Abstract
Genome editing tools have rapidly been adopted by plant scientists for gene function discovery and crop improvement. The current technical challenge is to efficiently induce precise and predictable targeted point mutations valuable for crop breeding purposes. Cytidine base editors (CBEs) are CRISPR/Cas9 derived tools recently developed to direct a C-to-T base conversion. Stable genomic integration of CRISPR/Cas9 components through Agrobacterium-mediated transformation is the most widely used approach in dicotyledonous plants. However, elimination of foreign DNA may be difficult to achieve, especially in vegetatively propagated plants. In this study, we targeted the acetolactate synthase (ALS) gene in tomato and potato by a CBE using Agrobacterium-mediated transformation. We successfully and efficiently edited the targeted cytidine bases, leading to chlorsulfuron-resistant plants with precise base edition efficiency up to 71% in tomato. More importantly, we produced 12.9% and 10% edited but transgene-free plants in the first generation in tomato and potato, respectively. Such an approach is expected to decrease deleterious effects due to the random integration of transgene(s) into the host genome. Our successful approach opens up new perspectives for genome engineering by the co-edition of the ALS with other gene(s), leading to transgene-free plants harboring new traits of interest.
1. Introduction
Genome editing tools, mostly based on the CRISPR/Cas9 system, have rapidly emerged in plants for gene function analysis and to improve agronomical traits. This technology relies on the creation of DNA double-strand breaks (DSBs) at predefined targeted sites in the plant genome. DSBs can be repaired by an error-prone non-homologous end-joining (NHEJ) mechanism that may result in small insertions or deletions (indels) in the targeted sequence, leading to gene knock-out, an essential tool for gene function analysis. A refinement of genome editing consists of the precise and predictable point mutations that are even more promising to decipher natural genomic variations and for crop breeding purposes. This could be achieved via homologous recombination (HR), but this repair mechanism suffers from low efficiency in plants and the delivery of donor DNA templates in plant cells, needed to promote the process, is still challenging [1]. More recently, base editors have been established based on either fusing an adenine or a cytidine deaminase to a Cas9 nickase (nCas9), leading to an A-to-G or a C-to-T substitution, respectively, without the introduction of a DSB. These base editing systems rely on the association of a single-guide RNA molecule (sgRNA) with the nCas9/deaminase fusion, driving the complex to the target locus and enabling deamination on the non-complementary strand [2]. To date, base editors have been successfully used in a number of crops, including rice, tomato, potato, wheat, maize, rape and watermelon [3,4,5,6,7,8,9,10,11].
For the delivery of CRISPR/Cas9 components into dicotyledonous plant cells, stable genomic integration of expression units through Agrobacterium-mediated transformation is the most widely used approach. When working on sexually propagated plants like tomato, the transfer DNA (T-DNA) can be eliminated thanks to Mendelian segregation in the subsequent generations, resulting in edited but transgene-free plants. However, this strategy cannot be applicable to vegetatively propagated and/or highly heterozygous plants like potato, as sexual reproduction would lead to the loss of desirable traits. To circumvent these limitations, Chen et al. [12] have recently developed a labor-intensive method based on the delivery of CRISPR/Cas9 reagents by Agrobacterium-mediated transformation followed by a high-throughput screening protocol in the tetraploid tobacco, leading to transgene-free mutants without selective pressure. Although some alternative delivery methods such as particle bombardment or protoplast transfection using plasmid DNA or ribonucleoprotein (RNP) complexes have been demonstrated to result in non-transgenic mutants, these approaches are often highly sensitive and limited to some species due to bottlenecks in the regeneration process. Regeneration from protoplasts has been recently shown to result in a substantial genome instability in the tetraploid potato, affecting the prospects for protoplast utilization in genome editing for this species [13]. Whether this syndrome may be reduced using regeneration from Agrobacterium-transformed explants is a question of upmost importance. Furthermore, if protoplast transfection could be considered “foreign-DNA-free”, the need for a large amount of plasmid DNA during protoplast transfection may result in the insertion of degraded DNA fragments into the target site or elsewhere into the genome [14,15,16,17].
The acetolactate synthase (ALS) gene encodes the enzyme that catalyses the initial step of the biosynthetic pathway for branched-chain amino acids. As many studies have shown that point mutations in this gene can confer dominant resistance to ALS-inhibitors, the targeting of the ALS gene constitutes a tool of choice for the selection of edited plants. The mutation of the Proline-197 residue (amino acid number standardized to the Arabidopsis sequence, corresponding to Proline-186 in tomato and potato ALS1) is one of the most commonly reported mutations conferring chlorsulfuron resistance [18]. According to the online database of sulfonylurea-resistant weed populations (http://www.weedscience.com/), chlorsulfuron resistance can be obtained by at least 11 different amino acids substitutions at Pro-197. In order to test and potentially improve the production of transgene-free edited plants that can be selected directly in vitro, we targeted the ALS locus in tomato and potato through Agrobacterium-mediated transformation using Target-AID (target-activation-induced cytidine deaminase), a cytidine base editor (CBE) [5].
2. Results and Discussion
2.1. Highly Efficient Production of Base Edited and Transgene-Free Tomatoes
The base editing strategy was firstly deployed on the diploid tomato. Because Agrobacterium is sometimes used for transient expression of transcriptional units located on the T-DNA [19], we developed a specific selection protocol in order to exploit this potentiality to obtain T-DNA-free events by transiently expressing the CBE. Based on the Solanum lycopersicum reference genome, we designed one sgRNA targeting the SlALS1 gene (Solyc03g044330), ensuring that nucleotides encoding the Pro186 (CCA) were located in the edition window of the CBE (Figure 1a, Figures S1 and S2). Because of the very high similarity between SlALS1 and SlALS2 genes, a single mismatch at the limit of the seed region of the sgRNA, at position -12 counting from the protospacer adjacent motif (PAM), was present with the SlALS2 gene (Solyc07g061940). The guide was cloned into the CBE binary vector [5], and Agrobacterium-mediated transformation was performed (Figure 1b). After one or two weeks of kanamycin selection pressure covering the transient expression period of Agrobacterium, plant tissues were transferred to a selective medium containing 40 ng mL−1 chlorsulfuron, so that only edited cells could grow and regenerate plantlets.
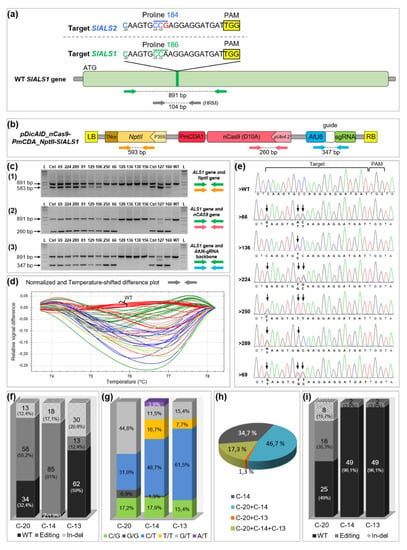
Figure 1.
Targeted modifications in tomato StALS1 gene. (a) SlALS1 and SlALS2 target region. The SlALS1 Pro186 codon is highlighted in green and the SlALS2 Pro184 codon in blue. The single mismatch in SlALS2 locus is written in red. The three targeted cytidines (C−20, C−14 and C−13) are written in green and in blue in the SlALS1 and StALS2 sequences, respectively. The protospacer adjacent motif (PAM) site is highlighted in yellow. Green and grey arrows indicate the relative positions of the PCR primers; (b) T-DNA physical map of the cytidine base editor (CBE) binary vector. Colored arrows indicate the relative positions of the PCR primers; (c) multiplex PCR analyses of 14 independent chlorsulfuron resistant plants, the wild-type (WT) and the positives controls (T+). L: molecular marker; (d) high resolution melting (HRM) assay using primers (grey arrows) flanking the targeted SlALS1 region. The color-label represents groups of plants (40 plantlets) that harbor similar melting curve shapes. The wild-type curves are colored in red. (e) chromatograms of the targeted region of the WT and of six independent chlorsulfuron resistant plants. The arrows indicate modifications; (f) histograms indicating the number of unedited plants (black), edited plants (grey) and plants with indels (in-del, striped), independently for C−20, C−14 or C−13 (not for the entire sequence), found on a total of 105 mutated plants in the targeted SlALS1 region; (g) percentage of each type of nucleotide changes found on cytidines C−20, C−14 or C−13. The total number of edited plants used in this analysis was 58 for C−20, 78 for C−14, 13 for C−13; (h) percentage of single, double and triple editing events in 75 base edited plants; (i) histogram indicating the number of unedited plants (in black), edited plants (in grey), and plants with indels (in-del, striped) in the SlALS2 locus, independently for C−20, C−14 or C−13 (not for the entire sequence), found on a total of 51 mutated plants on the SlALS1 locus.
Seventy-six percent of the treated cotyledons (289/378) were able to produce at least one plantlet with a strong resistance to chlorsulfuron. The first 232 plantlets that regenerated and rooted on chlorsulfuron media, all corresponding to independent transformation events, were first checked by polymerase chain reaction (PCR) for T-DNA integration (Figure 1c). Thirty plantlets out of the 232 chlorsulfuron resistant plants were found T-DNA-free (Figure S3). A hundred and five plants, out of the 232 chlorsulfuron resistant plants and including the 30 “T-DNA free” plants, were analysed for edition efficiency, firstly by High Resolution Melting analysis (HRM). All these plants displayed a mutated melting-curve shape compared to the wild-type at the SlALS1 targeted locus (Figure 1d). To characterize the base editing outcomes, the target site was then sequenced in these plants.
Counting starting from the PAM site, three cytidines are present in the edition window of the sgRNA sequence: C−20, C−14 and C−13, the last two corresponding to the Pro186 CCA codon. Ninety-nine percent (104/105) of the analysed sequences displayed mutation(s) at the SlALS1 locus. One plant, in which no C was found modified, died a few weeks after sequencing and was probably a chlorsulfuron selection escape. Up to 28.5% of the sequences showed indels in the target site, mostly originating from the edition window, where cytidine deamination is supposed to occur (Figure 1e,f and Figure S4). The formation of such a rate of undesired indels is in accordance with previously published results in tomato with the same base editor [5], and prevented a proper analysis of the editing outcomes at the C positions affected by indels. Seventy-five out of 105 plants (71.4%) were cleanly base edited at the SlALS1 Pro186 (CCA) codon (by extrapolating, ≈ 54% of the agroinoculated cotyledon explants). From these, 98.7% (74/75) were edited at position C−14. Any substitution (C-to-T, C-to-A or C-to-G) at this position is sufficient to change Pro186 amino acid to Ser, Ala or Thr residues that have been shown to confer chlorsulfuron resistance in tobacco (Figure 1e and Figure S5) [20]. More than 80% of edited C−14 were C-to-T changes, 16.7% being homozygous (Figure 1g; plant >250 Figure 1e). As expected, no C−20-to-T−20 homozygous modification was found as it would lead to amino acid change toward a stop codon, likely preventing plant regeneration. As a whole, most of the analysed plants were modified at several C positions (Figure 1h; plants >66, >224, >250, >69 Figure 1e). Only 34.7% of the analysed sequences showed a single C substitution, and exclusively at the C−14 targeted position.
In tobacco, natural mutations conferring chlorsulfuron resistance and centered on the Pro codon, equivalent to the tomato Pro186 and Pro184 ones, have been found in the ALS1, but also in the ALS2 gene [21]. It has been shown that off-targeting in potato ALS2 gene can occur, even with one mismatch within the seed region of the sgRNA [22]. Because of the presence of a mismatch at the limit of the seed region (N−12) between the sgRNA and the SlALS2 gene, we sequenced 51 plants at this locus, including most of the T-DNA free genotypes (Figure S6). Nineteen sequences were found with base editing events (37%) and eight with indels (16%) (Figure 1i). Most of the base editing events (18/19) were observed at position C−20 while two base conversion events were unexpectedly found at position C−24, upstream of the sgRNA sequence (Figures S6 and S7). The lower but nevertheless substantial amount of edition events at SlALS2 locus as compared to SlALS1 target site demonstrates that the off-target potential should be carefully estimated while designing target sequences [23]. Interestingly, among the 25 analysed T-DNA-free SlALS1 mutants, only four (16%) were edited at the SlALS2 locus (Figure S6). In contrast, the number of plants edited at the SlALS2 locus in the transgenic plants, 23 (88%) out of the 26 analysed, is significantly higher (Fisher exact test p = 0.003). These results suggest that limiting the expression of the CRISPR components to a few days strongly reduce the risk of off-target (5-fold decrease in our case) in tomato.
In summary, a very high base editing efficiency was obtained with the CBE strategy in tomato, allowing the recovery of T-DNA free edited plants at a reasonable rate (12.9% of the analysed chlorsulfuron-resistant plants), in the same range as a recently developed strategy in tobacco [12].
2.2. Production of Transgene-Free Base Edited Plants in the Tetraploid Potato
We have shown that our base editing strategy was efficient in the diploid tomato. Next, we aimed to apply the same approach in another Solanaceous species: the tetraploid, highly heterozygous and vegetatively propagated potato. The production of edited plants without stable integration of foreign DNA in such a species is an even more promising perspective, as transgene segregation through selfing cannot be performed without changing cultivar characteristics. Based on the potato reference genome, we simultaneously sequenced a portion of StALS1 (PGSC0003DMG400034102) and StALS2 (PGSC0003DMG400007078) homologs (96% protein identity; Figure S8) in the potato cultivar Desiree, using one couple of primers predicted to match the two genes (Figure 2a). Then, we designed one sgRNA targeting the StALS1 gene and covering the Pro-186 codon, while one mismatch was present at the limit of the seed region with some alleles of the StALS2 locus (Figure 2a). The guide was cloned into the CBE (Figure 2b) and an Agrobacterium-mediated transformation was performed in potato explants. After two weeks of kanamycin selection pressure, plant tissues were transferred to a selective medium containing 40 ng mL−1 chlorsulfuron.
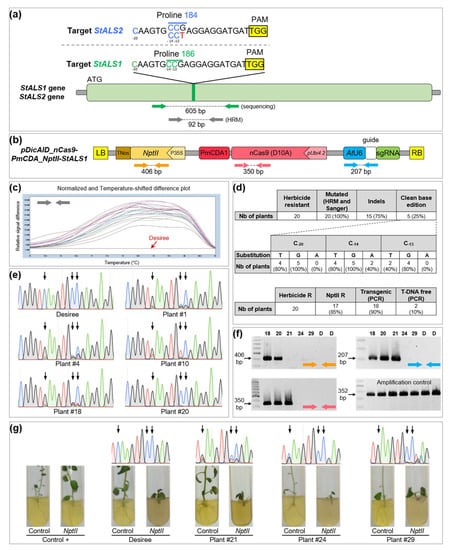
Figure 2.
Targeted modifications in potato StALS gene. (a) target region in the StALS1 and StALS2 genes of the cultivar Desiree. The StALS1 Pro186 codon is highlighted in green and the StALS2 Pro184 codon in blue. The single mismatch in the StALS2 locus is highlighted in red. Targeted C−20, C−14 and C−13 are represented in green and blue for StALS1 and StALS2, respectively. PAM site is highlighted in yellow. Green and grey arrows indicate the relative positions of the PCR primers; (b) T-DNA physical map of the CBE binary vector. Colored arrows indicate the relative positions of the PCR primers; (c) HRM analysis using primers targeting both StALS1 and StALS2 loci. Plants (20) are color-labeled according to their melting curve shape. The wild-type curve is colored in red; (d) summary of mutation efficiency and outcomes for 20 regenerated plants. The number of edited and transgenic or T-DNA-free plants is indicated; (e) Sanger chromatograms of the targeted region (StALS1 and StALS2) of five plants that do not harbor indels. The wild-type sequence is also provided. The black arrows indicate the localization of targeted cytidines; (f) PCR analyses of five chlorsulfuron resistant plants and the wild-type using primers matching the T-DNA (localization is indicated by the colored arrows); (g) Sanger chromatograms and rooting test of the three plants that did not amplify the NptII fragment. The wild-type and a positive control are also included. The black arrows indicate the localization of the targeted cytidines.
Twenty plants were regenerated and confirmed to be chlorsulfuron-resistant. Then, the HRM analysis showed that all the chlorsulfuron-resistant plants were mutated at the targeted locus (Figure 2c,d), showing that our editing selection strategy was highly efficient in potato. By contrast, base-editing efficiency in watermelon was 23% in the T0 generation, highlighting the advantage of selecting primary transformants on medium-containing an ALS-inhibitor [7]. All these plants were subjected to direct Sanger sequencing using primers matching both StALS1 and StALS2 genes. Sanger results confirmed that all plants harbored mutation(s) in the target sequence (Figure 2d). As previously observed in tomato, we noticed that a significant part of the potato plants (15 out of 20 mutated plants, 75%) showed indels in the target site (Figure 2d, Figures S9 and S10), which likely originate from uracil excision and downstream repair systems [24]. This substantial rate of indels is not surprising due to the number of targeted cytidines in each of the eight StALS alleles. Addition of an uracil DNA glycosylase inhibitor protein (UGI) to the deaminase function may prevent the formation of such undesired products [24], as observed in watermelon where the same ALS site was targeted using another cytidine base editor that harbored an UGI [7]. For a rigorous analysis of base editing, we decided to focus our analysis on the five plants without indels. Base conversion was mainly C-to-G and C-to-T, while C-to-A was much less frequent (Figure 2d,e). Based on a visual peak intensity analysis, we noticed that base conversion at C−20 and C−14 occurred more frequently than at C−13 (Figure 2e), as previously observed for tomato. For plant #10, chromatogram analysis showed that the C−20 was totally converted (Figure 2e), suggesting that the CBE is sufficiently efficient to mediate base conversion on the eight alleles.
To discriminate transgenic from non-transgenic edited plants, we performed PCR on the 20 StALS mutants using primers covering three regions in the T-DNA (Figure 2f). Two plants (10%) were considered to be T-DNA-free as they lacked all three PCR fragments. One plant harbored a truncated integration of the transgene, with a missing left border that contains the nptII cassette (Figure 2f). The growth of these three plants was strongly impaired under kanamycin treatment compared to transgenic plants, confirming our molecular analysis (Figure 2g).
3. Materials and Methods
3.1. Vector Cloning
Guide sequences targeting the ALS gene for tomato and potato were cloned into the pDicAID_nCas9-PmCDA_NptII_DELLA [5]. First, a 310 bp fragment containing the 3′ end of the AtU6-26 promoter, the 20 bp guide and the sgRNA scaffold were synthesized (Genscript, Piscataway, NJ, USA, for potato; IDT, Skokie, IL, USA, for tomato). BstXI and SpeI restriction sites were added at the 5′ and the 3′ end of the fragment, respectively. Synthesized fragment was subcloned into the pDONR207 using BP reaction (Thermo Fischer Scientific, Waltham, MA, USA). Then, the resulting pDONR207 and the binary plasmid were digested by the FastDigest restriction enzymes BstXI and SpeI (Thermo Fischer Scientific, Waltham, MA, USA). The synthesized fragment was ligated into the digested binary vector using T4 DNA ligase (New England Biolabs, Ipswich, MA, USA). Reaction mixture was transformed into One Shot™ TOP10 Chemically Competent E. coli (Thermo Fisher Scientific, USA) and bacteria were grown overnight at 37 °C on LB plates containing 100 µg mL−1 spectinomycin. Plasmids were Sanger sequenced (Genoscreen, Lille, France) and transferred into Agrobacterium tumefaciens by heat shock.
3.2. Agrobacterium-Mediated Transformation
Tomato plants were cultured in sterile conditions in a growth chamber with controlled temperatures of 22 °C/18 °C under a 16 h/8 h (day/night) photoperiod. Agrobacterium-mediated transformation using the C58 pGV2260 strain containing the CBE binary vector with SlALS1 sgRNA was performed on cotyledon segments from 8–12 day-old seedlings of the WVA106 tomato cultivar, as previously described [25]. After selection on kanamycin (100 mg L−1) for one or two weeks, the cotyledon pieces were transferred to fresh selective medium containing 40 ng mL−1 chlorsulfuron every two weeks.
The tetraploid potato cultivar Desiree (ZPC, the Netherlands) was in vitro propagated in 1X Murashige and Skoog (MS) medium (pH 5.8) including vitamins (Duchefa, the Netherlands), 0.4 mg L−1 thiamine hydrochloride (Sigma-Aldrich, Saint-Louis, MO, USA), 2.5% sucrose and 0.8% agar powder (VWR, Radnor, PA, USA). Plants were cultivated in a controlled environmental chamber at 19 °C under a 16 h light/8 h dark photoperiod. Stem and petiole tissues were cut from the top of 3 to 5-week-old plants, and placed overnight in the growth chamber on 1X Murashige and Skoog (MS) medium (pH 5.8) including vitamins (Duchefa, Haarlem, the Netherlands), 2.5% sucrose, 0.4 mg L−1 thiamine hydrochloride (Sigma-Aldrich, USA), 1 mg L−1 indole-3-acetic acid (Sigma-Aldrich, USA), 1 mg L−1 zeatin-riboside (Sigma-Aldrich, USA), 1 mg L−1 gibberellin A3 (Sigma-Aldrich, USA) and 0.7% agar powder (VWR, Radnor, PA, USA). A. tumefaciens C58pMP90 strain containing the CBE binary vector with the StALS1 sgRNA was grown overnight and the bacterial DO was set to ≈0.2. Potato tissues were co-cultured with Agrobacterium for 48 h at 25 °C in the dark, washed with sterile water and placed onto the culture medium described above, supplemented with 250 µg mL−1 cefotaxime, 100 µg mL−1 timentin® and 50 µg mL−1 kanamycin. After two weeks, explants were transferred onto a fresh culture medium with 40 ng mL−1 chlorsulfuron and reduced indole-3-acetic acid (0.1 mg L−1), and subcultured every three weeks.
3.3. Genotyping Analysis
Detection of the stable integration of the T-DNA was detected by PCR analysis from genomic DNA, using the GoTaq® G2 Flexi DNA Polymerase (Promega, Madison, WI, USA).
The High Resolution Melting-curve analysis was mostly performed as described in Veillet et al. [26]. Briefly, PCR amplification was carried out from genomic DNA using small amplification products (≈100 bp). For tomato, the experiment was carried out using the Precision Melt Supermix (BioRad, Hercules, CA, USA) with the CFX96™ Real-Time PCR Detection System (BioRad, Hercules, CA, USA), according to the manufacturer’s instructions. Melting-curves analysis was performed using the Precision Melt Analysis software (BioRad, Hercules, CA, USA). Sequence information was obtained by amplifying the target locus with GoTaq® G2 Flexi DNA Polymerase (Promega, Madison, WI, USA) and PCR products were purified and Sanger sequenced (Genoscreen, Lille, France). For potato, the analysis was performed using the High Resolution Melting Master (Roche, Mannheim, Germany) with the LightCycler® 480 II system (Roche, Mannheim, Germany), according to the manufacturer’s instructions. The analysis of results was done with the LightCycler® 480 Gene Scanning Software (Roche, Mannheim, Germany). For mutation detection, the spiking of all samples with 10–20% of wild type DNA was performed. For sequence information, target locus was amplified using Invitrogen Platinum SuperFi DNA Polymerase (Thermo Fischer Scientific, Waltham, MA, USA) and PCR products were purified and Sanger sequenced (Genoscreen, Lille, France).
Primers used in this study are listed in Tables S1 and S2.
4. Conclusions
In summary, we have established a straightforward, efficient and cost-effective strategy for the production of edited and transgene-free plants in tomato and potato, using Agrobacterium-mediated transient expression of a CBE. This system can be transferred to the other numerous species that are compatible with Agrobacterium transformation, expanding the scope of the CRISPR toolbox for generating foreign-DNA-free plants, especially in vegetatively propagated species and trees [27]. The use of another base editing tool, like the adenine base editor that displays much more cleanly base edition than cytidine base editors [8,9,10], should also be possible by targeting A-rich site(s) of the ALS gene that can confer resistance to ALS-inhibitors. As multiplex base editing has been reported in tomato and rice [5,9,28], the co-base-editing of the ALS gene with another gene of interest should allow the selection of base editing events lacking the undesired insertion of foreign DNA, and thus constitutes a promising prospect for using this tool, especially for vegetatively propagated species. Such a strategy will reduce the potentially deleterious effects of the random integration of the T-DNA into the host genome and will get rid of the constitutive expression of the base editor, thus limiting the risk for off-targets [23].
Supplementary Materials
Supplementary materials can be found at http://www.mdpi.com/1422-0067/20/2/402/s1.
Author Contributions
F.V., L.P., M.M. and F.N. designed the experiments. F.V., L.P., L.C., A.G.-D., M.-P.K. and M.M. performed the experiments. F.V., L.P., M.M. and F.N. wrote the article. F.V., L.P., L.C., J.-E.C., F.N., and M.M. discussed the data and revised the article. All authors approved the final manuscript.
Funding
This research was funded by the Investissement d’Avenir program of the French National Agency of Research for the project GENIUS (ANR-11-BTBR-0001_GENIUS) and by the Institut Carnot Plant2Pro program for the project POTATOCRISP. The Institut Jean-Pierre Bourgin (IJPB) benefits from the support of the LabEx Saclay Plant Sciences-SPS (ANR-10-LABX-0040-SPS).
Acknowledgments
We are grateful to Akihiko Kondo (Japan) who provided the Target-AID plasmid. We acknowledge the BrACySol BRC (INRA, Ploudaniel, France) that provided us with the plants that were used in this study. The authors thank Peter Rogowsky for his efficient management of the GENIUS project, Luc Jourdon for his technical support for in vitro culture and Emmanuel Botton for his precious help to take care of the plants, in vitro but also in growth chambers and greenhouses. We are thankful to Marina Perez Benitez for her help in the correction of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| ALS | Acetolactate synthase |
| CBE | Cytidine base editors |
| DSBs | DNA double-strand breaks |
| HR | Homologous recombination |
| HRM | High resolution melting |
| NHEJ | Non-homologous end-joining |
| PAM | Protospacer adjacent motif |
| PCR | Polymerase chain reaction |
| RNP | Ribonucleoprotein |
| T-DNA | Transfer DNA |
| Target-AID | Target-activation-induced cytidine deaminase |
| UGI | Uracil DNA glycosylase inhibitor protein |
References
- Schindele, P.; Wolter, F.; Puchta, H. Transforming plant biology and breeding with crispr/cas9, cas12 and cas13. FEBS Lett. 2018, 592, 1954–1967. [Google Scholar] [CrossRef] [PubMed]
- Kumlehn, J.; Pietralla, J.; Hensel, G.; Pacher, M.; Puchta, H. The crispr/cas revolution continues: From efficient gene editing for crop breeding to plant synthetic biology. J. Integr. Plant Biol. 2018, 60, 1127–1153. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Wang, Y.; Li, C.; Zhang, R.; Chen, K.; Ran, Y.; Qiu, J.L.; Wang, D.; Gao, C. Precise base editing in rice, wheat and maize with a cas9-cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 438–440. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Song, Q.; Li, C.; Jin, S.; Zhang, D.; Wang, Y.; Qiu, J.L.; Gao, C. Efficient c-to-t base editing in plants using a fusion of ncas9 and human apobec3a. Nat. Biotechnol. 2018, 36, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Shimatani, Z.; Kashojiya, S.; Takayama, M.; Terada, R.; Arazoe, T.; Ishii, H.; Teramura, H.; Yamamoto, T.; Komatsu, H.; Miura, K.; et al. Targeted base editing in rice and tomato using a crispr-cas9 cytidine deaminase fusion. Nat. Biotechnol. 2017, 35, 441–443. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zong, Y.; Wang, Y.; Jin, S.; Zhang, D.; Song, Q.; Zhang, R.; Gao, C. Expanded base editing in rice and wheat using a cas9-adenosine deaminase fusion. Genome Biol. 2018, 19, 59. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Jiang, L.; Cui, X.; Zhang, J.; Guo, S.; Li, M.; Zhang, H.; Ren, Y.; Gong, G.; Zong, M.; et al. Engineering herbicide-resistant watermelon variety through crispr/cas9-mediated base-editing. Plant Cell Rep. 2018, 37, 1353–1356. [Google Scholar] [CrossRef]
- Kang, B.C.; Yun, J.Y.; Kim, S.T.; Shin, Y.; Ryu, J.; Choi, M.; Woo, J.W.; Kim, J.S. Precision genome engineering through adenine base editing in plants. Nat. Plants 2018, 4, 427–431. [Google Scholar] [CrossRef]
- Hua, K.; Tao, X.; Yuan, F.; Wang, D.; Zhu, J.K. Precise a.T to g.C base editing in the rice genome. Mol. Plant 2018, 11, 627–630. [Google Scholar] [CrossRef]
- Yan, F.; Kuang, Y.; Ren, B.; Wang, J.; Zhang, D.; Lin, H.; Yang, B.; Zhou, X.; Zhou, H. Highly efficient a.T to g.C base editing by cas9n-guided trna adenosine deaminase in rice. Mol. Plant 2018, 11, 631–634. [Google Scholar] [CrossRef]
- Endo, M.; Mikami, M.; Endo, A.; Kaya, H.; Itoh, T.; Nishimasu, H.; Nureki, O.; Toki, S. Genome editing in plants by engineered crispr-cas9 recognizing ng pam. Nat. Plants 2018, 5, 14–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Li, W.; Katin-Grazzini, L.; Ding, J.; Gu, X.; Li, Y.; Gu, T.; Wang, R.; Lin, X.; Deng, Z.; et al. A method for the production and expedient screening of crispr/cas9-mediated non-transgenic mutant plants. Hortic. Res. 2018, 5, 13. [Google Scholar] [CrossRef]
- Fossi, M.; Comai, L. Widespread genome instability in solanum tuberosum plants regenerated from protoplasts. bioRxiv 2018, 382861. [Google Scholar] [CrossRef]
- Andersson, M.; Turesson, H.; Olsson, N.; Falt, A.S.; Ohlsson, P.; Gonzalez, M.N.; Samuelsson, M.; Hofvander, P. Genome editing in potato via crispr-cas9 ribonucleoprotein delivery. Physiol. Plant. 2018. [Google Scholar] [CrossRef]
- Clasen, B.M.; Stoddard, T.J.; Luo, S.; Demorest, Z.L.; Li, J.; Cedrone, F.; Tibebu, R.; Davison, S.; Ray, E.E.; Daulhac, A.; et al. Improving cold storage and processing traits in potato through targeted gene knockout. Plant Biotechnol. J. 2016, 14, 169–176. [Google Scholar] [CrossRef]
- Liang, Z.; Chen, K.; Li, T.; Zhang, Y.; Wang, Y.; Zhao, Q.; Liu, J.; Zhang, H.; Liu, C.; Ran, Y.; et al. Efficient DNA-free genome editing of bread wheat using crispr/cas9 ribonucleoprotein complexes. Nat. Commun. 2017, 8, 14261. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Kim, S.T.; Ryu, J.; Kang, B.C.; Kim, J.S.; Kim, S.G. Crispr/cpf1-mediated DNA-free plant genome editing. Nat. Commun. 2017, 8, 14406. [Google Scholar] [CrossRef]
- Yu, Q.; Han, H.; Vila-Aiub, M.M.; Powles, S.B. Ahas herbicide resistance endowing mutations: Effect on ahas functionality and plant growth. J. Exp. Bot. 2010, 61, 3925–3934. [Google Scholar] [CrossRef]
- Wu, H.-Y.; Liu, K.-H.; Wang, Y.-C.; Wu, J.-F.; Chiu, W.-L.; Chen, C.-Y.; Wu, S.-H.; Sheen, J.; Lai, E.-M. Agrobest: An efficient agrobacterium-mediated transient expression method for versatile gene function analyses in arabidopsis seedlings. Plant Methods 2014, 10, 19. [Google Scholar] [CrossRef]
- Kochevenko, A.; Willmitzer, L. Chimeric rna/DNA oligonucleotide-based site-specific modification of the tobacco acetolactate syntase gene. Plant Physiol. 2003, 132, 174–184. [Google Scholar] [CrossRef]
- Lee, K.Y.; Townsend, J.; Tepperman, J.; Black, M.; Chui, C.F.; Mazur, B.; Dunsmuir, P.; Bedbrook, J. The molecular basis of sulfonylurea herbicide resistance in tobacco. EMBO J. 1988, 7, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.M.; Atkins, P.A.; Voytas, D.F.; Douches, D.S. Generation and inheritance of targeted mutations in potato (Solanum tuberosum L.) using the crispr/cas system. PLoS ONE 2015, 10, e0144591. [Google Scholar] [CrossRef] [PubMed]
- Hahn, F.; Nekrasov, V. Crispr/cas precision: Do we need to worry about off-targeting in plants? Plant Cell Rep. 2018. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Arazoe, T.; Yachie, N.; Banno, S.; Kakimoto, M.; Tabata, M.; Mochizuki, M.; Miyabe, A.; Araki, M.; Hara, K.Y.; et al. Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 2016, 353, aaf8729. [Google Scholar] [CrossRef]
- Mazier, M.; Flamain, F.; Nicolai, M.; Sarnette, V.; Caranta, C. Knock-down of both eif4e1 and eif4e2 genes confers broad-spectrum resistance against potyviruses in tomato. PLoS ONE 2011, 6, e29595. [Google Scholar] [CrossRef]
- Veillet, F.; Gaillard, C.; Coutos-Thevenot, P.; La Camera, S. Targeting the atcwin1 gene to explore the role of invertases in sucrose transport in roots and during botrytis cinerea infection. Front. Plant Sci. 2016, 7, 1899. [Google Scholar] [CrossRef] [PubMed]
- Bewg, W.P.; Ci, D.; Tsai, C.J. Genome editing in trees: From multiple repair pathways to long-term stability. Front. Plant Sci. 2018, 9, 1732. [Google Scholar] [CrossRef]
- Shimatani, Z.; Fujikura, U.; Ishii, H.; Matsui, Y.; Suzuki, M.; Ueke, Y.; Taoka, K.I.; Terada, R.; Nishida, K.; Kondo, A. Inheritance of co-edited genes by crispr-based targeted nucleotide substitutions in rice. Plant Physiol. Biochem. 2018, 131, 78–83. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).