström, Å.; Hultmark, D. The immune response in Drosophila: Pattern of cecropin expression and biological activity. EMBO J. 1990, 9, 2969–2976. [Google Scholar] [CrossRef]
- Lu, X.; Shen, J.; Jin, X.; Ma, Y.; Huang, Y.; Mei, H.; Chu, F.; Zhu, J. Bactericidal activity of Musca domestica cecropin (Mdc) on multidrug-resistant clinical isolate of Escherichia coli. Appl. Microbiol. Biotechnol. 2012, 95, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.M.; Jin, X.B.; Zhu, J.Y.; Mei, H.F.; Ma, Y.; Chu, F.J.; Wang, Y.; Li, X.B. Expression of the antimicrobial peptide cecropin fused with human lysozyme in Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 87, 2169–2176. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Li, R.; Feng, Y.; Wang, S. Transmission electron microscopic morphological study and flow cytometric viability assessment of Acinetobacter baumannii susceptible to Musca domestica cecropin. Sci. World J. 2014, 2014, 657536. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boulanger, N.; Brun, R.; Ehret-Sabatier, L.; Kunz, C.; Bulet, P. Immunopeptides in the defense reactions of Glossina morsitans to bacterial and Trypanosoma brucei brucei infections. Insect Biochem. Mol. Biol. 2002, 32, 369–375. [Google Scholar] [CrossRef]
- Boulanger, N.; Munks, R.J.; Hamilton, J.V.; Vovelle, F.; Brun, R.; Lehane, M.J.; Bulet, P. Epithelial innate immunity. A novel antimicrobial peptide with antiparasitic activity in the blood-sucking insect Stomoxys calcitrans. J. Biol. Chem. 2002, 277, 49921–49926. [Google Scholar] [CrossRef]
- Okada, M.; Natori, S. Purification and characterization of an antibacterial protein from haemolymph of Sarcophaga peregrina (flesh-fly) larvae. Biochem. J. 1983, 211, 727–734. [Google Scholar] [CrossRef]
- Okada, M.; Natori, S. Mode of action of a bactericidal protein induced in the haemolymph of Sarcophaga peregrina (flesh-fly) larvae. Biochem. J. 1984, 222, 119–124. [Google Scholar] [CrossRef]
- Okada, M.; Natori, S. Primary structure of sarcotoxin I, an antibacterial protein induced in the hemolymph of Sarcophaga peregrina (flesh fly) larvae. J. Biol. Chem. 1985, 260, 7174–7177. [Google Scholar]
- Pöppel, A.K.; Vogel, H.; Wiesner, J.; Vilcinskas, A. Antimicrobial peptides expressed in medicinal maggots of the blow fly Lucilia sericata show combinatorial activity against bacteria. Antimicrob. Agents Chemother. 2015, 59, 2508–2514. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Bland, J.M.; Jacks, T.J.; Grimm, C.; Walsh, T.J. Fungicidal and binding properties of the natural peptides cecropin B and dermaseptin. Med. Mycol. 1998, 36, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Wachinger, M.; Kleinschmidt, A.; Winder, D.; von Pechmann, N.; Ludvigsen, A.; Neumann, M.; Holle, R.; Salmons, B.; Erfle, V.; Brack-Werner, R. Antimicrobial peptides melittin and cecropin inhibit replication of human immunodeficiency virus 1 by suppressing viral gene expression. J. Gen. Virol. 1998, 79, 731–740. [Google Scholar] [CrossRef] [PubMed]
- Andrä, J.; Berninghausen, O.; Leippe, M. Cecropins, antibacterial peptides from insects and mammals, are potently fungicidal against Candida albicans. Med. Microbiol. Immunol. 2001, 189, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Shin, A.; Kim, Y. Anti-inflammatory activities of cecropin a and its mechanism of action. Arch. Insect Biochem. Physiol. 2015, 88, 31–44. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.J.; Beazley, W.D.; Bibby, M.C.; Devine, D.A. Antimicrobial activity of cecropins. J. Antimicrob. Chemother. 1996, 37, 1077–1089. [Google Scholar] [CrossRef]
- Liu, X.; Guo, C.; Huang, Y.; Zhang, X.; Chen, Y. Inhibition of porcine reproductive and respiratory syndrome virus by Cecropin D in vitro. Infect. Genet. Evol. 2015, 34, 7–16. [Google Scholar] [CrossRef]
- Qu, Z.; Steiner, H.; Engström, A.; Bennich, H.; Boman, H.G. Insect immunity: Isolation and structure of cecropins B and D from pupae of the Chinese oak silk moth, Antheraea pernyi. Eur. J. Biochem. 1982, 127, 219–224. [Google Scholar] [CrossRef]
- Wang, J.; Ma, K.; Ruan, M.; Wang, Y.; Li, Y.; Fu, Y.V.; Song, Y.; Sun, H.; Wang, J. A novel cecropin B-derived peptide with antibacterial and potential anti-inflammatory properties. PeerJ 2018, 6, e5369. [Google Scholar] [CrossRef]
- Fang, S.L.; Wang, L.; Fang, Q.; Chen, C.; Zhao, X.S.; Qian, C.; Wei, G.Q.; Zhu, B.J.; Liu, C.L. Characterization and functional study of a Cecropin-like peptide from the Chinese oak silkworm, Antheraea pernyi. Arch. Insect Biochem. Physiol. 2017, 94, e21368. [Google Scholar] [CrossRef]
- Yang, W.; Cheng, T.; Ye, M.; Deng, X.; Yi, H.; Huang, Y.; Tan, X.; Han, D.; Wang, B.; Xiang, Z.; et al. Functional divergence among silkworm antimicrobial peptide paralogs by the activities of recombinant proteins and the induced expression profiles. PLoS ONE 2011, 6, e18109. [Google Scholar] [CrossRef]
- Lu, D.; Geng, T.; Hou, C.; Huang, Y.; Qin, G.; Guo, X. Bombyx mori cecropin A has a high antifungal activity to entomopathogenic fungus Beauveria bassiana. Gene 2016, 583, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Romoli, O.; Mukherjee, S.; Mohid, S.A.; Dutta, A.; Montali, A.; Franzolin, E.; Brady, D.; Zito, F.; Bergantino, E.; Rampazzo, C.; et al. Enhanced Silkworm Cecropin B Antimicrobial Activity against Pseudomonas aeruginosa from Single Amino Acid Variation. ACS Infect. Dis. 2019, 5, 1200–1213. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Mraheil, M.A.; Silva, S.; Müller, D.; Cemic, F.; Hemberger, J.; Hain, T.; Vilcinskas, A.; Chakraborty, T. Anti-Listeria activities of Galleria mellonella hemolymph proteins. Appl. Environ. Microbiol. 2011, 77, 4237–4240. [Google Scholar] [CrossRef] [PubMed]
- Oñate-Garzón, J.; Manrique-Moreno, M.; Trier, S.; Leidy, C.; Torres, R.; Patiño, E. Antimicrobial activity and interactions of cationic peptides derived from Galleria mellonella cecropin D-like peptide with model membranes. J. Antibiot. (Tokyo) 2017, 70, 238–245. [Google Scholar] [CrossRef]
- Kim, S.R.; Hong, M.Y.; Park, S.W.; Choi, K.H.; Yun, E.Y.; Goo, T.W.; Kang, S.W.; Suh, H.J.; Kim, I.; Hwang, J.S. Characterization and cDNA cloning of a cecropin-like antimicrobial peptide, papiliocin, from the swallowtail butterfly, Papilio xuthus. Mol. Cells 2010, 29, 419–423. [Google Scholar] [CrossRef]
- Kim, J.K.; Lee, E.; Shin, S.; Jeong, K.W.; Lee, J.Y.; Bae, S.Y.; Kim, S.H.; Lee, J.; Kim, S.R.; Lee, D.G.; et al. Structure and function of papiliocin with antimicrobial and anti-inflammatory activities isolated from the swallowtail butterfly, Papilio xuthus. J. Biol. Chem. 2011, 286, 41296–41311. [Google Scholar] [CrossRef]
- Lee, E.; Kim, J.-K.; Jeon, D.; Jeong, K.-W.; Shin, A.; Kim, Y. Functional roles of aromatic residues and helices of papiliocin in its antimicrobial and anti-inflammatory activities. Sci. Rep. 2015, 5, 12048. [Google Scholar] [CrossRef]
- Choi, C.-S.; Yoe, S.-M.; Kim, E.-S.; Chae, K.-S.; Kim, H.R. Purification and Characterization of Antibacterial Peptides, Spodopsin Ia and Ib Induced in the Larval Haemolymph of the Common Cutworm, Spodoptera Iitura. Korean J. Biol. Sci. 1997, 1, 457–462. [Google Scholar]
- Choi, C.S.; Lee, I.H.; Kim, E.; Kim, S.I.; Kim, H.R. Antibacterial properties and partial cDNA sequences of cecropin-like antibacterial peptides from the common cutworm, Spodoptera litura. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2000, 125, 287–297. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Du, C.; Chen, W.; Pang, Y. Characterization and expression of a cecropin-like gene from Helicoverpa armigera. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 148, 417–425. [Google Scholar] [CrossRef]
- Lockey, T.D.; Ourth, D.D. Formation of pores in Escherichia coli cell membranes by a cecropin isolated from hemolymph of Heliothis virescens larvae. Eur. J. Biochem. 1996, 236, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.R.; Lee, Y.H.; Bang, I.S.; Kim, E.S.; Kang, C.S.; Yun, C.Y.; Lee, I.H. cDNA cloning and antibacterial activities of cecropin D-like peptides from Agrius convolvuli. Arch. Insect Biochem. Physiol. 2000, 45, 149–155. [Google Scholar] [CrossRef]
- Park, S.I.; An, H.S.; Chang, B.S.; Yoe, S.M. Expression, cDNA cloning, and characterization of the antibacterial peptide cecropin D from Agrius convolvuli. Anim. Cells Syst. 2013, 17, 23–30. [Google Scholar] [CrossRef]
- Bang, I.S.; Son, S.Y.; Yoe, S.M. Hinnavin I, an antibacterial peptide from cabbage butterfly, Artogeia rapae. Mol. Cells 1997, 7, 509–513. [Google Scholar]
- Yoe, S.M.; Kang, C.S.; Han, S.S.; Bang, I.S. Characterization and cDNA cloning of hinnavin II, a cecropin family antibacterial peptide from the cabbage butterfly, Artogeia rapae. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 144, 199–205. [Google Scholar] [CrossRef]
- Duwadi, D.; Shrestha, A.; Yilma, B.; Kozlovski, I.; Sa-Eed, M.; Dahal, N.; Jukosky, J. Identification and screening of potent antimicrobial peptides in arthropod genomes. Peptides 2018, 103, 26–30. [Google Scholar] [CrossRef]
- Kylsten, P.; Samakovlis, C.; Hultmark, D. The cecropin locus in Drosophila; a compact gene cluster involved in the response to infection. EMBO J. 1990, 9, 217–224. [Google Scholar] [CrossRef]
- Tryselius, Y.; Samakovlis, C.; Kimbrell, D.A.; Hultmark, D. CecC, a cecropin gene expressed during metamorphosis in Drosophila pupae. Eur. J. Biochem. 1992, 204, 395–399. [Google Scholar] [CrossRef]
- Sackton, T.B.; Lazzaro, B.P.; Clark, A.G. Rapid expansion of immune-related gene families in the house fly, Musca domestica. Mol. Biol. Evol. 2017, 34, 857–872. [Google Scholar] [CrossRef]
- Gudmundsson, G.H.; Lidholm, D.A.; Asling, B.; Gan, R.; Boman, H.G. The cecropin locus. Cloning and expression of a gene cluster encoding three antibacterial peptides in Hyalophora cecropia. J. Biol. Chem. 1991, 266, 11510–11517. [Google Scholar]
- Ponnuvel, K.M.; Subhasri, N.; Sirigineedi, S.; Murthy, G.N.; Vijayaprakash, N.B. Molecular evolution of the cecropin multigene family in silkworm Bombyx mori. Bioinformation 2010, 5, 97–103. [Google Scholar] [CrossRef]
- Ntwasa, M.; Goto, A.; Kurata, S. Coleopteran antimicrobial peptides: Prospects for clinical applications. Int. J. Microbiol. 2012, 2012, 101989. [Google Scholar] [CrossRef]
- Zou, Z.; Evans, J.D.; Lu, Z.; Zhao, P.; Williams, M.; Sumathipala, N.; Hetru, C.; Hultmark, D.; Jiang, H. Comparative genomic analysis of the Tribolium immune system. Genome Biol. 2007, 8, R177. [Google Scholar] [CrossRef] [PubMed]
- Quesada, H.; Ramos-Onsins, S.E.; Aguadé, M. Birth-and-death evolution of the Cecropin multigene family in Drosophila. J. Mol. Evol. 2005, 60, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tassanakajon, A.; Somboonwiwat, K.; Amparyup, P. Sequence diversity and evolution of antimicrobial peptides in invertebrates. Dev. Comp. Immunol. 2015, 48, 324–341. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Onsins, S.; Aguade, M. Molecular evolution of the cecropin multigene family in Drosophila: Functional genes vs. pseudogenes. Genetics 1998, 150, 157–171. [Google Scholar]
- Unckless, R.L.; Lazzaro, B.P. The potential for adaptive maintenance of diversity in insect antimicrobial peptides. Phil. Tran. R. Soc. B 2016, 371, 20150291. [Google Scholar] [CrossRef]
- Tennessen, J.A. Molecular evolution of animal antimicrobial peptides: Widespread moderate positive selection. J. Evol. Biol. 2005, 18, 1387–1394. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Xia, L.-J.; Li, J.-Y.; Zhang, F.-C. CecropinXJ inhibits the proliferation of human gastric cancer BGC823 cells and induces cell death in vitro and in vivo. Int. J. Oncol. 2015, 46, 2181–2193. [Google Scholar] [CrossRef][Green Version]
- Price, M.N.; Dehal, P.S.; Arkin, A.P. FastTree 2–approximately maximum-likelihood trees for large alignments. PLoS ONE 2010, 5, e9490. [Google Scholar] [CrossRef]
- Uvell, H.; Engström, Y. A multilayered defense against infection: Combinatorial control of insect immune genes. Trends Genet. 2007, 23, 342–349. [Google Scholar] [CrossRef] [PubMed]
- Romoli, O.; Saviane, A.; Bozzato, A.; D’Antona, P.; Tettamanti, G.; Squartini, A.; Cappellozza, S.; Sandrelli, F. Differential sensitivity to infections and antimicrobial peptide-mediated immune response in four silkworm strains with different geographical origin. Sci. Rep. 2017, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Feix, J.B. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta. 2006, 1758, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Efimova, S.S.; Schagina, L.V.; Ostroumova, O.S. Channel-forming activity of cecropins in lipid bilayers: Effect of agents modifying the membrane dipole potential. Langmuir 2014, 30, 7884–7892. [Google Scholar] [CrossRef]
- Efimova, S.S.; Medvedev, R.Y.; Chulkov, E.G.; Schagina, L.V.; Ostroumova, O.S. Regulation of the Pore-Forming Activity of Cecropin A by Local Anesthetics. Cell Tiss. Biol. 2018, 12, 331–341. [Google Scholar] [CrossRef]
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55. [Google Scholar] [CrossRef]
- Andreu, D.; Merrifield, R.B.; Steiner, H.; Boman, H.G. N-terminal analogs of cecropin A: Synthesis, antibacterial activity, and conformational properties. Biochemistry 1985, 24, 1683–1688. [Google Scholar] [CrossRef]
- Christensen, B.; Fink, J.; Merrifield, R.B.; Mauzerall, D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc. Natl. Acad. Sci. USA 1988, 85, 5072–5076. [Google Scholar] [CrossRef]
- Durell, S.R.; Raghunathan, G.; Guy, H.R. Modeling the ion channel structure of cecropin. Biophys. J. 1992, 63, 1623–1631. [Google Scholar] [CrossRef]
- Tacconelli, E.; Magrini, N.; Kahlmeter, G.; Singh, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics; World Health Organization: Geneva, Switzerland, 2017; Volume 27. [Google Scholar]
- Jaynes, J.M.; Burton, C.A.; Barr, S.B.; Jeffers, G.W.; Julian, G.R.; White, K.L.; Enright, F.M.; Klei, T.R.; Laine, R.A. In vitro cytocidal effect of novel lytic peptides on Plasmodium falciparum and Trypanosoma cruzi. FASEB J. 1988, 2, 2878–2883. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Bland, J.M.; Vigo, C.B.; Jacks, T.J.; Peter, J.; Walsh, T.J. D-cecropin B: Proteolytic resistance, lethality for pathogenic fungi and binding properties. Med. Mycol. 2000, 38, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Boman, H.G.; Wade, D.; Boman, I.A.; Wåhlin, B.; Merrifield, R.B. Antibacterial and antimalarial properties of peptides that are cecropin-melittin hybrids. FEBS Lett. 1989, 259, 103–106. [Google Scholar] [CrossRef]
- Saugar, J.M.; Rodríguez-Hernández, M.J.; de la Torre, B.G.; Pachón-Ibañez, M.E.; Fernández-Reyes, M.; Andreu, D.; Pachón, J.; Rivas, L. Activity of cecropin A-melittin hybrid peptides against colistin-resistant clinical strains of Acinetobacter baumannii: Molecular basis for the differential mechanisms of action. Antimicrob. Agents Chemother. 2006, 50, 1251–1256. [Google Scholar] [CrossRef] [PubMed]
- Garbacz, K.; Kamysz, W.; Piechowicz, L. Activity of antimicrobial peptides, alone or combined with conventional antibiotics, against Staphylococcus aureus isolated from the airways of cystic fibrosis patients. Virulence 2017, 8, 94–100. [Google Scholar] [CrossRef] [PubMed]
- Geitani, R.; Moubareck, C.A.; Touqui, L.; Sarkis, D.K. Cationic antimicrobial peptides: Alternatives and/or adjuvants to antibiotics active against methicillin-resistant Staphylococcus aureus and multidrug-resistant Pseudomonas aeruginosa. BMC Microbiol. 2019, 19, 54. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Li, W.; Zhang, L.; Zhang, Y.; Cao, B. Cecropin A–melittin mutant with improved proteolytic stability and enhanced antimicrobial activity against bacteria and fungi associated with gastroenteritis in vitro. Biochem. Biophys. Res. Commun. 2014, 451, 650–655. [Google Scholar] [CrossRef]
- Chicharro, C.; Granata, C.; Lozano, R.; Andreu, D.; Rivas, L. N-terminal fatty acid substitution increases the leishmanicidal activity of CA (1-7) M (2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 2001, 45, 2441–2449. [Google Scholar] [CrossRef]
- Shin, S.Y.; Lee, M.K.; Kim, K.L.; Hahm, K.S. Structure-antitumor and hemolytic activity relationships of synthetic peptides derived from cecropin A-magainin 2 and cecropin A-melittin hybrid peptides. J. Pept. Res. 1997, 50, 279–285. [Google Scholar] [CrossRef]
- Oh, D.; Shin, S.Y.; Lee, S.; Kang, J.H.; Kim, S.D.; Ryu, P.D.; Hahm, K.S.; Kim, Y. Role of the hinge region and the tryptophan residue in the synthetic antimicrobial peptides, cecropin A (1–8)–magainin 2 (1–12) and its analogues, on their antibiotic activities and structures. Biochemistry 2000, 39, 11855–11864. [Google Scholar] [CrossRef]
- Jeong, K.-W.; Shin, S.-Y.; Kim, J.-K.; Kim, Y.-M. Antibacterial activity and synergism of the hybrid antimicrobial peptide, CAMA-syn. Bull. Korean Chem. Soc. 2009, 30, 1839–1844. [Google Scholar]
- Ryu, S.; Choi, S.Y.; Acharya, S.; Chun, Y.J.; Gurley, C.; Park, Y.; Armstrong, C.A.; Song, P.I.; Kim, B.J. Antimicrobial and anti-inflammatory effects of Cecropin A(1–8)-Magainin2(1–12) hybrid peptide analog p5 against Malassezia furfur infection in human keratinocytes. J. Invest. Dermatol. 2011, 131, 1677–1683. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.G.; Park, Y.; Jin, I.; Hahm, K.S.; Lee, H.H.; Moon, Y.H.; Woo, E.R. Structure-antiviral activity relationships of cecropin A-magainin 2 hybrid peptide and its analogues. J. Pept. Sci. 2004, 10, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Seo, C.H.; Luchian, T.; Park, Y. Antimicrobial Peptide CMA3 Derived from the CA-MA Hybrid Peptide: Antibacterial and Anti-inflammatory Activities with Low Cytotoxicity and Mechanism of Action in Escherichia coli. Antimicrob. Agents Chemother. 2015, 60, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.-B.; Wu, R.-J.; Si, D.-Y.; Liao, X.-D.; Zhang, L.-L.; Zhang, R.-J. Novel hybrid peptide cecropin A (1–8)-LL37 (17–30) with potential antibacterial activity. Int. J. Mol. Sci. 2016, 17, 983. [Google Scholar] [CrossRef]
- Liu, D.; Liu, J.; Li, J.; Xia, L.; Yang, J.; Sun, S.; Ma, J.; Zhang, F. A potential food biopreservative, CecXJ-37N, non-covalently intercalates into the nucleotides of bacterial genomic DNA beyond membrane attack. Food Chem. 2017, 217, 576–584. [Google Scholar] [CrossRef]
- Wei, L.; Huang, C.; Yang, H.; Li, M.; Yang, J.; Qiao, X.; Mu, L.; Xiong, F.; Wu, J.; Xu, W. A potent anti-inflammatory peptide from the salivary glands of horsefly. Parasites Vectors. 2015, 8, 556. [Google Scholar] [CrossRef][Green Version]
- Heredia, N.; García, S. Animals as sources of food-borne pathogens: A review. Anim. Nutr. 2018, 4, 250–255. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef]
- Kell, D.B.; Pretorius, E. On the translocation of bacteria and their lipopolysaccharides between blood and peripheral locations in chronic, inflammatory diseases: The central roles of LPS and LPS-induced cell death. Integr. Biol. (Camb.) 2015, 7, 1339–1377. [Google Scholar] [CrossRef]
- Kim, J.; Jacob, B.; Jang, M.; Kwak, C.; Lee, Y.; Son, K.; Lee, S.; Jung, I.D.; Jeong, M.S.; Kwon, S.H.; et al. Development of a novel short 12-meric papiliocin-derived peptide that is effective against Gram-negative sepsis. Sci. Rep. 2019, 9, 3817. [Google Scholar] [CrossRef]
- Giacometti, A.; Cirioni, O.; Ghiselli, R.; Viticchi, C.; Mocchegiani, F.; Riva, A.; Saba, V.; Scalise, G. Effect of mono-dose intraperitoneal cecropins in experimental septic shock. Crit. Care Med. 2001, 29, 1666–1669. [Google Scholar] [CrossRef]
- Zhang, L.; Gui, S.; Liang, Z.; Liu, A.; Chen, Z.; Tang, Y.; Xiao, M.; Chu, F.; Liu, W.; Jin, X.; et al. Musca domestica Cecropin (Mdc) Alleviates Salmonella typhimurium-Induced Colonic Mucosal Barrier Impairment: Associating With Inflammatory and Oxidative Stress Response, Tight Junction as Well as Intestinal Flora. Front. Microbiol. 2019, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Wang, W.; Smith, D.; Chan, S.C. Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim. Biophys. Acta 1997, 1336, 171–179. [Google Scholar] [CrossRef]
- Moore, A.J.; Devine, D.A.; Bibby, M.C. Preliminary experimental anticancer activity of cecropins. Pept. Res. 1994, 7, 265–269. [Google Scholar]
- Anghel, R.; Jitaru, D.; Bădescu, L.; Bădescu, M.; Ciocoiu, M. The cytotoxic effect of magainin II on the MDA-MB-231 and M14K tumour cell lines. Biomed. Res. Int. 2013, 2013, 831709. [Google Scholar] [CrossRef]
- Chan, S.-C.; Hui, L.; Chen, H.M. Enhancement of the cytolytic effect of anti-bacterial cecropin by the microvilli of cancer cells. Anticancer Res. 1998, 18, 4467–4474. [Google Scholar]
- Jin, X.; Mei, H.; Li, X.; Ma, Y.; Zeng, A.H.; Wang, Y.; Lu, X.; Chu, F.; Wu, Q.; Zhu, J. Apoptosis-inducing activity of the antimicrobial peptide cecropin of Musca domestica in human hepatocellular carcinoma cell line BEL-7402 and the possible mechanism. Acta Biochim. Biophys. Sin. 2010, 42, 259–265. [Google Scholar] [CrossRef]
- Suttmann, H.; Retz, M.; Paulsen, F.; Harder, J.; Zwergel, U.; Kamradt, J.; Wullich, B.; Unteregger, G.; Stöckle, M.; Lehmann, J. Antimicrobial peptides of the Cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008, 8, 5. [Google Scholar] [CrossRef]
- Hilchie, A.L.; Hoskin, D.W.; Coombs, M.R.P. Anticancer Activities of Natural and Synthetic Peptides. In Antimicrobial Peptides. Advances in Experimental Medicine and Biology; Matsuzaki, K., Ed.; Springer: Singapore, 2019; Volume 1117, pp. 131–147. [Google Scholar] [CrossRef]
- Xia, L.J.; Wu, Y.L.; Ma, J.; Zhang, F.C. Therapeutic effects of antimicrobial peptide on malignant ascites in a mouse model. Mol. Med. Rep. 2018, 17, 6245–6252. [Google Scholar] [CrossRef]
- Economou, V.; Gousia, P. Agriculture and food animals as a source of antimicrobial-resistant bacteria. Infect. Drug Resist. 2015, 8, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Jozefiak, A.; Engberg, R.M. Insect proteins as a potential source of antimicrobial peptides in livestock production. A review. J. Anim. Feed Sci. 2017, 26, 87–99. [Google Scholar] [CrossRef]
- Chiou, P.P.; Chen, M.J.; Lin, C.-M.; Khoo, J.; Larson, J.; Holt, R.; Leong, J.A.; Thorgarrd, G.; Chen, T.T. Production of homozygous transgenic rainbow trout with enhanced disease resistance. Mar. Biotechnol. 2014, 16, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Bundó, M.; Montesinos, L.; Izquierdo, E.; Campo, S.; Mieulet, D.; Guiderdoni, E.; Rossignol, M.; Badosa, E.; Montesinos, E.; San Segundo, B.; et al. Production of cecropin A antimicrobial peptide in rice seed endosperm. BMC Plant Biol. 2014, 14, 102. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, K.S.; Zareena, S.H.; Kumar, M.S.A. Assessment of salivary calcium, phosphate, magnesium, pH, and flow rate in healthy subjects, periodontitis, and dental caries. Contemp. Clin. Dent. 2015, 6, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Joris, L.; Dab, I.; Quinton, P.M. Elemental composition of human airway surface fluid in healthy and diseased airways. Am. Rev. Respir. Dis. 1993, 148, 1633–1637. [Google Scholar] [CrossRef]
- Shrestha, A.; Duwadi, D.; Jukosky, J.; Fiering, S.N. Cecropin-like antimicrobial peptide protects mice from lethal E. coli infection. PLoS ONE 2019, 14, e0220344. [Google Scholar] [CrossRef]
- Lewies, A.; Du Plessis, L.H.; Wentzel, J.F. Antimicrobial Peptides: The Achilles’ Heel of Antibiotic Resistance? Probiotics Antimicrob. Proteins 2019, 11, 370–381. [Google Scholar] [CrossRef]
- Rodríguez-Rojas, A.; Makarova, O.; Rolff, J. Antimicrobials, stress and mutagenesis. PLoS Pathog. 2014, 10, e1004445. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Dosler, S.; Karaaslan, E. Inhibition and destruction of Pseudomonas aeruginosa biofilms by antibiotics and antimicrobial peptides. Peptides 2014, 62, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Dostert, M.; Belanger, C.R.; Hancock, R.E.W. Design and assessment of anti-biofilm peptides: Steps toward clinical application. J. Innate Immun. 2019, 11, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Riool, M.; de Breij, A.; Drijfhout, J.W.; Nibbering, P.H.; Zaat, S.A.J. Antimicrobial peptides in biomedical device manufacturing. Front. Chem. 2017, 5, 63. [Google Scholar] [CrossRef]
- Mataraci, E.; Dosler, S. In vitro activities of antibiotics and antimicrobial cationic peptides alone and in combination against methicillin-resistant Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2012, 56, 6366–6371. [Google Scholar] [CrossRef]
- Cole, M.A.; Scott, T.F.; Mello, C.M. Bactericidal Hydrogels via Surface Functionalization with Cecropin A. ACS Biomater. Sci. Eng. 2016, 2, 1894–1904. [Google Scholar] [CrossRef]
- Querido, M.M.; Felgueiras, H.P.; Rai, A.; Costa, F.; Monteiro, C.; Borges, I.; Oliveira, D.; Ferreira, L.; Martins, M.C. Cecropin–Melittin Functionalized Polyurethane Surfaces Prevent Staphylococcus epidermidis Adhesion without Inducing Platelet Adhesion and Activation. Adv. Mater. Interfaces 2018, 5, 1801390. [Google Scholar] [CrossRef]
- Bai, L.; Zhu, L.; Min, S.; Liu, L.; Cai, Y.; Yao, J. Surface modification and properties of Bombyx mori silk fibroin films by antimicrobial peptide. Appl. Surf. Sci. 2008, 254, 2988–2995. [Google Scholar] [CrossRef]
- Saviane, A.; Romoli, O.; Bozzato, A.; Freddi, G.; Cappelletti, C.; Rosini, E.; Cappellozza, S.; Tettamanti, G.; Sandrelli, F. Intrinsic antimicrobial properties of silk spun by genetically modified silkworm strains. Transgenic Res. 2018, 27, 87–101. [Google Scholar] [CrossRef]
- Biswaro, L.S.; da Costa Sousa, M.G.; Rezende, T.; Dias, S.C.; Franco, O.L. Antimicrobial peptides and nanotechnology, recent advances and challenges. Front. Microbiol. 2018, 9, 855. [Google Scholar] [CrossRef]
- Rai, A.; Pinto, S.; Velho, T.R.; Ferreira, A.F.; Moita, C.; Trivedi, U.; Evangelista, M.; Comune, M.; Rumbaugh, K.P.; Simões, P.N.; et al. One-step synthesis of high-density peptide-conjugated gold nanoparticles with antimicrobial efficacy in a systemic infection model. Biomaterials 2016, 85, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Bédard, F.; Biron, E. Recent progress in the chemical synthesis of class II and S-glycosylated bacteriocins. Front. Microbiol. 2018, 9, 1048. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, K.E.; Joski, P.; Johnston, K.J. Antibiotic-resistant infection treatment costs have doubled since 2002, now exceeding $2 billion annually. Health Affairs 2018, 37, 662–669. [Google Scholar] [CrossRef] [PubMed]
| Insect | Species | Active Peptide (aa) | Antimicrobial Activity | Peptide conc. (μM) | |||
|---|---|---|---|---|---|---|---|
| Order | Virus | Bacteria | Fungi | Cytotox. | Hem Act. | ||
| Coleoptera | Oxysternon conspicillatum | Oxysterlin 1 (39) [19] | - | G+, G− | weak | >28 | >14 |
| Oxysterlin 2 (55) [19] | - | G− | NA | >19.75 | >19.75 | ||
| Oxysterlin 3 (39) [19] | - | G− | NA | >28 | >28 | ||
| Acalolepta luxuriosa | Cec (35) [20] | - | M. luteus, E. coli | - | - | - | |
| Paederus dermatitis | Sarcotoxin Pd (34) [21] | - | G+, G− | weak | - | 16 | |
| Diptera | Simulium bannaense | SibaCec (35) [22] | - | G+, G− | - | 58 | 58 |
| Anopheles gambiae | AngCec A (35) [23] | - | G+, G− | A | - | - | |
| Aedes aegypti | AeaeCec 1 (34) [24,25,26] | - | G+, G− | A | 50 [26] | 50 [26] | |
| AeaeCec 2–4 (34) [26] | - | - | - | 50 | 50 | ||
| AeaeCec 5 (34) [26] | - | - | - | 12.5 | 12.5 | ||
| Aedes albopictus | Cec A1 (35) [27,28] | - | E. coli, Francisella | - | - | - | |
| Cec B (35) [28] | - | Francisella | - | - | - | ||
| Culex pipens | Cec A (34) [28] | - | Francisella | - | - | - | |
| Cec B2 (34) [28] | - | Francisella | - | - | - | ||
| Tabanus yao | Cec TY1 (41) [29] | - | B. subtilis S. aureus E. coli | A | - | - | |
| Hermetia illucens | CLP1 (45) [30] | - | G− | - | - | - | |
| Drosophila melanogaster | Cec A (34) [23,24,31,32] | - | G+, G− | A | - | - | |
| Cec B (34) [31,32] | - | G− | A | - | - | ||
| Musca domestica | Mdc (40) [33,34,35] | - | G+, G− | - | - | - | |
| Glossina morsitans | Cec (39) [36] | - | M. luteus, E. coli | - | - | - | |
| Stomoxys calcitrans | Stomoxyn (42) [37] | - | G+, G− | A | - | >10 | |
| Sarcophaga peregrina | Sarcotoxins I A, B, C (39) [38,39,40] | - | G+, G− | - | - | - | |
| Lucilia sericata | Lser Cecs 1–6 (40) [41] | - | G− | NA | - | - | |
| LSerStomox1 (43) [41] | - | G− | NA | - | - | ||
| LSerStomox 2 (42) [41] | - | G− | NA | - | - | ||
| Lepidoptera | Hyalophora cecropia | Cec A (37) [8,9,10,42,43,44,45] | HIV | G+, G− | A | [44,45] | 100 [45] |
| Cec B (35) [9,42,44,46] | - | G+, G− | A | 30 [44] | 500 [46] | ||
| Cec D (36) [9,47] | PRRSV | G+, G− | - | - | - | ||
| Antheraea pernyi | Cec B (35) [48,49] | - | G+, G− | 25 [49] | 200 [49] | ||
| Cec D (36) [48] | - | G+, G− | - | - | - | ||
| ApCec (38) [50] | - | B. subtilis, E. coli | - | - | 62.5 | ||
| Bombyx mori | Cec A (35) [51,52] | - | G+, G− | A | - | - | |
| Cec B (35) [51,53] | - | G+, G− | NA | 200 [53] | 200 [53] | ||
| Cec D (36) [51] | - | G+, G− | - | - | - | ||
| Cec E (?) [51] | - | B. thuringiensis, G− | - | - | - | ||
| Galleria mellonella | Cec D (39) [54,55] | - | L. monocytogenes | - | - | >115 [55] | |
| Papilio xuthus | Papiliocin (38) [56,57,58] | - | G+, G− | A | 12.5 [58] | 100 [58] | |
| Spodoptera litura | Spodopsin Ia (35) [59] | - | G+, G− | NA | - | - | |
| Spodopsin Ib (35) [59] | - | G+, G− | NA | - | - | ||
| Cec A (35) [60] | - | G+, G− | - | - | - | ||
| Cec B (35) [60] | - | G+, G− | - | - | - | ||
| Helicoverpa armigera | Cec D (42) [61] | - | G+, G− | - | - | - | |
| Heliothis virescens | Cec B (35) [62] | - | E. coli | - | - | - | |
| Agrius convolvuli | AcCec D 1-3 (38) [63,64] | - | G+, G− | - | - | - | |
| Artogeia rapa | Hinnavin I (40) [65] | - | G+, G− | A | - | - | |
| (Pieris rapae) | Hinnavin II (38) [66] | - | G+, G− | A | - | - | |
| Danaus plexippus | DAN1 (37) [67] | - | G+ (weak), G− | - | - | 49.56 | |
| DAN2 (37) [67] | - | G+ (weak), G− | weak | - | 48.97 | ||
| Peptide (aa) | Source | Modification | Antimicrobial Activity | Peptide Conc. (μM) | ||||
|---|---|---|---|---|---|---|---|---|
| Virus | Bacteria | Fungi | Protozoa | Cytotox. | Hem Act. | |||
| SB-37 (38) [92] | H. cecropia Cec B | aa add./sub. | - | - | - | P. falciparum, T. cruzi | - | - |
| Shiva-1 (38) [46,92] | H. cecropia Cec B | aa add./sub. | - | G+, G− | NA | P. falciparum, T. cruzi | - | - |
| D-Cec B (35) [93] | A. pernyi Cec B | D-enantiomer | - | - | A | - | - | - |
| CecDH (32) [49] | A. pernyi Cec B | aa del. | - | G+, G− | - | - | 25 | 100 |
| ΔM1 (39) [55] | G melonella Cec D | N-term aa sub. | - | Sa (weak), Ec, Pa | - | - | - | 115 |
| ΔM2 (39) [55] | G melonella Cec D | N-term aa sub. | - | Sa, Ec, Pa | - | - | - | ~60 |
| Mdc–hly (?) [34] | M. domestica Mdc; human Lysozyme | Hybrid | - | G+, G− | - | - | - | - |
| CAMs (≤26) [94,95,96,97,98] | H. cecropia Cec A; A. mellifera Mellitin | Hybrids | - | G+, G− | A | Plasmodium | 9 [96] | [98] |
| Ac-CAMs (15) [99] | H. cecropia Cec A; A. mellifera Mellitin | N-term fatty acid acylation | - | Sa, Ec, Ab | - | L. pifanoi | - | - |
| CAM-W (26) [98] | H. cecropia Cec A; A. mellifera Mellitin | aa sub. | - | G+, G− | A | - | - | 3.12 |
| CA-MAs (≤20) [100,101,102,103,104,105] | H-cecropia Cec A; X. laevis Magainin 2 | Hybrids with aa sub. | virus–cell fusion inhibition | G+, G− | A | - | [105] | [105] |
| CA-LL37 (22) [106] | H-cecropia Cec A; human LL37 | Hybrid | - | G+, G− | - | - | - | [106] |
| CecXJ-37C (37) [107] | B. mori Cec B | C-term aa add. | - | G+, G− | - | - | 20 | 19 |
| CecXJ-37N (37) [107] | B. mori Cec B | C-term aa add. | - | G+, G− | - | - | 20 | 33 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brady, D.; Grapputo, A.; Romoli, O.; Sandrelli, F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. Int. J. Mol. Sci. 2019, 20, 5862. https://doi.org/10.3390/ijms20235862
Brady D, Grapputo A, Romoli O, Sandrelli F. Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. International Journal of Molecular Sciences. 2019; 20(23):5862. https://doi.org/10.3390/ijms20235862
Chicago/Turabian StyleBrady, Daniel, Alessandro Grapputo, Ottavia Romoli, and Federica Sandrelli. 2019. "Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications" International Journal of Molecular Sciences 20, no. 23: 5862. https://doi.org/10.3390/ijms20235862
APA StyleBrady, D., Grapputo, A., Romoli, O., & Sandrelli, F. (2019). Insect Cecropins, Antimicrobial Peptides with Potential Therapeutic Applications. International Journal of Molecular Sciences, 20(23), 5862. https://doi.org/10.3390/ijms20235862