Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level
Abstract
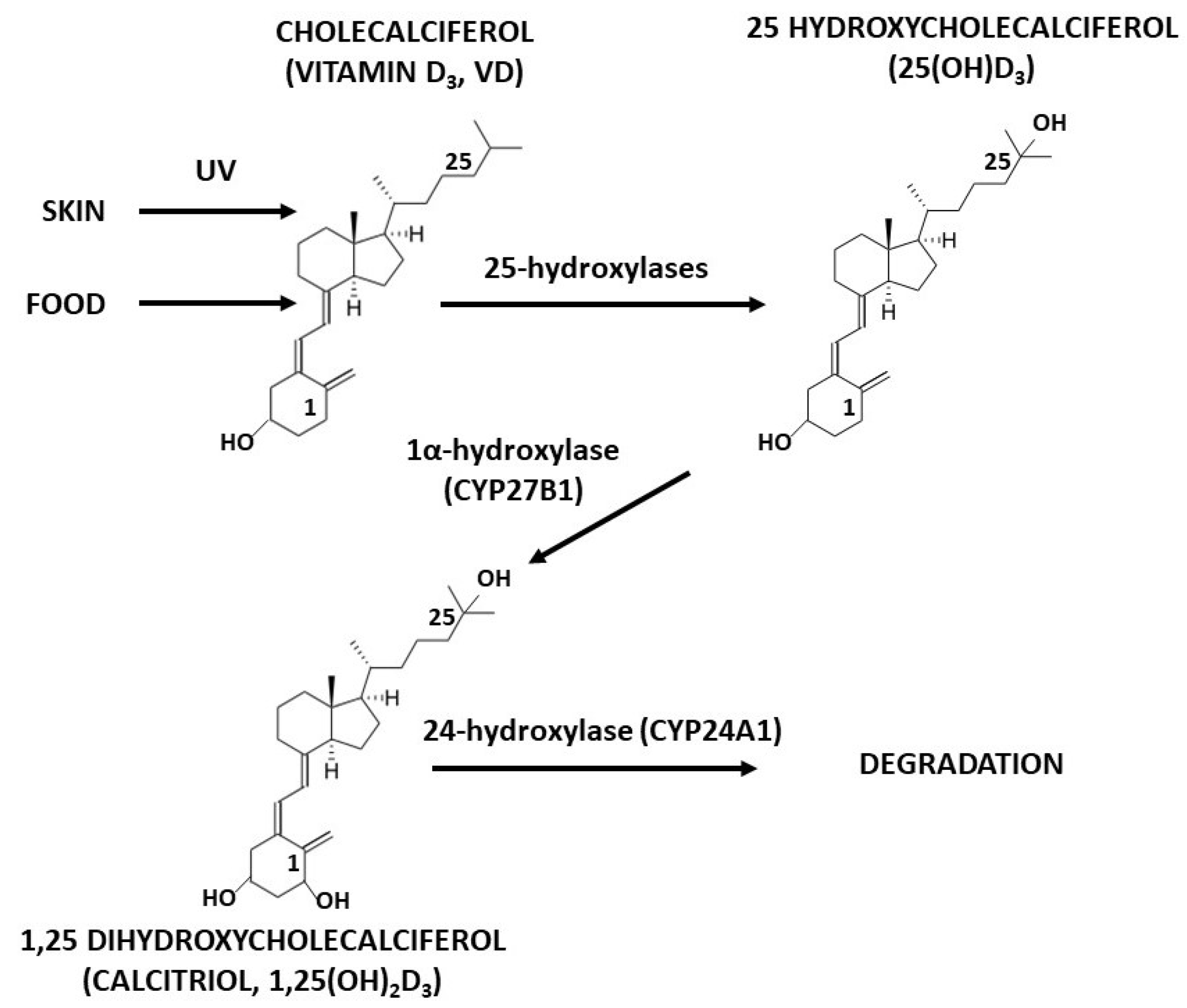
:1. Introduction
2. Results
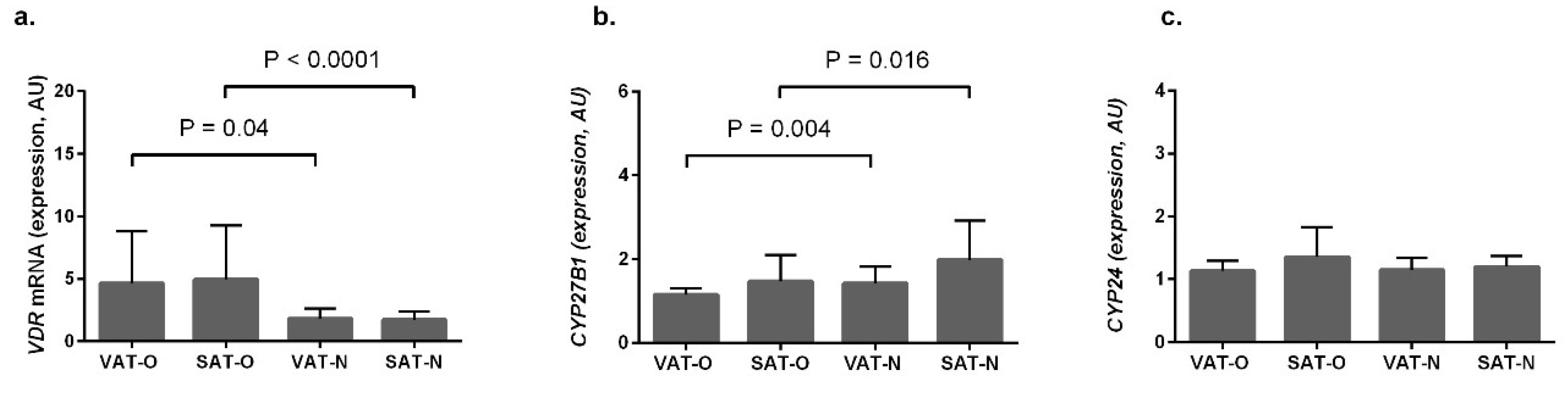
2.1. VDR and CYP27B1 Have Opposite Expression Profiles in Adipose Tissues
2.2. VDR and CYP27B1 Expression Profiles Do Not Depend on Vitamin D Metabolites’ Serum Levels
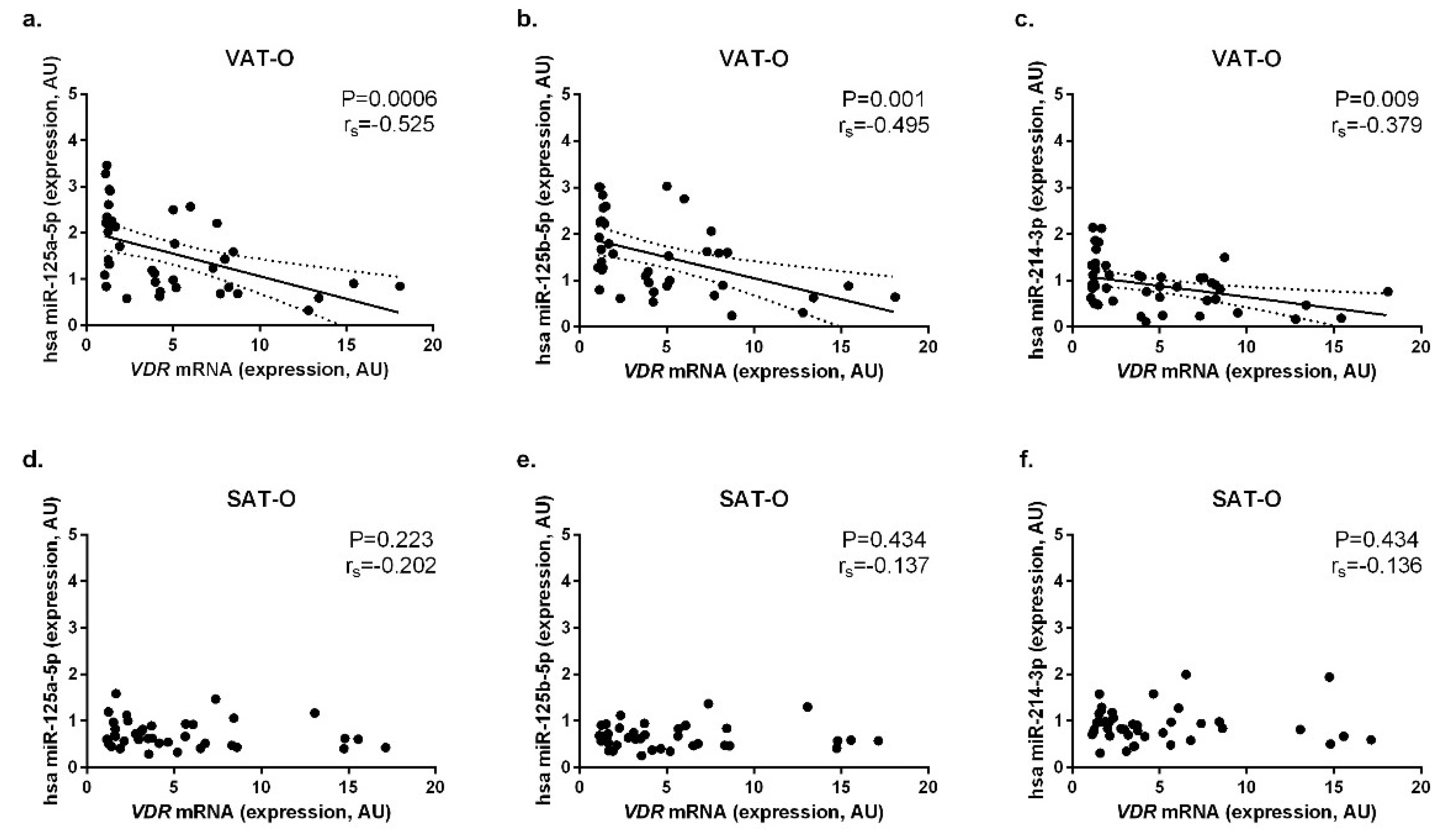
2.3. Expression of the Selected miRNAs Correlates Negatively with VDR mRNA Levels in Adipose Tissues
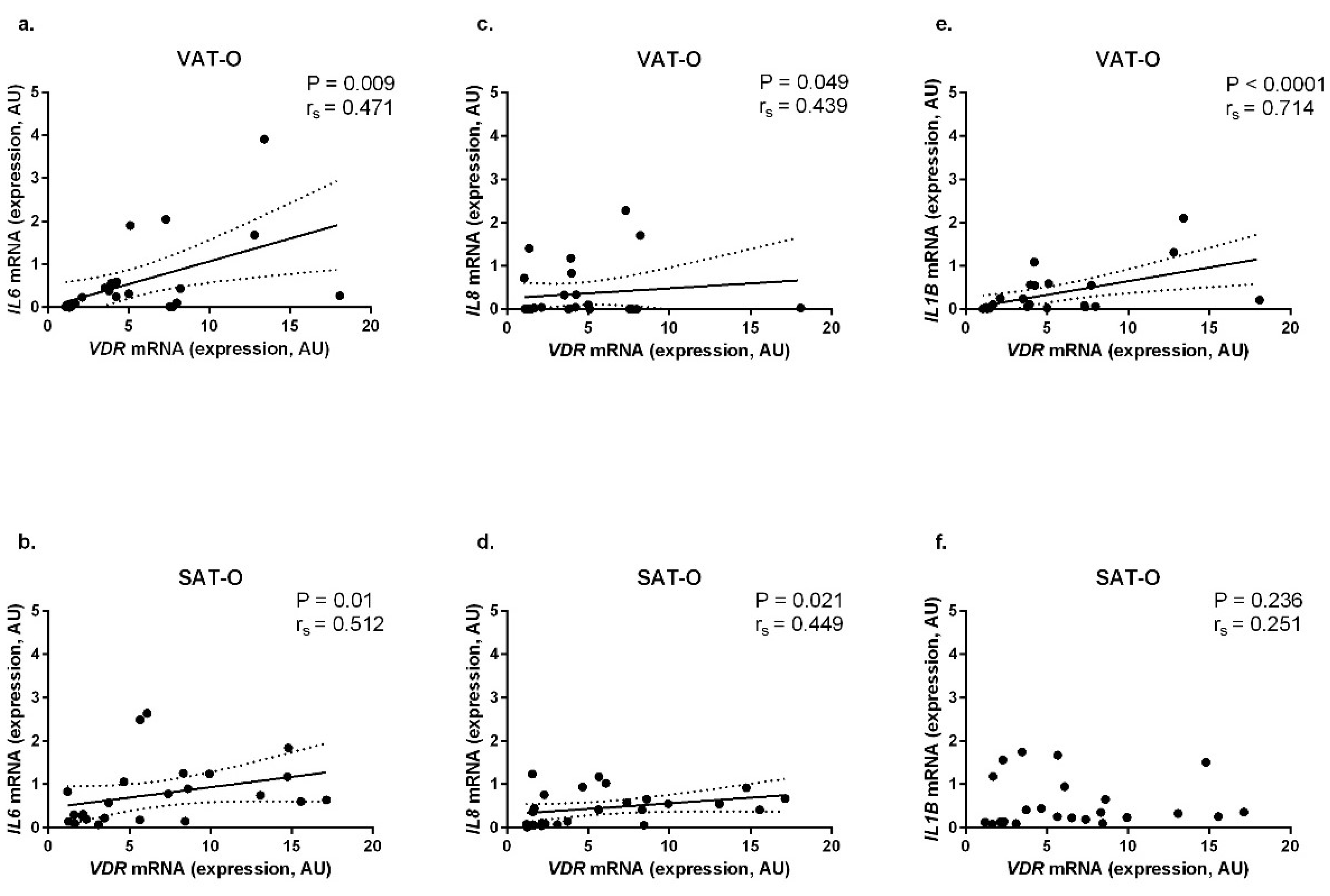
2.4. VDR mRNA Levels Correlate Positively with the Expression of Genes Encoding Pro-Inflammatory Cytokines in Adipose Tissues of Obese Individuals
3. Discussion
4. Materials and Methods
4.1. Studied Groups and Tissues
4.2. Measurement of 25(OH)D3 and 1,25(OH)2D3 Concentrations in Sera
4.3. RNA Isolation, Reverse Transcription and Real-Time PCR
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Choi, H.; Myung, K. Calcitriol enhances fat synthesis factors and calpain activity in co-cultured cells. Cell Biol. Int. 2014, 38, 910–917. [Google Scholar] [CrossRef] [PubMed]
- Mutt, S.J.; Hyppönen, E.; Saarnio, J.; Järvelin, M.R.; Herzig, K.H. Vitamin D and adipose tissue-more than storage. Front. Physiol. 2014, 5, 228. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.F.; Karkeni, E.; Marcotorchino, J.; Bonnet, L.; Tourniaire, F. Vitamin D modulates adipose tissue biology: Possible consequences for obesity? Proc. Nutr. Soc. 2016, 75, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Nimitphong, H.; Holick, M.F.; Fried, S.K.; Lee, M.J. 25-hydroxyvitamin D₃ and 1,25-dihydroxyvitamin D₃ promote the differentiation of human subcutaneous preadipocytes. PLoS ONE 2012, 7, e52171. [Google Scholar] [CrossRef] [PubMed]
- Lorente-Cebrián, S.; Eriksson, A.; Dunlop, T.; Mejhert, N.; Dahlman, I.; Aström, G.; Sjölin, E.; Wåhlén, K.; Carlberg, C.; Laurencikiene, J.; et al. Differential effects of 1α,25-dihydroxycholecalciferol on MCP-1 and adiponectin production in human white adipocytes. Eur. J. Nutr. 2012, 51, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Narvaez, C.J.; Simmons, K.M.; Brunton, J.; Salinero, A.; Chittur, S.V.; Welsh, J.E. Induction of STEAP4 correlates with 1,25-dihydroxyvitamin D3 stimulation of adipogenesis in mesenchymal progenitor cells derived from human adipose tissue. J. Cell. Physiol. 2013, 228, 2024–2036. [Google Scholar] [CrossRef]
- Sun, X.; Morris, K.L.; Zemel, M.B. Role of calcitriol and cortisol on human adipocyte proliferation and oxidative and inflammatory stress: A microarray study. J. Nutrigenet. Nutrigenomics 2008, 1, 30–48. [Google Scholar] [CrossRef]
- Wamberg, L.; Cullberg, K.B.; Rejnmark, L.; Richelsen, B.; Pedersen, S.B. Investigations of the anti-inflammatory effects of vitamin D in adipose tissue: Results from an in vitro study and a randomized controlled trial. Horm. Metab. Res. 2013, 45, 456–462. [Google Scholar] [CrossRef]
- Gao, D.; Trayhurn, P.; Bing, C. 1,25-Dihydroxyvitamin D3 inhibits the cytokine-induced secretion of MCP-1 and reduces monocyte recruitment by human preadipocytes. Int. J. Obes. 2013, 37, 357–365. [Google Scholar] [CrossRef]
- Ding, C.; Wilding, J.P.; Bing, C. 1,25-dihydroxyvitamin D3 protects against macrophage-induced activation of NFκB and MAPK signalling and chemokine release in human adipocytes. PLoS ONE 2013, 8, e61707. [Google Scholar] [CrossRef]
- Gregor, M.F.; Hotamisligil, G.S. Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 2011, 29, 415–445. [Google Scholar] [CrossRef] [PubMed]
- Shu, J.; Matarese, A.; Santulli, G. Diabetes, body fat, skeletal muscle, and hypertension: The ominous chiasmus? J. Clin. Hypertens. 2019, 21, 239–242. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Santulli, G. Integrating diet and inflammation to calculate cardiovascular risk. Atherosclerosis 2016, 253, 258–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordeiro, A.; Santos, A.; Bernardes, M.; Ramalho, A.; Martins, M.J. Vitamin D metabolism in human adipose tissue: Could it explain low vitamin D status in obesity? Horm. Mol. Biol. Clin. Investig. 2017, 33, 2. [Google Scholar] [CrossRef] [PubMed]
- Mohri, T.; Nakajima, M.; Takagi, S.; Komagata, S.; Yokoi, T. MicroRNA regulates human vitamin D receptor. Int. J. Cancer 2009, 125, 1328–1333. [Google Scholar] [CrossRef]
- Singh, R.; Yadav, V.; Kumar, S.; Saini, N. MicroRNA-195 inhibits proliferation, invasion and metastasis in breast cancer cells by targeting FASN, HMGCR, ACACA and CYP27B1. Sci. Rep. 2015, 5, 17454. [Google Scholar] [CrossRef]
- Komagata, S.; Nakajima, M.; Takagi, S.; Mohri, T.; Taniya, T.; Yokoi, T. Human CYP24 catalyzing the inactivation of calcitriol is post-transcriptionally regulated by miR-125b. Mol. Pharmacol. 2009, 76, 702–709. [Google Scholar] [CrossRef]
- Rusińska, A.; Płudowski, P.; Walczak, M.; Borszewska-Kornacka, M.K.; Bossowski, A.; Chlebna-Sokół, D.; Czech-Kowalska, J.; Dobrzańska, A.; Franek, E.; Helwich, E.; et al. Vitamin D Supplementation Guidelines for General Population and Groups at Risk of Vitamin D Deficiency in Poland-Recommendations of the Polish Society of Pediatric Endocrinology and Diabetes and the Expert Panel With Participation of National Specialist Consultants and Representatives of Scientific Societies-2018 Update. Front. Endocrinol. 2018, 9, 246. [Google Scholar]
- Kuryłowicz, A.; Wicik, Z.; Owczarz, M.; Jonas, M.I.; Kotlarek, M.; Świerniak, M.; Lisik, W.; Jonas, M.; Noszczyk, B.; Puzianowska-Kuźnicka, M. NGS Reveals Molecular Pathways Affected by Obesity and Weight Loss-Related Changes in miRNA Levels in Adipose Tissue. Int. J. Mol. Sci. 2017, 19, 66. [Google Scholar] [CrossRef]
- Kurylowicz, A.; Owczarz, M.; Polosak, J.; Jonas, M.I.; Lisik, W.; Jonas, M.; Chmura, A.; Puzianowska-Kuznicka, M. SIRT1 and SIRT7 expression in adipose tissues of obese and normal-weight individuals is regulated by microRNAs but not by methylation status. Int. J. Obes. 2016, 40, 1635–1642. [Google Scholar] [CrossRef]
- Xu, J.; Wang, Y.; Zhang, Y.; Dang, S.; He, S. Astemizole promotes the anti-tumor effect of vitamin D through inhibiting miR-125a-5p-meidated regulation of VDR in HCC. Biomed. Pharmacother. 2018, 107, 1682–1691. [Google Scholar] [CrossRef]
- Alimirah, F.; Peng, X.; Gupta, A.; Yuan, L.; Welsh, J.; Cleary, M.; Mehta, R.G. Crosstalk between the vitamin D receptor (VDR) and miR-214 in regulating SuFu, a hedgehog pathway inhibitor in breast cancer cells. Exp. Cell Res. 2016, 349, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Postigo, M.; Muñoz-Garach, A.; Serrano, M.; Garrido-Sánchez, L.; Bernal-López, M.R.; Fernández-García, D.; Moreno-Santos, I.; Garriga, N.; Castellano-Castillo, D.; Camargo, A.; et al. Serum 25-hydroxyvitamin D and adipose tissue vitamin D receptor gene expression: Relationship with obesity and type 2 diabetes. J. Clin. Endocrinol. Metab. 2015, 100, E591–E595. [Google Scholar] [CrossRef] [PubMed]
- Yuzbashian, E.; Asghari, G.; Hedayati, M.; Zarkesh, M.; Mirmiran, P.; Khalaj, A. Determinants of vitamin D receptor gene expression in visceral and subcutaneous adipose tissue in non-obese, obese, and morbidly obese subjects. J. Steroid Biochem. Mol. Biol. 2019, 187, 82–87. [Google Scholar] [CrossRef]
- Castellano-Castillo, D.; Morcillo, S.; Clemente-Postigo, M.; Crujeiras, A.B.; Fernandez-García, J.C.; Torres, E.; Tinahones, F.J.; Macias-Gonzalez, M. Adipose tissue inflammation and VDR expression and methylation in colorectal cancer. Clin. Epigenet. 2018, 10, 60. [Google Scholar] [CrossRef]
- Price, N.L.; Fernández-Hernando, C. miRNA regulation of white and brown adipose tissue differentiation and function. Biochim. Biophys. Acta 2016, 1861, 2104–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, T.; Adams, B.D. The regulatory role of miRNAs on VDR in breast cancer. Transcription 2017, 8, 232–241. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.M.; Meyer, M.B.; Benkusky, N.A.; O’Brien, C.A.; Pike, J.W. Mechanisms of Enhancer-mediated Hormonal Control of Vitamin D Receptor Gene Expression in Target Cells. J. Biol. Chem. 2015, 290, 30573–30586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Nisio, A.; De Toni, L.; Sabovic, I.; Rocca, M.S.; De Filippis, V.; Opocher, G.; Azzena, B.; Vettor, R.; Plebani, M.; Foresta, C. Impaired Release of Vitamin D in Dysfunctional Adipose Tissue: New Cues on Vitamin D Supplementation in Obesity. J. Clin. Endocrinol. Metab. 2017, 102, 2564–2574. [Google Scholar] [CrossRef]
- Jonas, M.I.; Kurylowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Wierzbicki, Z.; Chmura, A.; Pruszczyk, P.; Puzianowska-Kuznicka, M. Interleukins 6 and 15 Levels Are Higher in Subcutaneous Adipose Tissue, but Obesity is Associated with Their Increased Content in Visceral Fat Depots. Int. J. Mol. Sci. 2015, 16, 25817–25830. [Google Scholar] [CrossRef] [PubMed]
- Gerhard, G.S.; Styer, A.M.; Strodel, W.E.; Roesch, S.L.; Yavorek, A.; Carey, D.J.; Wood, G.C.; Petrick, A.T.; Gabrielsen, J.; Ibele, A.; et al. Gene expression profiling in subcutaneous, visceral and epigastric adipose tissues of patients with extreme obesity. Int. J. Obes. 2014, 38, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Spoto, B.; Di Betta, E.; Mattace-Raso, F.; Sijbrands, E.; Vilardi, A.; Parlongo, R.M.; Pizzini, P.; Pisano, A.; Vermi, W.; Testa, A.; et al. Pro- and anti-inflammatory cytokine gene expression in subcutaneous and visceral fat in severe obesity. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Guillot, X.; Semerano, L.; Saidenberg-Kermanac’h, N.; Falgarone, G.; Boissier, M.C. Vitamin D and inflammation. Jt. Bone Spine 2010, 77, 552–557. [Google Scholar] [CrossRef]
- Mawer, E.B.; Backhouse, J.; Holman, C.A.; Lumb, G.A.; Stanbury, S.W. The distribution and storage of vitamin D and its metabolites in human tissues. Clin. Sci. 1972, 43, 413–431. [Google Scholar] [CrossRef]
- Neville, M.J.; Collins, J.M.; Gloyn, A.L.; McCarthy, M.I.; Karpe, F. Comprehensive human adipose tissue mRNA and microRNA endogenous control selection for quantitative real-time-PCR normalization. Obesity 2011, 19, 888–892. [Google Scholar] [CrossRef]
| Parameter | Obese Individuals (n = 55) | Normal-Weight Controls (n = 31) | p | ||
|---|---|---|---|---|---|
| Mean ± SD | Min–Max | Mean ± SD | Min–Max | ||
| Age (years) | 41.49 ± 10.40 | 20–59 | 45.76 ± 14.81 | 23–62 | 0.167 |
| Weight (kg) | 131.68 ± 18.09 | 100–198.6 | 67.71 ± 11.23 | 50–90 | <0.0001 |
| BMI (kg/m2) | 46.85 ± 4.74 | 40.35–59.26 | 23.42 ± 1.66 | 20.07–24.95 | <0.0001 |
| Adipose tissue (% body mass) | 47.96 ± 4.99 | 32,63–57.23 | – | – | |
| Waist circumference (m) | 1.23 ± 0.18 | 97–167 | – | – | |
| CRP (nmol/L) | 96.86 ± 45.14 | 11.43–184.67 | 72.0 ± 16.45 | 1.9–90.11 | 0.228 |
| Glucose (mmol/L) | 5.61 ± 1.46 | 3.38–10.16 | 5.21 ± 0.22 | 4.22–5.44 | 0.677 |
| Total cholesterol (mmol/L) | 5.21 ± 1.07 | 3.13–7.87 | 4.85 ± 0.20 | 3.8–4.92 | 0.845 |
| LDL (mmol/L) | 3.27 ± 1.06 | 1.25–5.64 | 2.77 ± 018 | 2.64–2.90 | 0.486 |
| HDL (mmol/L) | 1.21 ± 0.22 | 0.78–1.79 | 1.70 ± 0.64 | 1.24–2.14 | 0.179 |
| Triglycerides (mmol/L) | 1.65 ± 1.31 | 1.22–7.62 | 1.32 ± 0.19 | 1.08–1.46 | 0.792 |
| TSH (mIU/L) | 1.74 ± 0.86 | 0.33–3.65 | 1.22 ± 0.18 | 1.09–1.35 | 0.506 |
| 25(OH)D3 (nmol /L) | 39.65 ± 30.02 | 7.5–174.17 | 41.48 ± 25.23 | 9–86.1 | 0.978 |
| 1,25(OH)2D3 (pmol /L) | 130.41 ± 46.30 | 42.7–260.4 | 133.99 ± 46.29 | 59.22–214.71 | 0.638 |
| Co-morbidities | |||||
| Type 2 DM/IGT | 26 (47.3%) | none | |||
| Hypertension | 32 (58.2%) | none | |||
| Hyperlipidemia | 34 (61.8%) | none | |||
| Metabolic syndrome * | 33 (60.0%) | none | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonas, M.I.; Kuryłowicz, A.; Bartoszewicz, Z.; Lisik, W.; Jonas, M.; Kozniewski, K.; Puzianowska-Kuznicka, M. Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level. Int. J. Mol. Sci. 2019, 20, 5272. https://doi.org/10.3390/ijms20215272
Jonas MI, Kuryłowicz A, Bartoszewicz Z, Lisik W, Jonas M, Kozniewski K, Puzianowska-Kuznicka M. Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level. International Journal of Molecular Sciences. 2019; 20(21):5272. https://doi.org/10.3390/ijms20215272
Chicago/Turabian StyleJonas, Marta Izabela, Alina Kuryłowicz, Zbigniew Bartoszewicz, Wojciech Lisik, Maurycy Jonas, Krzysztof Kozniewski, and Monika Puzianowska-Kuznicka. 2019. "Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level" International Journal of Molecular Sciences 20, no. 21: 5272. https://doi.org/10.3390/ijms20215272
APA StyleJonas, M. I., Kuryłowicz, A., Bartoszewicz, Z., Lisik, W., Jonas, M., Kozniewski, K., & Puzianowska-Kuznicka, M. (2019). Vitamin D Receptor Gene Expression in Adipose Tissue of Obese Individuals is Regulated by miRNA and Correlates with the Pro-Inflammatory Cytokine Level. International Journal of Molecular Sciences, 20(21), 5272. https://doi.org/10.3390/ijms20215272







