Upgrading the Repertoire of miRNAs in Gastric Adenocarcinoma to Provide a New Resource for Biomarker Discovery
Abstract
1. Introduction
2. Results
2.1. Discovery of Novel miRNA Sequences in Gastric Tumor and Nonsalignant Samples
2.2. Context and Tissue-Specific Expression Patterns of Novel miRNAs
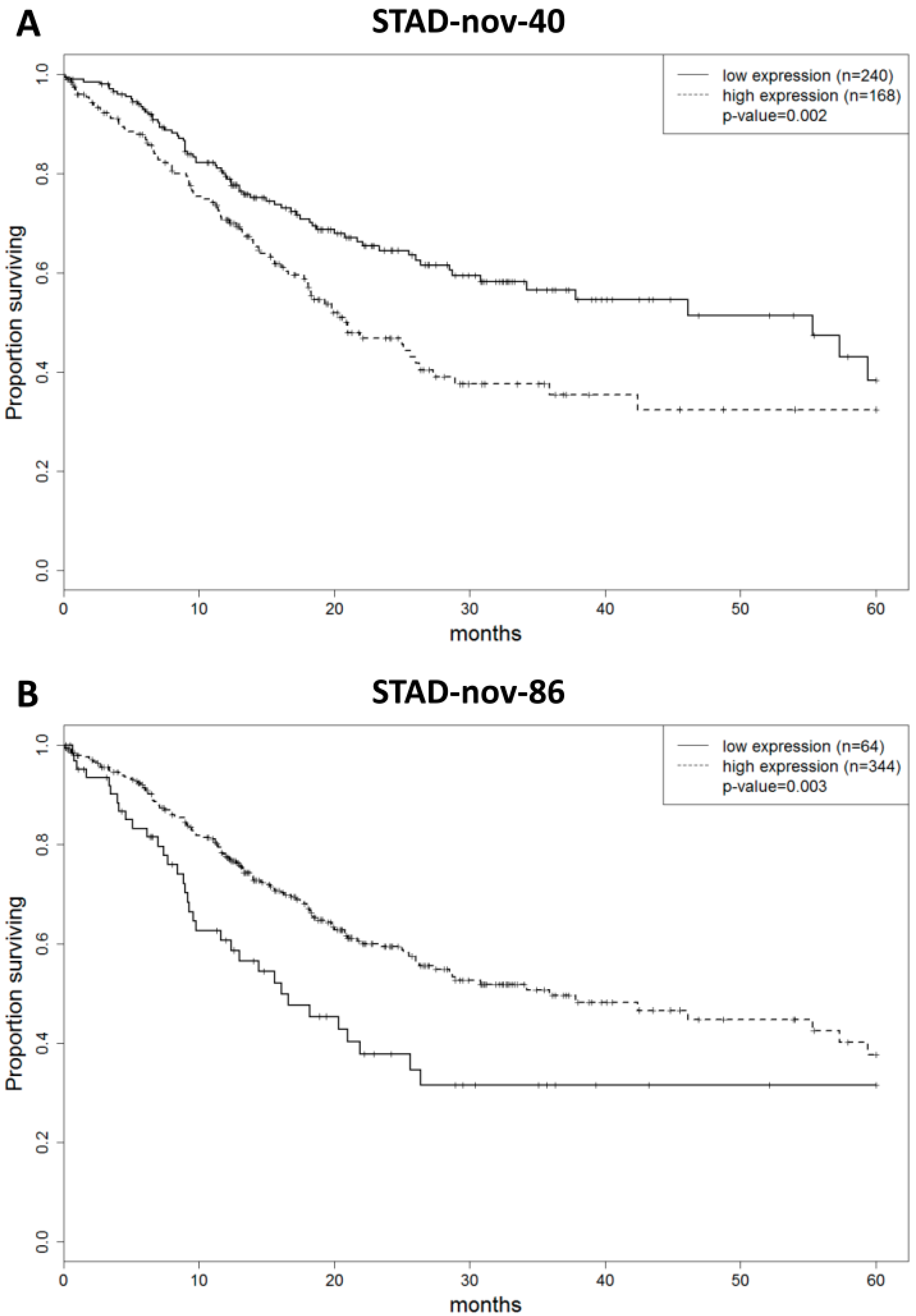
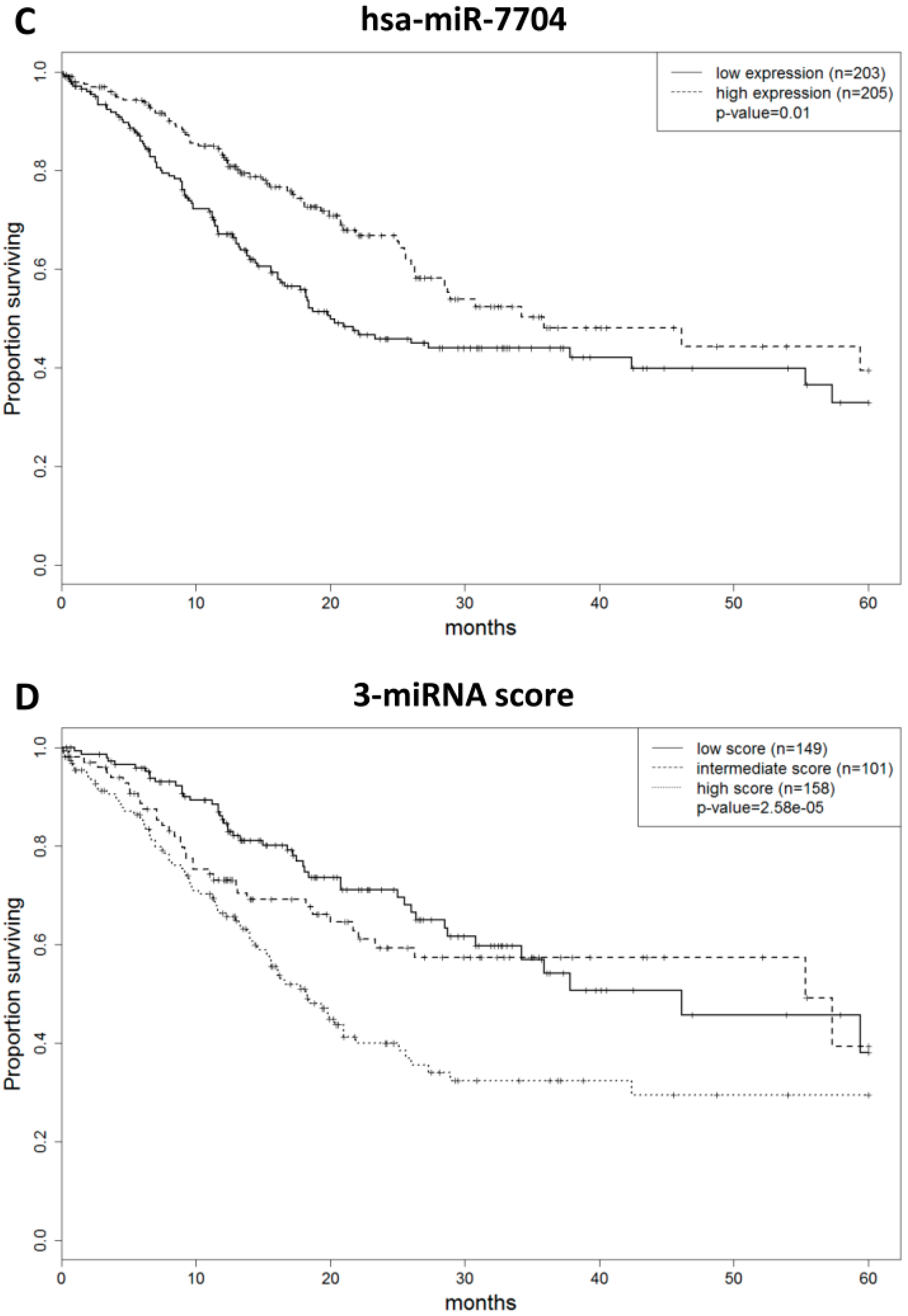
2.3. MicroRNA Prognostic Score to Predict Gastric Adenocarcinoma Patient Outcome
3. Discussion
4. Materials and Methods
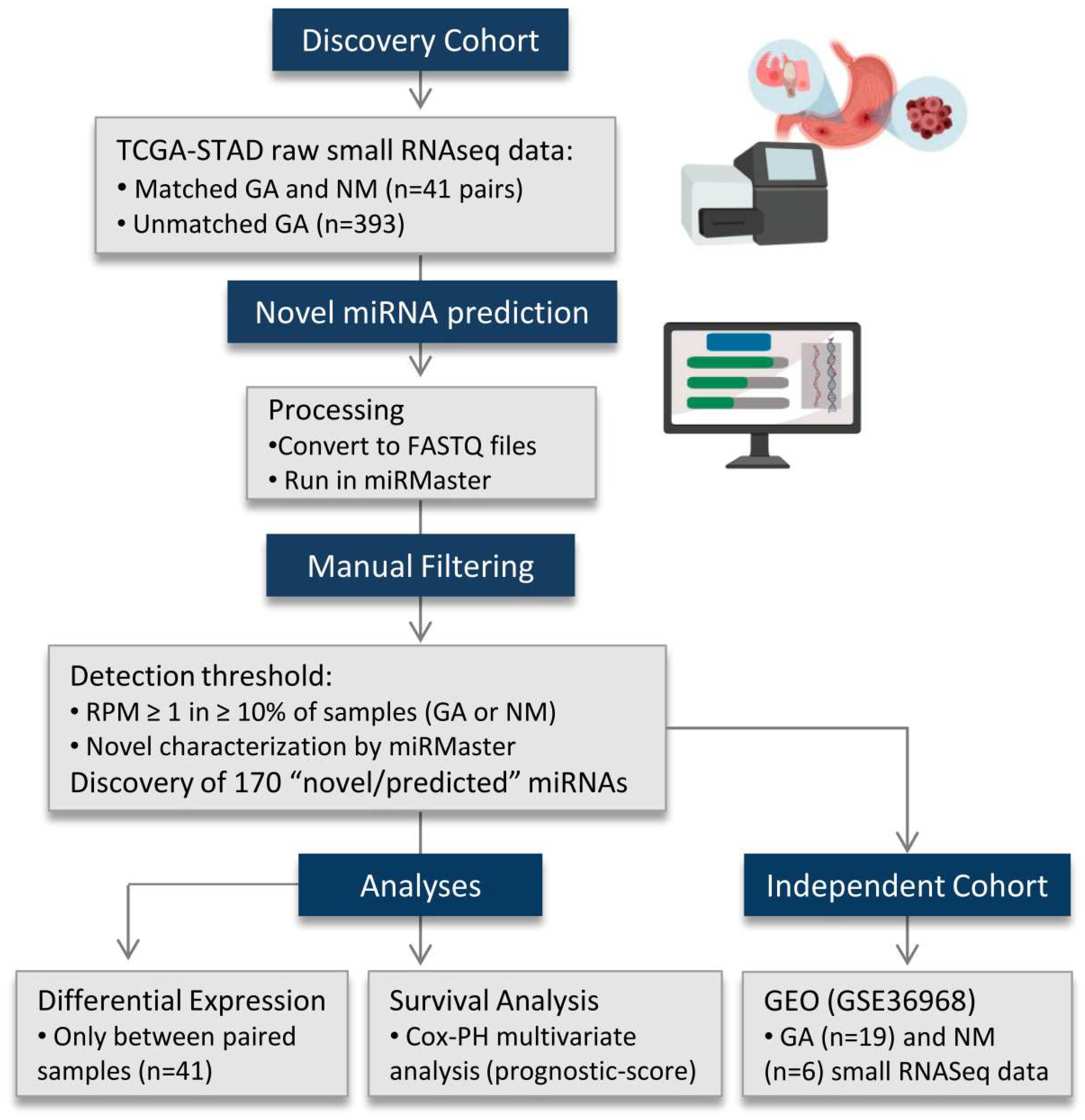
4.1. Data Processing and miRNA Discovery
4.2. Assessment of Tissue and Context Specificity of the Novel miRNAs
4.3. Development of an miRNA-Prognostic Score
4.4. Target Prediction and Pathway Enrichment Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BAM | Binary Alignment Map |
| BH | Benjamini–Hochberg correction |
| CI95 | 95% Confidence interval |
| GA | Gastric adenocarcinoma |
| GEO | Gene Expression Omnibus |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| miRNA | microRNA |
| NM | Adjacent non-malignant tissue |
| RPM | Read per million |
| sncRNAs | Small non-coding RNAs |
| TCGA | The Cancer Genome Atlas |
| t-SNE | t-Distributed stochastic neighbor embedding |
| UTR | Untranslated regions |
References
- Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 2014, 513, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Arun, K.; Arunkumar, G.; Bennet, D.; Chandramohan, S.M.; Murugan, A.K.; Munirajan, A.K. Comprehensive analysis of aberrantly expressed lncRNAs and construction of ceRNA network in gastric cancer. Oncotarget 2018, 9, 18386–18399. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.D.; Enfield, K.S.S.; Rowbotham, D.A.; Lam, W.L. An atlas of gastric PIWI-interacting RNA transcriptomes and their utility for identifying signatures of gastric cancer recurrence. Gastric Cancer 2016, 19, 660–665. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.; Min, B.H.; Jang, J.; Kang, S.Y.; Bae, H.; Jang, S.S.; Kim, J.-I.; Kim, K.-M. MicroRNA Expression Profiles in Gastric Carcinogenesis. Sci. Rep. 2018, 8, 14393. [Google Scholar] [CrossRef]
- Han, X.; Zhang, J.J.; Han, Z.Q.; Zhang, H.B.; Wang, Z.A. Let-7b attenuates cisplatin resistance and tumor growth in gastric cancer by targeting AURKB. Cancer Gene Ther. 2018, 25, 300–308. [Google Scholar] [CrossRef]
- Li, F.; Yoshizawa, J.M.; Kim, K.M.; Kanjanapangka, J.; Grogan, T.R.; Wang, X.; Elashoff, D.E.; Ishikawa, S.; Chia, D.; Liao, W.; et al. Discovery and Validation of Salivary Extracellular RNA Biomarkers for Noninvasive Detection of Gastric Cancer. Clin. Chem. 2018, 64, 1513–1521. [Google Scholar] [CrossRef]
- Chen, X.; Xie, D.; Zhao, Q.; You, Z.H. MicroRNAs and complex diseases: From experimental results to computational models. Brief. Bioinform. 2019, 20, 515–539. [Google Scholar] [CrossRef]
- Friedländer, M.R.; Mackowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. miRDeep2 accurately identifies known and hundreds of novel microRNA genes in seven animal clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Londin, E.; Loher, P.; Telonis, A.G.; Quann, K.; Clark, P.; Jing, Y.; Hatzmichael, E.; Kirino, Y.; Honda, S.; Lally, M.; et al. Analysis of 13 cell types reveals evidence for the expression of numerous novel primate- and tissue-specific microRNAs. Proc. Natl. Acad. Sci. USA 2015, 112, E1106–E1115. [Google Scholar] [CrossRef]
- Minatel, B.C.; Martinez, V.D.; Ng, K.W.; Sage, A.P.; Tokar, T.; Marshall, E.A.; Anderson, C.; Enfield, K.S.S.; Stewart, G.L.; Reis, P.P.; et al. Large-scale discovery of previously undetected microRNAs specific to human liver. Hum. Genom. 2018, 12, 16. [Google Scholar] [CrossRef]
- Barros-Filho, M.C.; Pewarchuk, M.; Minatel, B.C.; Sage, A.P.; Marshall, E.A.; Martinez, V.D.; Rock, L.D.; MacAulay, G.; Kowalski, L.P.; Rogatto, S.R.; et al. Previously undescribed thyroid-specific miRNA sequences in papillary thyroid carcinoma. J. Hum. Genet. 2019, 64, 505–508. [Google Scholar] [CrossRef] [PubMed]
- Sage, A.P.; Minatel, B.C.; Marshall, E.A.; Martinez, V.D.; Stewart, G.L.; Enfield, K.S.S.; Lam, W.L. Expanding the miRNA Transcriptome of Human Kidney and Renal Cell Carcinoma. Int. J. Genom. 2018, 2018, 6972397. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, X.; Zou, H.; Bai, R.; Yang, K.; Tian, Z. Hypoxia Promotes Gastric Cancer Malignancy Partly through the HIF-1α Dependent Transcriptional Activation of the Long Non-coding RNA GAPLINC. Front. Physiol. 2016, 7, 420. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Chen, C.; Zhou, Z.H.; Gao, S.T.; Tee, T.J.; Yang, L.Q.; Xu, Y.Y.; Pang, T.H.; Xu, X.Y.; Sun, Q.; et al. Hypoxia-inducible factor-1 alpha Correlates with Tumor-Associated Macrophages Infiltration, Influences Survival of Gastric Cancer Patients. J. Cancer 2017, 8, 1818–1825. [Google Scholar] [CrossRef]
- Wang, Y.W.; Chen, X.; Gao, J.W.; Zhang, H.; Ma, R.R.; Gao, Z.H.; Gao, P. High expression of cAMP-responsive element-binding protein 1 (CREB1) is associated with metastasis, tumor stage and poor outcome in gastric cancer. Oncotarget 2015, 6, 10646–10657. [Google Scholar] [CrossRef]
- Bibi, F.; Naseer, M.I.; Alvi, S.A.; Yasir, M.; Jiman-Fatani, A.A.; Sawan, A.; Abuzenadah, A.M.; Ai-Qahtaniet, M.H.; Azhar, E.I. microRNA analysis of gastric cancer patients from Saudi Arabian population. BMC Genomics 2016, 17, 751. [Google Scholar] [CrossRef]
- Chen, W.; Lin, G.; Yao, Y.; Chen, J.; Shui, H.; Yang, Q.; Wang, X.Y.; Weng, X.Y.; Sun, L.; Chen, F.; et al. MicroRNA hsa-let-7e-5p as a potential prognosis marker for rectal carcinoma with liver metastases. Oncol. Lett. 2018, 15, 6913–6924. [Google Scholar] [CrossRef]
- Backes, C.; Meder, B.; Hart, M.; Ludwig, N.; Leidinger, P.; Vogel, B.; Galata, V.; Roth, P.; Menegatti, J.; Grasser, F.; et al. Prioritizing and selecting likely novel miRNAs from NGS data. Nucleic Acids Res. 2016, 44, e53. [Google Scholar] [CrossRef][Green Version]
- Martinez, V.D.; Marshall, E.A.; Anderson, C.; Ng, K.W.; Minatel, B.C.; Sage, A.P.; Enfield, K.S.S.; Xu, Z.; Lam, W.L. Discovery of Previously Undetected MicroRNAs in Mesothelioma and Their Use as Tissue-of-Origin Markers. Am. J. Respir. Cell Mol. Biol. 2019, 61, 266–268. [Google Scholar] [CrossRef]
- Fehlmann, T.; Backes, C.; Kahraman, M.; Haas, J.; Ludwig, N.; Posch, A.E.; Wurstle, M.L.; Hubenthal, M.; Franke, A.; Meder, B.; et al. Web-based NGS data analysis using miRMaster: A large-scale meta-analysis of human miRNAs. Nucleic Acids Res. 2017, 45, 8731–8744. [Google Scholar] [CrossRef]
- Kozomara, A.; Griffiths-Jones, S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014, 42, D68–D73. [Google Scholar] [CrossRef] [PubMed]
- Enright, A.J.; John, B.; Gaul, U.; Tuschl, T.; Sander, C.; Marks, D.S. MicroRNA targets in Drosophila. Genome Biol. 2003, 5, R1. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, S.; Abovsky, M.; Pastrello, C.; Jurisica, I. pathDIP: An annotated resource for known and predicted human gene-pathway associations and pathway enrichment analysis. Nucleic Acids Res. 2017, 45, D419–D426. [Google Scholar] [CrossRef]

| Features 1 | Number (%) |
|---|---|
| Gender | |
| Male | 280 (64.5) |
| Female | 154 (35.5) |
| Age | |
| Median | 67 |
| ≥55 | 362 (83.4) |
| <55 | 69 (15.9) |
| N/A | 3 |
| Tumor Histologic Grade | |
| G1 | 10 (2.3) |
| G2 | 155 (35.7) |
| G3 | 260 (59.9) |
| GX | 9 (2.1) |
| Distant Metastasis (M) | |
| M0 | 382 (88) |
| M1 | 30 (6.9) |
| MX | 22 (5.1) |
| Regional Lymph Nodes (N) | |
| N0 | 129 (29.7) |
| N1 | 116 (26.7) |
| N2 | 85 (19.6) |
| N3 | 86 (19.8) |
| NX | 17 (3.9) |
| Discrepancy | 1 (0.2) |
| Primary Tumor (T) | |
| T1 | 23 (5.3) |
| T2 | 91 (21) |
| T3 | 192 (44.2) |
| T4 | 118 (27.2) |
| TX | 10 (2.3) |
| Stage | |
| I | 58 (13.4) |
| II | 128 (29.5) |
| III | 182 (41.9) |
| IV | 42 (9.7) |
| N/A | 16 (3.7) |
| Discrepancy | 8 (1.8) |
| H. pylori | |
| Yes | 19 (4.4) |
| No | 161 (37.1) |
| N/A | 254 (58.5) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pewarchuk, M.E.; Barros-Filho, M.C.; Minatel, B.C.; Cohn, D.E.; Guisier, F.; Sage, A.P.; Marshall, E.A.; Stewart, G.L.; Rock, L.D.; Garnis, C.; et al. Upgrading the Repertoire of miRNAs in Gastric Adenocarcinoma to Provide a New Resource for Biomarker Discovery. Int. J. Mol. Sci. 2019, 20, 5697. https://doi.org/10.3390/ijms20225697
Pewarchuk ME, Barros-Filho MC, Minatel BC, Cohn DE, Guisier F, Sage AP, Marshall EA, Stewart GL, Rock LD, Garnis C, et al. Upgrading the Repertoire of miRNAs in Gastric Adenocarcinoma to Provide a New Resource for Biomarker Discovery. International Journal of Molecular Sciences. 2019; 20(22):5697. https://doi.org/10.3390/ijms20225697
Chicago/Turabian StylePewarchuk, Michelle E., Mateus C. Barros-Filho, Brenda C. Minatel, David E. Cohn, Florian Guisier, Adam P. Sage, Erin A. Marshall, Greg L. Stewart, Leigha D. Rock, Cathie Garnis, and et al. 2019. "Upgrading the Repertoire of miRNAs in Gastric Adenocarcinoma to Provide a New Resource for Biomarker Discovery" International Journal of Molecular Sciences 20, no. 22: 5697. https://doi.org/10.3390/ijms20225697
APA StylePewarchuk, M. E., Barros-Filho, M. C., Minatel, B. C., Cohn, D. E., Guisier, F., Sage, A. P., Marshall, E. A., Stewart, G. L., Rock, L. D., Garnis, C., & Lam, W. L. (2019). Upgrading the Repertoire of miRNAs in Gastric Adenocarcinoma to Provide a New Resource for Biomarker Discovery. International Journal of Molecular Sciences, 20(22), 5697. https://doi.org/10.3390/ijms20225697





