The Novel Perspectives of Adipokines on Brain Health
Abstract
1. Introduction
2. Effects of Conventional and Novel Adipokines on the Brain
2.1. Leptin
2.2. Adiponectin
2.3. Chemerin
2.4. Apelin
2.5. Visfatin (Nicotinamide phosphoribosyltransferase, NAMPT)
3. Novel Adipocyte-Derived Messengers on Brain Health
3.1. Palmitoleic Acid (16:1n7)
3.2. Lysophosphatidic Acid
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kershaw, E.E.; Flier, J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004, 89, 2548–2556. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Sun, H.M.; Hwang, K.C.; Kim, S.W. Adipose-derived stromal vascular fraction cells: Update on clinical utility and efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef] [PubMed]
- Warner, A.; Mittag, J. Brown fat and vascular heat dissipation: The new cautionary tail. Adipocyte 2014, 3, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Cedikova, M.; Kripnerova, M.; Dvorakova, J.; Pitule, P.; Grundmanova, M.; Babuska, V.; Mullerova, D.; Kuncova, J. Mitochondria in white, brown, and beige adipocytes. Stem Cells Int. 2016, 2016, 6067349. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.E.; Ahmadian, M.; Jaworski, K.; Sarkadi-Nagy, E.; Sul, H.S. Regulation of lipolysis in adipocytes. Annu. Rev. Nutr. 2007, 27, 79–101. [Google Scholar] [CrossRef] [PubMed]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose tissue remodeling: Its role in energy metabolism and metabolic disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Cook, K.S.; Min, H.Y.; Johnson, D.; Chaplinsky, R.J.; Flier, J.S.; Hunt, C.R.; Spiegelman, B.M. Adipsin: A circulating serine protease homolog secreted by adipose tissue and sciatic nerve. Science 1987, 237, 402–405. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Rosen, E.D.; Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, K.; Sypniewska, G. Diabetes as a complication of adipose tissue dysfunction. Is there a role for potential new biomarkers? Clin. Chem. Lab. Med. 2013, 51, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Flier, J.S.; Maratos-Flier, E. Lasker lauds leptin. Cell 2010, 143, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Solovyova, N.; Irving, A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- De la Monte, S.M.; Wands, J.R. Review of insulin and insulin-like growth factor expression, signaling, and malfunction in the central nervous system: Relevance to Alzheimer’s disease. J. Alzheimers Dis. 2005, 7, 45–61. [Google Scholar] [CrossRef] [PubMed]
- Steen, E.; Terry, B.M.; Rivera, E.J.; Cannon, J.L.; Neely, T.R.; Tavares, R.; Xu, X.J.; Wands, J.R.; de la Monte, S.M. Impaired insulin and insulin-like growth factor expression and signaling mechanisms in Alzheimer’s disease—Is this type 3 diabetes? J. Alzheimers Dis. 2005, 7, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Schioth, H.B.; Craft, S.; Brooks, S.J.; Frey, W.H., 2nd; Benedict, C. Brain insulin signaling and Alzheimer’s disease: Current evidence and future directions. Mol. Neurobiol. 2012, 46, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Craft, S. Alzheimer disease: Insulin resistance and AD—Extending the translational path. Nat. Rev. Neurol. 2012, 8, 360–362. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Scherer, P.E. Adiponectin, driver or passenger on the road to insulin sensitivity? Mol. Metab. 2013, 2, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Maeda, N.; Shimomura, I.; Kishida, K.; Nishizawa, H.; Matsuda, M.; Nagaretani, H.; Furuyama, N.; Kondo, H.; Takahashi, M.; Arita, Y.; et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat. Med. 2002, 8, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Ng, R.C.; Cheng, O.Y.; Jian, M.; Kwan, J.S.; Ho, P.W.; Cheng, K.K.; Yeung, P.K.; Zhou, L.L.; Hoo, R.L.; Chung, S.K.; et al. Chronic adiponectin deficiency leads to Alzheimer’s disease-like cognitive impairments and pathologies through AMPK inactivation and cerebral insulin resistance in aged mice. Mol. Neurodegener. 2016, 11, 71. [Google Scholar] [CrossRef] [PubMed]
- Yaffe, K. Metabolic syndrome and cognitive decline. Curr. Alzheimer Res. 2007, 4, 123–126. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef] [PubMed]
- Stoeckel, L.E.; Arvanitakis, Z.; Gandy, S.; Small, D.; Kahn, C.R.; Pascual-Leone, A.; Sherwin, R.; Smith, P. Complex mechanisms linking neurocognitive dysfunction to insulin resistance and other metabolic dysfunction. F1000Research 2016, 5, 353. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, D. Adiposity indices and dementia. Lancet Neurol. 2006, 5, 713–720. [Google Scholar] [CrossRef]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J. Biol. Chem. 2008, 283, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Yau, S.Y.; Li, A.; Hoo, R.L.; Ching, Y.P.; Christie, B.R.; Lee, T.M.; Xu, A.; So, K.F. Physical exercise-induced hippocampal neurogenesis and antidepressant effects are mediated by the adipocyte hormone adiponectin. Proc. Natl. Acad. Sci. USA 2014, 111, 15810–15815. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Jiang, H.; Xu, X.; Duan, W.; Mattson, M.P. Leptin-mediated cell survival signaling in hippocampal neurons mediated by JAK STAT3 and mitochondrial stabilization. J. Biol. Chem. 2008, 283, 1754–1763. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kang, S.M.; Kim, E.; Kim, C.H.; Song, H.T.; Lee, J.E. Adiponectin receptor-mediated signaling ameliorates cerebral cell damage and regulates the neurogenesis of neural stem cells at high glucose concentrations: An in vivo and in vitro study. Cell Death Dis. 2015, 6, e1844. [Google Scholar] [CrossRef] [PubMed]
- Formolo, D.A.; Lee, T.H.; Yau, S.Y. Increasing adiponergic system activity as a potential treatment for depressive disorders. Mol. Neurobiol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Wang, Y.; Gan, X.; Fang, D.; Zhong, C.; Wu, L.; Hu, G.; Sosunov, A.A.; McKhann, G.M.; Yu, H.; et al. Drp1-mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes 2015, 64, 1728–1742. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Guo, H.; Li, X.; Yue, L.; Liu, H.; Zhao, L.; Bai, H.; Liu, X.; Wu, X.; Qu, Y. Adiponectin attenuates oxygen-glucose deprivation-induced mitochondrial oxidative injury and apoptosis in hippocampal HT22 cells via the JAK2/STAT3 pathway. Cell Transpl. 2018, 27, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Qiu, G.; Wan, R.; Hu, J.; Mattson, M.P.; Spangler, E.; Liu, S.; Yau, S.Y.; Lee, T.M.; Gleichmann, M.; Ingram, D.K.; et al. Adiponectin protects rat hippocampal neurons against excitotoxicity. Age 2011, 33, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Choi, S.M.; Whitcomb, D.J.; Kim, B.C. Adiponectin controls the apoptosis and the expression of tight junction proteins in brain endothelial cells through AdipoR1 under beta amyloid toxicity. Cell Death Dis. 2017, 8, e3102. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Cazareth, J.; Zarif, H.; Guyon, A.; Heurteaux, C.; Chabry, J.; Petit-Paitel, A. Globular adiponectin limits microglia pro-inflammatory phenotype through an AdipoR1/NF-kappaB signaling pathway. Front. Cell Neurosci. 2017, 11, 352. [Google Scholar] [CrossRef] [PubMed]
- Zuloaga, K.L.; Johnson, L.A.; Roese, N.E.; Marzulla, T.; Zhang, W.; Nie, X.; Alkayed, F.N.; Hong, C.; Grafe, M.R.; Pike, M.M.; et al. High fat diet-induced diabetes in mice exacerbates cognitive deficit due to chronic hypoperfusion. J. Cereb. Blood Flow Metab. 2016, 36, 1257–1270. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hao, S.; Yin, H.; Gao, J.; Yang, Z. Autophagy ameliorates cognitive impairment through activation of PVT1 and apoptosis in diabetes mice. Behav. Brain Res. 2016, 305, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Du, F.; Wu, L.; Zhang, Z.; Zhong, C.; Yu, Q.; Wang, Y.; Lue, L.F.; Walker, D.G.; Douglas, J.T.; et al. F1F0 ATP synthase-cyclophilin D interaction contributes to diabetes-induced synaptic dysfunction and cognitive decline. Diabetes 2016, 65, 3482–3494. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.J.; Wu, J.H.; Sun, S.Y.; Ma, L.L.; Zhou, J.Q. Liraglutide ameliorates cognitive decline by promoting autophagy via the AMP-activated protein kinase/mammalian target of rapamycin pathway in a streptozotocin-induced mouse model of diabetes. Neuropharmacology 2018, 131, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Song, F.; Xu, K.; Liu, Z.; Han, S.; Li, F.; Sun, Y. Irisin attenuates neuroinflammation and prevents the memory and cognitive deterioration in streptozotocin-induced diabetic mice. Mediators Inflamm. 2019, 2019, 1567179. [Google Scholar] [CrossRef] [PubMed]
- Saravia, F.E.; Beauquis, J.; Revsin, Y.; Homo-Delarche, F.; de Kloet, E.R.; De Nicola, A.F. Hippocampal neuropathology of diabetes mellitus is relieved by estrogen treatment. Cell Mol. Neurobiol. 2006, 26, 943–957. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Deng, J.; Sheng, W.; Zuo, Z. Metformin attenuates Alzheimer’s disease-like neuropathology in obese, leptin-resistant mice. Pharmacol. Biochem. Behav. 2012, 101, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Hayashi-Park, E.; Ozment, B.N.; Griffith, C.M.; Zhang, H.; Patrylo, P.R.; Rose, G.M. Experimentally induced diabetes worsens neuropathology, but not learning and memory, in middle aged 3xTg mice. Behav. Brain Res. 2017, 322, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Leuner, B.; Gould, E. Structural plasticity and hippocampal function. Annu. Rev. Psychol. 2010, 61, 111–140. [Google Scholar] [CrossRef] [PubMed]
- Brunner, L.; Nick, H.P.; Cumin, F.; Chiesi, M.; Baum, H.P.; Whitebread, S.; Stricker-Krongrad, A.; Levens, N. Leptin is a physiologically important regulator of food intake. Int. J. Obes. Relat. Metab. Disord. 1997, 21, 1152–1160. [Google Scholar] [CrossRef] [PubMed]
- Considine, R.V.; Sinha, M.K.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Nyce, M.R.; Ohannesian, J.P.; Marco, C.C.; McKee, L.J.; Bauer, T.L.; et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N. Engl. J. Med. 1996, 334, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Licinio, J.; Mantzoros, C.; Negrao, A.B.; Cizza, G.; Wong, M.L.; Bongiorno, P.B.; Chrousos, G.P.; Karp, B.; Allen, C.; Flier, J.S.; et al. Human leptin levels are pulsatile and inversely related to pituitary-adrenal function. Nat. Med. 1997, 3, 575–579. [Google Scholar] [CrossRef] [PubMed]
- Sinha, M.K.; Ohannesian, J.P.; Heiman, M.L.; Kriauciunas, A.; Stephens, T.W.; Magosin, S.; Marco, C.; Caro, J.F. Nocturnal rise of leptin in lean, obese, and non-insulin-dependent diabetes mellitus subjects. J. Clin. Investig. 1996, 97, 1344–1347. [Google Scholar] [CrossRef] [PubMed]
- Boden, G.; Chen, X.; Mozzoli, M.; Ryan, I. Effect of fasting on serum leptin in normal human subjects. J. Clin. Endocrinol. Metab. 1996, 81, 3419–3423. [Google Scholar] [PubMed]
- Chan, J.L.; Heist, K.; DePaoli, A.M.; Veldhuis, J.D.; Mantzoros, C.S. The role of falling leptin levels in the neuroendocrine and metabolic adaptation to short-term starvation in healthy men. J. Clin. Investig. 2003, 111, 1409–1421. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.L.; Mantzoros, C.S. Role of leptin in energy-deprivation states: Normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. Lancet 2005, 366, 74–85. [Google Scholar] [CrossRef]
- Myers, M.G.; Cowley, M.A.; Munzberg, H. Mechanisms of leptin action and leptin resistance. Annu. Rev. Physiol. 2008, 70, 537–556. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Hanefeld, M.; Haffner, S.M.; Fusch, C.; Schwanebeck, U.; Kohler, C.; Fucker, K.; Julius, U. Insulin-resistant patients with type 2 diabetes mellitus have higher serum leptin levels independently of body fat mass. Acta Diabetol. 2002, 39, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.; Zhou, J.; Redmann, S.M., Jr.; Smagin, G.N.; Smith, S.R.; Rodgers, E.; Zachwieja, J.J. A leptin dose-response study in obese (ob/ob) and lean (+/?) mice. Endocrinology 1998, 139, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Berglund, E.D.; Vianna, C.R.; Donato, J., Jr.; Kim, M.H.; Chuang, J.C.; Lee, C.E.; Lauzon, D.A.; Lin, P.; Brule, L.J.; Scott, M.M.; et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J. Clin. Investig. 2012, 122, 1000–1009. [Google Scholar] [CrossRef] [PubMed]
- Cummings, B.P.; Bettaieb, A.; Graham, J.L.; Stanhope, K.L.; Dill, R.; Morton, G.J.; Haj, F.G.; Havel, P.J. Subcutaneous administration of leptin normalizes fasting plasma glucose in obese type 2 diabetic UCD-T2DM rats. Proc. Natl. Acad. Sci. USA 2011, 108, 14670–14675. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Gelling, R.W.; Niswender, K.D.; Morrison, C.D.; Rhodes, C.J.; Schwartz, M.W. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005, 2, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Coppari, R.; Ichinose, M.; Lee, C.E.; Pullen, A.E.; Kenny, C.D.; McGovern, R.A.; Tang, V.; Liu, S.M.; Ludwig, T.; Chua, S.C., Jr.; et al. The hypothalamic arcuate nucleus: A key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005, 1, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Enriori, P.J.; Evans, A.E.; Sinnayah, P.; Jobst, E.E.; Tonelli-Lemos, L.; Billes, S.K.; Glavas, M.M.; Grayson, B.E.; Perello, M.; Nillni, E.A.; et al. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007, 5, 181–194. [Google Scholar] [CrossRef] [PubMed]
- El-Haschimi, K.; Pierroz, D.D.; Hileman, S.M.; Bjorbaek, C.; Flier, J.S. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J. Clin. Investig. 2000, 105, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Munzberg, H.; Flier, J.S.; Bjorbaek, C. Region-specific leptin resistance within the hypothalamus of diet-induced obese mice. Endocrinology 2004, 145, 4880–4889. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Thomas, T.C.; Storlien, L.H.; Huang, X.F. Development of high fat diet-induced obesity and leptin resistance in C57Bl/6J mice. Int. J. Obes. Relat. Metab. Disord. 2000, 24, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Mittendorfer, B.; Horowitz, J.F.; DePaoli, A.M.; McCamish, M.A.; Patterson, B.W.; Klein, S. Recombinant human leptin treatment does not improve insulin action in obese subjects with type 2 diabetes. Diabetes 2011, 60, 1474–1477. [Google Scholar] [CrossRef] [PubMed]
- Moon, H.S.; Matarese, G.; Brennan, A.M.; Chamberland, J.P.; Liu, X.; Fiorenza, C.G.; Mylvaganam, G.H.; Abanni, L.; Carbone, F.; Williams, C.J.; et al. Efficacy of metreleptin in obese patients with type 2 diabetes: Cellular and molecular pathways underlying leptin tolerance. Diabetes 2011, 60, 1647–1656. [Google Scholar] [CrossRef] [PubMed]
- Funahashi, H.; Yada, T.; Suzuki, R.; Shioda, S. Distribution, function, and properties of leptin receptors in the brain. Int. Rev. Cytol. 2003, 224, 1–27. [Google Scholar] [PubMed]
- Zhou, Y.; Rui, L. Leptin signaling and leptin resistance. Front. Med. 2013, 7, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Morrison, C.D. Leptin signaling in brain: A link between nutrition and cognition? Biochim. Biophys. Acta 2009, 1792, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Farr, O.M.; Tsoukas, M.A.; Mantzoros, C.S. Leptin and the brain: Influences on brain development, cognitive functioning and psychiatric disorders. Metabolism 2015, 64, 114–130. [Google Scholar] [CrossRef] [PubMed]
- Coll, A.P.; Farooqi, I.S.; O’Rahilly, S. The hormonal control of food intake. Cell 2007, 129, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Cristino, L.; Busetto, G.; Imperatore, R.; Ferrandino, I.; Palomba, L.; Silvestri, C.; Petrosino, S.; Orlando, P.; Bentivoglio, M.; Mackie, K.; et al. Obesity-driven synaptic remodeling affects endocannabinoid control of orexinergic neurons. Proc. Natl. Acad. Sci. USA 2013, 110, E2229–E2238. [Google Scholar] [CrossRef] [PubMed]
- Cason, A.M.; Smith, R.J.; Tahsili-Fahadan, P.; Moorman, D.E.; Sartor, G.C.; Aston-Jones, G. Role of orexin/hypocretin in reward-seeking and addiction: Implications for obesity. Physiol. Behav. 2010, 100, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Hay-Schmidt, A.; Helboe, L.; Larsen, P.J. Leptin receptor immunoreactivity is present in ascending serotonergic and catecholaminergic neurons of the rat. Neuroendocrinology 2001, 73, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Figlewicz, D.P.; Evans, S.B.; Murphy, J.; Hoen, M.; Baskin, D.G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain Res. 2003, 964, 107–115. [Google Scholar] [CrossRef]
- Balland, E.; Cowley, M.A. New insights in leptin resistance mechanisms in mice. Front. Neuroendocrinol. 2015, 39, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Hara, J.; Beuckmann, C.T.; Nambu, T.; Willie, J.T.; Chemelli, R.M.; Sinton, C.M.; Sugiyama, F.; Yagami, K.; Goto, K.; Yanagisawa, M.; et al. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 2001, 30, 345–354. [Google Scholar] [CrossRef]
- Harris, G.C.; Wimmer, M.; Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 2005, 437, 556–559. [Google Scholar] [CrossRef] [PubMed]
- Narita, M.; Nagumo, Y.; Hashimoto, S.; Narita, M.; Khotib, J.; Miyatake, M.; Sakurai, T.; Yanagisawa, M.; Nakamachi, T.; Shioda, S.; et al. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. J. Neurosci. 2006, 26, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Bello, N.T.; Pang, Z.P. Presynaptic regulation of leptin in a defined lateral hypothalamus-ventral tegmental area neurocircuitry depends on energy state. J. Neurosci. 2017, 37, 11854–11866. [Google Scholar] [CrossRef] [PubMed]
- Salamone, J.D.; Correa, M.; Mingote, S.M.; Weber, S.M. Beyond the reward hypothesis: Alternative functions of nucleus accumbens dopamine. Curr. Opin. Pharmacol. 2005, 5, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Kelley, A.E.; Baldo, B.A.; Pratt, W.E.; Will, M.J. Corticostriatal-hypothalamic circuitry and food motivation: Integration of energy, action and reward. Physiol. Behav. 2005, 86, 773–795. [Google Scholar] [CrossRef] [PubMed]
- Schultz, W. Behavioral dopamine signals. Trends Neurosci. 2007, 30, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Coccurello, R.; Maccarrone, M. Hedonic eating and the “Delicious circle”: From lipid-derived mediators to brain dopamine and back. Front. Neurosci. 2018, 12, 271. [Google Scholar] [CrossRef] [PubMed]
- Leinninger, G.M.; Jo, Y.H.; Leshan, R.L.; Louis, G.W.; Yang, H.; Barrera, J.G.; Wilson, H.; Opland, D.M.; Faouzi, M.A.; Gong, Y.; et al. Leptin acts via leptin receptor-expressing lateral hypothalamic neurons to modulate the mesolimbic dopamine system and suppress feeding. Cell Metab. 2009, 10, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.F.; Choi, D.L.; Schurdak, J.D.; Fitzgerald, M.F.; Clegg, D.J.; Lipton, J.W.; Figlewicz, D.P.; Benoit, S.C. Leptin regulates energy balance and motivation through action at distinct neural circuits. Biol. Psychiatry 2011, 69, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Fulton, S.; Pissios, P.; Manchon, R.P.; Stiles, L.; Frank, L.; Pothos, E.N.; Maratos-Flier, E.; Flier, J.S. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 2006, 51, 811–822. [Google Scholar] [CrossRef] [PubMed]
- Korotkova, T.M.; Brown, R.E.; Sergeeva, O.A.; Ponomarenko, A.A.; Haas, H.L. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur. J. Neurosci. 2006, 23, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Krugel, U.; Schraft, T.; Kittner, H.; Kiess, W.; Illes, P. Basal and feeding-evoked dopamine release in the rat nucleus accumbens is depressed by leptin. Eur. J. Pharmacol. 2003, 482, 185–187. [Google Scholar] [CrossRef] [PubMed]
- Morton, G.J.; Blevins, J.E.; Kim, F.; Matsen, M.; Figlewicz, D.P. The action of leptin in the ventral tegmental area to decrease food intake is dependent on Jak-2 signaling. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E202–E210. [Google Scholar] [CrossRef] [PubMed]
- Scarpace, P.J.; Matheny, M.; Kirichenko, N.; Gao, Y.X.; Tumer, N.; Zhang, Y. Leptin overexpression in VTA trans-activates the hypothalamus whereas prolonged leptin action in either region cross-desensitizes. Neuropharmacology 2013, 65, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Hommel, J.D.; Trinko, R.; Sears, R.M.; Georgescu, D.; Liu, Z.W.; Gao, X.B.; Thurmon, J.J.; Marinelli, M.; DiLeone, R.J. Leptin receptor signaling in midbrain dopamine neurons regulates feeding. Neuron 2006, 51, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Jiang, C.; Liu, P.; Wang, F.; Ma, L. Mesolimbic leptin signaling negatively regulates cocaine-conditioned reward. Transl. Psychiatry 2016, 6, e972. [Google Scholar] [CrossRef] [PubMed]
- Haass-Koffler, C.L.; Aoun, E.G.; Swift, R.M.; de la Monte, S.M.; Kenna, G.A.; Leggio, L. Leptin levels are reduced by intravenous ghrelin administration and correlated with cue-induced alcohol craving. Transl. Psychiatry 2015, 5, e646. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Bocchio Chiavetto, L.; Bignotti, S.; Battisa Tura, G.; Pioli, R.; Boin, F.; Kenis, G.; Bosmans, E.; de Jongh, R.; Lin, A.; et al. Effects of atypical antipsychotics on the inflammatory response system in schizophrenic patients resistant to treatment with typical neuroleptics. Eur. Neuropsychopharmacol. 2000, 10, 119–124. [Google Scholar] [CrossRef]
- Coccurello, R.; Moles, A. Potential mechanisms of atypical antipsychotic-induced metabolic derangement: Clues for understanding obesity and novel drug design. Pharmacol. Ther. 2010, 127, 210–251. [Google Scholar] [CrossRef] [PubMed]
- Sapra, M.; Lawson, D.; Iranmanesh, A.; Varma, A. Adiposity-independent hypoadiponectinemia as a potential marker of insulin resistance and inflammation in schizophrenia patients treated with second generation antipsychotics. Schizophr. Res. 2016, 174, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Flowers, S.A.; Evans, S.J.; Ward, K.M.; McInnis, M.G.; Ellingrod, V.L. Interaction between atypical antipsychotics and the gut microbiome in a bipolar disease cohort. Pharmacotherapy 2017, 37, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, X.F.; Shao, R.; Chen, C.; Deng, C. Molecular mechanisms of antipsychotic drug-induced diabetes. Front. Neurosci. 2017, 11, 643. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bansal, Y.; Medhi, B.; Kuhad, A. Antipsychotics-induced metabolic alterations: Recounting the mechanistic insights, therapeutic targets and pharmacological alternatives. Eur. J. Pharmacol. 2019, 844, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Zhuang, X. Atypical antipsychotics-induced metabolic syndrome and nonalcoholic fatty liver disease: A critical review. Neuropsychiatr. Dis. Treat. 2019, 15, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Haack, M.; Schuld, A.; Hinze-Selch, D.; Kühn, M.; Uhr, M.; Pollmächer, T. Body weight and leptin plasma levels during treatment with antipsychotic drugs. Am. J. Psychiatry 1999, 156, 312–314. [Google Scholar] [PubMed]
- McIntyre, R.S.; Mancini, D.A.; Basile, V.S.; Srinivasan, J.; Kennedy, S.H. Antipsychotic-induced weight gain: Bipolar disorder and leptin. J. Clin. Psychopharmacol. 2003, 23, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Popovic, V.; Doknic, M.; Maric, N.; Pekic, S.; Damjanovic, A.; Miljic, D.; Popovic, S.; Miljic, N.; Djurovic, M.; Jasovic-Gasic, M.; et al. Changes in neuroendocrine and metabolic hormones induced by atypical antipsychotics in normal-weight patients with schizophrenia. Neuroendocrinology 2007, 85, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Potvin, S.; Zhornitsky, S.; Stip, E. Antipsychotic-induced changes in blood levels of leptin in schizophrenia: A meta-analysis. Can. J. Psychiatry 2015, 60 (Suppl. 2), S26–S34. [Google Scholar]
- Ota, M.; Mori, K.; Nakashima, A.; Kaneko, Y.S.; Fujiwara, K.; Itoh, M.; Nagasaka, A.; Ota, A. Peripheral injection of risperidone, an atypical antipsychotic, alters the bodyweight gain of rats. Clin. Exp. Pharmacol. Physiol. 2002, 29, 980–989. [Google Scholar] [CrossRef] [PubMed]
- Albaugh, V.L.; Henry, C.R.; Bello, N.T.; Hajnal, A.; Lynch, S.L.; Halle, B.; Lynch, C.J. Hormonal and metabolic effects of olanzapine and clozapine related to body weight in rodents. Obesity 2006, 14, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Minet-Ringuet, J.; Even, P.C.; Goubern, M.; Tome, D.; de Beaurepaire, R. Long term treatment with olanzapine mixed with the food in male rats induces body fat deposition with no increase in body weight and no thermogenic alteration. Appetite 2006, 46, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Minet-Ringuet, J.; Even, P.C.; Lacroix, M.; Tome, D.; de Beaurepaire, R. A model for antipsychotic-induced obesity in the male rat. Psychopharmacology 2006, 187, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.C.; Wyler, S.C.; Wan, R.; Castorena, C.M.; Ahmed, N.; Mathew, D.; Lee, S.; Liu, C.; Elmquist, J.K. The atypical antipsychotic olanzapine causes weight gain by targeting serotonin receptor, 2C. J. Clin. Investig. 2017, 127, 3402–3406. [Google Scholar] [CrossRef] [PubMed]
- Kusumi, I.; Boku, S.; Takahashi, Y. Psychopharmacology of atypical antipsychotic drugs: From the receptor binding profile to neuroprotection and neurogenesis. Psychiatry Clin. Neurosci. 2015, 69, 243–258. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y. What’s atypical about atypical antipsychotic drugs? Curr. Opin. Pharmacol. 2004, 4, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Basile, V.S.; Masellis, M.; McIntyre, R.S.; Meltzer, H.Y.; Lieberman, J.A.; Kennedy, J.L. Genetic dissection of atypical antipsychotic-induced weight gain: Novel preliminary data on the pharmacogenetic puzzle. J. Clin. Psychiatry 2001, 62 (Suppl. 23), 45–66. [Google Scholar] [PubMed]
- Starrenburg, F.C.; Bogers, J.P. How can antipsychotics cause Diabetes Mellitus? Insights based on receptor-binding profiles, humoral factors and transporter proteins. Eur. Psychiatry 2009, 24, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Gettys, T.W.; Harkness, P.J.; Watson, P.M. The beta 3-adrenergic receptor inhibits insulin-stimulated leptin secretion from isolated rat adipocytes. Endocrinology 1996, 137, 4054–4057. [Google Scholar] [CrossRef] [PubMed]
- Munzberg, H. Differential leptin access into the brain—A hierarchical organization of hypothalamic leptin target sites? Physiol. Behav. 2008, 94, 664–669. [Google Scholar] [CrossRef] [PubMed]
- Morash, B.; Li, A.; Murphy, P.R.; Wilkinson, M.; Ur, E. Leptin gene expression in the brain and pituitary gland. Endocrinology 1999, 140, 5995–5998. [Google Scholar] [CrossRef] [PubMed]
- Ur, E.; Wilkinson, D.A.; Morash, B.A.; Wilkinson, M. Leptin immunoreactivity is localized to neurons in rat brain. Neuroendocrinology 2002, 75, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A.; Clever, C.M.; Farrell, C.L. Partial saturation and regional variation in the blood-to-brain transport of leptin in normal weight mice. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E1158–E1165. [Google Scholar] [CrossRef] [PubMed]
- Van Doorn, C.; Macht, V.A.; Grillo, C.A.; Reagan, L.P. Leptin resistance and hippocampal behavioral deficits. Physiol. Behav. 2017, 176, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Dhar, M.; Zhu, M.; Impey, S.; Lambert, T.J.; Bland, T.; Karatsoreos, I.N.; Nakazawa, T.; Appleyard, S.M.; Wayman, G.A. Leptin induces hippocampal synaptogenesis via CREB-regulated microRNA-132 suppression of, p.2.5.0.G.A.P. Mol. Endocrinol. 2014, 28, 1073–1087. [Google Scholar] [CrossRef] [PubMed]
- Li, X.L.; Aou, S.; Oomura, Y.; Hori, N.; Fukunaga, K.; Hori, T. Impairment of long-term potentiation and spatial memory in leptin receptor-deficient rodents. Neuroscience 2002, 113, 607–615. [Google Scholar] [CrossRef]
- Gerges, N.Z.; Aleisa, A.M.; Alkadhi, K.A. Impaired long-term potentiation in obese zucker rats: Possible involvement of presynaptic mechanism. Neuroscience 2003, 120, 535–539. [Google Scholar] [CrossRef]
- Guo, M.; Lu, Y.; Garza, J.C.; Li, Y.; Chua, S.C.; Zhang, W.; Lu, B.; Lu, X.Y. Forebrain glutamatergic neurons mediate leptin action on depression-like behaviors and synaptic depression. Transl. Psychiatry 2012, 2, e83. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Lowe, C.; Pretz, D.; Steger, J.; Williams, L.M.; Tups, A. High-fat diet induces leptin resistance in leptin-deficient mice. J. Neuroendocrinol. 2014, 26, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chandrasekera, P.C.; Pippin, J.J. Leptin- and leptin receptor-deficient rodent models: Relevance for human type 2 diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, D.; MacDonald, N.; Mizielinska, S.; Connolly, C.N.; Irving, A.J.; Harvey, J. Leptin promotes rapid dynamic changes in hippocampal dendritic morphology. Mol. Cell. Neurosci. 2007, 35, 559–572. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Garza, J.C.; Bronner, J.; Kim, C.S.; Zhang, W.; Lu, X.Y. Acute administration of leptin produces anxiolytic-like effects: A comparison with fluoxetine. Psychopharmacology 2010, 207, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Banks, W.A.; Morley, J.E. Effects of leptin on memory processing. Peptides 2006, 27, 1420–1425. [Google Scholar] [CrossRef] [PubMed]
- Kanoski, S.E.; Hayes, M.R.; Greenwald, H.S.; Fortin, S.M.; Gianessi, C.A.; Gilbert, J.R.; Grill, H.J. Hippocampal leptin signaling reduces food intake and modulates food-related memory processing. Neuropsychopharmacology 2011, 36, 1859–1870. [Google Scholar] [CrossRef] [PubMed]
- Suarez, A.N.; Noble, E.E.; Kanoski, S.E. Regulation of memory function by feeding-relevant biological systems: following the breadcrumbs to the hippocampus. Front. Mol. Neurosci. 2019, 12, 101. [Google Scholar] [CrossRef] [PubMed]
- Bliss, T.V.; Collingridge, G.L. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature 1993, 361, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Wayner, M.J.; Armstrong, D.L.; Phelix, C.F.; Oomura, Y. Orexin-A (Hypocretin-1) and leptin enhance LTP in the dentate gyrus of rats in vivo. Peptides 2004, 25, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Malenka, R.C. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron 1991, 6, 53–60. [Google Scholar] [CrossRef]
- Greco, S.J.; Bryan, K.J.; Sarkar, S.; Zhu, X.; Smith, M.A.; Ashford, J.W.; Johnston, J.M.; Tezapsidis, N.; Casadesus, G. Leptin reduces pathology and improves memory in a transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2010, 19, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.Q.; Zhang, J.; Hao, M.; Yang, J.; Han, Y.F.; Liu, X.J.; Shi, H.; Wu, M.N.; Liu, Q.S.; Qi, J.S. Leptin attenuates the detrimental effects of beta-amyloid on spatial memory and hippocampal later-phase long term potentiation in rats. Horm. Behav. 2015, 73, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, Y.; Liu, J.; Yin, F. Geniposide attenuates the level of Aβ1-42 via enhancing leptin signaling in cellular and APP/PS1 transgenic mice. Arch. Pharmacal Res. 2017, 40, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Tanida, M.; Kasahara, R.; Sobue, K.; Suzuki, K. Leptin inhibits amyloid beta-protein fibrillogenesis by decreasing GM1 gangliosides on the neuronal cell surface through PI3K/Akt/mTOR pathway. J. Neurochem. 2014, 131, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Chandran, M.; Phillips, S.A.; Ciaraldi, T.; Henry, R.R. Adiponectin: More than just another fat cell hormone? Diabetes Care 2003, 26, 2442–2450. [Google Scholar] [CrossRef] [PubMed]
- Kawano, J.; Arora, R. The role of adiponectin in obesity, diabetes, and cardiovascular disease. J. Cardiometab. Syndr. 2009, 4, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed]
- Guenther, M.; James, R.; Marks, J.; Zhao, S.; Szabo, A.; Kidambi, S. Adiposity distribution influences circulating adiponectin levels. Transl. Res. 2014, 164, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; van de Wall, E.; Laplante, M.; Azzara, A.; Trujillo, M.E.; Hofmann, S.M.; Schraw, T.; Durand, J.L.; Li, H.; Li, G.; et al. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J. Clin. Investig. 2007, 117, 2621–2637. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ricchiuti, V.; Lian, B.Q.; Yao, T.M.; Coutinho, P.; Romero, J.R.; Li, J.; Williams, G.H.; Adler, G.K. Mineralocorticoid receptor blockade reverses obesity-related changes in expression of adiponectin, peroxisome proliferator-activated receptor-gamma, and proinflammatory adipokines. Circulation 2008, 117, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Saleh, S.; El-Maraghy, N.; Reda, E.; Barakat, W. Modulation of diabetes and dyslipidemia in diabetic insulin-resistant rats by mangiferin: Role of adiponectin and TNF-alpha. An. Acad. Bras. Cienc. 2014, 86, 1935–1948. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Geloen, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Holmes, R.M.; Yi, Z.; De Filippis, E.; Berria, R.; Shahani, S.; Sathyanarayana, P.; Sherman, V.; Fujiwara, K.; Meyer, C.; Christ-Roberts, C.; et al. Increased abundance of the adaptor protein containing pleckstrin homology domain, phosphotyrosine binding domain and leucine zipper motif (APPL1) in patients with obesity and type 2 diabetes: Evidence for altered adiponectin signalling. Diabetologia 2011, 54, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Felder, T.K.; Hahne, P.; Soyal, S.M.; Miller, K.; Hoffinger, H.; Oberkofler, H.; Krempler, F.; Patsch, W. Hepatic adiponectin receptors (ADIPOR) 1 and 2 mRNA and their relation to insulin resistance in obese humans. Int. J. Obes. 2010, 34, 846–851. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Okamoto, Y.; Folco, E.J.; Minami, M.; Wara, A.K.; Feinberg, M.W.; Sukhova, G.K.; Colvin, R.A.; Kihara, S.; Funahashi, T.; Luster, A.D.; et al. Adiponectin inhibits the production of CXC receptor 3 chemokine ligands in macrophages and reduces T-lymphocyte recruitment in atherogenesis. Circ. Res. 2008, 102, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.S.; Jeng, C.Y.; Wu, T.J.; Tanaka, S.; Funahashi, T.; Matsuzawa, Y.; Wang, J.P.; Chen, C.L.; Tai, T.Y.; Chuang, L.M. Synthetic peroxisome proliferator-activated receptor-gamma agonist, rosiglitazone, increases plasma levels of adiponectin in type 2 diabetic patients. Diabetes Care 2002, 25, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Fry, M.; Smith, P.M.; Hoyda, T.D.; Duncan, M.; Ahima, R.S.; Sharkey, K.A.; Ferguson, A.V. Area postrema neurons are modulated by the adipocyte hormone adiponectin. J. Neurosci. 2006, 26, 9695–9702. [Google Scholar] [CrossRef] [PubMed]
- Hoyda, T.D.; Fry, M.; Ahima, R.S.; Ferguson, A.V. Adiponectin selectively inhibits oxytocin neurons of the paraventricular nucleus of the hypothalamus. J. Physiol. 2007, 585, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Neumeier, M.; Weigert, J.; Buettner, R.; Wanninger, J.; Schaffler, A.; Muller, A.M.; Killian, S.; Sauerbruch, S.; Schlachetzki, F.; Steinbrecher, A.; et al. Detection of adiponectin in cerebrospinal fluid in humans. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E965–E969. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pacheco, F.; Martinez-Fuentes, A.J.; Tovar, S.; Pinilla, L.; Tena-Sempere, M.; Dieguez, C.; Castano, J.P.; Malagon, M.M. Regulation of pituitary cell function by adiponectin. Endocrinology 2007, 148, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Thundyil, J.; Tang, S.C.; Okun, E.; Shah, K.; Karamyan, V.T.; Li, Y.I.; Woodruff, T.M.; Taylor, S.M.; Jo, D.G.; Mattson, M.P.; et al. Evidence that adiponectin receptor 1 activation exacerbates ischemic neuronal death. Exp. Transl. Stroke Med. 2010, 2, 15. [Google Scholar] [CrossRef] [PubMed]
- Repunte-Canonigo, V.; Berton, F.; Cottone, P.; Reifel-Miller, A.; Roberts, A.J.; Morales, M.; Francesconi, W.; Sanna, P.P. A potential role for adiponectin receptor 2 (AdipoR2) in the regulation of alcohol intake. Brain Res. 2010, 1339, 11–17. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, D.; Guo, M.; Zhang, W.; Lu, X.Y. Adiponectin stimulates proliferation of adult hippocampal neural stem/progenitor cells through activation of p38 mitogen-activated protein kinase (p38MAPK)/glycogen synthase kinase 3beta (GSK-3beta)/beta-catenin signaling cascade. J. Biol. Chem. 2011, 286, 44913–44920. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Guo, M.; Zhang, D.; Cheng, S.Y.; Liu, M.; Ding, J.; Scherer, P.E.; Liu, F.; Lu, X.Y. Adiponectin is critical in determining susceptibility to depressive behaviors and has antidepressant-like activity. Proc. Natl. Acad. Sci. USA 2012, 109, 12248–12253. [Google Scholar] [CrossRef] [PubMed]
- Varhelyi, Z.P.; Kalman, J.; Olah, Z.; Ivitz, E.V.; Fodor, E.K.; Santha, M.; Datki, Z.L.; Pakaski, M. Adiponectin receptors are less sensitive to stress in a transgenic mouse model of Alzheimer’s disease. Front. Neurosci. 2017, 11, 199. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.E.; Lowe, C.; Legler, K.; Benzler, J.; Boucsein, A.; Bottiger, G.; Grattan, D.R.; Williams, L.M.; Tups, A. Central adiponectin acutely improves glucose tolerance in male mice. Endocrinology 2014, 155, 1806–1816. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Cheng, K.K. Hypothalamic AMPK as a mediator of hormonal regulation of energy balance. Int. J. Mol. Sci. 2018, 19, 3552. [Google Scholar] [CrossRef] [PubMed]
- Kubota, N.; Yano, W.; Kubota, T.; Yamauchi, T.; Itoh, S.; Kumagai, H.; Kozono, H.; Takamoto, I.; Okamoto, S.; Shiuchi, T.; et al. Adiponectin stimulates AMP-activated protein kinase in the hypothalamus and increases food intake. Cell Metab. 2007, 6, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Gao, Y.; Yao, T.; Huang, Y.; He, Z.; Kong, X.; Yu, K.J.; Wang, R.T.; Guo, H.; Yan, J.; et al. Adiponectin potentiates the acute effects of leptin in arcuate Pomc neurons. Mol. Metab. 2016, 5, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Takahashi, N.; Hileman, S.M.; Patel, H.R.; Berg, A.H.; Pajvani, U.B.; Scherer, P.E.; Ahima, R.S. Adiponectin acts in the brain to decrease body weight. Nat. Med. 2004, 10, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Maekawa, F.; Maejima, Y.; Kubota, N.; Kadowaki, T.; Yada, T. Glucose level determines excitatory or inhibitory effects of adiponectin on arcuate POMC neuron activity and feeding. Sci. Rep. 2016, 6, 30796. [Google Scholar] [CrossRef] [PubMed]
- Suyama, S.; Lei, W.; Kubota, N.; Kadowaki, T.; Yada, T. Adiponectin at physiological level glucose-independently enhances inhibitory postsynaptic current onto NPY neurons in the hypothalamic arcuate nucleus. Neuropeptides 2017, 65, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hanssens, L.; van Winkel, R.; Wampers, M.; Van Eyck, D.; Scheen, A.; Reginster, J.Y.; Collette, J.; Peuskens, J.; De Hert, M. A cross-sectional evaluation of adiponectin plasma levels in patients with schizophrenia and schizoaffective disorder. Schizophr. Res. 2008, 106, 308–314. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Crocamo, C.; Clerici, M.; Carra, G. Second-generation antipsychotics and adiponectin levels in schizophrenia: A comparative meta-analysis. Eur. Neuropsychopharmacol. 2015, 25, 1767–1774. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Schreurs, V.; Sweers, K.; Van Eyck, D.; Hanssens, L.; Sinko, S.; Wampers, M.; Scheen, A.; Peuskens, J.; van Winkel, R. Typical and atypical antipsychotics differentially affect long-term incidence rates of the metabolic syndrome in first-episode patients with schizophrenia: a retrospective chart review. Schizophr. Res. 2008, 101, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.M.; Chen, T.T.; Yang, W.S.; Chi, Y.C.; Lin, C.C.; Liou, Y.J.; Wang, Y.C.; Su, T.P.; Chou, P.; Chen, J.Y. Association of adiponectin and metabolic syndrome among patients taking atypical antipsychotics for schizophrenia: A cohort study. Schizophr. Res. 2009, 111, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Weston-Green, K.; Huang, X.F.; Deng, C. Alterations to melanocortinergic, GABAergic and cannabinoid neurotransmission associated with olanzapine-induced weight gain. PLoS ONE 2012, 7, e33548. [Google Scholar] [CrossRef] [PubMed]
- Murashita, M.; Inoue, T.; Kusumi, I.; Nakagawa, S.; Itoh, K.; Tanaka, T.; Izumi, T.; Hosoda, H.; Kangawa, K.; Koyama, T. Glucose and lipid metabolism of long-term risperidone monotherapy in patients with schizophrenia. Psychiatry Clin. Neurosci. 2007, 61, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Wampers, M.; Hanssens, L.; van Winkel, R.; Heald, A.; Collette, J.; Peuskens, J.; Reginster, J.Y.; Scheen, A.; De Hert, M. Differential effects of olanzapine and risperidone on plasma adiponectin levels over time: Results from a 3-month prospective open-label study. Eur. Neuropsychopharmacol. 2012, 22, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Bartoli, F.; Lax, A.; Crocamo, C.; Clerici, M.; Carra, G. Plasma adiponectin levels in schizophrenia and role of second-generation antipsychotics: A meta-analysis. Psychoneuroendocrinology 2015, 56, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Sun, F.; Lei, Y.; You, J.; Li, C.; Sun, L.; Garza, J.; Zhang, D.; Guo, M.; Scherer, P.E.; Lodge, D.; et al. Adiponectin modulates ventral tegmental area dopamine neuron activity and anxiety-related behavior through AdipoR1. Mol. Psychiatry 2019, 24, 126–144. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Carlezon, W.A., Jr. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry 2006, 59, 1151–1159. [Google Scholar] [CrossRef] [PubMed]
- Coque, L.; Mukherjee, S.; Cao, J.L.; Spencer, S.; Marvin, M.; Falcon, E.; Sidor, M.M.; Birnbaum, S.G.; Graham, A.; Neve, R.L.; et al. Specific role of VTA dopamine neuronal firing rates and morphology in the reversal of anxiety-related, but not depression-related behavior in the ClockDelta19 mouse model of mania. Neuropsychopharmacology 2011, 36, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Tye, K.M.; Mirzabekov, J.J.; Warden, M.R.; Ferenczi, E.A.; Tsai, H.C.; Finkelstein, J.; Kim, S.Y.; Adhikari, A.; Thompson, K.R.; Andalman, A.S.; et al. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature 2013, 493, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Chaudhury, D.; Walsh, J.J.; Friedman, A.K.; Juarez, B.; Ku, S.M.; Koo, J.W.; Ferguson, D.; Tsai, H.C.; Pomeranz, L.; Christoffel, D.J.; et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013, 493, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Kahl, K.G.; Schweiger, U.; Correll, C.; Muller, C.; Busch, M.L.; Bauer, M.; Schwarz, P. Depression, anxiety disorders, and metabolic syndrome in a population at risk for type 2 diabetes mellitus. Brain Behav. 2015, 5, e00306. [Google Scholar] [CrossRef] [PubMed]
- Turer, A.T.; Khera, A.; Ayers, C.R.; Turer, C.B.; Grundy, S.M.; Vega, G.L.; Scherer, P.E. Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia 2011, 54, 2515–2524. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Kihara, S.; Funahashi, T.; Matsuzawa, Y.; Libby, P. Adiponectin: A key adipocytokine in metabolic syndrome. Clin. Sci. (Lond.) 2006, 110, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Casares, N.; Garcia-Arnes, J.A.; Rioja, J.; Ariza, M.J.; Gutierrez, A.; Alfaro, F.; Nabrozidis, A.; Gonzalez-Alegre, P.; Gonzalez-Santos, P. Alzheimer’s like brain changes correlate with low adiponectin plasma levels in type 2 diabetic patients. J. Diabetes Complicat. 2016, 30, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Diniz, B.S.; Campos, A.C.; Miranda, A.S.; Rocha, N.P.; Talib, L.L.; Gattaz, W.F.; Forlenza, O.V. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer’s disease. Neuromolecular. Med. 2013, 15, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Wang, X.; Lu, X.Y. Adiponectin Exerts Neurotrophic Effects on Dendritic Arborization, Spinogenesis, and Neurogenesis of the Dentate Gyrus of Male Mice. Endocrinology 2016, 157, 2853–2869. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.W.; Abid, N.B.; Jo, M.H.; Jo, M.G.; Yoon, G.H.; Kim, M.O. Suppression of adiponectin receptor 1 promotes memory dysfunction and Alzheimer’s disease-like pathologies. Sci. Rep. 2017, 7, 12435. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Veyssiere, J.; Gandin, C.; Zsurger, N.; Pietri, M.; Heurteaux, C.; Glaichenhaus, N.; Petit-Paitel, A.; Chabry, J. Neurogenesis-independent antidepressant-like effects of enriched environment is dependent on adiponectin. Psychoneuroendocrinology 2015, 57, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Chabry, J.; Nicolas, S.; Cazareth, J.; Murris, E.; Guyon, A.; Glaichenhaus, N.; Heurteaux, C.; Petit-Paitel, A. Enriched environment decreases microglia and brain macrophages inflammatory phenotypes through adiponectin-dependent mechanisms: Relevance to depressive-like behavior. Brain Behav. Immun. 2015, 50, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Li, C.; Lei, Y.; Xu, S.; Zhao, D.; Lu, X.Y. Role of the adipose PPARgamma-adiponectin axis in susceptibility to stress and depression/anxiety-related behaviors. Mol. Psychiatry 2017, 22, 1056–1068. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, N.; Argueta, J.G.; Masuhiro, Y.; Kagishita, M.; Nonaka, K.; Saito, T.; Hanazawa, S.; Yamashita, Y. Adiponectin inhibits Toll-like receptor family-induced signaling. FEBS Lett. 2005, 579, 6821–6826. [Google Scholar] [CrossRef] [PubMed]
- Mandal, P.; Pratt, B.T.; Barnes, M.; McMullen, M.R.; Nagy, L.E. Molecular mechanism for adiponectin-dependent M2 macrophage polarization: Link between the metabolic and innate immune activity of full-length adiponectin. J. Biol. Chem. 2011, 286, 13460–13469. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Gan, G.S.; Liu, Y.M.; Xiao, J.S.; Liu, H.X.; Mei, B.; Zhang, J.J. Adiponectin Attenuates Streptozotocin-Induced Tau Hyperphosphorylation and Cognitive Deficits by Rescuing PI3K/Akt/GSK-3beta Pathway. Neurochem. Res. 2018, 43, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Jian, M.; Kwan, J.S.; Bunting, M.; Ng, R.C.; Chan, K.H. Adiponectin suppresses amyloid-beta oligomer (AbetaO)-induced inflammatory response of microglia via AdipoR1-AMPK-NF-kappaB signaling pathway. J. Neuroinflamm. 2019, 16, 110. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Choi, S.M.; Kim, B.C. Adiponectin Regulates the Polarization and Function of Microglia via PPAR-gamma Signaling Under Amyloid beta Toxicity. Front. Cell Neurosci. 2017, 11, 64. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Jo, J.; Song, J. Adiponectin improves long-term potentiation in the 5XFAD mouse brain. Sci. Rep. 2019, 9, 8918. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Dranse, H.J.; Sinal, C.J. Towards an integrative approach to understanding the role of chemerin in human health and disease. Obesity Rev. 2013, 14, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Silverio, A.M.; Butcher, E.C. Chemokine-like receptor 1 expression and chemerin-directed chemotaxis distinguish plasmacytoid from myeloid dendritic cells in human blood. J. Immunol. 2005, 174, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Nakae, S.; Zuniga, L.; Kim, J.Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef] [PubMed]
- Barnea, G.; Strapps, W.; Herrada, G.; Berman, Y.; Ong, J.; Kloss, B.; Axel, R.; Lee, K.J. The genetic design of signaling cascades to record receptor activation. Proc. Natl. Acad. Sci. USA 2008, 105, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A. Tazarotene-induced gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Investig. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Curran, J.E.; Stocker, C.J.; Zaibi, M.S.; Segal, D.; Konstantopoulos, N.; Morrison, S.; Carless, M.; Dyer, T.D.; Cole, S.A.; et al. Chemerin, a novel adipokine in the regulation of angiogenesis. J. Clin. Endocrinol. Metab. 2010, 95, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar] [CrossRef] [PubMed]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Sell, H.; Laurencikiene, J.; Taube, A.; Eckardt, K.; Cramer, A.; Horrighs, A.; Arner, P.; Eckel, J. Chemerin is a novel adipocyte-derived factor inducing insulin resistance in primary human skeletal muscle cells. Diabetes 2009, 58, 2731–2740. [Google Scholar] [CrossRef] [PubMed]
- Chakaroun, R.; Raschpichler, M.; Kloting, N.; Oberbach, A.; Flehmig, G.; Kern, M.; Schon, M.R.; Shang, E.; Lohmann, T.; Dressler, M.; et al. Effects of weight loss and exercise on chemerin serum concentrations and adipose tissue expression in human obesity. Metabolism 2012, 61, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Wargent, E.T.; Zaibi, M.S.; O’Dowd, J.F.; Cawthorne, M.A.; Wang, S.J.; Arch, J.R.; Stocker, C.J. Evidence from studies in rodents and in isolated adipocytes that agonists of the chemerin receptor CMKLR1 may be beneficial in the treatment of type 2 diabetes. PeerJ 2015, 3, e753. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Issa, M.; Goralski, K.B.; Sinal, C.J. Chemerin exacerbates glucose intolerance in mouse models of obesity and diabetes. Endocrinology 2010, 151, 1998–2007. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Okimura, Y.; Iguchi, G.; Nishizawa, H.; Yamamoto, M.; Suda, K.; Kitazawa, R.; Fujimoto, W.; Takahashi, K.; Zolotaryov, F.N.; et al. Chemerin regulates beta-cell function in mice. Sci. Rep. 2011, 1, 123. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.C.; Haidl, I.D.; Zuniga, L.A.; Dranse, H.J.; Rourke, J.L.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J. Disruption of the chemokine-like receptor-1 (CMKLR1) gene is associated with reduced adiposity and glucose intolerance. Endocrinology 2012, 153, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, M.; Ren, L.; Xiang, L.; Chen, J.; Li, M.; Xiao, T.; Ren, P.; Xiong, L.; Zhang, J.V. CMKLR1 deficiency influences glucose tolerance and thermogenesis in mice on high fat diet. Biochem. Biophys. Res. Commun. 2016, 473, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Rourke, J.L.; Muruganandan, S.; Dranse, H.J.; McMullen, N.M.; Sinal, C.J. Gpr1 is an active chemerin receptor influencing glucose homeostasis in obese mice. J. Endocrinol. 2014, 222, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Helfer, G.; Ross, A.W.; Thomson, L.M.; Mayer, C.D.; Stoney, P.N.; McCaffery, P.J.; Morgan, P.J. A neuroendocrine role for chemerin in hypothalamic remodelling and photoperiodic control of energy balance. Sci. Rep. 2016, 6, 26830. [Google Scholar] [CrossRef] [PubMed]
- Perumalsamy, S.; Aqilah Mohd Zin, N.A.; Widodo, R.T.; Wan Ahmad, W.A.; Vethakkan, S.; Huri, H.Z. Chemokine Like Receptor-1 (CMKLR-1) Receptor: A Potential Therapeutic Target in Management of Chemerin Induced Type 2 Diabetes Mellitus and Cancer. Curr. Pharm. Des. 2017, 23, 3689–3698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, N.; Ding, Y.; Doycheva, D.M.; Zhang, Y.; Li, Q.; Flores, J.; Haghighiabyaneh, M.; Tang, J.; Zhang, J.H. Chemerin reverses neurological impairments and ameliorates neuronal apoptosis through ChemR23/CAMKK2/AMPK pathway in neonatal hypoxic-ischemic encephalopathy. Cell Death Dis. 2019, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Yu, Y.; Liu, J.; Li, S.; He, H.; Cheng, N.; Ye, R.D. The chemerin receptor CMKLR1 is a functional receptor for amyloid-beta peptide. J. Alzheimers Dis. 2015, 43, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Fu, Y.; Xu, Y.; Weng, S.; Liu, D.; Cui, D.; Yu, S.; Liu, X.; Jiang, K.; Dong, Y. Chronic mild restraint stress rats decreased CMKLR1 expression in distinct brain region. Neurosci. Lett. 2012, 524, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Deyama, S.; Shimoda, K.; Suzuki, H.; Ishikawa, Y.; Ishimura, K.; Fukuda, H.; Hitora-Imamura, N.; Ide, S.; Satoh, M.; Kaneda, K.; et al. Resolvin E1/E2 ameliorate lipopolysaccharide-induced depression-like behaviors via ChemR23. Psychopharmacology 2018, 235, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Li, Q.; Wang, W.; Yu, P.; Pan, H.; Li, P.; Sun, Y.; Zhang, J. Apelin inhibits insulin secretion in pancreatic beta-cells by activation of PI3-kinase-phosphodiesterase 3B. Endocr Res. 2009, 34, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Valet, P.; Castan-Laurell, I. Apelin and energy metabolism. Front. Physiol. 2015, 6, 115. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Chen, L.; Li, L. Apelin/APJ system: A novel promising therapy target for pathological angiogenesis. Clin. Chim. Acta 2017, 466, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Yamaleyeva, L.M.; Shaltout, H.A.; Varagic, J. Apelin-13 in blood pressure regulation and cardiovascular disease. Curr. Opin. Nephrol. Hypertens. 2016, 25, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Valle, A.; Hoggard, N.; Adams, A.C.; Roca, P.; Speakman, J.R. Chronic central administration of apelin-13 over 10 days increases food intake, body weight, locomotor activity and body temperature in C57BL/6 mice. J. Neuroendocrinol. 2008, 20, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Vinel, C.; Lukjanenko, L.; Batut, A.; Deleruyelle, S.; Pradere, J.P.; Le Gonidec, S.; Dortignac, A.; Geoffre, N.; Pereira, O.; Karaz, S.; et al. The exerkine apelin reverses age-associated sarcopenia. Nat. Med. 2018, 24, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Castan-Laurell, I.; Dray, C.; Knauf, C.; Kunduzova, O.; Valet, P. Apelin, a promising target for type 2 diabetes treatment? Trends Endocrinol. Metab. 2012, 23, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Pope, G.R.; Roberts, E.M.; Lolait, S.J.; O’Carroll, A.M. Central and peripheral apelin receptor distribution in the mouse: Species differences with rat. Peptides 2012, 33, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Jin, H.; Xu, S.; Aillaud, M.; Deng, A.C.; Azuma, J.; Kundu, R.K.; Reaven, G.M.; Quertermous, T.; Tsao, P.S. Apelin decreases lipolysis via G(q), G(i), and AMPK-Dependent Mechanisms. Endocrinology 2011, 152, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Yue, P.; Jin, H.; Aillaud, M.; Deng, A.C.; Azuma, J.; Asagami, T.; Kundu, R.K.; Reaven, G.M.; Quertermous, T.; Tsao, P.S. Apelin is necessary for the maintenance of insulin sensitivity. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E59–E67. [Google Scholar] [CrossRef] [PubMed]
- Dray, C.; Knauf, C.; Daviaud, D.; Waget, A.; Boucher, J.; Buleon, M.; Cani, P.D.; Attane, C.; Guigne, C.; Carpene, C.; et al. Apelin stimulates glucose utilization in normal and obese insulin-resistant mice. Cell Metab. 2008, 8, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Attane, C.; Foussal, C.; Le Gonidec, S.; Benani, A.; Daviaud, D.; Wanecq, E.; Guzman-Ruiz, R.; Dray, C.; Bezaire, V.; Rancoule, C.; et al. Apelin treatment increases complete Fatty Acid oxidation, mitochondrial oxidative capacity, and biogenesis in muscle of insulin-resistant mice. Diabetes 2012, 61, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Than, A.; Cheng, Y.; Foh, L.C.; Leow, M.K.; Lim, S.C.; Chuah, Y.J.; Kang, Y.; Chen, P. Apelin inhibits adipogenesis and lipolysis through distinct molecular pathways. Mol. Cell Endocrinol. 2012, 362, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Sun, F.; Li, W.; Cao, Y.; Wang, C.; Wang, Y.; Liang, D.; Zhang, R.; Zhang, S.; Wang, H.; et al. Apelin stimulates glucose uptake through the PI3K/Akt pathway and improves insulin resistance in 3T3-L1 adipocytes. Mol. Cell Biochem. 2011, 353, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Chen, J.; Bai, B.; Xin, Q. Neuroprotection of apelin and its signaling pathway. Peptides 2012, 37, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Xin, Q.; Cheng, B.; Pan, Y.; Liu, H.; Yang, C.; Chen, J.; Bai, B. Neuroprotective effects of apelin-13 on experimental ischemic stroke through suppression of inflammation. Peptides 2015, 63, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Yang, X.; Huang, C.; Gao, Z.; Tang, Y.; Dong, Q. Apelin-13 Protects against Ischemic Blood-Brain Barrier Damage through the Effects of Aquaporin-4. Cerebrovasc. Dis. 2017, 44, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Lee, J.; Gu, X.; Wei, L.; Yu, S.P. Intranasal Delivery of Apelin-13 Is Neuroprotective and Promotes Angiogenesis After Ischemic Stroke in Mice. ASN Neuro 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Bao, H.J.; Zhang, L.; Han, W.C.; Dai, D.K. Apelin-13 attenuates traumatic brain injury-induced damage by suppressing autophagy. Neurochem. Res. 2015, 40, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 Suppresses Neuroinflammation Against Cognitive Deficit in a Streptozotocin-Induced Rat Model of Alzheimer’s Disease Through Activation of BDNF-TrkB Signaling Pathway. Front. Pharmacol. 2019, 10, 395. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Deng, H.; Wang, B.; Fu, W.; You, Y.; Tian, S. Apelin-13 exerts antidepressant-like and recognition memory improving activities in stressed rats. Eur. Neuropsychopharmacol. 2016, 26, 420–430. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.Y.; Wang, B.; Fu, W.; Jin, X.; You, Y.; Tian, S.W.; Kuang, X. The Hippocampus is a Critical Site Mediating Antidepressant-like Activity of Apelin-13 in Rats. Neuroscience 2018, 375, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.T.; Wang, B.; Xiao, Z.Y.; You, Y.; Tian, S.W. Apelin-13 Upregulates BDNF Against Chronic Stress-induced Depression-like Phenotypes by Ameliorating HPA Axis and Hippocampal Glucocorticoid Receptor Dysfunctions. Neuroscience 2018, 390, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J. Biol. Chem. 2004, 279, 50754–50763. [Google Scholar] [CrossRef] [PubMed]
- Garten, A.; Schuster, S.; Penke, M.; Gorski, T.; de Giorgis, T.; Kiess, W. Physiological and pathophysiological roles of NAMPT and NAD metabolism. Nat. Rev. Endocrinol. 2015, 11, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, J.; Mills, K.F.; Yoon, M.J.; Imai, S. Nicotinamide mononucleotide, a key NAD(+) intermediate, treats the pathophysiology of diet- and age-induced diabetes in mice. Cell Metab. 2011, 14, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Chalkiadaki, A.; Guarente, L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Bruno, J.; Easlon, E.; Lin, S.J.; Cheng, H.L.; Alt, F.W.; Guarente, L. Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 2008, 22, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Ke, S.F.; Zhou, C.C.; Zhang, S.L.; Guan, Y.F.; Xu, T.Y.; Sheng, C.Q.; Wang, P.; Miao, C.Y. Nicotinamide phosphoribosyltransferase is required for the calorie restriction-mediated improvements in oxidative stress, mitochondrial biogenesis, and metabolic adaptation. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Stromsdorfer, K.L.; Yamaguchi, S.; Yoon, M.J.; Moseley, A.C.; Franczyk, M.P.; Kelly, S.C.; Qi, N.; Imai, S.; Yoshino, J. NAMPT-Mediated NAD(+) Biosynthesis in Adipocytes Regulates Adipose Tissue Function and Multi-organ Insulin Sensitivity in Mice. Cell Rep. 2016, 16, 1851–1860. [Google Scholar] [CrossRef] [PubMed]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S. SIRT1-Mediated eNAMPT Secretion from Adipose Tissue Regulates Hypothalamic NAD+ and Function in Mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Jing, Z.; Xing, J.; Chen, X.; Stetler, R.A.; Weng, Z.; Gan, Y.; Zhang, F.; Gao, Y.; Chen, J.; Leak, R.K.; et al. Neuronal NAMPT is released after cerebral ischemia and protects against white matter injury. J. Cereb Blood Flow Metab. 2014, 34, 1613–1621. [Google Scholar] [CrossRef] [PubMed]
- Erfani, S.; Khaksari, M.; Oryan, S.; Shamsaei, N.; Aboutaleb, N.; Nikbakht, F. Nampt/PBEF/visfatin exerts neuroprotective effects against ischemia/reperfusion injury via modulation of Bax/Bcl-2 ratio and prevention of caspase-3 activation. J. Mol. Neurosci. 2015, 56, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Guan, Y.F.; Zhou, X.M.; Li, G.Q.; Li, Z.Y.; Zhou, C.C.; Wang, P.; Miao, C.Y. Regenerative Neurogenesis After Ischemic Stroke Promoted by Nicotinamide Phosphoribosyltransferase-Nicotinamide Adenine Dinucleotide Cascade. Stroke 2015, 46, 1966–1974. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, X.Z.; Tian, W.W.; Guan, Y.F.; Wang, P.; Miao, C.Y. Extracellular visfatin has nicotinamide phosphoribosyltransferase enzymatic activity and is neuroprotective against ischemic injury. CNS Neurosci. Ther. 2014, 20, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ryu, D.; Wu, Y.; Gariani, K.; Wang, X.; Luan, P.; D’Amico, D.; Ropelle, E.R.; Lutolf, M.P.; Aebersold, R.; et al. NAD(+) repletion improves mitochondrial and stem cell function and enhances life span in mice. Science 2016, 352, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Fricker, R.A.; Green, E.L.; Jenkins, S.I.; Griffin, S.M. The Influence of Nicotinamide on Health and Disease in the Central Nervous System. Int. J. Tryptophan Res. 2018, 11, 1178646918776658. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, X.; Yang, Y.; Takata, T.; Sakurai, T. Nicotinamide mononucleotide protects against beta-amyloid oligomer-induced cognitive impairment and neuronal death. Brain Res. 2016, 1643, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Gong, B.; Pan, Y.; Vempati, P.; Zhao, W.; Knable, L.; Ho, L.; Wang, J.; Sastre, M.; Ono, K.; Sauve, A.A.; et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-gamma coactivator 1alpha regulated beta-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging 2013, 34, 1581–1588. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Gerhold, K.; Mayers, J.R.; Wiest, M.M.; Watkins, S.M.; Hotamisligil, G.S. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell 2008, 134, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.; George-Hyslop, P.H.; Pericak-Vance, M.A.; Joo, S.H.; Rosi, B.L.; Gusella, J.F.; Crapper-MacLachlan, D.R.; Alberts, M.J.; et al. Association of apolipoprotein E allele epsilon 4 with late-onset familial and sporadic Alzheimer’s disease. Neurology 1993, 43, 1467–1472. [Google Scholar] [CrossRef] [PubMed]
- Cutler, R.G.; Kelly, J.; Storie, K.; Pedersen, W.A.; Tammara, A.; Hatanpaa, K.; Troncoso, J.C.; Mattson, M.P. Involvement of oxidative stress-induced abnormalities in ceramide and cholesterol metabolism in brain aging and Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2004, 101, 2070–2075. [Google Scholar] [CrossRef] [PubMed]
- Lukiw, W.J.; Pappolla, M.; Pelaez, R.P.; Bazan, N.G. Alzheimer’s disease--a dysfunction in cholesterol and lipid metabolism. Cell Mol. Neurobiol. 2005, 25, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Calon, F.; Julien, C.; Winkler, J.W.; Petasis, N.A.; Lukiw, W.J.; Bazan, N.G. Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 2011, 6, e15816. [Google Scholar] [CrossRef]
- Sanchez-Mejia, R.O.; Newman, J.W.; Toh, S.; Yu, G.Q.; Zhou, Y.; Halabisky, B.; Cisse, M.; Scearce-Levie, K.; Cheng, I.H.; Gan, L.; et al. Phospholipase A2 reduction ameliorates cognitive deficits in a mouse model of Alzheimer’s disease. Nat. Neurosci. 2008, 11, 1311–1318. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.O.; Grimm, H.S.; Patzold, A.J.; Zinser, E.G.; Halonen, R.; Duering, M.; Tschape, J.A.; De Strooper, B.; Muller, U.; Shen, J.; et al. Regulation of cholesterol and sphingomyelin metabolism by amyloid-beta and presenilin. Nat. Cell Biol. 2005, 7, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Furuhashi, N.; Kuromori, Y.; Miyashita, M.; Iwata, F.; Harada, K. Plasma palmitoleic acid content and obesity in children. Am. J. Clin. Nutr. 2005, 82, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Paillard, F.; Catheline, D.; Duff, F.L.; Bouriel, M.; Deugnier, Y.; Pouchard, M.; Daubert, J.C.; Legrand, P. Plasma palmitoleic acid, a product of stearoyl-coA desaturase activity, is an independent marker of triglyceridemia and abdominal adiposity. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 436–440. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Ohrvall, M.; Vessby, B. Fatty acid composition and estimated desaturase activities are associated with obesity and lifestyle variables in men and women. Nutr. Metab. Cardiovasc. Dis. 2006, 16, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Cao, H.; King, I.B.; Lemaitre, R.N.; Song, X.; Siscovick, D.S.; Hotamisligil, G.S. Circulating palmitoleic acid and risk of metabolic abnormalities and new-onset diabetes. Am. J. Clin. Nutr. 2010, 92, 1350–1358. [Google Scholar] [CrossRef] [PubMed]
- Ferry, G.; Tellier, E.; Try, A.; Gres, S.; Naime, I.; Simon, M.F.; Rodriguez, M.; Boucher, J.; Tack, I.; Gesta, S.; et al. Autotaxin is released from adipocytes, catalyzes lysophosphatidic acid synthesis, and activates preadipocyte proliferation. Up-regulated expression with adipocyte differentiation and obesity. J. Biol. Chem. 2003, 278, 18162–18169. [Google Scholar] [CrossRef] [PubMed]
- Gesta, S.; Simon, M.F.; Rey, A.; Sibrac, D.; Girard, A.; Lafontan, M.; Valet, P.; Saulnier-Blache, J.S. Secretion of a lysophospholipase D activity by adipocytes: Involvement in lysophosphatidic acid synthesis. J. Lipid Res. 2002, 43, 904–910. [Google Scholar] [PubMed]
- Boucher, J.; Quilliot, D.; Praderes, J.P.; Simon, M.F.; Gres, S.; Guigne, C.; Prevot, D.; Ferry, G.; Boutin, J.A.; Carpene, C.; et al. Potential involvement of adipocyte insulin resistance in obesity-associated up-regulation of adipocyte lysophospholipase D/autotaxin expression. Diabetologia 2005, 48, 569–577. [Google Scholar] [CrossRef] [PubMed]
- Dusaulcy, R.; Rancoule, C.; Gres, S.; Wanecq, E.; Colom, A.; Guigne, C.; van Meeteren, L.A.; Moolenaar, W.H.; Valet, P.; Saulnier-Blache, J.S. Adipose-specific disruption of autotaxin enhances nutritional fattening and reduces plasma lysophosphatidic acid. J. Lipid Res. 2011, 52, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Dusaulcy, R.; Treguer, K.; Gres, S.; Guigne, C.; Quilliot, D.; Valet, P.; Saulnier-Blache, J.S. Depot-specific regulation of autotaxin with obesity in human adipose tissue. J. Physiol. Biochem. 2012, 68, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Rachakonda, V.P.; Reeves, V.L.; Aljammal, J.; Wills, R.C.; Trybula, J.S.; DeLany, J.P.; Kienesberger, P.C.; Kershaw, E.E. Serum autotaxin is independently associated with hepatic steatosis in women with severe obesity. Obesity (Silver Spring) 2015, 23, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Reeves, V.L.; Trybula, J.S.; Wills, R.C.; Goodpaster, B.H.; Dube, J.J.; Kienesberger, P.C.; Kershaw, E.E. Serum Autotaxin/ENPP2 correlates with insulin resistance in older humans with obesity. Obesity (Silver Spring) 2015, 23, 2371–2376. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, K.; Kane, D.A.; Touaibia, M.; Kershaw, E.E.; Pulinilkunnil, T.; Kienesberger, P.C. Autotaxin Is Regulated by Glucose and Insulin in Adipocytes. Endocrinology 2017, 158, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Rancoule, C.; Attane, C.; Gres, S.; Fournel, A.; Dusaulcy, R.; Bertrand, C.; Vinel, C.; Treguer, K.; Prentki, M.; Valet, P.; et al. Lysophosphatidic acid impairs glucose homeostasis and inhibits insulin secretion in high-fat diet obese mice. Diabetologia 2013, 56, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Yung, Y.C.; Stoddard, N.C.; Chun, J. LPA receptor signaling: Pharmacology, physiology, and pathophysiology. J. Lipid Res. 2014, 55, 1192–1214. [Google Scholar] [CrossRef] [PubMed]
- Gimenez da Silva-Santi, L.; Masetto Antunes, M.; Mori, M.A.; Biesdorf de Almeida-Souza, C.; Vergilio Visentainer, J.; Carbonera, F.; Rabello Crisma, A.; Nunes Masi, L.; Massao Hirabara, S.; Curi, R.; et al. Brain Fatty Acid Composition and Inflammation in Mice Fed with High-Carbohydrate Diet or High-Fat Diet. Nutrients 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Park, S.M.; Kim, I.Y.; Sung, H.; Seong, J.K.; Moon, M.H. High-fat diet-induced lipidome perturbations in the cortex, hippocampus, hypothalamus, and olfactory bulb of mice. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, R.; Gomez-Pinilla, F. ‘Metabolic syndrome’ in the brain: Deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J. Physiol. 2012, 590, 2485–2499. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Zhuang, Y.; Gomez-Pinilla, F. High-fat diet transition reduces brain DHA levels associated with altered brain plasticity and behaviour. Sci. Rep. 2012, 2, 431. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Morishita, R. The roles of lipid and glucose metabolism in modulation of beta-amyloid, tau, and neurodegeneration in the pathogenesis of Alzheimer disease. Front. Aging Neurosci. 2015, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.; Jansen, D.; Mutsaers, M.P.; Dederen, P.J.; Geenen, B.; Mulder, M.T.; Kiliaan, A.J. The Effect of a High-Fat Diet on Brain Plasticity, Inflammation and Cognition in Female ApoE4-Knockin and ApoE-Knockout Mice. PLoS ONE 2016, 11, e0155307. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.B.; Zhang, Z.; Luo, P.; Wang, S.S.; Peng, Y.; Chu, S.F.; Chen, N.H. Lipid metabolism in Alzheimer’s disease. Brain Res. Bull. 2019, 144, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Del Olmo, N.; Ruiz-Gayo, M. Influence of High-Fat Diets Consumed During the Juvenile Period on Hippocampal Morphology and Function. Front. Cell Neurosci. 2018, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic Acid: Physiological Role, Metabolism and Nutritional Implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Strable, M.S.; Ntambi, J.M. Genetic control of de novo lipogenesis: Role in diet-induced obesity. Crit. Rev. Biochem. Mol. Biol. 2010, 45, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Silbernagel, G.; Kovarova, M.; Cegan, A.; Machann, J.; Schick, F.; Lehmann, R.; Haring, H.U.; Stefan, N.; Schleicher, E.; Fritsche, A.; et al. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J. Clin. Endocrinol. Metab. 2012, 97, E2288–E2292. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Balu, D.; Melrose, J.; Chan, C. Brain region-specificity of palmitic acid-induced abnormalities associated with Alzheimer’s disease. BMC Res. Notes 2008, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.H.; Lin, C.I.; Liao, H.; Chen, Y.H.; Lin, S.H. Palmitic acid-induced neuron cell cycle G2/M arrest and endoplasmic reticular stress through protein palmitoylation in SH-SY5Y human neuroblastoma cells. Int. J. Mol. Sci. 2014, 15, 20876–20899. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Melrose, J.; Chan, C. Involvement of astroglial ceramide in palmitic acid-induced Alzheimer-like changes in primary neurons. Eur. J. Neurosci. 2007, 26, 2131–2141. [Google Scholar] [CrossRef] [PubMed]
- Frigolet, M.E.; Gutierrez-Aguilar, R. The Role of the Novel Lipokine Palmitoleic Acid in Health and Disease. Adv. Nutr. 2017, 8, 173S–181S. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Li, H.; Xu, H.; Halim, V.; Zhang, W.; Wang, H.; Ong, K.T.; Woo, S.L.; Walzem, R.L.; Mashek, D.G.; et al. Palmitoleate induces hepatic steatosis but suppresses liver inflammatory response in mice. PLoS ONE 2012, 7, e39286. [Google Scholar] [CrossRef] [PubMed]
- Ouchi, N.; Parker, J.L.; Lugus, J.J.; Walsh, K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011, 11, 85–97. [Google Scholar] [CrossRef] [PubMed]
- Bolsoni-Lopes, A.; Festuccia, W.T.; Farias, T.S.; Chimin, P.; Torres-Leal, F.L.; Derogis, P.B.; de Andrade, P.B.; Miyamoto, S.; Lima, F.B.; Curi, R.; et al. Palmitoleic acid (n-7) increases white adipocyte lipolysis and lipase content in a PPARalpha-dependent manner. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1093–E1102. [Google Scholar] [CrossRef] [PubMed]
- Dimopoulos, N.; Watson, M.; Sakamoto, K.; Hundal, H.S. Differential effects of palmitate and palmitoleate on insulin action and glucose utilization in rat L6 skeletal muscle cells. Biochem. J. 2006, 399, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Kuda, O.; Stankova, B.; Tvrzicka, E.; Hensler, M.; Jelenik, T.; Rossmeisl, M.; Flachs, P.; Kopecky, J. Prominent role of liver in elevated plasma palmitoleate levels in response to rosiglitazone in mice fed high-fat diet. J. Physiol. Pharmacol. 2009, 60, 135–140. [Google Scholar] [PubMed]
- Furuhashi, M.; Hotamisligil, G.S. Fatty acid-binding proteins: Role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 2008, 7, 489–503. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, M.; Ishimura, S.; Ota, H.; Miura, T. Lipid chaperones and metabolic inflammation. Int. J. Inflam. 2011, 2011, 642612. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.C.; Wat, N.M.; Tam, S.C.; Janus, E.D.; Lam, T.H.; Lam, K.S. C-reactive protein predicts the deterioration of glycemia in chinese subjects with impaired glucose tolerance. Diabetes Care 2003, 26, 2323–2328. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Y.; Xydakis, A.M.; Hoogeveen, R.C.; Jones, P.H.; Smith, E.O.; Nelson, K.W.; Ballantyne, C.M. Multiplexed analysis of biomarkers related to obesity and the metabolic syndrome in human plasma, using the Luminex-100 system. Clin. Chem. 2005, 51, 1102–1109. [Google Scholar] [CrossRef] [PubMed]
- Degawa-Yamauchi, M.; Bovenkerk, J.E.; Juliar, B.E.; Watson, W.; Kerr, K.; Jones, R.; Zhu, Q.; Considine, R.V. Serum resistin (FIZZ3) protein is increased in obese humans. J. Clin. Endocrinol. Metab. 2003, 88, 5452–5455. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Wang, Y.; Xu, J.Y.; Stejskal, D.; Tam, S.; Zhang, J.; Wat, N.M.; Wong, W.K.; Lam, K.S. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin. Chem. 2006, 52, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Kralisch, S.; Ebert, T.; Lossner, U.; Jessnitzer, B.; Stumvoll, M.; Fasshauer, M. Adipocyte fatty acid-binding protein is released from adipocytes by a non-conventional mechanism. Int J. Obes. (Lond.) 2014, 38, 1251–1254. [Google Scholar] [CrossRef] [PubMed]
- Erbay, E.; Babaev, V.R.; Mayers, J.R.; Makowski, L.; Charles, K.N.; Snitow, M.E.; Fazio, S.; Wiest, M.M.; Watkins, S.M.; Linton, M.F.; et al. Reducing endoplasmic reticulum stress through a macrophage lipid chaperone alleviates atherosclerosis. Nat. Med. 2009, 15, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Coleman, S.L.; Park, Y.K.; Lee, J.Y. Unsaturated fatty acids repress the expression of adipocyte fatty acid binding protein via the modulation of histone deacetylation in RAW 264.7 macrophages. Eur. J. Nutr. 2011, 50, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Uysal, K.T.; Scheja, L.; Wiesbrock, S.M.; Bonner-Weir, S.; Hotamisligil, G.S. Improved glucose and lipid metabolism in genetically obese mice lacking aP2. Endocrinology 2000, 141, 3388–3396. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Cao, H.; Kono, K.; Gorgun, C.Z.; Furuhashi, M.; Uysal, K.T.; Cao, Q.; Atsumi, G.; Malone, H.; Krishnan, B.; et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005, 1, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Maeda, K.; Gorgun, C.Z.; Kim, H.J.; Park, S.Y.; Shulman, G.I.; Kim, J.K.; Hotamisligil, G.S. Regulation of metabolic responses by adipocyte/macrophage Fatty Acid-binding proteins in leptin-deficient mice. Diabetes 2006, 55, 1915–1922. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyn, P.; Jazurek, M.; Dobrzyn, A. Stearoyl-CoA desaturase and insulin signaling--what is the molecular switch? Biochim. Biophys. Acta 2010, 1797, 1189–1194. [Google Scholar] [CrossRef] [PubMed]
- Astarita, G.; Jung, K.M.; Vasilevko, V.; Dipatrizio, N.V.; Martin, S.K.; Cribbs, D.H.; Head, E.; Cotman, C.W.; Piomelli, D. Elevated stearoyl-CoA desaturase in brains of patients with Alzheimer’s disease. PLoS ONE 2011, 6, e24777. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.; Miyazaki, M.; Socci, N.D.; Hagge-Greenberg, A.; Liedtke, W.; Soukas, A.A.; Sharma, R.; Hudgins, L.C.; Ntambi, J.M.; Friedman, J.M. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science 2002, 297, 240–243. [Google Scholar] [CrossRef] [PubMed]
- Corpeleijn, E.; Feskens, E.J.; Jansen, E.H.; Mensink, M.; Saris, W.H.; de Bruin, T.W.; Blaak, E.E. Improvements in glucose tolerance and insulin sensitivity after lifestyle intervention are related to changes in serum fatty acid profile and desaturase activities: The SLIM study. Diabetologia 2006, 49, 2392–2401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Warensjo, E.; Ingelsson, E.; Lundmark, P.; Lannfelt, L.; Syvanen, A.C.; Vessby, B.; Riserus, U. Polymorphisms in the SCD1 gene: Associations with body fat distribution and insulin sensitivity. Obesity (Silver Spring) 2007, 15, 1732–1740. [Google Scholar] [CrossRef] [PubMed]
- Warensjo, E.; Riserus, U.; Vessby, B. Fatty acid composition of serum lipids predicts the development of the metabolic syndrome in men. Diabetologia 2005, 48, 1999–2005. [Google Scholar] [CrossRef] [PubMed]
- Mar-Heyming, R.; Miyazaki, M.; Weissglas-Volkov, D.; Kolaitis, N.A.; Sadaat, N.; Plaisier, C.; Pajukanta, P.; Cantor, R.M.; de Bruin, T.W.; Ntambi, J.M.; et al. Association of stearoyl-CoA desaturase 1 activity with familial combined hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Snigdha, S.; Astarita, G.; Piomelli, D.; Cotman, C.W. Effects of diet and behavioral enrichment on free fatty acids in the aged canine brain. Neuroscience 2012, 202, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Srodulski, S.; Sharma, S.; Bachstetter, A.B.; Brelsfoard, J.M.; Pascual, C.; Xie, X.S.; Saatman, K.E.; Van Eldik, L.J.; Despa, F. Neuroinflammation and neurologic deficits in diabetes linked to brain accumulation of amylin. Mol. Neurodegener. 2014, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.A.; Spencer, S.J. Obesity and neuroinflammation: A pathway to cognitive impairment. Brain Behav. Immun. 2014, 42, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Posey, K.A.; Clegg, D.J.; Printz, R.L.; Byun, J.; Morton, G.J.; Vivekanandan-Giri, A.; Pennathur, S.; Baskin, D.G.; Heinecke, J.W.; Woods, S.C.; et al. Hypothalamic proinflammatory lipid accumulation, inflammation, and insulin resistance in rats fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E1003–E1012. [Google Scholar] [CrossRef] [PubMed]
- Pistell, P.J.; Morrison, C.D.; Gupta, S.; Knight, A.G.; Keller, J.N.; Ingram, D.K.; Bruce-Keller, A.J. Cognitive impairment following high fat diet consumption is associated with brain inflammation. J. Neuroimmunol. 2010, 219, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Brandon, J.A.; Kraemer, M.; Vandra, J.; Halder, S.; Ubele, M.; Morris, A.J.; Smyth, S.S. Adipose-derived autotaxin regulates inflammation and steatosis associated with diet-induced obesity. PLoS ONE 2019, 14, e0208099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Natarajan, V. Lysophosphatidic acid (LPA) and its receptors: Role in airway inflammation and remodeling. Biochim. Biophys. Acta 2013, 1831, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ramakrishnan, D.P.; Ren, B. Regulation of angiogenesis by phospholipid lysophosphatidic acid. Front. Biosci. (Landmark Ed.) 2013, 18, 852–861. [Google Scholar] [PubMed]
- Estivill-Torrus, G.; Llebrez-Zayas, P.; Matas-Rico, E.; Santin, L.; Pedraza, C.; De Diego, I.; Del Arco, I.; Fernandez-Llebrez, P.; Chun, J.; De Fonseca, F.R. Absence of LPA1 signaling results in defective cortical development. Cereb. Cortex 2008, 18, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Matas-Rico, E.; Garcia-Diaz, B.; Llebrez-Zayas, P.; Lopez-Barroso, D.; Santin, L.; Pedraza, C.; Smith-Fernandez, A.; Fernandez-Llebrez, P.; Tellez, T.; Redondo, M.; et al. Deletion of lysophosphatidic acid receptor LPA1 reduces neurogenesis in the mouse dentate gyrus. Mol. Cell Neurosci. 2008, 39, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Herr, D.R.; Chun, J. Diversity of lysophosphatidic acid receptor-mediated intracellular calcium signaling in early cortical neurogenesis. J. Neurosci. 2010, 30, 7300–7309. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, N.; Shano, S.; Moriyama, R.; Chun, J. Lysophosphatidic acid stimulates neuronal differentiation of cortical neuroblasts through the LPA1-G(i/o) pathway. Neurochem. Int. 2007, 50, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Furuta, D.; Yamane, M.; Tsujiuchi, T.; Moriyama, R.; Fukushima, N. Lysophosphatidic acid induces neurite branch formation through LPA3. Mol. Cell Neurosci. 2012, 50, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Pilpel, Y.; Segal, M. The role of LPA1 in formation of synapses among cultured hippocampal neurons. J. Neurochem. 2006, 97, 1379–1392. [Google Scholar] [CrossRef] [PubMed]
- Goldin, M.; Segal, M. Protein kinase C and ERK involvement in dendritic spine plasticity in cultured rodent hippocampal neurons. Eur. J. Neurosci. 2003, 17, 2529–2539. [Google Scholar] [CrossRef] [PubMed]
- Pilpel, Y.; Segal, M. Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur. J. Neurosci. 2004, 19, 3151–3164. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.M.; Reavill, C.; Brown, G.; Brown, J.T.; Cluderay, J.E.; Crook, B.; Davies, C.H.; Dawson, L.A.; Grau, E.; Heidbreder, C.; et al. LPA1 receptor-deficient mice have phenotypic changes observed in psychiatric disease. Mol. Cell Neurosci. 2003, 24, 1170–1179. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Winter, P.; Shilliam, C.S.; Hughes, Z.A.; Langmead, C.; Maycox, P.R.; Dawson, L.A. Neurochemical changes in LPA1 receptor deficient mice--a putative model of schizophrenia. Neurochem. Res. 2005, 30, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Castilla-Ortega, E.; Sanchez-Lopez, J.; Hoyo-Becerra, C.; Matas-Rico, E.; Zambrana-Infantes, E.; Chun, J.; De Fonseca, F.R.; Pedraza, C.; Estivill-Torrus, G.; Santin, L.J. Exploratory, anxiety and spatial memory impairments are dissociated in mice lacking the LPA1 receptor. Neurobiol. Learn. Mem. 2010, 94, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Santin, L.J.; Bilbao, A.; Pedraza, C.; Matas-Rico, E.; Lopez-Barroso, D.; Castilla-Ortega, E.; Sanchez-Lopez, J.; Riquelme, R.; Varela-Nieto, I.; de la Villa, P.; et al. Behavioral phenotype of maLPA1-null mice: Increased anxiety-like behavior and spatial memory deficits. Genes Brain Behav. 2009, 8, 772–784. [Google Scholar] [CrossRef] [PubMed]
- Shano, S.; Moriyama, R.; Chun, J.; Fukushima, N. Lysophosphatidic acid stimulates astrocyte proliferation through LPA1. Neurochem. Int. 2008, 52, 216–220. [Google Scholar] [CrossRef] [PubMed]
- TC, E.S.; Dezonne, R.S.; Rehen, S.K.; Gomes, F.C. Astrocytes treated by lysophosphatidic acid induce axonal outgrowth of cortical progenitors through extracellular matrix protein and epidermal growth factor signaling pathway. J. Neurochem. 2011, 119, 113–123. [Google Scholar] [CrossRef]
- Schilling, T.; Repp, H.; Richter, H.; Koschinski, A.; Heinemann, U.; Dreyer, F.; Eder, C. Lysophospholipids induce membrane hyperpolarization in microglia by activation of IKCa1 Ca(2+)-dependent K(+) channels. Neuroscience 2002, 109, 827–835. [Google Scholar] [CrossRef]
- Schilling, T.; Stock, C.; Schwab, A.; Eder, C. Functional importance of Ca2+-activated K+ channels for lysophosphatidic acid-induced microglial migration. Eur. J. Neurosci. 2004, 19, 1469–1474. [Google Scholar] [CrossRef] [PubMed]
- Awada, R.; Rondeau, P.; Gres, S.; Saulnier-Blache, J.S.; Lefebvre d’Hellencourt, C.; Bourdon, E. Autotaxin protects microglial cells against oxidative stress. Free Radic. Biol. Med. 2012, 52, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Awada, R.; Saulnier-Blache, J.S.; Gres, S.; Bourdon, E.; Rondeau, P.; Parimisetty, A.; Orihuela, R.; Harry, G.J.; d’Hellencourt, C.L. Autotaxin downregulates LPS-induced microglia activation and pro-inflammatory cytokines production. J. Cell Biochem. 2014, 115, 2123–2132. [Google Scholar] [CrossRef] [PubMed]
- Moller, T.; Musante, D.B.; Ransom, B.R. Lysophosphatidic acid-induced calcium signals in cultured rat oligodendrocytes. Neuroreport 1999, 10, 2929–2932. [Google Scholar] [CrossRef] [PubMed]
- Nogaroli, L.; Yuelling, L.M.; Dennis, J.; Gorse, K.; Payne, S.G.; Fuss, B. Lysophosphatidic acid can support the formation of membranous structures and an increase in MBP mRNA levels in differentiating oligodendrocytes. Neurochem. Res. 2009, 34, 182–193. [Google Scholar] [CrossRef] [PubMed]
- Castanon, N.; Luheshi, G.; Laye, S. Role of neuroinflammation in the emotional and cognitive alterations displayed by animal models of obesity. Front. Neurosci. 2015, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- McLimans, K.E.; Willette, A.A.; Alzheimer’s Disease Neuroimaging, I. Autotaxin is Related to Metabolic Dysfunction and Predicts Alzheimer’s Disease Outcomes. J. Alzheimers Dis. 2017, 56, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Dong, Y.; Cui, M.Z.; Xu, X. Lysophosphatidic acid induces increased BACE1 expression and Abeta formation. Biochim. Biophys. Acta 2013, 1832, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Kinouchi, T.; Sorimachi, H.; Maruyama, K.; Mizuno, K.; Ohno, S.; Ishiura, S.; Suzuki, K. Conventional protein kinase C (PKC)-alpha and novel PKC epsilon, but not -delta, increase the secretion of an N-terminal fragment of Alzheimer’s disease amyloid precursor protein from PKC cDNA transfected 3Y1 fibroblasts. FEBS Lett. 1995, 364, 203–206. [Google Scholar] [PubMed]
- Jolly-Tornetta, C.; Wolf, B.A. Regulation of amyloid precursor protein (APP) secretion by protein kinase calpha in human ntera 2 neurons (NT2N). Biochemistry 2000, 39, 7428–7435. [Google Scholar] [CrossRef] [PubMed]
- Rossner, S.; Mendla, K.; Schliebs, R.; Bigl, V. Protein kinase Calpha and beta1 isoforms are regulators of alpha-secretory proteolytic processing of amyloid precursor protein in vivo. Eur J. Neurosci. 2001, 13, 1644–1648. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, D.; Lin, Y.H.; McMahon, T.; Koo, E.H.; Messing, R.O. Protein kinase C epsilon suppresses Abeta production and promotes activation of alpha-secretase. Biochem. Biophys. Res. Commun. 2001, 285, 997–1006. [Google Scholar] [CrossRef] [PubMed]
- Blois, J.T.; Mataraza, J.M.; Mecklenbrauker, I.; Tarakhovsky, A.; Chiles, T.C. B cell receptor-induced cAMP-response element-binding protein activation in B lymphocytes requires novel protein kinase Cdelta. J. Biol. Chem. 2004, 279, 30123–30132. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Moreno-Flores, M.T.; Avila, J.; Wandosell, F. The neurite retraction induced by lysophosphatidic acid increases Alzheimer’s disease-like Tau phosphorylation. J. Biol. Chem. 1999, 274, 37046–37052. [Google Scholar] [CrossRef] [PubMed]
- Sayas, C.L.; Avila, J.; Wandosell, F. Regulation of neuronal cytoskeleton by lysophosphatidic acid: Role of GSK-3. Biochim. Biophys. Acta 2002, 1582, 144–153. [Google Scholar] [CrossRef]
- Stranahan, A.M. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience 2015, 309, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, X.; Lin, H.; Fu, X.; Lin, W.; Li, M.; Zeng, X.; Gao, Q. Metabolic syndrome and stroke: A meta-analysis of prospective cohort studies. J. Clin. Neurosci. 2017, 40, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat. Rev. Neurosci. 2019, 20, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Pousti, F.; Ahmadi, R.; Mirahmadi, F.; Hosseinmardi, N.; Rohampour, K. Adiponectin modulates synaptic plasticity in hippocampal dentate gyrus. Neurosci. Lett. 2018, 662, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Forny-Germano, L.; De Felice, F.G.; Vieira, M. The Role of Leptin and Adiponectin in Obesity-Associated Cognitive Decline and Alzheimer’s Disease. Front. Neurosci. 2018, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Irving, A.J.; Harvey, J. Leptin regulation of hippocampal synaptic function in health and disease. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130155. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.N.; Liu, X.L. Functions of adiponectin signaling in regulating neural plasticity and its application as the therapeutic target to neurological and psychiatric diseases. Rev. Neurosci. 2019, 30, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Ghavami, S.; Shojaei, S.; Yeganeh, B.; Ande, S.R.; Jangamreddy, J.R.; Mehrpour, M.; Christoffersson, J.; Chaabane, W.; Moghadam, A.R.; Kashani, H.H.; et al. Autophagy and apoptosis dysfunction in neurodegenerative disorders. Prog. Neurobiol. 2014, 112, 24–49. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, J.; Nutma, E.; van der Valk, P.; Amor, S. Inflammation in CNS neurodegenerative diseases. Immunology 2018, 154, 204–219. [Google Scholar] [CrossRef] [PubMed]
- Radak, D.; Katsiki, N.; Resanovic, I.; Jovanovic, A.; Sudar-Milovanovic, E.; Zafirovic, S.; Mousad, S.A.; Isenovic, E.R. Apoptosis and Acute Brain Ischemia in Ischemic Stroke. Curr. Vasc. Pharmacol. 2017, 15, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Jayaraj, R.L.; Azimullah, S.; Beiram, R.; Jalal, F.Y.; Rosenberg, G.A. Neuroinflammation: Friend and foe for ischemic stroke. J. Neuroinflamm. 2019, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Parimisetty, A.; Dorsemans, A.C.; Awada, R.; Ravanan, P.; Diotel, N.; Lefebvre d’Hellencourt, C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J. Neuroinflamm. 2016, 13, 67. [Google Scholar] [CrossRef] [PubMed]
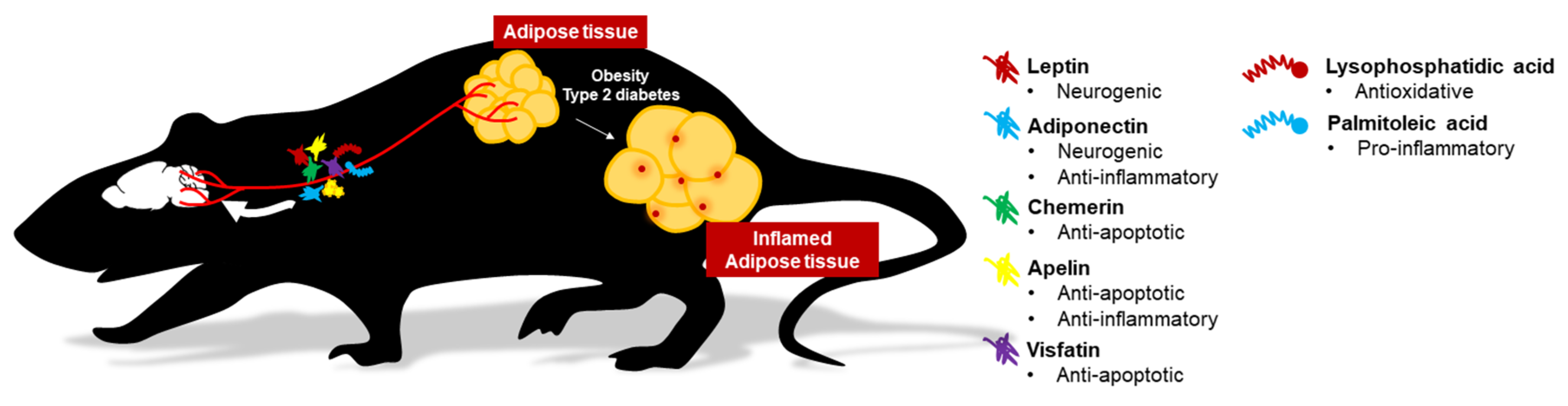
| Animal Model | Treatment | Delivery Route | Neurological Effects | References | Year | |
|---|---|---|---|---|---|---|
| Leptin | ||||||
| C57BL/6J (healthy) | mice | 14 days 1 mg/kg recombinant rat leptin | i.p. |
| Garza et al. [24] | 2008 |
| C57BL/6JJ (healthy) | mice | 30 min after injecting 1 mg/kg leptin | i.p. |
| Liu et al. [127] | 2010 |
| 4 and 12 m.o. SAM-P8 | mice | 0.25–0.5 µg mouse recombinant leptin post-training | Intra-hippo |
| Farr et al. [118] | 2006 |
| Sprague-DawleyJ (healthy) | rats | 1.0 µM leptin | Intra-DG |
| Wayner et al. [131] | 2004 |
| 6 m.o. TgCRND8 AD | mice | 8 weeks 20 µg leptin daily | Systemic infusion by osmotic pump |
| Greco et al. [133] | 2010 |
| Aβ1-42-assaulted | rat | 10 days 1 µg leptin daily | i.c.v. |
| Tong et al. [134] | 2015 |
| Adiponectin | ||||||
| HFD-induced stress and naïve C57BL/6J | mice | 0.1–0.3 µg globular adiponectin | i.c.v |
| Liu et al. [158] | 2012 |
| C57BL/6J (healthy) | mice | Overexpression of adiponectin by adenovirus | i.c.v |
| Yau et al. [25] | 2014 |
| Sedentary or environmentally enriched C57BL/6J | mice | 1 mg/kg globular adiponectin | i.v. |
| Nicolas et al. [187] | 2015 |
| Corticosterone-induced stress C57BL/6J | mice | 0.3 µg globular adiponectin | i.c.v. |
| Chabry et al. [188] | 2015 |
| Adipo+/+ & +/- C57BL/6J mice | mice | 10 mg/kg rosiglitazone 1 or 3 h before test | i.p. |
| Guo et al. [189] | 2017 |
| STZ-infused Sprague–Dawley | rats | 0.1 µg/g adiponectin | i.c.v. |
| Xu et al. [192] | 2018 |
| Chemerin | ||||||
| Hypoxic-ischemic encephalopathy in P10 Sprague-Dawley | rats | 9 μg/kg rh-chemerin, 3 doses | i.n. |
| Zhang et al. [215] | 2019 |
| LPS-induced depression BALB/c | mice | 500 ng chemerin | i.c.v. |
| Deyama et al. [218] | 2018 |
| LPS-induced depression BALB/c | mice | Resolvin E1 | i.c.v. (1 ng) and bilateral intra-mPFC and intra-DG (50 pg/side) |
| Deyama et al. [218] | 2018 |
| LPS-induced depression BALB/c | mice | 10 ng resolvin E2 | i.c.v. |
| Deyama et al. [218] | 2018 |
| Apelin | ||||||
| Cerebral I/R Wistar | rats | 50 ng/kg apelin-13 post-ischemia | i.c.v. |
| Xin et al. [234] | 2015 |
| Cerebral I/R AQP4+/+ & -/- | mice | 50 ng/kg apelin-13 post-ischemia | i.c.v. |
| Chu et al. [235] | 2017 |
| Cerebral I/R C57BL/6J | mice | 4 mg/kg, 3 doses for three days post-ischemia | i.n. |
| Chen et al. [236] | 2015 |
| TBI CD-1 | mice | 50 µg apelin-13 | i.c.v. |
| Bao et al. [237] | 2015 |
| STZ-infused AD Sprague–Dawley | rats | 2 µg, daily for 4 weeks | i.c.v. |
| Luo et al. [238] | 2019 |
| Forced-swimming stressed Wistar | rats | 2 µg, three doses in 24 h before FST | i.c.v. |
| Li et al. [238] | 2016 |
| Chronic water-immersion restraint stress Wistar | rats | 2 µg, daily for 7 days | i.c.v. |
| Dai et al. [241] | 2018 |
| Visfatin | ||||||
| Cerebral I/R | mice | pThy1:NAMPT | transgenic |
| Jing et al. [250] | 2014 |
| Cerebral I/R Wistar | rats | 100 ng visfatin during reperfusion | Intra-CA |
| Erfani et al. [251] | 2015 |
| Cerebral I/R C57BL/6J | mice | pIRES2::NAMPT | transgenic |
| Zhao et al. [252] | 2015 |
| Cerebral I/R C57BL/6J | mice | 500 mg/kg NMN daily for 7 days | i.p. |
| Zhao et al. [252] | 2015 |
| Aβ-infused AD Sprague-Dawley | rats | 500 mg/kg NMN daily for 10 days | i.p. |
| Wang et al. [256] | 2016 |
| Tg2576 AD | mice | 250 mg/kg nicotinamide riboside daily for 3 months | i.p. |
| Gong et al. [257] | 2013 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, T.H.-y.; Cheng, K.K.-y.; Hoo, R.L.-c.; Siu, P.M.-f.; Yau, S.-y. The Novel Perspectives of Adipokines on Brain Health. Int. J. Mol. Sci. 2019, 20, 5638. https://doi.org/10.3390/ijms20225638
Lee TH-y, Cheng KK-y, Hoo RL-c, Siu PM-f, Yau S-y. The Novel Perspectives of Adipokines on Brain Health. International Journal of Molecular Sciences. 2019; 20(22):5638. https://doi.org/10.3390/ijms20225638
Chicago/Turabian StyleLee, Thomas Ho-yin, Kenneth King-yip Cheng, Ruby Lai-chong Hoo, Parco Ming-fai Siu, and Suk-yu Yau. 2019. "The Novel Perspectives of Adipokines on Brain Health" International Journal of Molecular Sciences 20, no. 22: 5638. https://doi.org/10.3390/ijms20225638
APA StyleLee, T. H.-y., Cheng, K. K.-y., Hoo, R. L.-c., Siu, P. M.-f., & Yau, S.-y. (2019). The Novel Perspectives of Adipokines on Brain Health. International Journal of Molecular Sciences, 20(22), 5638. https://doi.org/10.3390/ijms20225638