Recent Advances in Graphene Oxide Membranes for Gas Separation Applications
Abstract
1. Introduction
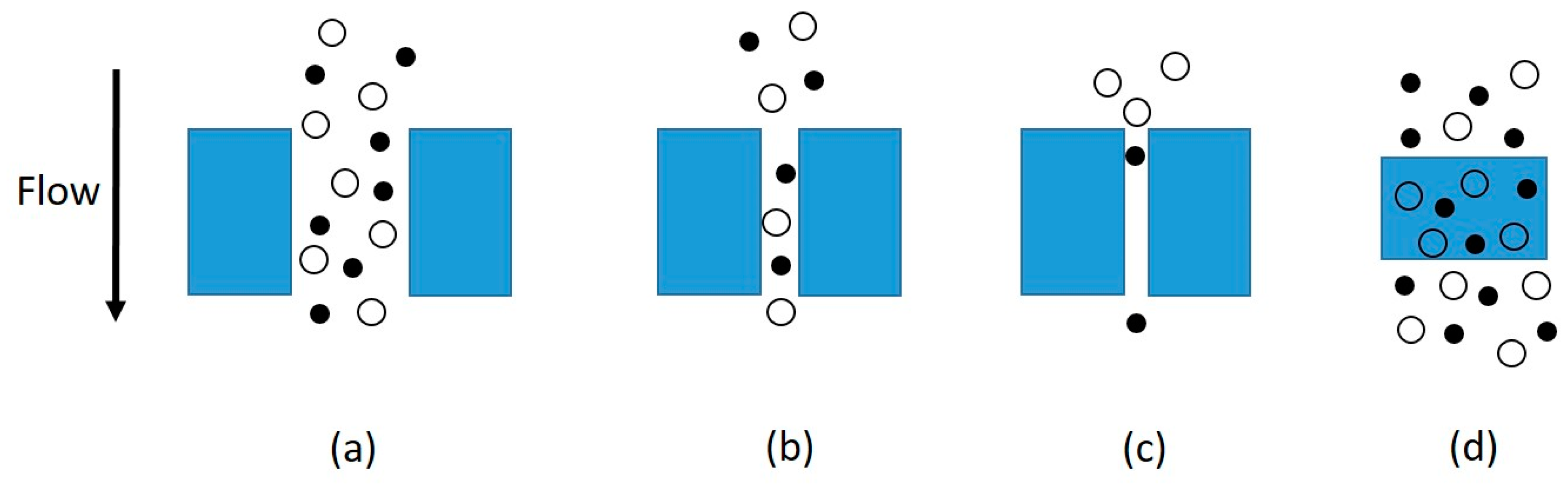
2. Mechanisms of Gas Transport through Membranes
3. Preparation and Characterization of GO Membranes
3.1. Preparation of GO Membranes
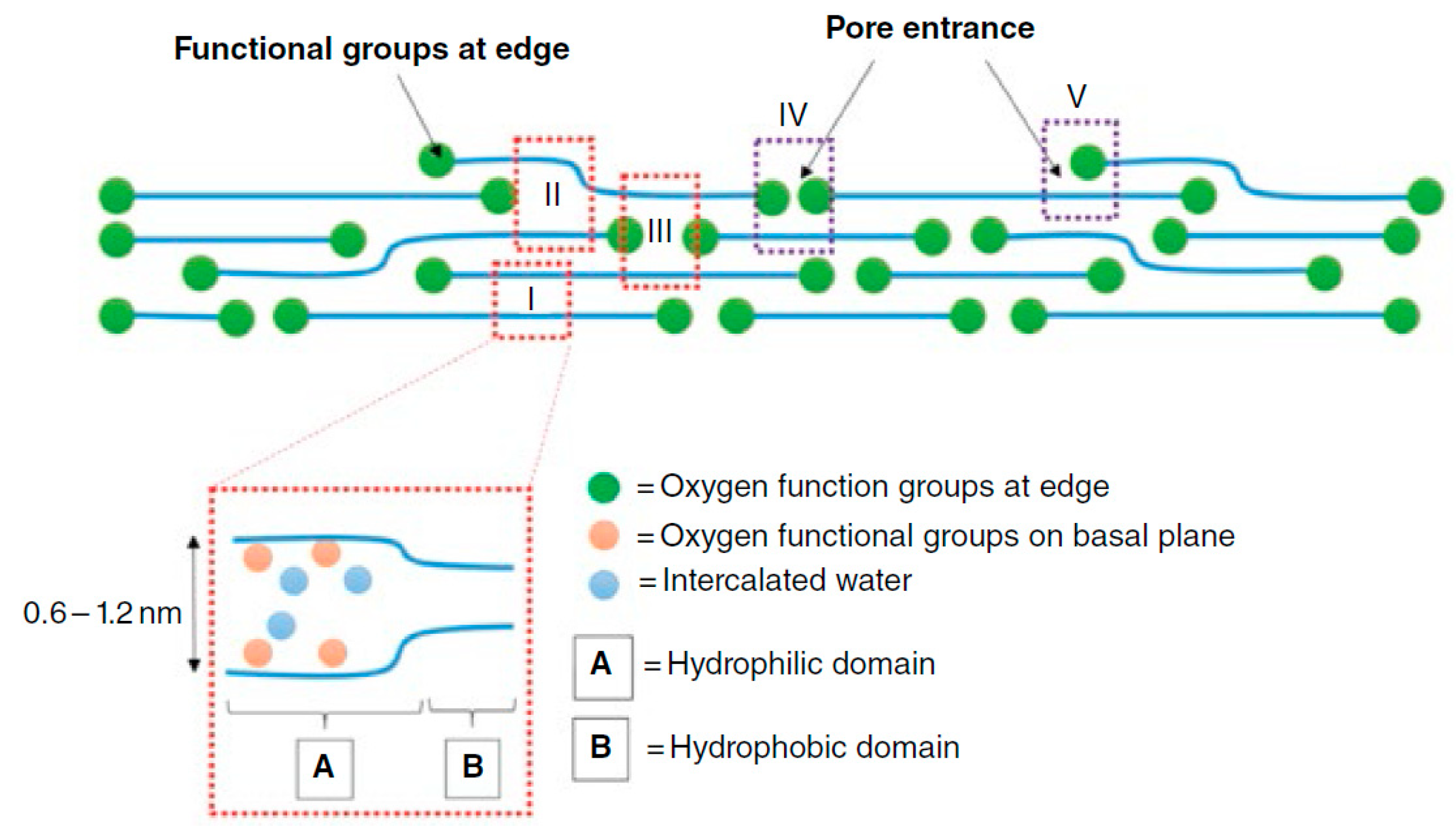
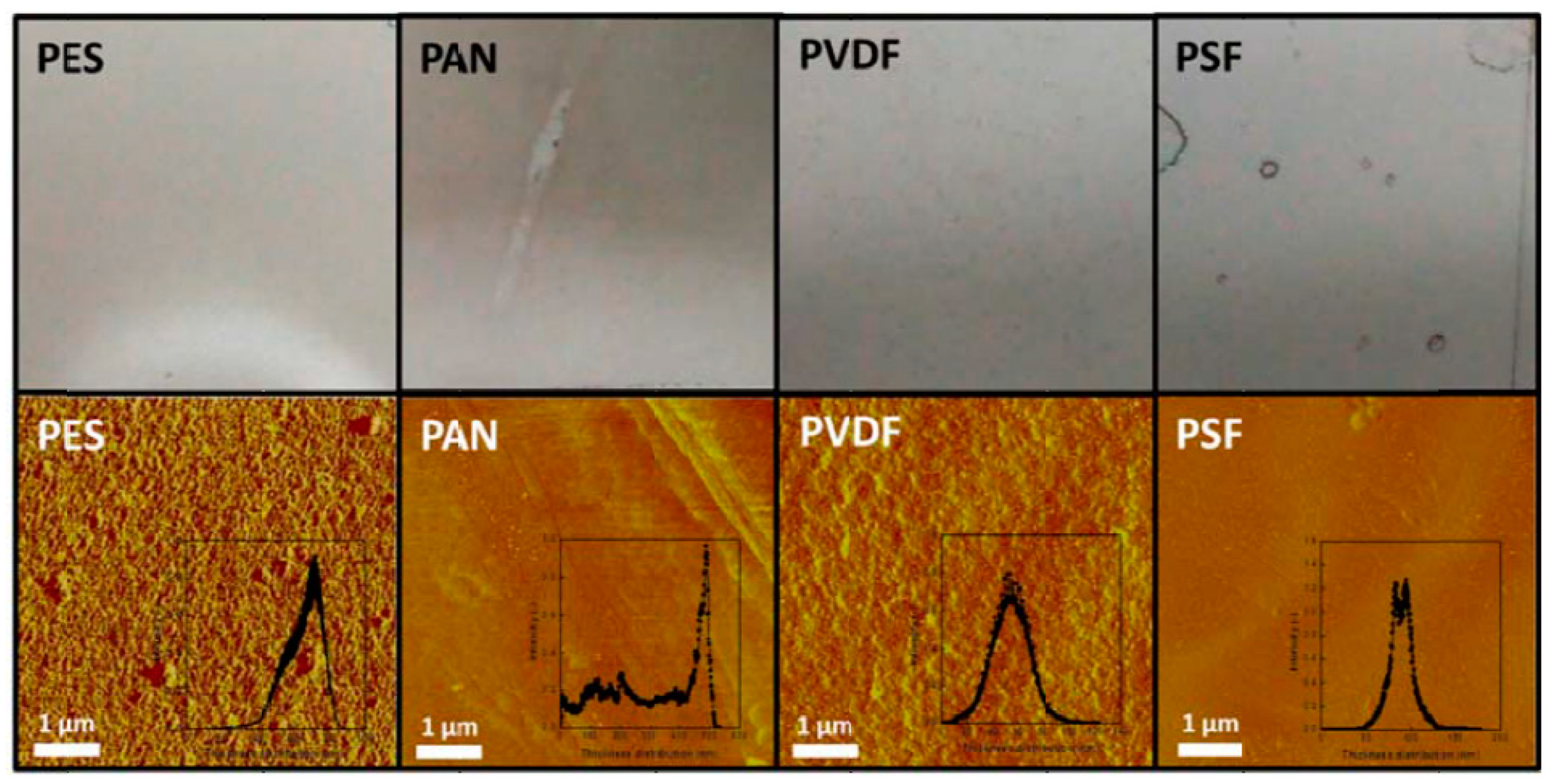
3.2. Characterization of GO Membranes
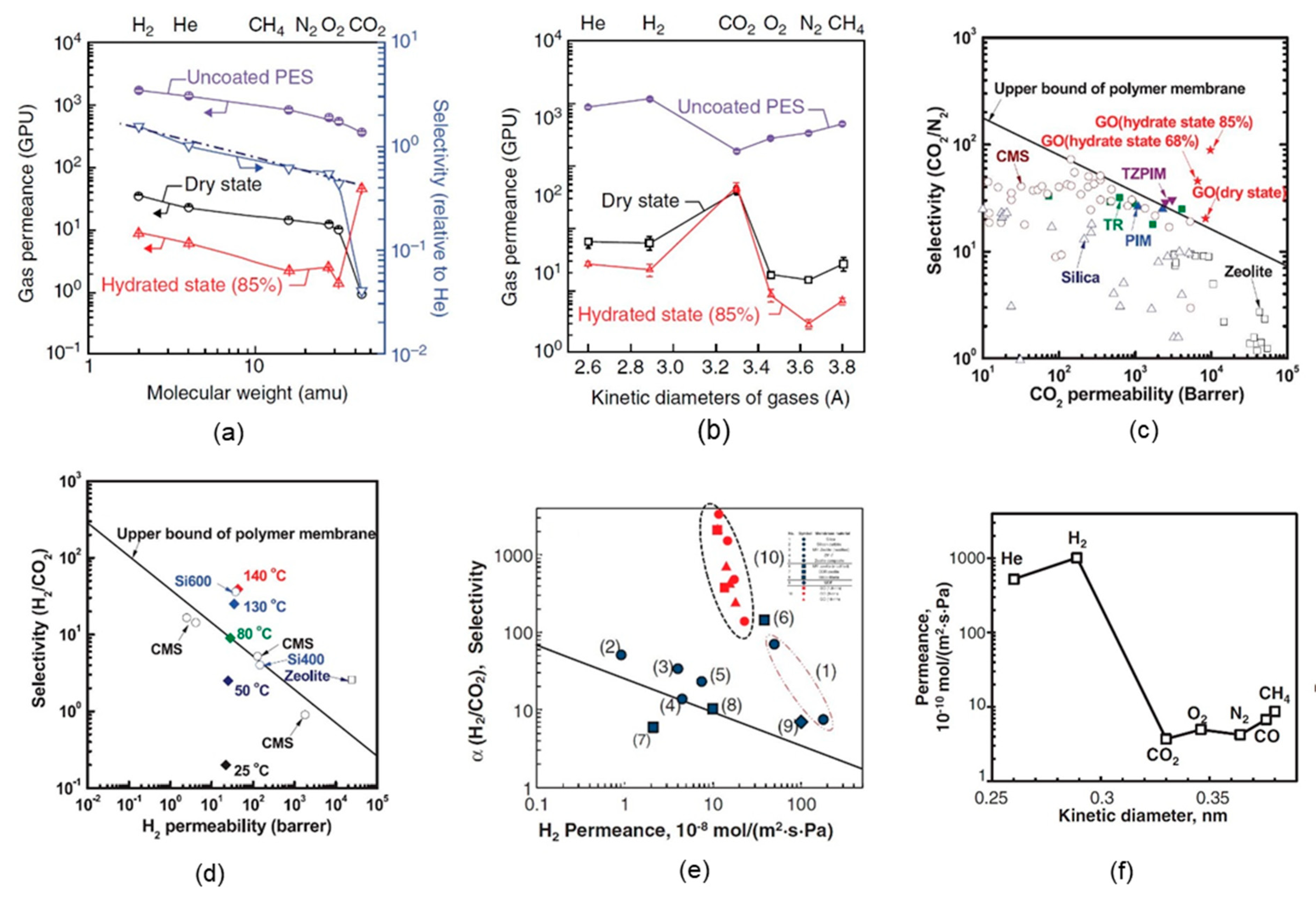
4. Gas Separation Performance of GO Membranes
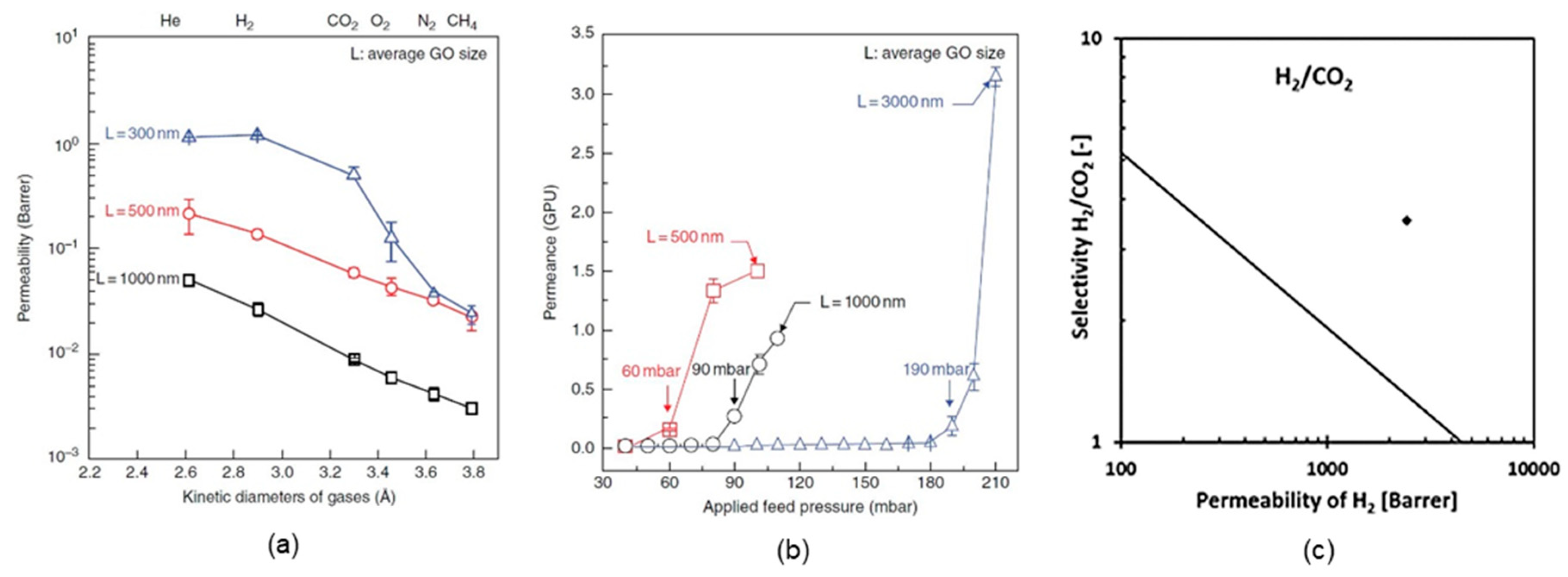
4.1. Supported GO Membranes
4.2. Self-Standing GO Membranes
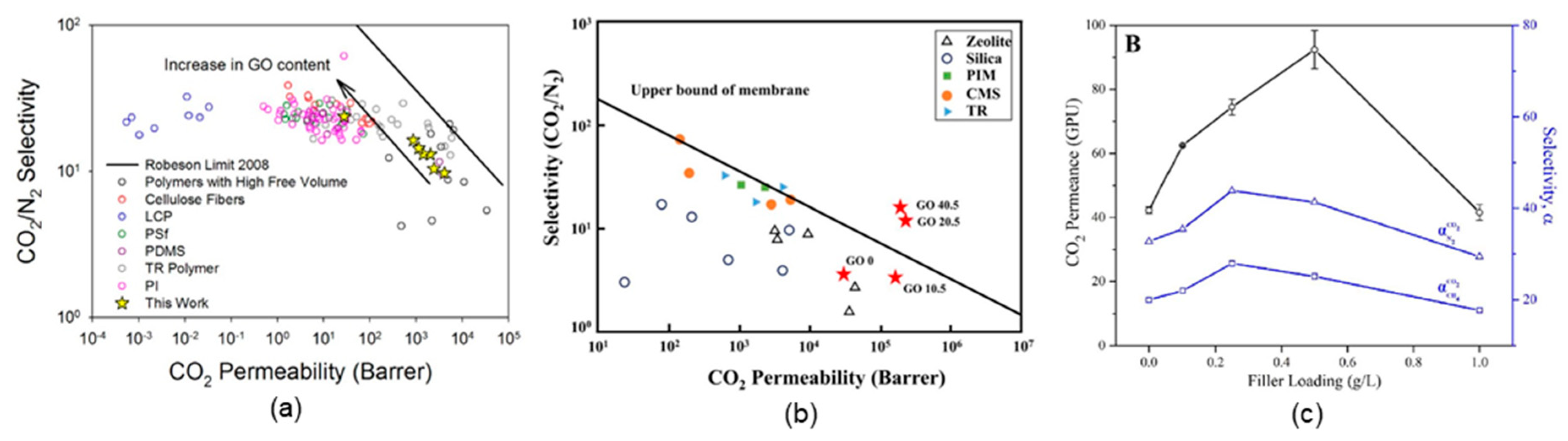
4.3. GO-Based Mixed-Matrix Membranes
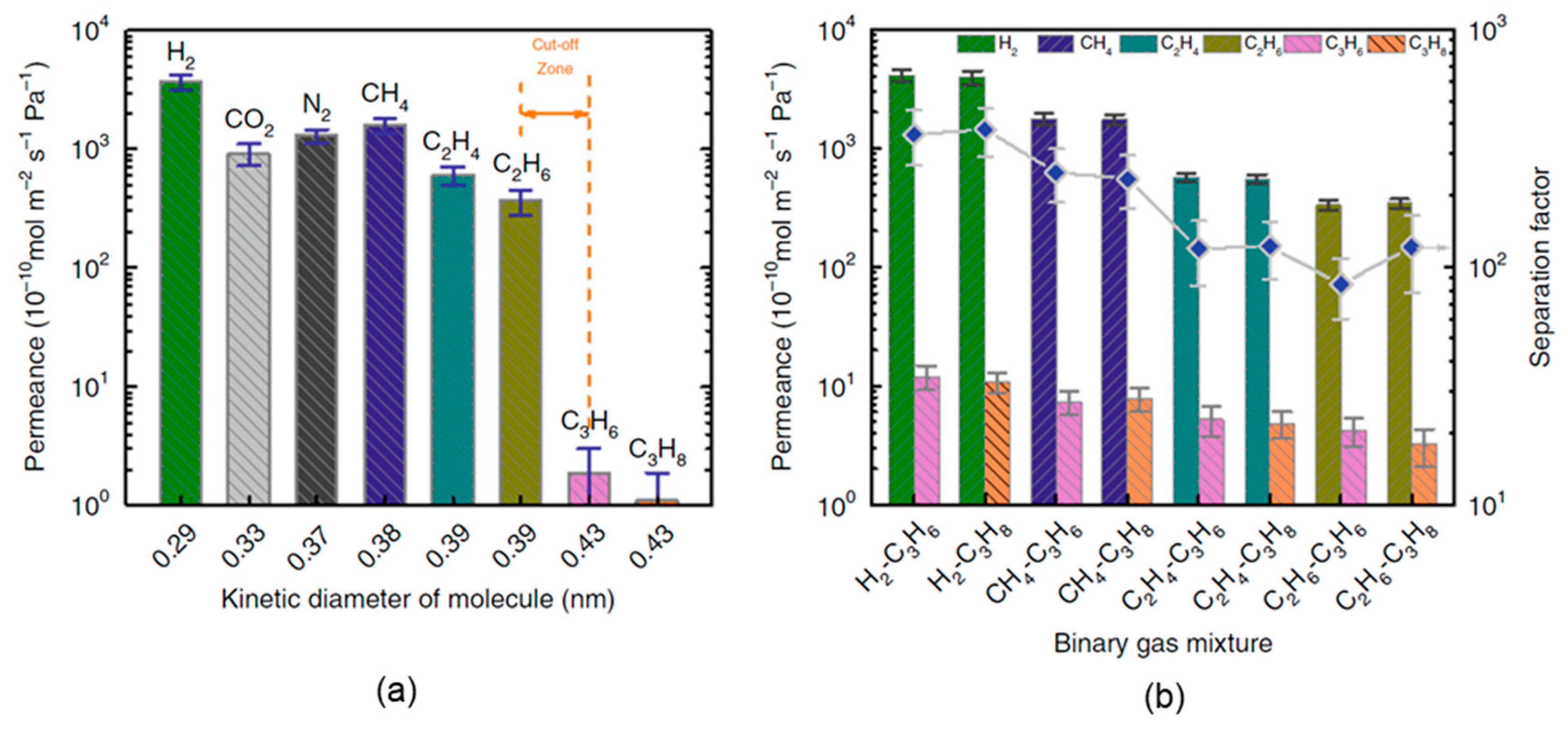
4.4. GO Membranes for Organics and Hydrocarbon Separation
5. Conclusions
- Graphene oxide-supported membranes can be prepared by a facile method through the deposition of GO on various support materials, such as a porous polymer, porous metal, or porous ceramic. They can provide enhanced gas separation performance while having reasonable mechanical stability. Proper selection of the membrane support materials, with consideration given to their porosity, thickness, thermal stability, wettability, and compatibility with the application environment, is critically important. Polyethersulfone is among the best flexible porous polymeric support materials identified in this review. Porous stainless-steel substrates and porous ceramic membranes could also be used as suitable support materials for the fabrication of GO-supported membranes. However, the relatively large thicknesses of these nonpolymeric support materials might pose a limit to the flux of gas transport through the membrane.
- The self-standing GO membrane concept was developed based on the target of achieving an ultrathin membrane. However, the main issue that needs to be resolved is the mechanical stability and robustness of self-standing membranes.
- Nanocomposite GO membranes, prepared by a composite of GO nanosheets and a suitable polymeric or inorganic membrane material, is one emerging technology used to develop membranes with higher permeability and higher selectivity beyond the upper bounds of existing polymeric and nanoporous membranes. The preparation of ultrathin nanocomposite membranes might be challenging, considering the limits for preparing thin inorganic membranes with acceptable mechanical properties.
- A GO membrane in a dry state has an interlayer spacing of 0.43 nm, which can be further reduced by the controlled reduction and removal of oxygen functionalities or can be increased by intercalation with water or other molecules. This approach would enable the GO membrane to be tailored for specific gas separation applications.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AAO | anodic aluminum oxide |
| AFM | atomic force microscopy |
| CMS | carbon molecular sieve |
| DLS | dynamic light scattering |
| ED | electrophoresis deposition |
| FE-SEM | field emission scanning electron microscopy |
| FTIR | Fourier-transform infrared spectroscopy |
| GO | graphene oxide |
| LB | Langmuir–Blodgett |
| LBL | layer by layer |
| MMM | mixed-matrix membranes |
| MOF | metal−organic frameworks |
| MOF-5 | zinc-based metal−organic frameworks |
| PBT | poly(butylene terephthalate) |
| PDAC | poly(diallyldimethylammonium chloride) |
| PDMS | poly(dimethylsiloxane) |
| PEI | polyethylenimine |
| PEO | poly(ethylene oxide) |
| PES | polyethersulfone |
| PIM | polymer of intrinsic microporosity |
| PSS | polystyrene sulfonate |
| PSSHF | porous stainless steel hollow fibers |
| SEM | scanning electron microscopy |
| TEM | transmission electron microscopy |
| TFN | thin film nanocomposite |
| TR polymer | thermally rearranged polymer |
| TGA | thermogravimetric analysis |
| TZPIM | tetrazole polymer of intrinsic microporosity |
| UiO-66-NH2 | functionalized zirconium-based porous metal–organic frameworks |
| WCA | water contact angle |
| XPS | X-ray photoelectron spectroscopy |
| XRD | X-ray diffraction |
References
- Nair, R.R.; Wu, H.A.; Jayaram, P.N.; Grigorieva, I.V.; Geim, A.K. Unimpeded permeation of water through helium-leak-tight graphene-based membranes. Science 2012, 335, 442–444. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.W.; Yoon, H.W.; Yoon, S.-M.; Yoo, B.M.; Ahn, B.K.; Cho, Y.H.; Shin, H.J.; Yang, H.; Paik, U.; Kwon, S.; et al. Selective gas transport through few-layered graphene and graphene oxide membranes. Science 2013, 342, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, molecular-sieving graphene oxide membranes for selective hydrogen separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Bouša, D.; Friess, K.; Pilnáček, K.; Vopička, O.; Lanč, M.; Fónod, K.; Pumera, M.; Sedmidubský, D.; Luxa, J.; Sofer, Z. Thin, high-flux, self-standing, graphene oxide membranes for efficient hydrogen separation from gas mixtures. Chem. Eur. J. 2017, 23, 11416–11422. [Google Scholar] [CrossRef]
- Qi, B.; He, X.; Zeng, G.; Pan, Y.; Li, G.; Liu, G. Strict molecular sieving over electrodeposited 2D-interspacing-narrowed graphene oxide membranes. Nat. Commun. 2017, 8, 825. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Park, H.B.; Yoon, H.W.; Cho, Y.H. Graphene oxide membrane for molecular separation. In Graphene Oxide: Fundamentals and Applications; Dimiev, A.M., Eigler, S., Eds.; John Wiley & Sons: New York, NY, USA, 2016; pp. 296–313. [Google Scholar]
- Pandey, P.; Chauhan, R. Membranes for gas separation. Prog. Polym. Sci. 2001, 26, 853–893. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Ma, J.; Ping, D.; Dong, X. Recent developments of graphene oxide-based membranes: A review. Membranes 2017, 7, 1–29. [Google Scholar]
- Xu, Q.; Zhang, W. Next-generation graphene-based membranes for gas separation and water purifications. In Advances in Carbon Nanostructures; Silva, A., Carabineiro, S., Eds.; IntechOpen: London, UK, 2016; pp. 417–424. [Google Scholar]
- Zhao, J.; Liu, L.; Li, F. (Eds.) Application of GO in environmental science. In Graphene Oxide: Physics and Applications; Springer: Heidelberg, Germany, 2015; pp. 119–135. [Google Scholar]
- Koenig, S.P.; Wang, L.; Pellegrino, J.; Bunch, J.S. Selective molecular sieving through porous graphene. Nat. Nanotechnol. 2012, 7, 728–732. [Google Scholar] [CrossRef]
- Xu, Q.; Xu, H.; Chen, J.; Lv, Y.; Dong, C.; Sreeprasad, T.S. Graphene and graphene oxide: Advanced membranes for gas separation and water purification. Inorg. Chem. Front. 2015, 2, 417–424. [Google Scholar] [CrossRef]
- Sun, C.; Wen, B.; Bai, B. Recent advances in nanoporous graphene membrane for gas separation and water purification. Sci. Bull. 2015, 60, 1807–1823. [Google Scholar] [CrossRef]
- Wang, L.; Boutilier, M.S.H.; Kidambi, P.R.; Jang, D.; Hadjiconstantinou, N.G.; Karnik, R. Fundamental transport mechanisms, fabrication and potential applications of nanoporous atomically thin membranes. Nat. Nanotechnol. 2017, 12, 509–522. [Google Scholar] [CrossRef]
- Shen, X.; Lin, X.; Yousefi, N.; Jia, J.; Kim, J.-K. Wrinkling in graphene sheets and graphene oxide papers. Carbon 2014, 66, 84–92. [Google Scholar] [CrossRef]
- Bunch, J.S.; Verbridge, S.S.; Alden, J.S.; Zande, A.M.; Parpia, J.M.; Craighead, H.G.; McEuen, P.L. Impermeable atomic membranes from graphene sheets. Nano Lett. 2008, 8, 2458–2462. [Google Scholar] [CrossRef] [PubMed]
- O’Hern, S.C.; Boutilier, M.S.; Idrobo, J.; Song, Y.; Kong, J.; Laoui, T.; Atieh, M.; Karnik, R. Selective ionic transport through tunable subnanometer pores in single-layer graphene membranes. Nano Lett. 2014, 14, 1234–1241. [Google Scholar] [CrossRef]
- Yoo, B.M.; Shin, J.E.; Lee, H.D.; Park, H.B. Graphene and graphene oxide membranes for gas separation applications. Curr. Opin. Chem. Eng. 2017, 16, 39–47. [Google Scholar] [CrossRef]
- Zhang, J.; Li, Z.; Zhan, K.; Sun, R.; Sheng, Z.; Wang, M.; Wang, S.; Hou, X. Two dimensional nanomaterial-based separation membranes. Electrophoresis 2019, 40, 2029–2040. [Google Scholar] [CrossRef]
- Du, Y.-C.; Huang, L.-J.; Wang, Y.-X.; Yang, K.; Tang, J.-G.; Wang, Y.; Cheng, M.-M.; Zhang, Y.; Kipper, M.J.; Belfiore, L.A.; et al. Recent developments in graphene-based polymer composite membranes: Preparation, mass transfer mechanism, and applications. J. Appl. Polym. Sci. 2019. [Google Scholar] [CrossRef]
- Huang, L.; Lin, H. Engineering sub-nanometer channels in two-dimensional materials for membrane gas separation. Membranes 2018, 8, 100. [Google Scholar] [CrossRef]
- Wikimedia Commons. Mechanisms of Transport in Membranes. Available online: https://commons.wikimedia.org/wiki/File:Mechanisms_of_transport_in_membranes.jpg (accessed on 4 November 2019).
- Sun, P.; Zhu, M.; Wang, K.; Zhong, M.; Wei, J.; Wu, D.; Xu, Z.; Zhu, H. Selective ion penetration of graphene oxide membranes. ACS Nano 2012, 7, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Joshi, R.K.; Carbone, P.; Wang, F.C.; Kravets, V.G.; Su, Y.; Grigorieva, I.V.; Wu, H.A.; Geim, A.K.; Nair, R.R. Precise and ultrafast molecular sieving through graphene oxide membranes. Science 2014, 343, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Abraham, J.; Vasu, K.S.; Williams, C.D.; Gopinadhan, K.; Su, Y.; Cherian, C.T.; Dix, J.; Prestat, E.; Haigh, S.J.; Grigorieva, I.V.; et al. Tunable sieving of ions using graphene oxide membranes. Nat. Nanotechnol. 2017, 12, 546–550. [Google Scholar] [CrossRef]
- Chen, L.; Shi, G.; Shen, J.; Peng, B.; Zhang, B.; Wang, Y.; Bian, F.; Wang, J.; Li, D.; Qian, Z.; et al. Ion sieving in graphene oxide membranes via cationic control of interlayer spacing. Nature 2017, 550, 380–383. [Google Scholar] [CrossRef] [PubMed]
- Brodie, B.C. XIII. On the atomic weight of graphite. Philos. Trans. R. Soc. Lond. 1859, 149, 49–259. [Google Scholar]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.-B.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Heo, J.; Choi, M.; Chang, J.; Ji, D.; Kang, S.W.; Hong, J. Highly permeable graphene oxide/polyelectrolytes hybrid thin films for enhanced CO2/N2 separation performance. Sci. Rep. 2017, 7, 1–8. [Google Scholar] [CrossRef]
- Chi, C.; Wang, X.; Peng, Y.; Qian, Y.; Hu, Z.; Dong, J.; Zhao, D. Facile preparation of graphene oxide membranes for gas separation. Chem. Mater. 2016, 28, 2921–2927. [Google Scholar] [CrossRef]
- Yang, Y.-H.; Bolling, L.; Priolo, M.A.; Grunlan, J.C. Graphene: Super gas barrier and selectivity of graphene oxide-polymer multilayer thin films. Adv. Mater. 2013, 25, 493. [Google Scholar] [CrossRef]
- Karunakaran, M.; Shevate, R.; Kumar, M.; Peinemann, K.-V. CO2-selective PEO–PBT (PolyActive™)/graphene oxide composite membranes. Chem. Commun. 2015, 51, 14187–14190. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Goh, P.S.; Ismail, A.F. Highly permeable and selective graphene oxide-enabled thin film nanocomposite for carbon dioxide separation. Int. J. Greenh. Gas Control 2017, 64, 257–266. [Google Scholar] [CrossRef]
- Jia, M.; Feng, Y.; Liu, S.; Qiu, J.; Yao, J. Graphene oxide gas separation membranes intercalated by UiO-66-NH2 with enhanced hydrogen separation performance. J. Membr. Sci. 2017, 539, 172–177. [Google Scholar] [CrossRef]
- Yang, Q.; Su, Y.; Chi, C.; Cherian, C.T.; Huang, K.; Kravets, V.G.; Wang, F.C.; Zhang, J.C.; Pratt, A.; Grigorenko, A.N.; et al. Ultrathin graphene-based membrane with precise molecular sieving and ultrafast solvent permeation. Nat. Mater. 2017, 16, 1198–1202. [Google Scholar] [CrossRef]
- Huang, K.; Liu, G.; Lou, Y.; Dong, Z.; Shen, J.; Jin, W. A graphene oxide membrane with highly selective molecular separation of aqueous organic solution. Angew. Chem. 2014, 126, 7049–7052. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, P.; Xu, L.; Chen, Z.; Xiao, L.; Sun, J.; Zhang, J.; Huang, Y.; Bielawski, C.W.; Geng, J. Tuning the surface properties of graphene oxide by surface-initiated polymerization of epoxides: An efficient method for enhancing gas separation. ACS Appl. Mater. Interfaces 2017, 9, 4998–5005. [Google Scholar] [CrossRef]
- Zheng, S.; Tu, Q.; Urban, J.J.; Li, S.; Mi, B. Swelling of graphene oxide membranes in aqueous solution: Characterization of interlayer spacing and insight into water transport mechanisms. ACS Nano 2017, 11, 6440–6450. [Google Scholar] [CrossRef]
- Zheng, H.; Zhu, L.; He, D.; Guo, T.; Li, X.; Chang, X.; Xue, Q. Two-dimensional graphene oxide membrane for H2/CH4 separation: Insights from molecular dynamics simulations. Int. J. Hydrog. Energy 2017, 42, 30653–30660. [Google Scholar] [CrossRef]
- Ha, H.; Park, J.; Ando, S.; Kim, C.B.; Nagai, K.; Freeman, B.D.; Ellison, C.J. Gas permeation and selectivity of poly(dimethylsiloxane)/graphene oxide composite elastomer membranes. J. Membr. Sci. 2016, 518, 131–140. [Google Scholar] [CrossRef]
- Castarlenas, S.; Téllez, C.; Coronas, J. Gas separation with mixed matrix membranes obtained from MOF UiO-66-graphite oxide hybrids. J. Membr. Sci. 2017, 526, 205–211. [Google Scholar] [CrossRef]
| GO/Support Material | Preparation Method | Reference |
|---|---|---|
| GO/PES | Dip casting, drop coating | [2] |
| GO/AAO | Vacuum filtration | [3] |
| GO/Cu | Spin coating, spray coating, vacuum filtration | [1] |
| Highly laminated GO | Ultrasonic exfoliation, stepwise separation | [39] |
| Self-standing GO | Gravitation assembly | [4] |
| GO/ceramic hollow fiber | Vacuum suction | [40] |
| Characterization Approach | Measurements | References | Major Findings |
|---|---|---|---|
| SEM (FE-SEM) | Size and distribution of the GO flakes | [2,3,4,5,41] | GO particle size of 0.1–20 µm |
| TEM | Thinness and curliness of the GO nanosheets | [2,5,41] | Effectiveness of the method of exfoliation |
| AFM | Surface morphology and depth profile of the GO membrane | [2,3,4,5,34,41] | Thickness of a single GO layer of 0.7–0.9 nm |
| XPS | Surface chemical composition | [3,4,5] | Density of different bonds, e.g., C = C (28.04%), C–C (13.79%), C = O (27.11%), C–O (14.62%), O–C = O (12.52%) |
| XRD | Amount of GO content and curvature in the polymeric support for GO membranes | [4,5,41] | GO has a peak 2θ at 11.1° |
| FTIR | Analysis of the functional groups | [4,5,41] | Functional groups in the form of stretching vibrations of different bonds, e.g., O–H (3400 cm−1), C = O (1720 cm−1), C = C skeletal (1620 cm−1), C–O carboxyl (1390 cm−1), C–O epoxy (1195 cm−1), and C–O alkoxy (1035 cm−1) |
| Raman spectroscopy | Structural integrity of the GO membrane | [3,4,5,41] | GO powder: G band at 1585 cm−1 and D band at 1338 cm−1 |
| WCA | Wettability of the GO membrane | [5] | GO membrane WCA of 46.7 ± 1.1° |
| DLS | Particle size distribution | [34] | Particle size distribution of the GO membrane, e.g., 10 µm (30%), 20 µm (40%) |
| TGA | Weight percentage of GO or a GO–polymer composite with variation in temperature | [41] | Weight percentage of GO or a GO–polymer composite at different temperatures |
| Ideal Selectivity (row/col.) a | H2 (0.29 nm) | CO2 (0.33 nm) | N2 (0.37 nm) | CH4 (0.38 nm) | C2H4 (0.39 nm) | C2H6 (0.39 nm) | C3H6 (0.43 nm) |
|---|---|---|---|---|---|---|---|
| CO2 (0.33 nm) | 4.1 | ||||||
| N2 (0.37 nm) | 2.9 | 0.7 | |||||
| CH4 (0.38 nm) | 2.3 | 0.6 | 0.8 | ||||
| C2H4 (0.39 nm) | 6.1 | 1.5 | 2.1 | 2.6 | |||
| C2H6 (0.39 nm) | 10.1 | 2.5 | 3.6 | 4.3 | 1.7 | ||
| C3H6 (0.43 nm) | 1949.2 (361.5) b | 478.9 | 683.8 | 836.0 (249.7) b | 319.1 (119.6) b | 192.3 (84.6) b | |
| C3H8 (0.43 nm) | 3366.8 (378.7) b | 827.2 | 1181.1 | 1443.9 (234.7) b | 551.1 (121.8) b | 332.2 (121.2) b | 1.7 |
| Type of GO Membranes | GO/Supporting Material | Application | Membrane Performance | Reference |
|---|---|---|---|---|
| Polymer-supported GO membranes | GO/PES | CO2 separation | a. CO2/N2 selectivity: 20 | [2] |
| b. CO2 permeability: 8500 barrer (1 barrer = 1 × 10−10 cm3⋅cm/cm2·sec·cmHg) | ||||
| GO/AAO | H2 separation | a. H2/CO2 selectivity: 3400 | [3] | |
| b. H2/N2 selectivity: 900 | ||||
| Self-standing GO membranes | GO (~5 µm) | H2 separation | CO2 permeability: <0.5 barrer | [2] |
| GO (15–20 µm) | H2 separation | a. H2/CO2 selectivity: 3400 | [4] | |
| b. CO2 permeability: 685 barrer | ||||
| GO-based mixed-matrix membranes | PEI/GO | CO2 separation | a. H2/CO2 selectivity: >383 | [35] |
| b. O2 permeability: 2.5 × 10−20 cm3⋅cm⋅cm−2⋅s−1 Pa−1 | ||||
| PEO-PBT/GO | CO2 separation | a. CO2/N2 selectivity: 73 | [36] | |
| b. CO2 permeability: 143 barrer | ||||
| PDMS/GO | CO2 separation | a. CO2/N2 selectivity: 24 | [44] | |
| b. CO2 permeability: <1000 GPU | ||||
| (PDAC/PSS)/(GO/GO) | CO2 separation | a. CO2/N2 selectivity: 73 | [33] | |
| b. CO2 permeability: 143 barrer | ||||
| UiO-66-NH2/GO | H2 separation | a. H2/N2 selectivity: 9.75 | [38] | |
| b. CO2 permeability: 6.1 × 10−9 mol⋅m−2⋅s−1⋅Pa−1 | ||||
| TFN/GO | CO2 separation | a. CO2/N2 selectivity: 41 | [34] | |
| b. CO2 permeability: 92.4 GPU | ||||
| Hydrocarbon mixture separation | GO/PSSHF | Hydrocarbon separation | a. Ideal selectivity between methane and propane 1443.9 | [3] |
| b. Separation factor of methane/propane from binary gas mixture 234.7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alen, S.K.; Nam, S.; Dastgheib, S.A. Recent Advances in Graphene Oxide Membranes for Gas Separation Applications. Int. J. Mol. Sci. 2019, 20, 5609. https://doi.org/10.3390/ijms20225609
Alen SK, Nam S, Dastgheib SA. Recent Advances in Graphene Oxide Membranes for Gas Separation Applications. International Journal of Molecular Sciences. 2019; 20(22):5609. https://doi.org/10.3390/ijms20225609
Chicago/Turabian StyleAlen, Saif Khan, SungWoo Nam, and Seyed A. Dastgheib. 2019. "Recent Advances in Graphene Oxide Membranes for Gas Separation Applications" International Journal of Molecular Sciences 20, no. 22: 5609. https://doi.org/10.3390/ijms20225609
APA StyleAlen, S. K., Nam, S., & Dastgheib, S. A. (2019). Recent Advances in Graphene Oxide Membranes for Gas Separation Applications. International Journal of Molecular Sciences, 20(22), 5609. https://doi.org/10.3390/ijms20225609






