The Involvement of the RhoA/ROCK Signaling Pathway in Hypersensitivity Reactions Induced by Paclitaxel Injection
Abstract
:1. Introduction
2. Results
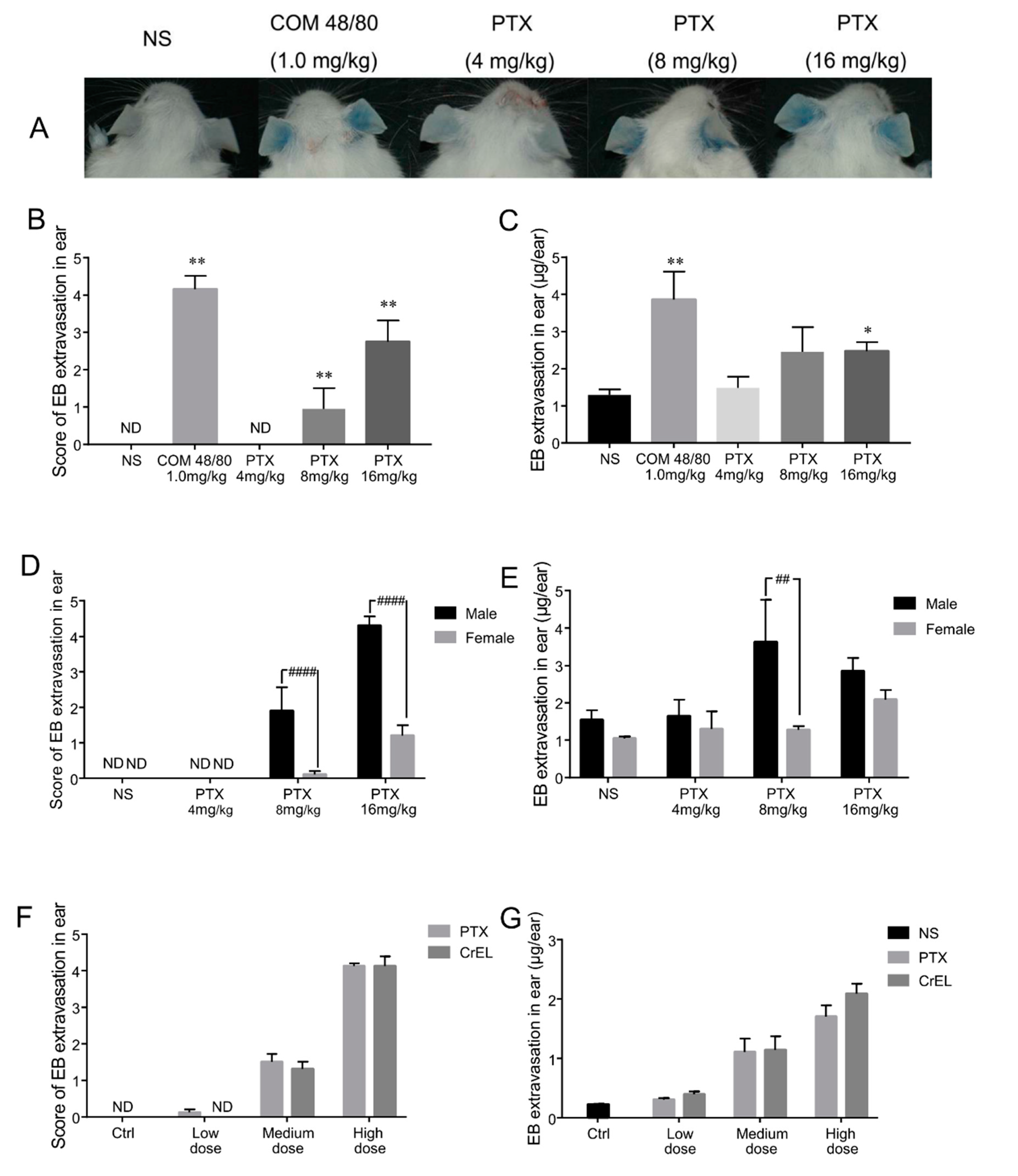
2.1. First Exposure of Paclitaxel Injection Could Cause Hypersensitivity Reactions, Which Are Mainly Induced by Cremophor EL
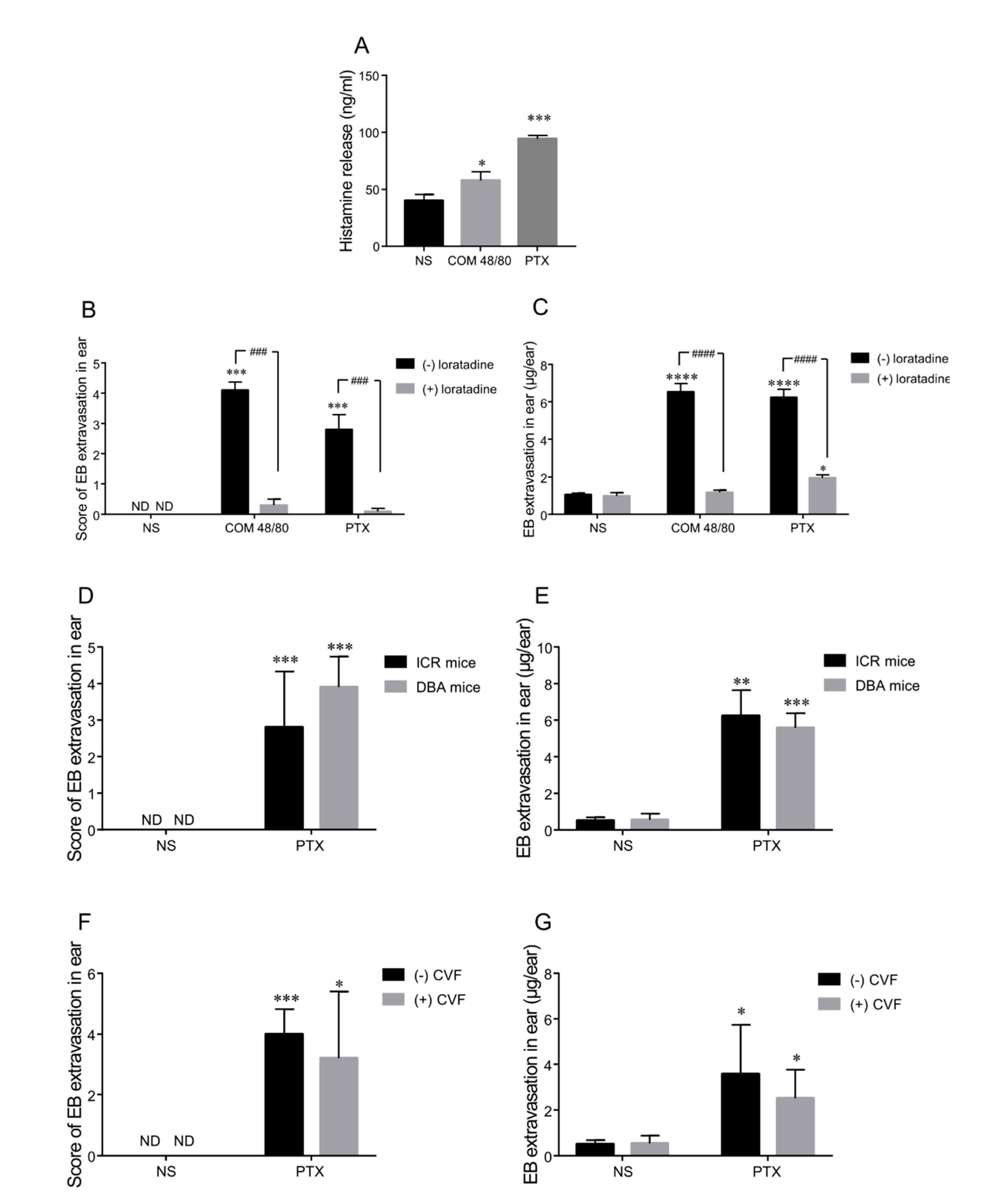
2.2. Histamine Release Contributes to Paclitaxel-Induced Hypersensitivity Reactions
2.3. Complement Is Not the Main Mediator of Paclitaxel Injection-Induced Hypersensitivity Reactions in Mice
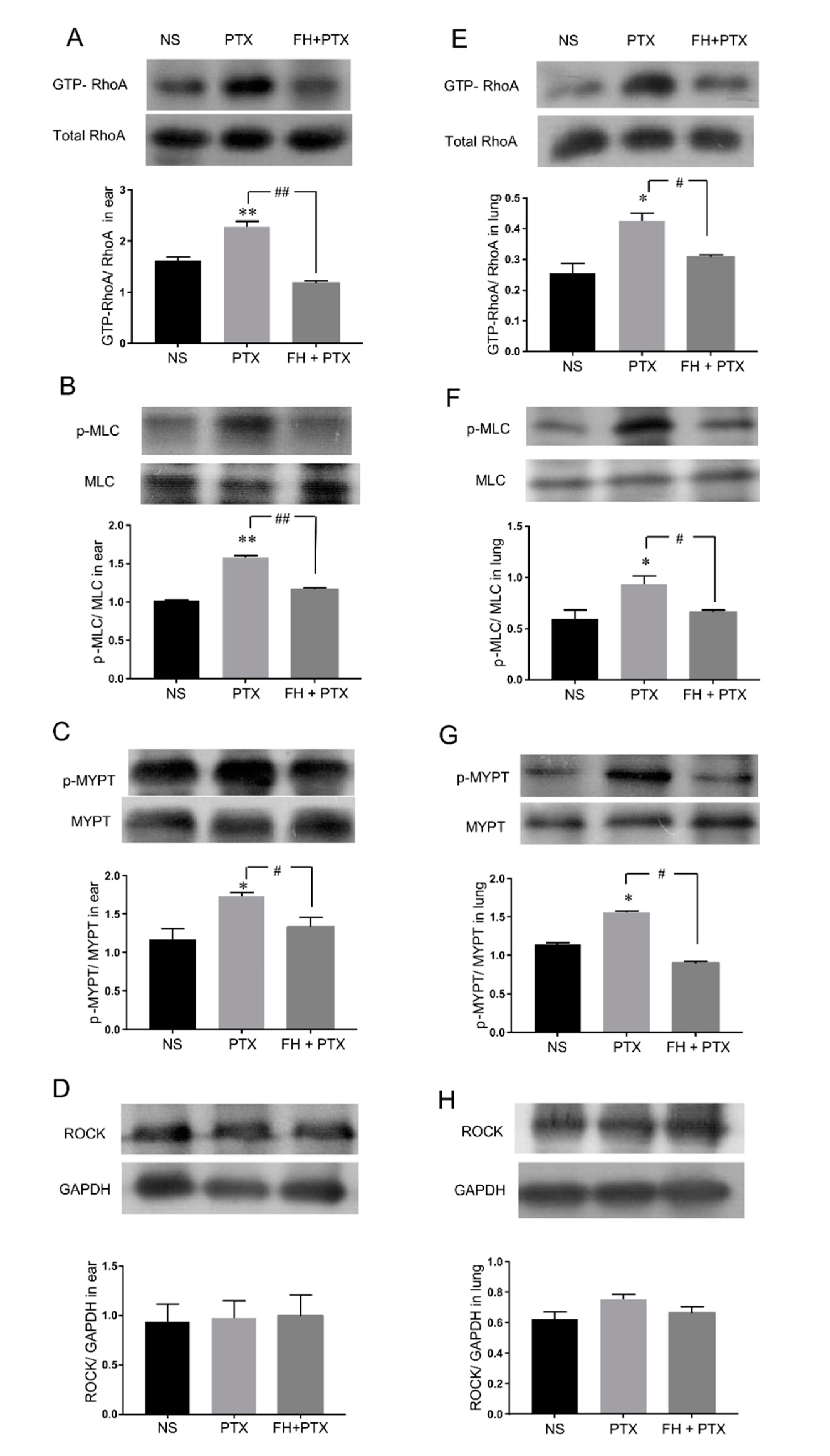
2.4. Paclitaxel Injection-Induced Hypersensitivity Reactions Were Associated with RhoA/ROCK Signaling Pathway Activation
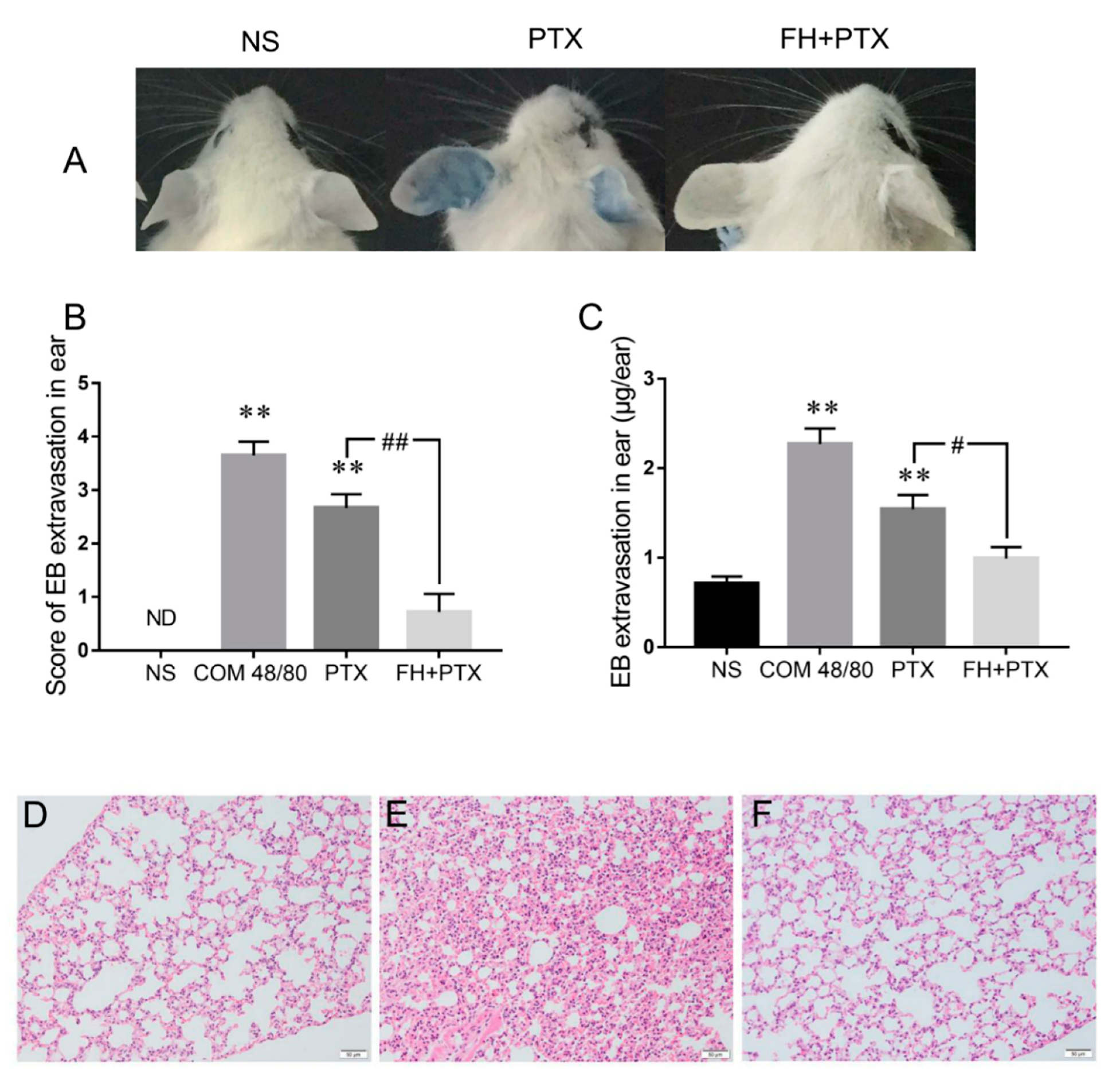
2.5. Fasudil Inhibits Paclitaxel Injection Hypersensitivity Reactions in Mice
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Reagents and Antibodies
4.3. Assessment of Vascular Leakage in ICR Mice
4.4. Histamine Assays and Anti-Histamine Treatment in ICR Mice
4.5. Vascular Permeability Assay in Complement-Depleted Mice
4.6. Paclitaxel Injection Induced RhoA/ROCK Signaling Pathway Activation
4.7. Effect of ROCK Inhibitor on Paclitaxel Injection-Induced Hypersensitive Reactions
4.8. Statistical Analyses
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| HSRs | Hypersensitivity reactions |
| IgE | Immunoglobulin E |
| iv | Intravenous injection |
| ip | Intraperitoneal injection |
| EB | Evans blue |
| NS | Normal saline |
| COM 48/80 | Compound 48/80 |
| PTX | Paclitaxel |
| CVF | Cobra venom factor |
| C3 | Complement component 3 |
| C5 | Complement component 5 |
| ELISA | Enzyme-linked immunosorbent assays |
References
- Moos, P.J.; Fitzpatrick, F.A. Taxanes propagate apoptosis via two cell populations with distinctive cytological and molecular traits. Cell Growth Differ. 1998, 9, 687–697. [Google Scholar] [PubMed]
- Okano, J.I.; Rustgi, A.K. Paclitaxel Induces Prolonged Activation of the Ras/MEK/ERK Pathway Independently of Activating the Programmed Cell Death Machinery. J. Biol. Chem. 2001, 276, 19555–19564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadoyama, K.; Kuwahara, A.; Yamamori, M.; Brown, J.B.; Sakaeda, T.; Okuno, Y. Hypersensitivity reactions to anticancer agents: Data mining of the public version of the FDA adverse event reporting system, AERS. J. Exp. Clin. Cancer Res. 2011, 30, 93. [Google Scholar] [CrossRef] [PubMed]
- Lenz, H.J. Management and Preparedness for Infusion and Hypersensitivity Reactions. Oncologist 2007, 12, 601–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Markman, M.; Kennedy, A.; Webster, K.; Kulp, B.; Peterson, G.; Belinson, J. Paclitaxel-associated hypersensitivity reactions: Experience of the Gynecologic Oncology Program of the Cleveland Clinic Cancer Center. J. Clin. Oncol. 2000, 18, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Feldweg, A.M.; Lee, C.W.; Matulonis, U.A.; Castells, M. Rapid desensitization for hypersensitivity reactions to paclitaxel and docetaxel: A new standard protocol used in 77 successful treatments. Gynecol. Oncol. 2005, 96, 824–829. [Google Scholar] [CrossRef] [PubMed]
- Ardavanis, A.; Tryfonopoulos, D.; Yiotis, I.; Gerasimidis, G.; Baziotis, N.; Rigatos, G. Non-allergic nature of docetaxel-induced acute hypersensitivity reactions. Anticancer Drugs 2004, 15, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Weiss, R.B. Hypersensitivity reactions from taxol. J. Clin. Oncol. 1990, 8, 1263–1268. [Google Scholar] [CrossRef] [PubMed]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Szebeni, J.; Muggia, F.M.; Alving, C.R. Complement activation by Cremophor EL as a possible contributor to hypersensitivity to paclitaxel: An in vitro study. J. Natl. Cancer Inst. 1998, 90, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Nuijen, B.; Bouma, M.; Schellens, J.H.M.; Beijnen, J.H. Progress in the development of alternative pharmaceutical formulations of taxanes. Investig. New Drugs 2001, 19, 143–153. [Google Scholar] [CrossRef]
- Dauchel, H.; Julen, N.; Lemercier, C.; Daveau, M.; Ozanne, D.; Fontaine, M.; Ripoche, J. Expression of complement alternative pathway proteins by endothelial cells. Differential regulation by interleukin 1 and glucocorticoids. Eur. J. Immunol. 1990, 20, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Berger, M.J.; Phillips, G.S.; Shapiro, C.L.; Rettig, A.E.; Lustberg, M.B.; Dunlea, L.J. Feasibility of stopping paclitaxel premedication after two doses in patients not experiencing a previous infusion hypersensitivity reaction. Support. Care Cancer 2011, 20, 1991–1997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikelis, C.M.; Simaan, M.; Ando, K.; Fukuhara, S.; Sakurai, A.; Amornphimoltham, P.; Masedunskas, A.; Weigert, R.; Chavakis, T.; Adams, R.H.; et al. RhoA and ROCK mediate histamine-induced vascular leakage and anaphylactic shock. Nat. Commun. 2015, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.B.; Ashworth, S.L.; Wean, S.; Hosford, M.; Sandoval, R.M.; Hallett, M.A.; Atkinson, S.J.; Molitoris, B.A. Cytokine-induced F-actin reorganization in endothelial cells involves RhoA activation. Am. J. Physiol. Ren. Physiol. 2009, 296, F487–F495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bryan, B.A.; Dennstedt, E.; Mitchell, D.C.; Walshe, T.E.; Noma, K.; Loureiro, R.; Saint-Geniez, M.; Campaigniac, J.P.; Liao, J.K.; D’Amore, P.A. RhoA/ROCK signaling is essential for multiple aspects of VEGF-mediated angiogenesis. FASEB J. 2010, 24, 3186–3195. [Google Scholar] [CrossRef] [PubMed]
- Spindler, V.; Schlegel, N.; Waschke, J. Role of GTPases in control of microvascular permeability. Cardiovasc. Res. 2010, 87, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Nair, A.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27. [Google Scholar] [CrossRef]
- Bates, D.O. Vascular endothelial growth factors and vascular permeability. Cardiovasc. Res. 2010, 87, 262–271. [Google Scholar] [CrossRef] [Green Version]
- Vasiadi, M.; Kempuraj, D.; Boucher, W.; Kalogeromitros, D.; Theoharides, T.C. Progesterone inhibits mast cell secretion. Int. J. Immunopathol. Pharmacol. 2006, 19, 787–794. [Google Scholar] [CrossRef]
- Etienne-Manneville, S.; Hall, A. Rho GTPases in cell biology. Nature 2002, 420, 629–635. [Google Scholar] [CrossRef] [PubMed]
- Amin, E.; Dubey, B.N.; Zhang, S.C.; Gremer, L.; Dvorsky, R.; Moll, J.M.; Taha, M.S.; Nagel-Steger, L.; Piekorz, R.P.; Somlyo, A.V.; et al. Rho-kinase: Regulation, (dys)function, and inhibition. Biol. Chem. 2013, 394, 1399–1410. [Google Scholar] [CrossRef] [PubMed]
- Nunes, K.P.; Rigsby, C.S.; Webb, R.C. RhoA/Rho-kinase and vascular diseases: What is the link? Cell. Mol. Life Sci. 2010, 67, 3823–3836. [Google Scholar] [CrossRef] [PubMed]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton 2010, 67, 545–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marupudi, N.I.; Han, J.E.; Li, K.W.; Renard, V.M.; Tyler, B.M.; Brem, H. Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 2007, 6, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Yi, Y.; Li, C.; Zhang, Y.; Wang, L.; Zhao, Y.; Pan, C.; Liang, A. Involvement of histamine and rhoa/rock in penicillin immediate hypersensitivity reactions. Sci. Rep. 2016, 6, 33192. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhao, Y.; Zhang, Y.; Li, C.; Yi, Y.; Pan, C.; Tian, J.; Yang, Y.; Cui, H.; Wang, L.; et al. RhoA/ROCK signaling pathway mediates Shuanghuanglian injection-induced pseudo-allergic reactions. Front. Pharmacol. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sjöberg, A.P.; Trouw, L.A.; Blom, A.M. Complement activation and inhibition: A delicate balance. Trends Immunol. 2009, 30, 83–90. [Google Scholar] [CrossRef]
- Szebeni, J.; Alving, C.R.; Savay, S.; Barenholz, Y.; Priev, A.; Danino, D.; Talmon, Y. Formation of complement-activating particles in aqueous solutions of Taxol: Possible role in hypersensitivity reactions. Int. Immunopharmacol. 2001, 1, 721–735. [Google Scholar] [CrossRef]
- Rozo, C.; Chinenov, Y.; Maharaj, R.K.; Gupta, S.; Leuenberger, L.; Kirou, K.A.; Bykerk, V.P.; Goodman, S.M.; Salmon, J.E.; Pernis, A.B. Targeting the RhoA-ROCK pathway to reverse T-cell dysfunction in SLE. Ann. Rheum. Dis. 2017, 76, 740–747. [Google Scholar] [CrossRef]
- Song, J.; Xi, J.Y.; Yu, W.B.; Yan, C.; Luo, S.S.; Zhou, L.; Zhu, W.H.; Lu, J.H.; Dong, Q.; Xiao, B.G.; et al. Inhibition of ROCK activity regulates the balance of Th1, Th17 and Treg cells in myasthenia gravis. Clin. Immunol. 2019, 203, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Kataoka, C.; Egashira, K.; Inoue, S.; Takemoto, M.; Ni, W.; Koyanagi, M.; Kitamoto, S.; Usui, M.; Kaibuchi, K.; Shimokawa, H.; et al. Important role of Rho-kinase in the pathogenesis of cardiovascular inflammation and remodeling induced by long-term blockade of nitric oxide synthesis in rats. Hypertension 2002, 39, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Possa, S.S.; Charafeddine, H.T.; Righetti, R.F.; da Silva, P.A.; Almeida-Reis, R.; Saraiva-Romanholo, B.M.; Perini, A.; Prado, C.M.; Leick-Maldonado, E.A.; Martins, M.A.; et al. Rho-kinase inhibition attenuates airway responsiveness, inflammation, matrix remodeling, and oxidative stress activation induced by chronic inflammation. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012, 303, 939–953. [Google Scholar] [CrossRef] [PubMed]
- Wallez, Y.; Huber, P. Endothelial adherens and tight junctions in vascular homeostasis, inflammation and angiogenesis. Biochim. Biophys. Acta Biomembr. 2008, 1778, 794–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiroto, T.; Yasuda, S.; Tsuburaya, R.; Ito, Y.; Takahashi, J.; Ito, K.; Ishibashi-Ueda, H.; Shimokawa, H. Role of Rho-Kinase in the Pathogenesis of Coronary Hyperconstricting Responses Induced by Drug-Eluting Stents in Pigs in Vivo. J. Am. Coll. Cardiol. 2009, 54, 2321–2329. [Google Scholar] [CrossRef] [PubMed]
- Kolluru, G.K.; Majumder, S.; Chatterjee, S. Rho-kinase as a therapeutic target in vascular diseases: Striking nitric oxide signaling. Nitric Oxide Biol. Chem. 2014, 43, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.C.; Zeidan, A.; Javadov, S.; Kilić, A.; Rajapurohitam, V.; Karmazyn, M. Nitric oxide inhibits endothelin-1-induced neonatal cardiomyocyte hypertrophy via a RhoA-ROCK-dependent pathway. J. Mol. Cell. Cardiol. 2009, 47, 810–818. [Google Scholar] [CrossRef]
- Paskas, S.; Mazzon, E.; Basile, M.S.; Cavalli, E.; Al-Abed, Y.; He, M.; Rakocevic, S.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Lopinavir-NO, a nitric oxide-releasing HIV protease inhibitor, suppresses the growth of melanoma cells in vitro and in vivo. Investig. New Drugs 2019. [Google Scholar] [CrossRef]
- Basile, M.S.; Mazzon, E.; Krajnovic, T.; Draca, D.; Cavalli, E.; Al-Abed, Y.; Bramanti, P.; Nicoletti, F.; Mijatovic, S.; Maksimovic-Ivanic, D. Anticancer and differentiation properties of the nitric oxide derivative of lopinavir in human glioblastoma cells. Molecules 2018, 23, 2463. [Google Scholar] [CrossRef]
- Paskaš, S.; Krajnović, T.; Basile, M.S.; Dunđerović, D.; Cavalli, E.; Mangano, K.; Mammana, S.; Al-Abed, Y.; Nicoletti, F.; Mijatović, S.; et al. Senescence as a main mechanism of Ritonavir and Ritonavir-NO action against melanoma. Mol. Carcinog. 2019, 58, 1362–1375. [Google Scholar] [CrossRef]
- Kupczyk, M.; Kupryś, I.; Górski, P.; Kuna, P. The effect of montelukast (10 mg daily) and loratadine (10 mg daily) on wheal, flare and itching reactions in skin prick tests. Pulm. Pharmacol. Ther. 2007, 20, 85–89. [Google Scholar] [CrossRef] [PubMed]
- Harvill, E.T.; Lee, G.; Grippe, V.K.; Merkel, T.J. Complement depletion renders C57BL/6 mice sensitive to the Bacillus anthracis sterne strain. Infect. Immun. 2005, 73, 4420–4422. [Google Scholar] [CrossRef] [PubMed]
- Singhal, R.; Ganey, P.E.; Roth, R.A. Complement Activation in Acetaminophen-Induced Liver Injury in Mice. J. Pharmacol. Exp. Ther. 2012, 341, 377–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, C.; Zhang, Y.-S.; Han, J.-Y.; Li, C.-Y.; Yi, Y.; Zhao, Y.; Wang, L.-M.; Tian, J.-Z.; Liu, S.-Y.; Li, G.-Q.; et al. The Involvement of the RhoA/ROCK Signaling Pathway in Hypersensitivity Reactions Induced by Paclitaxel Injection. Int. J. Mol. Sci. 2019, 20, 4988. https://doi.org/10.3390/ijms20204988
Pan C, Zhang Y-S, Han J-Y, Li C-Y, Yi Y, Zhao Y, Wang L-M, Tian J-Z, Liu S-Y, Li G-Q, et al. The Involvement of the RhoA/ROCK Signaling Pathway in Hypersensitivity Reactions Induced by Paclitaxel Injection. International Journal of Molecular Sciences. 2019; 20(20):4988. https://doi.org/10.3390/ijms20204988
Chicago/Turabian StylePan, Chen, Yu-Shi Zhang, Jia-Yin Han, Chun-Ying Li, Yan Yi, Yong Zhao, Lian-Mei Wang, Jing-Zhuo Tian, Su-Yan Liu, Gui-Qin Li, and et al. 2019. "The Involvement of the RhoA/ROCK Signaling Pathway in Hypersensitivity Reactions Induced by Paclitaxel Injection" International Journal of Molecular Sciences 20, no. 20: 4988. https://doi.org/10.3390/ijms20204988





