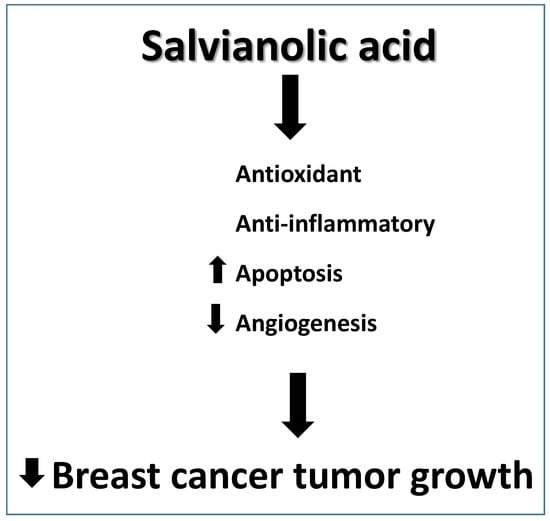
Salvianolic Acid B Slows the Progression of Breast Cancer Cell Growth via Enhancement of Apoptosis and Reduction of Oxidative Stress, Inflammation, and Angiogenesis
Abstract
1. Introduction
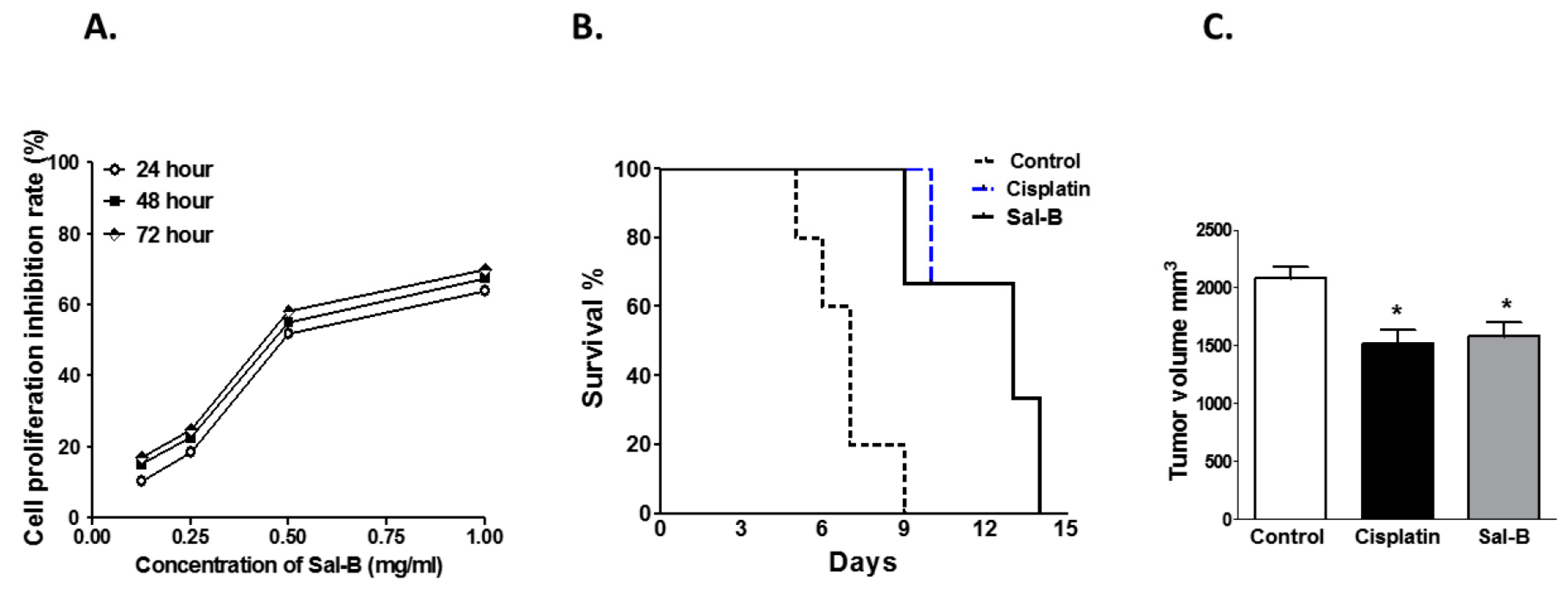
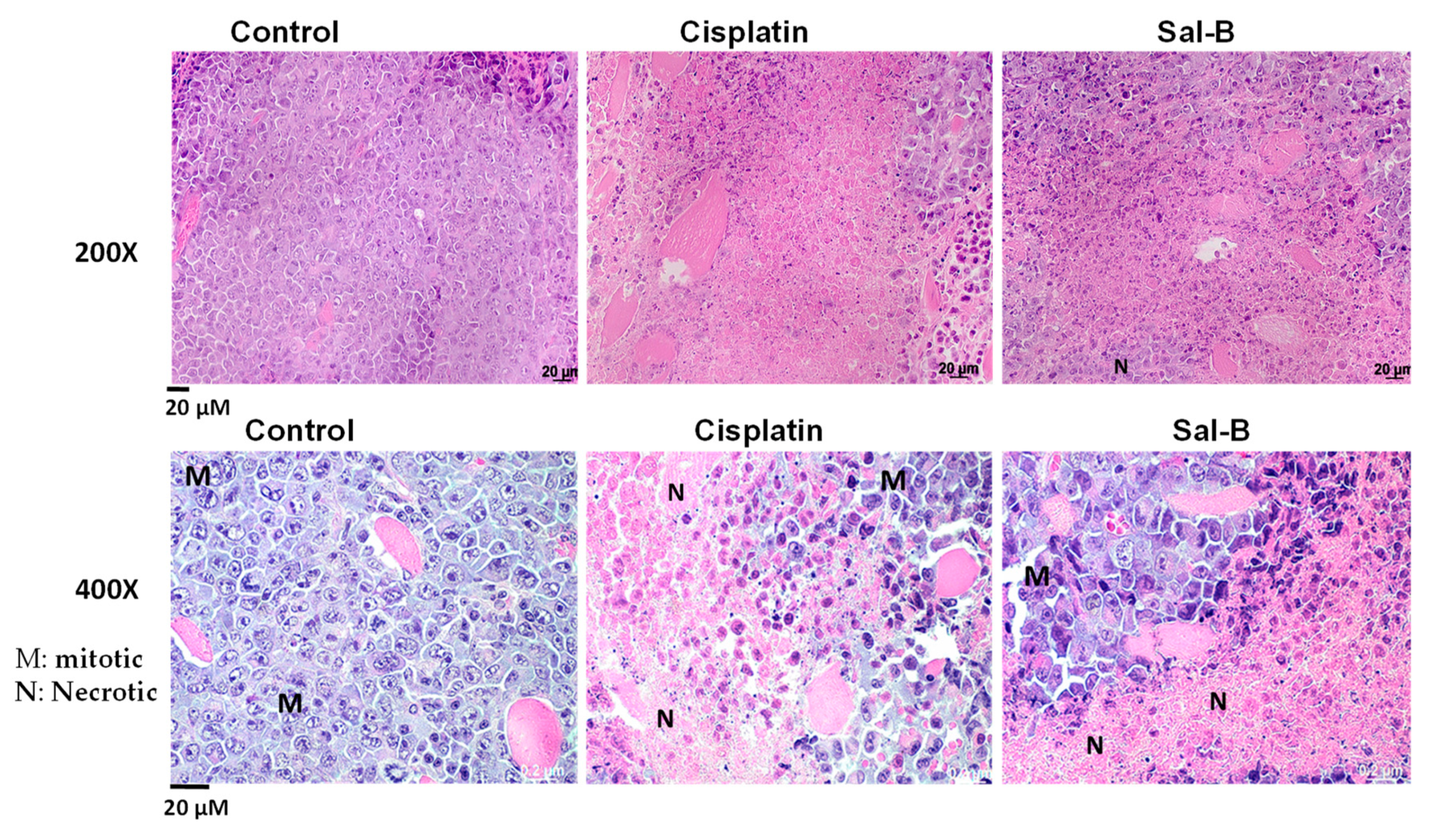
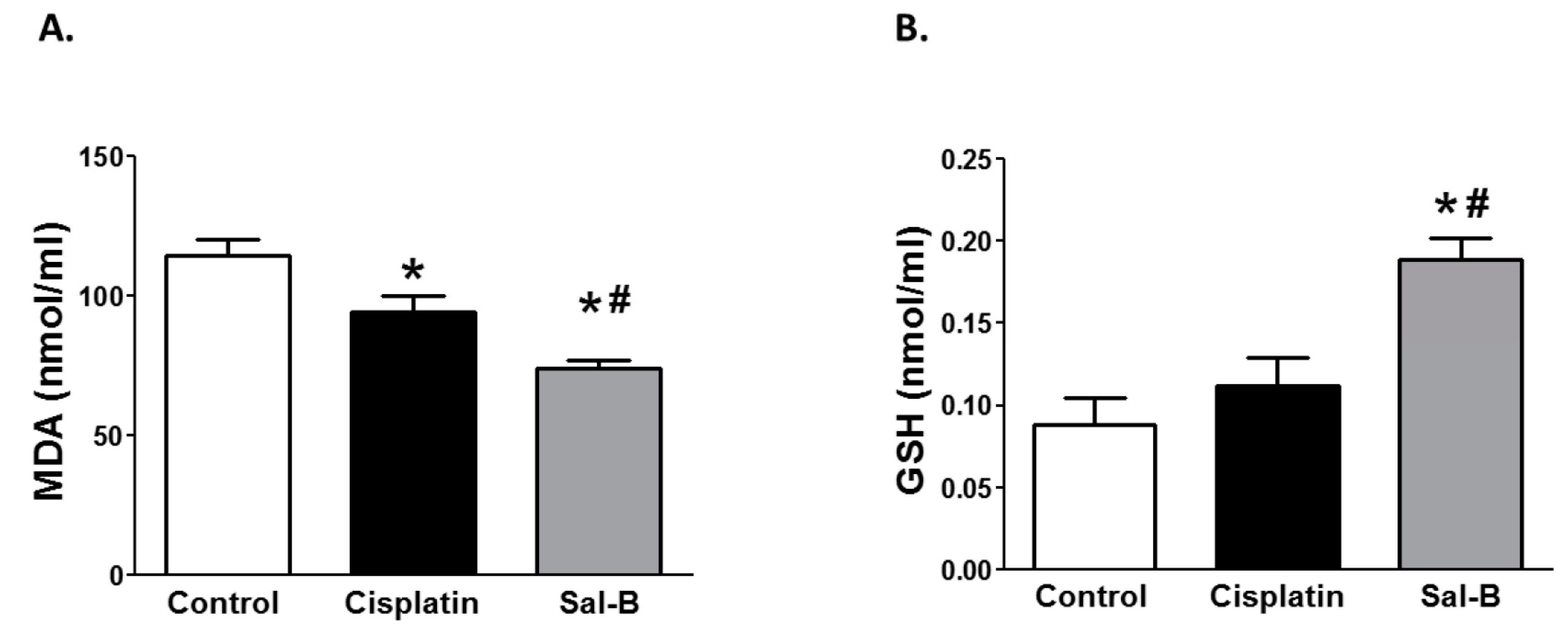
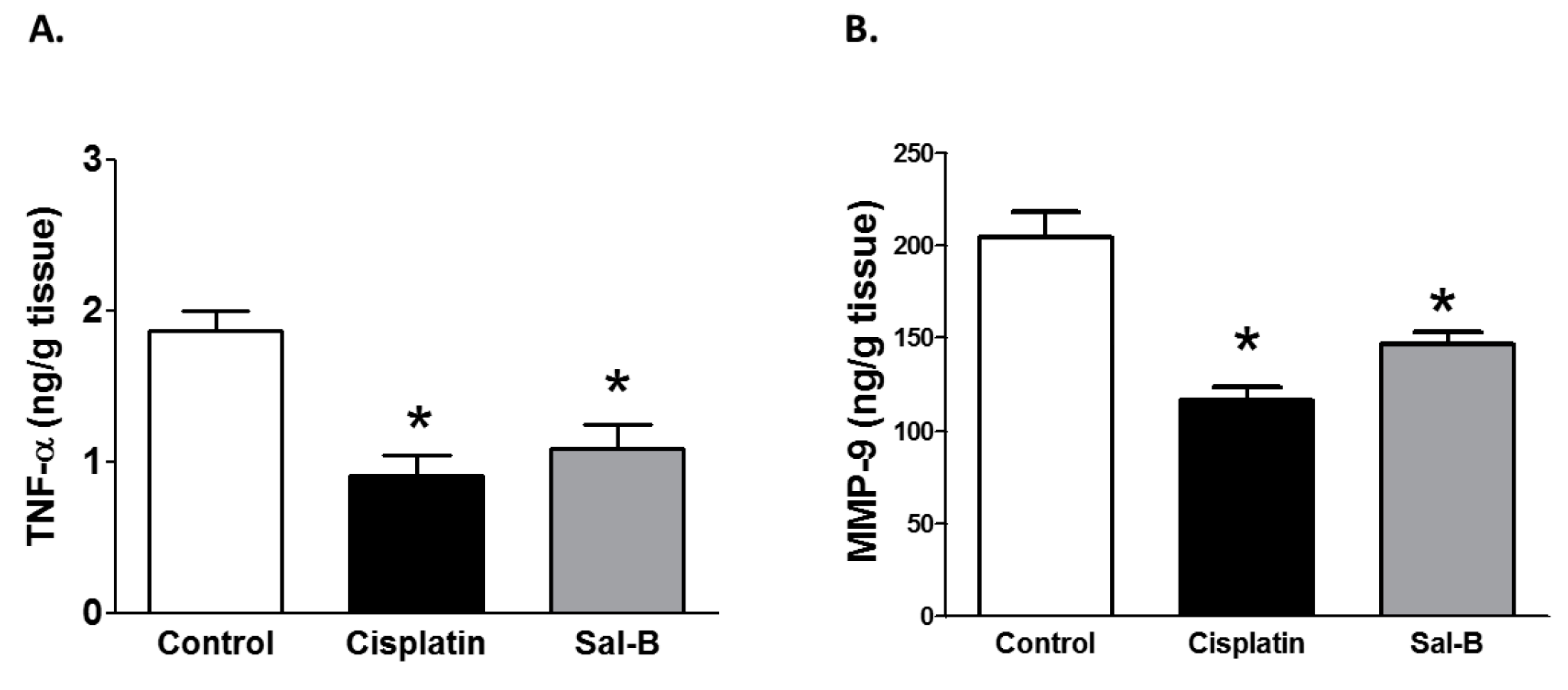
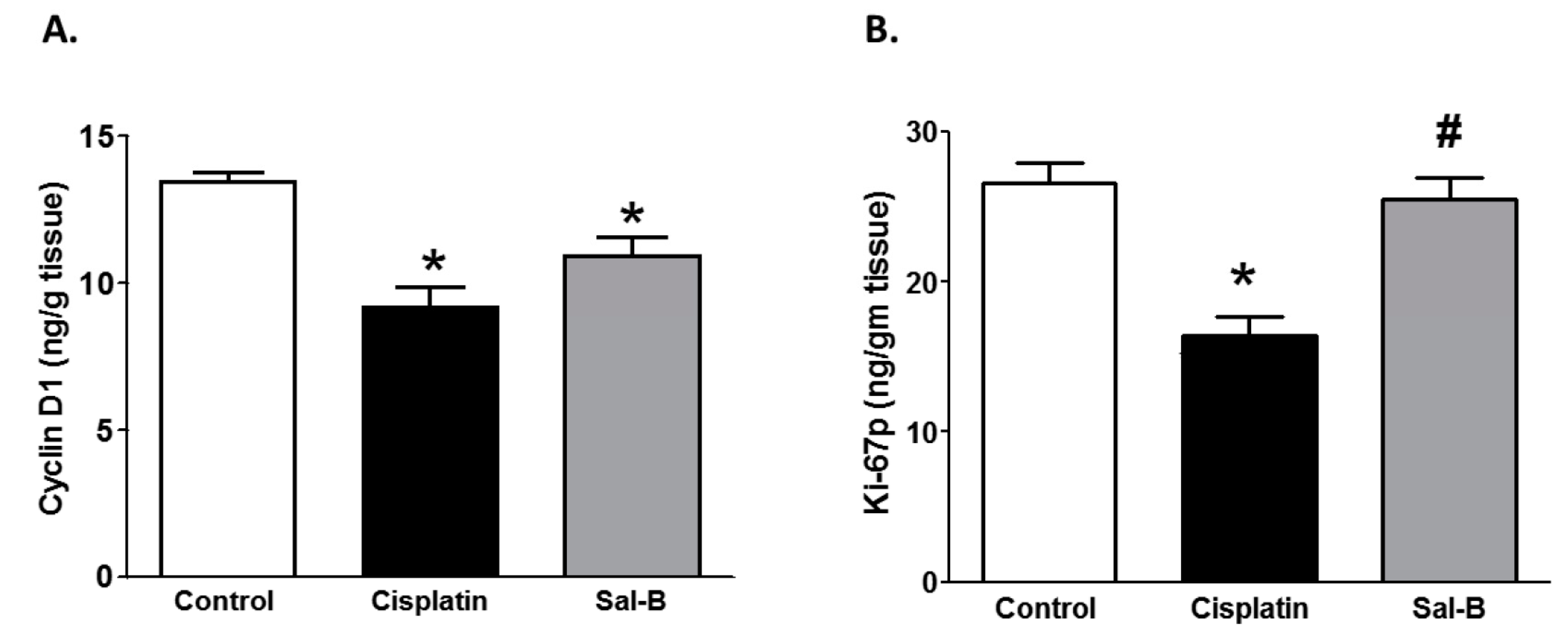
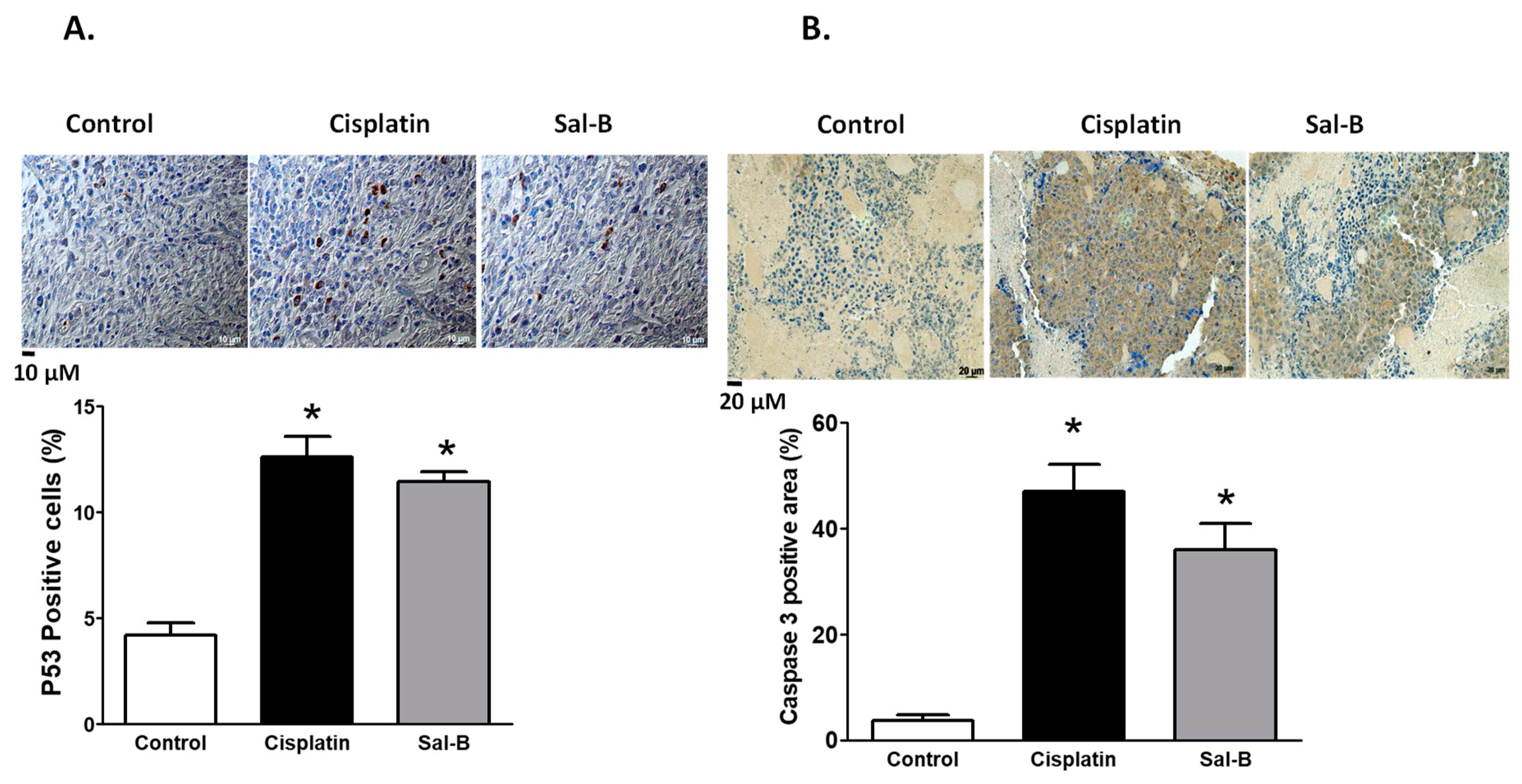
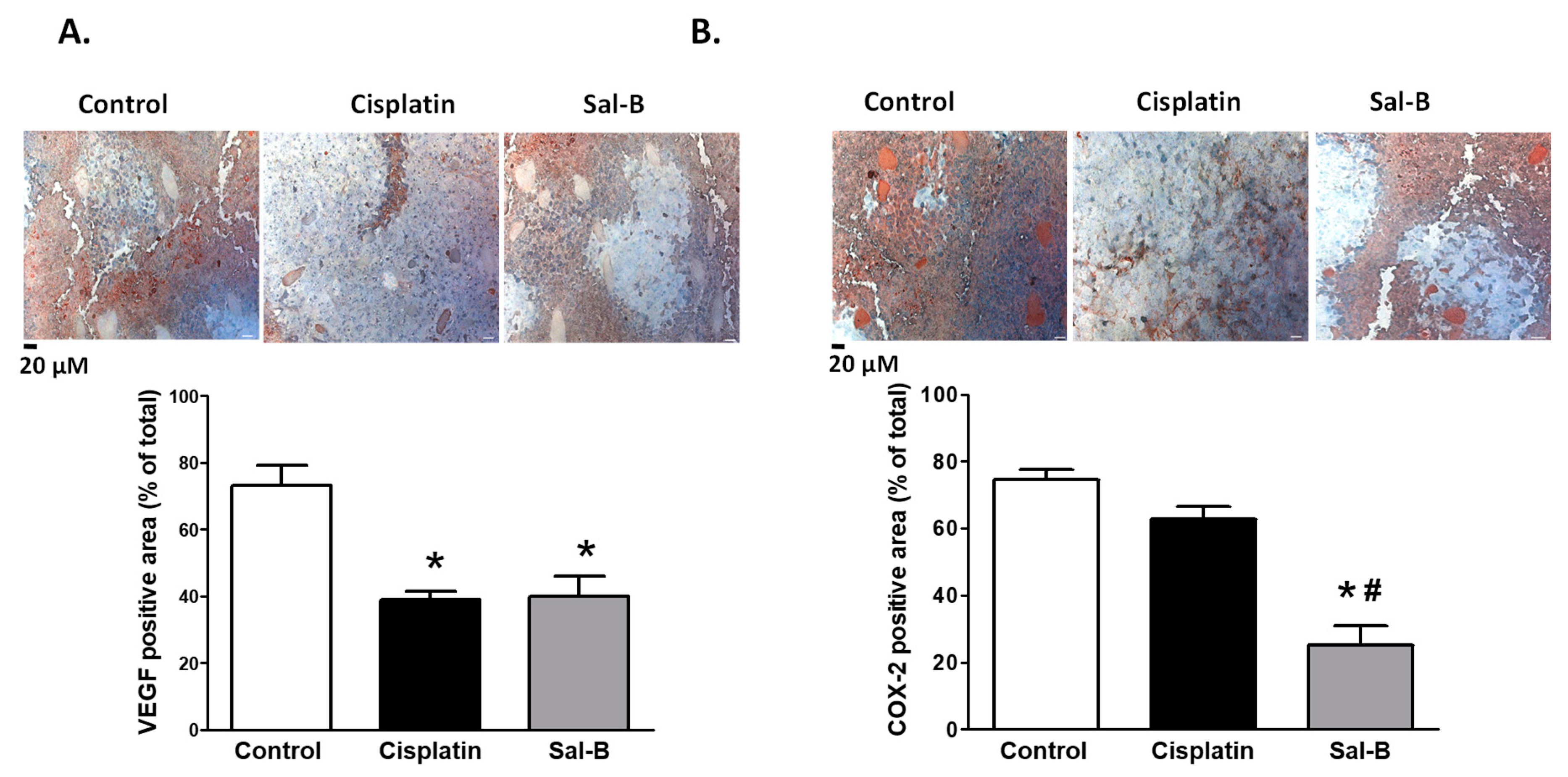
2. Results
3. Discussion
4. Material and Methods
4.1. MCF-7 Cell Culture
4.2. Determination of Cell Proliferation Inhibition Rate by MTT Assay
4.3. Animals Studies
- Group 1: Control Ehrlich Solid Carcinoma (ESC)—Mice Were Injected subcutaneous (S.C.) with 0.2 mL of Ehrlich Cell Line (1 × 106 Cells) Obtained from the Pharmacology and Experimental Oncology Unit of the National Cancer Institute, Cairo University, Egypt) into the Left Hind Leg.
- Group 2: Cisplatin— Mice were injected S.C. with 0.2 mL Ehrlich cell line (1 × 106 cells) into the left hind leg. On the seventh day after tumor implantation, mice received intraperitoneal (I.P.) injection of cisplatin (Sigma, St. Louis, MO, USA) as a single dose of 3.5 mg/kg, as previously prescribed [59].
- Group 3: Salvianolic acid B (Sal-B)—Mice were injected S.C. with 0.2 mL of Ehrlich cell line (1 × 106 cells) into the left hind leg. On the seventh day after tumor implantation, mice received I.P. injection of Sal-B (Sigma) 25 mg/kg [60] daily for two weeks.
4.4. Assessment of the Tumor Volume
4.5. Assessment of the Biochemical Parameters
4.6. Histopathological Examination
4.7. Immuno-Histochemical Examination
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.; Ma, J.; Zou, Z.; Jemal, A. Cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 9–29. [Google Scholar] [CrossRef] [PubMed]
- Sha, W.; Zhou, Y.; Ling, Z.Q.; Xie, G.; Pang, X.; Wang, P.; Gu, X. Antitumor properties of Salvianolic acid B against triple-negative and hormone receptor-positive breast cancer cells via ceramide-mediated apoptosis. Oncotarget 2018, 9, 36331–36343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Diana, A.; Franzese, E.; Centonze, S.; Carlino, F.; Della Corte, C.M.; Ventriglia, J.; Petrillo, A.; De Vita, F.; Alfano, R.; Ciardiello, F.; et al. Triple-negative breast cancers: Systematic review of the literature on molecular and clinical features with a focus on treatment with innovative drugs. Curr. Oncol. Rep. 2018, 20, 76. [Google Scholar] [CrossRef] [PubMed]
- Bahr, H.I.; Toraih, E.A.; Mohammed, E.A.; Mohammad, H.M.; Ali, E.A.; Zaitone, S.A. Chemopreventive effect of leflunomide against Ehrlich’s solid tumor grown in mice: Effect on EGF and EGFR expression and tumor proliferation. Life Sci. 2015, 141, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Barakat, W.; Elshazly, S.M.; Mahmoud, A.A. Spirulina platensis lacks antitumor effect against solid ehrlich carcinoma in female mice. Adv. Pharmacol. Sci. 2015, 2015, 132873. [Google Scholar] [PubMed]
- Sherif, I.O.; Al-Gayyar, M.M. Antioxidant, anti-inflammatory and hepatoprotective effects of silymarin on hepatic dysfunction induced by sodium nitrite. Eur. Cytokine Netw. 2013, 24, 114–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.F.; Zhang, J.; Li, R. Salvianolic acid B attenuates lung inflammation induced by cigarette smoke in mice. Eur. J. Pharmacol. 2015, 761, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guo, Y.; Gu, X. Salvianolic Acid B, a potential chemopreventive agent, for head and neck squamous cell cancer. J. Oncol. 2011, 2011, 534548. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, F.; Chen, J.; Shen, X.; Deng, Y.; Xu, L.; Yin, J.; Chen, H.; Teng, F.; Liu, X.; et al. Matrix metalloproteinase-9 induces cardiac fibroblast migration, collagen and cytokine secretion: Inhibition by salvianolic acid B from Salvia miltiorrhiza. Phytomedicine 2011, 19, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Yue, J.; Li, B.; Jing, Q.; Guan, Q. Salvianolic acid B accelerated ABCA1-dependent cholesterol efflux by targeting PPAR-gamma and LXRalpha. Biochem. Biophys. Res. Commun. 2015, 462, 233–238. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Sun, G.; Wu, P.; Chen, R.; Yao, F.; Qin, M.; Luo, Y.; Sun, H.; Zhang, Q.; Dong, X.; et al. Salvianolic acid B prevents arsenic trioxide-induced cardiotoxicity in vivo and enhances its anticancer activity in vitro. Evid. Based Complement. Altern. Med. 2013, 2013, 759483. [Google Scholar]
- Hao, Y.; Xie, T.; Korotcov, A.; Zhou, Y.; Pang, X.; Shan, L.; Ji, H.; Sridhar, R.; Wang, P.; Califano, J.; et al. Salvianolic acid B inhibits growth of head and neck squamous cell carcinoma in vitro and in vivo via cyclooxygenase-2 and apoptotic pathways. Int. J. Cancer 2009, 124, 2200–2209. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lei, C.K.; Shan, M. [Study of growth inhibitory effect and apoptosis induced by different matches of Tanshinone IIA and Salvianolic Acid B on Acute Promyelocytic Leukemia cells (HL-60)]. Zhong Yao Cai 2008, 31, 1512–1514. [Google Scholar] [PubMed]
- Wang, Z.S.; Luo, P.; Dai, S.H.; Liu, Z.B.; Zheng, X.R.; Chen, T. Salvianolic acid B induces apoptosis in human glioma U87 cells through p38-mediated ROS generation. Cell. Mol. Neurobiol. 2013, 33, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Karasawa, T.; Steyger, P.S. An integrated view of cisplatin-induced nephrotoxicity and ototoxicity. Toxicol. Lett. 2015, 237, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Pruefer, F.G.; Lizarraga, F.; Maldonado, V.; Melendez-Zajgla, J. Participation of Omi Htra2 serine-protease activity in the apoptosis induced by cisplatin on SW480 colon cancer cells. J. Chemother. 2008, 20, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Ikitimur-Armutak, E.I.; Sonmez, K.; Akgun-Dar, K.; Sennazli, G.; Kapucu, A.; Yigit, F.; Yilmaz, V.T.; Ulukaya, E. Anticancer effect of a novel palladium-saccharinate complex of terpyridine by inducing apoptosis on Ehrlich ascites carcinoma (EAC) in Balb-C mice. Anticancer Res. 2015, 35, 1491–1497. [Google Scholar] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nakagawara, A. p53: The attractive tumor suppressor in the cancer research field. J. Biomed. Biotechnol. 2011, 2011, 603925. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. VEGF as a key mediator of angiogenesis in cancer. Oncology 2005, 69 (Suppl. 3), 4–10. [Google Scholar] [CrossRef] [PubMed]
- Fosslien, E. Review: Molecular pathology of cyclooxygenase-2 in cancer-induced angiogenesis. Ann. Clin. Lab. Sci. 2001, 31, 325–348. [Google Scholar] [PubMed]
- Wei, J.; Xie, G.; Ge, S.; Qiu, Y.; Liu, W.; Lu, A.; Chen, T.; Li, H.; Zhou, Z.; Jia, W. Metabolic transformation of DMBA-induced carcinogenesis and inhibitory effect of salvianolic acid b and breviscapine treatment. J. Proteome Res. 2012, 11, 1302–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.T.; Yang, Y.; Ge, J.P. The preventive effect of salvianolic acid B on malignant transformation of DMBA-induced oral premalignant lesion in hamsters. Carcinogenesis 2006, 27, 826–832. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.L.; Wang, X.J.; Sun, Y.N.; Li, C.R.; Xing, Y.L.; Zhao, H.B.; Duan, M.; Zhou, Z.; Wang, S.Q. Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6-hydroxydopamine induced apoptosis in SH-SY5Y cells. Int. J. Biochem. Cell Biol. 2008, 40, 409–422. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Lambert, J.D.; Sang, S. Antioxidative and anti-carcinogenic activities of tea polyphenols. Arch. Toxicol. 2009, 83, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Cheng, Y.; Hu, J.F.; Zhang, W.; Chen, N.H.; Zhang, J.T. Comparison of antioxidant activities between salvianolic acid B and Ginkgo biloba extract (EGb 761). Acta Pharmacol. Sin. 2006, 27, 1137–1145. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhu, H.; Wang, J.; Liu, Z.; Bi, J. Isolation and purification of salvianolic acid A and salvianolic acid B from Salvia miltiorrhiza by high-speed counter-current chromatography and comparison of their antioxidant activity. J. Chromatogr. B 2009, 877, 733–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.H.; Du, G.H.; Zhang, J.T. Salvianolic acid B protects brain against injuries caused by ischemia-reperfusion in rats. Acta Pharmacol. Sin. 2000, 21, 463–466. [Google Scholar] [PubMed]
- Wang, X.; Zhu, G. [Study on protective effect of salvianolic acid B on glutamate-induced excitotoxicity in pheochromocytoma PC12 cells]. Zhongguo Zhong Yao Za Zhi 2012, 37, 353–357. [Google Scholar] [PubMed]
- Pelicano, H.; Carney, D.; Huang, P. ROS stress in cancer cells and therapeutic implications. Drug Resist. Updates 2004, 7, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rahman, M.N.; Kabel, A.M. Comparative study between the effect of methotrexate and valproic acid on solid Ehrlich tumour. J. Egypt. Natl. Cancer Inst. 2012, 24, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Xu, M.; Dai, X.; Ni, T.; Li, D.; Jin, F.; Wang, H.; Tao, L.; Pan, B.; Woodgett, J.R.; et al. Polypharmacological profiles underlying the antitumor property of salvia miltiorrhiza root (Danshen) interfering with NOX-dependent neutrophil extracellular traps. Oxid. Med. Cell. Longev. 2018, 2018, 4908328. [Google Scholar] [CrossRef] [PubMed]
- Tsai, M.K.; Lin, Y.L.; Huang, Y.T. Effects of salvianolic acids on oxidative stress and hepatic fibrosis in rats. Toxicol. Appl. Pharmacol. 2010, 242, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Yao, G.; Xu, L.; Wu, X.; Xu, L.; Yang, J.; Chen, H. Preventive effects of salvianolic acid B on transforming growth factor-beta1-induced epithelial-to-mesenchymal transition of human kidney cells. Biol. Pharm. Bull. 2009, 32, 882–886. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Pang, M.; Rong, L.; Liu, C.; Wang, J.; Zhou, W.; Wang, X.; Liu, B. Protective effects and mechanisms of salvianolic acid b against H2O2-induced injury in induced pluripotent stem cell-derived neural stem cells. Neurochem. Res. 2015, 40, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Seo, M.; Lee, E.J. Salvianolic acid B inhibits atherogenesis of vascular cells through induction of Nrf2-dependent heme oxygenase-1. Curr. Med. Chem. 2014, 21, 3095–3106. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Wang, S.; Liang, W.; Wang, W.; Wang, H.; Zhao, M.; Liu, X. Salvianolic acid B reverses multidrug resistance in HCT8/VCR human colorectal cancer cells by increasing ROS levels. Mol. Med. Rep. 2017, 15, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Schumacker, P.T. Reactive oxygen species in cancer cells: Live by the sword, die by the sword. Cancer Cell 2006, 10, 175–176. [Google Scholar] [CrossRef] [PubMed]
- Geronikaki, A.A.; Gavalas, A.M. Antioxidants and inflammatory disease: Synthetic and natural antioxidants with anti-inflammatory activity. Comb. Chem. High Throughput Screen. 2006, 9, 425–442. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Hao, Y.; Ji, H.; Fang, Y.; Guo, Y.; Sha, W.; Zhou, Y.; Pang, X.; Southerland, W.M.; Califano, J.A.; et al. Combination effects of salvianolic acid B with low-dose celecoxib on inhibition of head and neck squamous cell carcinoma growth in vitro and in vivo. Cancer Prev. Res. (Phila) 2010, 3, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.X.; Hu, L.M.; Gao, X.M.; Guo, H.; Fan, G.W. Anti-inflammatory activity of salvianolic acid B in microglia contributes to its neuroprotective effect. Neurochem. Res. 2010, 35, 1029–1037. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Ye, T.X.; Zhao, G.R.; Yuan, Y.J.; Guo, Z.X. Aqueous extract of Salvia miltiorrhiza attenuates increased endothelial permeability induced by tumor necrosis factor-alpha. Int. Immunopharmacol. 2005, 5, 1641–1651. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Hu, C.S.; Lin, F.Y.; Chen, Y.H.; Sheu, L.M.; Ku, H.H.; Shiao, M.S.; Chen, J.W.; Lin, S.J. Salvianolic acid B attenuates cyclooxygenase-2 expression in vitro in LPS-treated human aortic smooth muscle cells and in vivo in the apolipoprotein-E-deficient mouse aorta. J. Cell. Biochem. 2006, 98, 618–631. [Google Scholar] [CrossRef] [PubMed]
- Luo, P.; Tan, Z.; Zhang, Z.; Li, H.; Mo, Z. Inhibitory effects of salvianolic acid B on the high glucose-induced mesangial proliferation via NF-kappaB-dependent pathway. Biol. Pharm. Bull. 2008, 31, 1381–1386. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Luo, J.L.; Maeda, S.; Hsu, L.C.; Yagita, H.; Karin, M. Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 2004, 6, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Wang, L.; Shen, J.; Hao, S.; Ming, A.; Wang, X.; Su, F.; Zhang, Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 2015, 115, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Altiok, N.; Mezzadra, H.; Patel, P.; Koyuturk, M.; Altiok, S. A plant oxylipin, 12-oxo-phytodienoic acid, inhibits proliferation of human breast cancer cells by targeting cyclin D1. Breast Cancer Res. Treat. 2008, 109, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Wilson, T.C.; Robinson, R.A. Basal cell adenocarcinoma and Basal cell adenoma of the salivary glands: A clinicopathological review of seventy tumors with comparison of morphologic features and growth control indices. Head Neck Pathol. 2015, 9, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Yang, Z.; Xu, Y.; Chen, Y.; Yu, Q. Apoptosis, autophagy, necroptosis, and cancer metastasis. Mol. Cancer 2015, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Greenhough, A.; Smartt, H.J.; Moore, A.E.; Roberts, H.R.; Williams, A.C.; Paraskeva, C.; Kaidi, A. The COX-2/PGE2 pathway: Key roles in the hallmarks of cancer and adaptation to the tumour microenvironment. Carcinogenesis 2009, 30, 377–386. [Google Scholar] [CrossRef] [PubMed]
- Yan, F. Effects of salvianolic acid B on growth inhibition and apoptosis induction of ovarian cancer SKOV3. Eur. J. Gynaecol. Oncol. 2016, 37, 653–656. [Google Scholar] [PubMed]
- Jing, Z.; Fei, W.; Zhou, J.; Zhang, L.; Chen, L.; Zhang, X.; Liang, X.; Xie, J.; Fang, Y.; Sui, X.; et al. Salvianolic acid B, a novel autophagy inducer, exerts antitumor activity as a single agent in colorectal cancer cells. Oncotarget 2016, 7, 61509–61519. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Di, C.; Xia, X.; Wang, J.; Chen, G.; Shi, J.; Chen, P.; Xu, H.; Zhang, W. AKT/mTOR signaling pathway is involved in salvianolic acid B-induced autophagy and apoptosis in hepatocellular carcinoma cells. Int. J. Oncol. 2016, 49, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Sadick, H.; Naim, R.; Gossler, U.; Hormann, K.; Riedel, F. Angiogenesis in hereditary hemorrhagic telangiectasia: VEGF165 plasma concentration in correlation to the VEGF expression and microvessel density. Int. J. Mol. Med. 2005, 15, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Ge, P.J.; Jiang, L.; Li, F.L.; Zhu, Q.Y. Modulation of growth and angiogenic potential of oral squamous carcinoma cells in vitro using salvianolic acid B. BMC Complementary Altern. Med. 2011, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Gately, S.; Li, W.W. Multiple roles of COX-2 in tumor angiogenesis: A target for antiangiogenic therapy. Semin. Oncol. 2004, 31, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Dhamija, I.; Kumar, N.; Manjula, S.N.; Parihar, V.; Setty, M.M.; Pai, K.S. Preliminary evaluation of in vitro cytotoxicity and in vivo antitumor activity of Premna herbacea Roxb. In Ehrlich ascites carcinoma model and Dalton’s lymphoma ascites model. Exp. Toxicol. Pathol. 2013, 65, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, Y.; Zhai, X.; Lin, M.; Chen, Z.; Tian, X.; Zhang, F.; Gao, D.; Ma, X.; Lv, L.; et al. Salvianolic acid A preconditioning confers protection against concanavalin A-induced liver injury through SIRT1-mediated repression of p66shc in mice. Toxicol. Appl. Pharmacol. 2013, 273, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Qin, T.; Rasul, A.; Sarfraz, A.; Sarfraz, I.; Hussain, G.; Anwar, H.; Riaz, A.; Liu, S.; Wei, W.; Li, J.; et al. Salvianolic acid A & B: Potential cytotoxic polyphenols in battle against cancer via targeting multiple signaling pathways. Int. J. Biol. Sci. 2019, 15, 2256–2264. [Google Scholar] [PubMed]
- Li, H.; Shi, L.; Wei, J.; Zhang, C.; Zhou, Z.; Wu, L.; Liu, W. Cellular uptake and anticancer activity of salvianolic acid B phospholipid complex loaded nanoparticles in head and neck cancer and precancer cells. Colloids Surf. B Biointerfaces 2016, 147, 65–72. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katary, M.A.; Abdelsayed, R.; Alhashim, A.; Abdelhasib, M.; Elmarakby, A.A. Salvianolic Acid B Slows the Progression of Breast Cancer Cell Growth via Enhancement of Apoptosis and Reduction of Oxidative Stress, Inflammation, and Angiogenesis. Int. J. Mol. Sci. 2019, 20, 5653. https://doi.org/10.3390/ijms20225653
Katary MA, Abdelsayed R, Alhashim A, Abdelhasib M, Elmarakby AA. Salvianolic Acid B Slows the Progression of Breast Cancer Cell Growth via Enhancement of Apoptosis and Reduction of Oxidative Stress, Inflammation, and Angiogenesis. International Journal of Molecular Sciences. 2019; 20(22):5653. https://doi.org/10.3390/ijms20225653
Chicago/Turabian StyleKatary, Mohamed A., Rafik Abdelsayed, Abdulmohsin Alhashim, Mohamed Abdelhasib, and Ahmed A. Elmarakby. 2019. "Salvianolic Acid B Slows the Progression of Breast Cancer Cell Growth via Enhancement of Apoptosis and Reduction of Oxidative Stress, Inflammation, and Angiogenesis" International Journal of Molecular Sciences 20, no. 22: 5653. https://doi.org/10.3390/ijms20225653
APA StyleKatary, M. A., Abdelsayed, R., Alhashim, A., Abdelhasib, M., & Elmarakby, A. A. (2019). Salvianolic Acid B Slows the Progression of Breast Cancer Cell Growth via Enhancement of Apoptosis and Reduction of Oxidative Stress, Inflammation, and Angiogenesis. International Journal of Molecular Sciences, 20(22), 5653. https://doi.org/10.3390/ijms20225653