Emerging Roles of DDB2 in Cancer
Abstract
:1. Introduction
2. DDB2: A New Potent Tumor Suppressor?
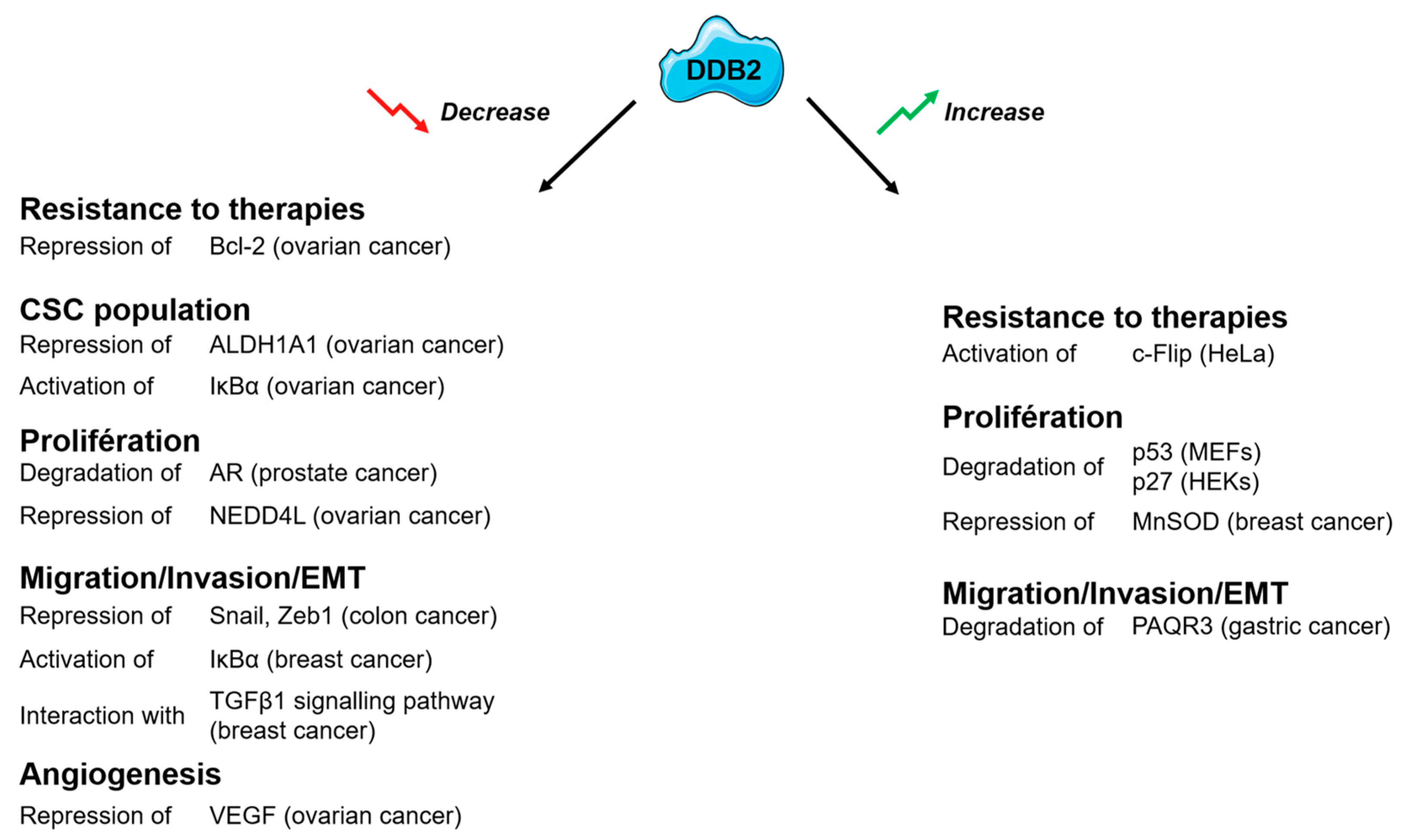
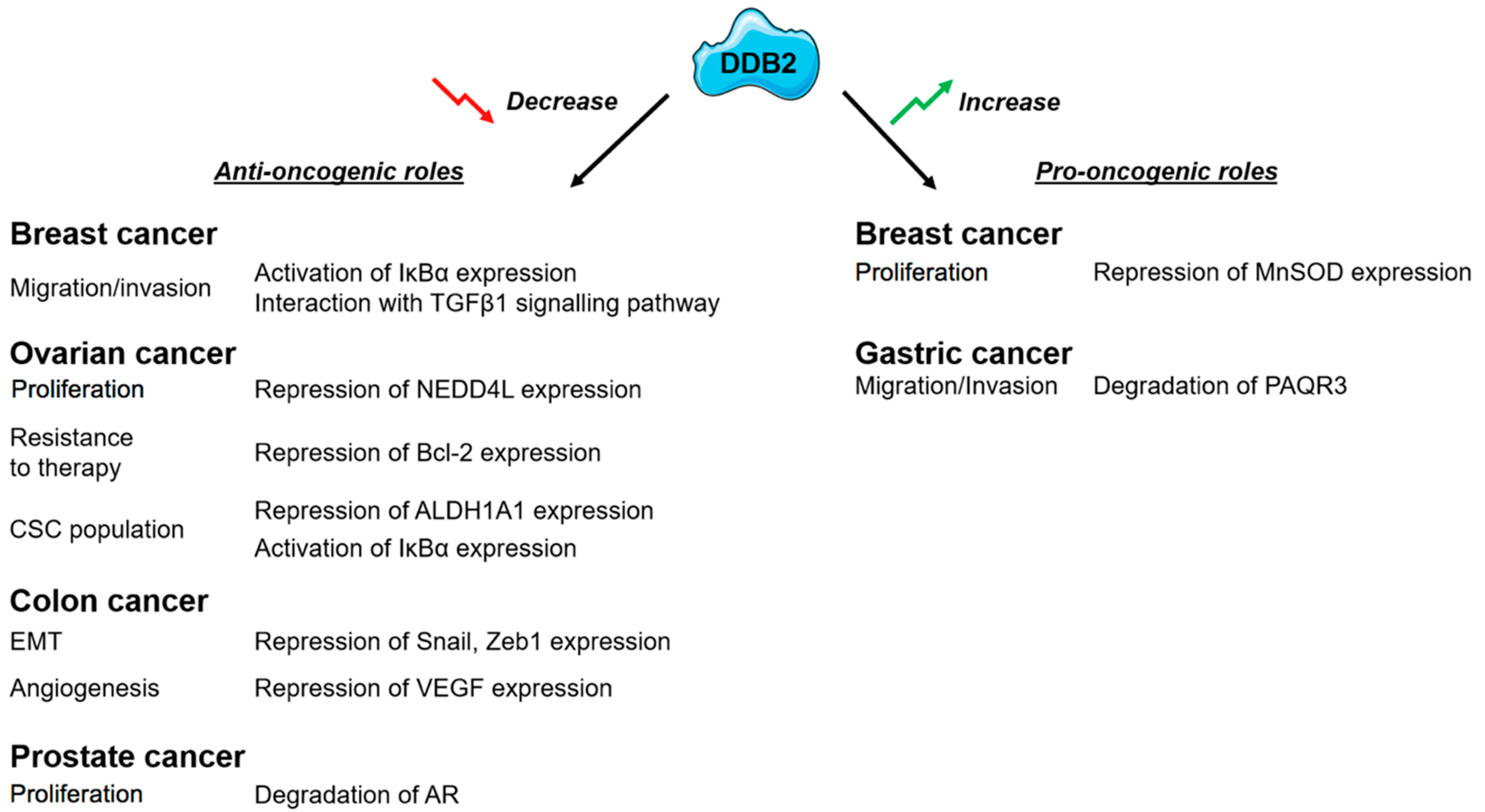
3. DDB2 Has a Dual Activity on Cancer Cell Proliferation
4. DDB2 Confers Resistance to Radiation and PARP Inhibitors and Sensitizes Cancer Cells to Chemotherapy-Induced Apoptosis
5. DDB2 Influences Epithelial to Mesenchymal Transition (EMT) and Cancer Cell Migration and Invasion
6. DDB2 Affects Cancer Stem Cell Populations
7. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 6-4PPs | 6-4 pyrimidine-pyrimidone photoproducts |
| ALDH1A1 | Aldehyde Dehydrogenase 1 family member A1 |
| AR | Androgen receptor |
| Bcl-2 | B-cell lymphoma 2 |
| BRCA1 | Breast Cancer Associated protein 1 |
| c-Flip | Cellular FLICE-like inhibitory protein |
| CBP | CREB-binding protein |
| CDK | Cyclin-dependent kinase |
| CDPs | Cyclobutane pyrimidine dimers |
| CREB | cAMP-response-element-binding protein |
| CRL | Cullin4A-Regulator of Cullins-1 E3 ubiquitin ligase |
| CSC | Cancer stem cells |
| Cul4A | Cullin4A |
| DCAF | DDB1 and CUL4-associated factors |
| DDB1 | Damage-specific DNA-binding protein 1 |
| DDB2 | Damage-specific DNA-binding protein 2 |
| E2F | Transcription factor E2F |
| EMT | Epithelial to mesenchymal transition |
| ER | Estrogen receptor |
| EZH2 | Enhancer of zeste homolog 2 |
| GG-NER | Global genomic nucleotide excision repair |
| HDAC1 | Histone deacetylase 1 |
| HEKs | Human epidermal keratinocytes |
| IκBα | Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor, alpha |
| IRES | Internal ribosome entry site |
| MEFs | Mouse embryonic fibroblasts |
| MnSOD | Manganese superoxide dismutase |
| NEDD4L | Neural precursor cell expressed developmentally downregulated gene 4-like |
| NF-κB | Nuclear factor κB |
| NFKBIA | NFKB Inhibitor Alpha |
| NF1 | Neurofibromin 1 |
| NRIP | Nuclear receptor-interacting protein 1 |
| NSCLC | Non-small-cell lung carcinoma |
| PAQR3 | Progestin and adipoQ receptor family member III |
| PARP | Poly(ADP-ribose) polymerases |
| PARP1 | Poly [ADP-ribose] polymerase 1 |
| PARylation | Protein poly ADP-ribosylation |
| PCNA | Proliferating cell nuclear antigen |
| PIP | PCNA-interacting protein |
| Rnf43 | Ring Finger protein 43 |
| ROC1 | Regulator of Cullins-1 |
| ROS | Reactive oxygen species |
| SMAD | Mothers against decapentaplegic homolog |
| SOD2 | Superoxide dismutase 2 |
| Sp1 | Transcription factor Sp1 |
| STAGA | SPT3-TAFII31-GCN5L acetylase |
| Suv39h | Suppressor of variegation 3-9 homolog 1 |
| SWI/SNF | SWItch/Sucrose Non Fermenting |
| TAp63γ | Tumor protein 63 isoform gamma |
| TGF-β | Transforming growth factor beta |
| UV-DDB | UV-damaged DNA-binding protein |
| VEGF | Vascular Endothelial Growth Factor |
| XPC | Xeroderma pigmentosum group C |
| XP-E | Xeroderma pigmentosum group E |
| ZEB1 | Zinc finger E-box Binding homeobox 1 |
References
- Tan, T.; Chu, G. p53 Binds and Activates the Xeroderma Pigmentosum DDB2 Gene in Humans but Not Mice. Mol. Cell. Biol. 2002, 22, 3247–3254. [Google Scholar] [CrossRef]
- Scrima, A.; Konícková, R.; Czyzewski, B.K.; Kawasaki, Y.; Jeffrey, P.D.; Groisman, R.; Nakatani, Y.; Iwai, S.; Pavletich, N.P.; Thomä, N.H. Structural basis of UV DNA-damage recognition by the DDB1-DDB2 complex. Cell 2008, 135, 1213–1223. [Google Scholar] [CrossRef]
- Nag, A.; Bondar, T.; Shiv, S.; Raychaudhuri, P. The xeroderma pigmentosum group E gene product DDB2 is a specific target of cullin 4A in mammalian cells. Mol. Cell. Biol. 2001, 21, 6738–6747. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Lin, M.; Zhang, C.; Wang, D.; Feng, Z.; Hu, W. TAp63γ enhances nucleotide excision repair through transcriptional regulation of DNA repair genes. DNA Repair 2012, 11, 167–176. [Google Scholar] [CrossRef] [PubMed]
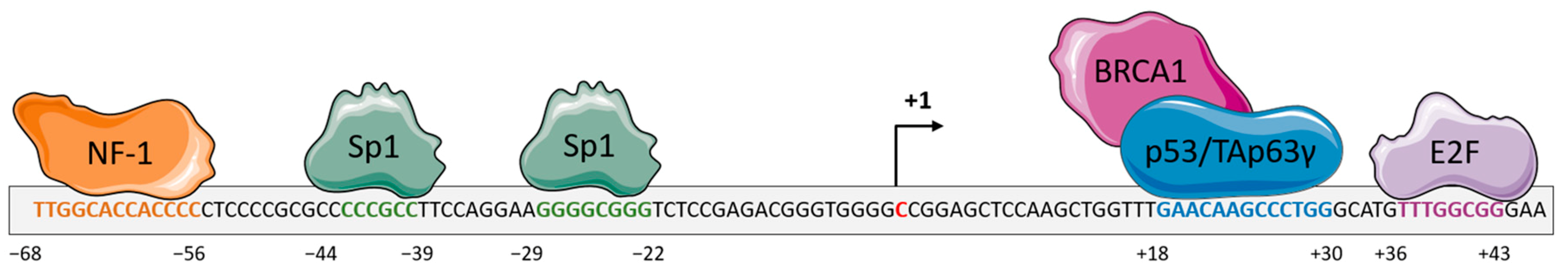
- Nichols, A.F.; Itoh, T.; Zolezzi, F.; Hutsell, S.; Linn, S. Basal transcriptional regulation of human damage-specific DNA-binding protein genes DDB1 and DDB2 by Sp1, E2F, N-myc and NF1 elements. Nucleic Acids Res. 2003, 31, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Ma, W.; Li, Q.; Tao, Y.; Ding, P.; Zhu, R.; Jin, J. The 5′-UTR of DDB2 harbors an IRES element and upregulates translation during stress conditions. Gene 2015, 573, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Luijsterburg, M.S.; Lindh, M.; Acs, K.; Vrouwe, M.G.; Pines, A.; van Attikum, H.; Mullenders, L.H.; Dantuma, N.P. DDB2 promotes chromatin decondensation at UV-induced DNA damage. J. Cell Biol. 2012, 197, 267–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cazzalini, O.; Perucca, P.; Mocchi, R.; Sommatis, S.; Prosperi, E.; Stivala, L.A. DDB2 association with PCNA is required for its degradation after UV-induced DNA damage. Cell Cycle Georget. Tex 2014, 13, 240–248. [Google Scholar] [CrossRef]
- Perucca, P.; Sommatis, S.; Mocchi, R.; Prosperi, E.; Stivala, L.A.; Cazzalini, O. A DDB2 mutant protein unable to interact with PCNA promotes cell cycle progression of human transformed embryonic kidney cells. Cell Cycle 2015, 14, 3920–3928. [Google Scholar] [CrossRef]
- Marteijn, J.A.; Lans, H.; Vermeulen, W.; Hoeijmakers, J.H.J. Understanding nucleotide excision repair and its roles in cancer and ageing. Nat. Rev. Mol. Cell Biol. 2014, 15, 465–481. [Google Scholar] [CrossRef]
- Scrima, A.; Fischer, E.S.; Lingaraju, G.M.; Böhm, K.; Cavadini, S.; Thomä, N.H. Detecting UV-lesions in the genome: The modular CRL4 ubiquitin ligase does it best! FEBS Lett. 2011, 585, 2818–2825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, L.; Nakajima, S.; Kapetanaki, M.G.; Hsieh, C.L.; Fagerburg, M.; Thickman, K.; Rodriguez-Collazo, P.; Leuba, S.H.; Levine, A.S.; Rapić-Otrin, V. Monoubiquitinated histone H2A destabilizes photolesion-containing nucleosomes with concomitant release of UV-damaged DNA-binding protein E3 ligase. J. Biol. Chem. 2012, 287, 12036–12049. [Google Scholar] [CrossRef] [PubMed]
- Lans, H.; Marteijn, J.A.; Vermeulen, W. ATP-dependent chromatin remodeling in the DNA-damage response. Epigenetics Chromatin 2012, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Chu, G. Xeroderma pigmentosum complementation group E and UV-damaged DNA-binding protein. DNA Repair 2002, 1, 601–616. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Wang, Q.E.; Ray, A.; Wani, G.; Han, C.; Milum, K.; Wani, A.A. Modulation of nucleotide excision repair by mammalian SWI/SNF chromatin-remodeling complex. J. Biol. Chem. 2009, 284, 30424–30432. [Google Scholar] [CrossRef] [PubMed]
- Kattan, Z.; Marchal, S.; Brunner, E.; Ramacci, C.; Leroux, A.; Merlin, J.L.; Domenjoud, L.; Dauça, M.; Becuwe, P. Damaged DNA binding protein 2 plays a role in breast cancer cell growth. PLoS ONE 2008, 3, e2002. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, X.; Legerski, R.J. Artemis interacts with the Cul4A-DDB1DDB2 ubiquitin E3 ligase and regulates degradation of the CDK inhibitor p27. Cell Cycle 2011, 10, 4098–4109. [Google Scholar] [CrossRef]
- Sharma, P.; Nag, A. CUL4A ubiquitin ligase: A promising drug target for cancer and other human diseases. Open Biol. 2014, 4, 130217. [Google Scholar] [CrossRef]
- Yoon, T.; Chakrabortty, A.; Franks, R.; Valli, T.; Kiyokawa, H.; Raychaudhuri, P. Tumor-prone phenotype of the DDB2-deficient mice. Oncogene 2005, 24, 469–478. [Google Scholar] [CrossRef]
- Bagchi, S.; Raychaudhuri, P. Damaged-DNA Binding Protein-2 Drives Apoptosis Following DNA Damage. Cell Div. 2010, 5, 3. [Google Scholar] [CrossRef]
- Chen, H.-H.; Fan, P.; Chang, S.W.; Tsao, Y.P.; Huang, H.P.; Chen, S.L. NRIP/DCAF6 stabilizes the androgen receptor protein by displacing DDB2 from the CUL4A-DDB1 E3 ligase complex in prostate cancer. Oncotarget 2017, 8, 21501–21515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.; Liu, J.; Jing, J.; Wang, Z.; Li, Y.; Gou, K.; Feng, X.; Yuan, Y.; Xing, C. Expression of DDB2 Protein in the Initiation, Progression, and Prognosis of Colorectal Cancer. Dig. Dis. Sci. 2018, 63, 2959–2968. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, H.; Sun, L.; Feng, X.; Wang, Z.; Yuan, Y.; Xing, C. The Differential Expression of Core Genes in Nucleotide Excision Repair Pathway Indicates Colorectal Carcinogenesis and Prognosis. BioMed Res. Int. 2018, 2018, 9651320. [Google Scholar] [CrossRef] [PubMed]
- Stoyanova, T.; Roy, N.; Bhattacharjee, S.; Kopanja, D.; Valli, T.; Bagchi, S.; Raychaudhuri, P. p21 cooperates with DDB2 protein in suppression of ultraviolet ray-induced skin malignancies. J. Biol. Chem. 2012, 287, 3019–3028. [Google Scholar] [CrossRef] [PubMed]
- Bommi, P.V.; Ravindran, S.; Raychaudhuri, P.; Bagchi, S. DDB2 regulates Epithelial-to-Mesenchymal Transition (EMT) in Oral/Head and Neck Squamous Cell Carcinoma. Oncotarget 2018, 9, 34708–34718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Zhao, R.; Liu, X.; Srivastava, A.; Gong, L.; Mao, H.; Qu, M.; Zhao, W.; Yu, J.; Wang, Q.E. DDB2 suppresses tumorigenicity by limiting the cancer stem cell population in ovarian cancer. Mol. Cancer Res. 2014, 12, 784–794. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, J.F.; Torrieri, R.; Serafim, R.B.; Di Cristofaro, L.F.; Escanfella, F.D.; Ribeiro, R.; Zanette, D.L.; Paçó-Larson, M.L.; da Silva, W.A., Jr.; Tirapelli, D.P.; et al. Expression signatures of DNA repair genes correlate with survival prognosis of astrocytoma patients. Tumor Biol. 2017, 39, 1010428317694552. [Google Scholar] [CrossRef] [PubMed]
- Becuwe, P.; Ennen, M.; Klotz, R.; Barbieux, C.; Grandemange, S. Manganese superoxide dismutase in breast cancer: From molecular mechanisms of gene regulation to biological and clinical significance. Free Radic. Biol. Med. 2014, 77, 139–151. [Google Scholar] [CrossRef]
- Zhao, R.; Cui, T.; Han, C.; Zhang, X.; He, J.; Srivastava, A.K.; Yu, J.; Wani, A.A.; Wang, Q.E. DDB2 modulates TGF-β signal transduction in human ovarian cancer cells by downregulating NEDD4L. Nucleic Acids Res. 2015, 43, 7838–7849. [Google Scholar] [CrossRef]
- Chang, S.W.; Su, C.H.; Chen, H.H.; Huang, C.W.; Tsao, L.P.; Tsao, Y.; Chen, S.L. DDB2 is a novel AR interacting protein and mediates AR ubiquitination/degradation. Int. J. Biochem. Cell Biol. 2012, 44, 1952–1961. [Google Scholar] [CrossRef]
- Huang, S.; Fantini, D.; Merrill, B.J.; Bagchi, S.; Guzman, G.; Raychaudhuri, P. DDB2 Is a Novel Regulator of Wnt Signaling in Colon Cancer. Cancer Res. 2017, 77, 6562–6575. [Google Scholar] [CrossRef] [PubMed]
- Zou, N.; Xie, G.; Cui, T.; Srivastava, A.K.; Qu, M.; Yang, L.; Wei, S.; Zheng, Y.; Wang, Q.E. DDB2 increases radioresistance of NSCLC cells by enhancing DNA damage responses. Tumour Biol. 2016, 37, 14183–14191. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Si, C.-S.; Yu, Y.; Lu, J.-W.; Zhuang, Y. Depletion of DNA damage binding protein 2 sensitizes triple-negative breast cancer cells to poly ADP-ribose polymerase inhibition by destabilizing Rad51. Cancer Sci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Han, C.; Eisenhauer, E.; Kroger, J.; Zhao, W.; Yu, J.; Selvendiran, K.; Liu, X.; Wani, A.A.; Wang, Q.E. DNA Damage-Binding Complex Recruits HDAC1 to Repress Bcl-2 Transcription in Human Ovarian Cancer Cells. Mol. Cancer Res. 2014, 12, 370–380. [Google Scholar] [CrossRef] [Green Version]
- Itoh, T.; O’Shea, C.; Linn, S. Impaired regulation of tumor suppressor p53 caused by mutations in the xeroderma pigmentosum DDB2 gene: Mutual regulatory interactions between p48(DDB2) and p53. Mol. Cell. Biol. 2003, 23, 7540–7553. [Google Scholar] [CrossRef]
- Sun, N.K.; Sun, C.L.; Lin, C.H.; Pai, L.M.; Chao, C.C. Damaged DNA-binding protein 2 (DDB2) protects against UV irradiation in human cells and Drosophila. J. Biomed. Sci. 2010, 17, 27. [Google Scholar] [CrossRef]
- Barbieux, C.; Bacharouche, J.; Soussen, C.; Hupont, S.; Razafitianamaharavo, A.; Klotz, R.; Pannequin, R.; Brie, D.; Bécuwe, P.; Francius, G.; et al. DDB2 (damaged-DNA binding 2) protein: A new modulator of nanomechanical properties and cell adhesion of breast cancer cells. Nanoscale 2016, 8, 5268–5279. [Google Scholar] [CrossRef]
- Qiao, S.; Guo, W.; Liao, L.; Wang, L.; Wang, Z.; Zhang, R.; Xu, D.; Zhang, Y.; Pan, Y.; Wang, Z.; et al. DDB2 is involved in ubiquitination and degradation of PAQR3 and regulates tumorigenesis of gastric cancer cells. Biochem. J. 2015, 469, 469–480. [Google Scholar] [CrossRef]
- Cui, T.; Srivastava, A.K.; Han, C.; Wu, D.; Wani, N.; Liu, L.; Gao, Z.; Qu, M.; Zou, N.; Zhang, X.; et al. DDB2 represses ovarian cancer cell dedifferentiation by suppressing ALDH1A1. Cell Death Dis. 2018, 9, 561. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilson, P.; Drouot, G.; Witz, A.; Merlin, J.-L.; Becuwe, P.; Harlé, A. Emerging Roles of DDB2 in Cancer. Int. J. Mol. Sci. 2019, 20, 5168. https://doi.org/10.3390/ijms20205168
Gilson P, Drouot G, Witz A, Merlin J-L, Becuwe P, Harlé A. Emerging Roles of DDB2 in Cancer. International Journal of Molecular Sciences. 2019; 20(20):5168. https://doi.org/10.3390/ijms20205168
Chicago/Turabian StyleGilson, Pauline, Guillaume Drouot, Andréa Witz, Jean-Louis Merlin, Philippe Becuwe, and Alexandre Harlé. 2019. "Emerging Roles of DDB2 in Cancer" International Journal of Molecular Sciences 20, no. 20: 5168. https://doi.org/10.3390/ijms20205168
APA StyleGilson, P., Drouot, G., Witz, A., Merlin, J.-L., Becuwe, P., & Harlé, A. (2019). Emerging Roles of DDB2 in Cancer. International Journal of Molecular Sciences, 20(20), 5168. https://doi.org/10.3390/ijms20205168




