Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation
Abstract
1. Introduction
2. Cl− as an Essential Micronutrient
3. Cl− as a Beneficial Macronutrient
3.1. Charge–Balance, Osmoregulation, Turgor, Cell Volume, and Growth
3.2. Cell Water Balance and Tissue Hydration
3.3. Whole-Plant Water Relations, Photosynthesis, and Water-Use Efficiency
3.4. Energy Efficiency and Increase of Dry Biomass
3.5. Cl−/NO3− Interaction and Nitrogen-Use Efficiency
3.6. Chloroplast and Organellar Performance
3.7. Other Functions: Electrical Signals, Circulating Ion Currents, and Plant Immunity
3.8. Relevance of Cl− for Crop Yield
3.9. Cl− and Salinity
4. Regulation of Cl− Homeostasis
4.1. Cl− Influx and Net Cl− Uptake in the Root
4.1.1. Cl− Influx
4.1.2. Cl− Efflux
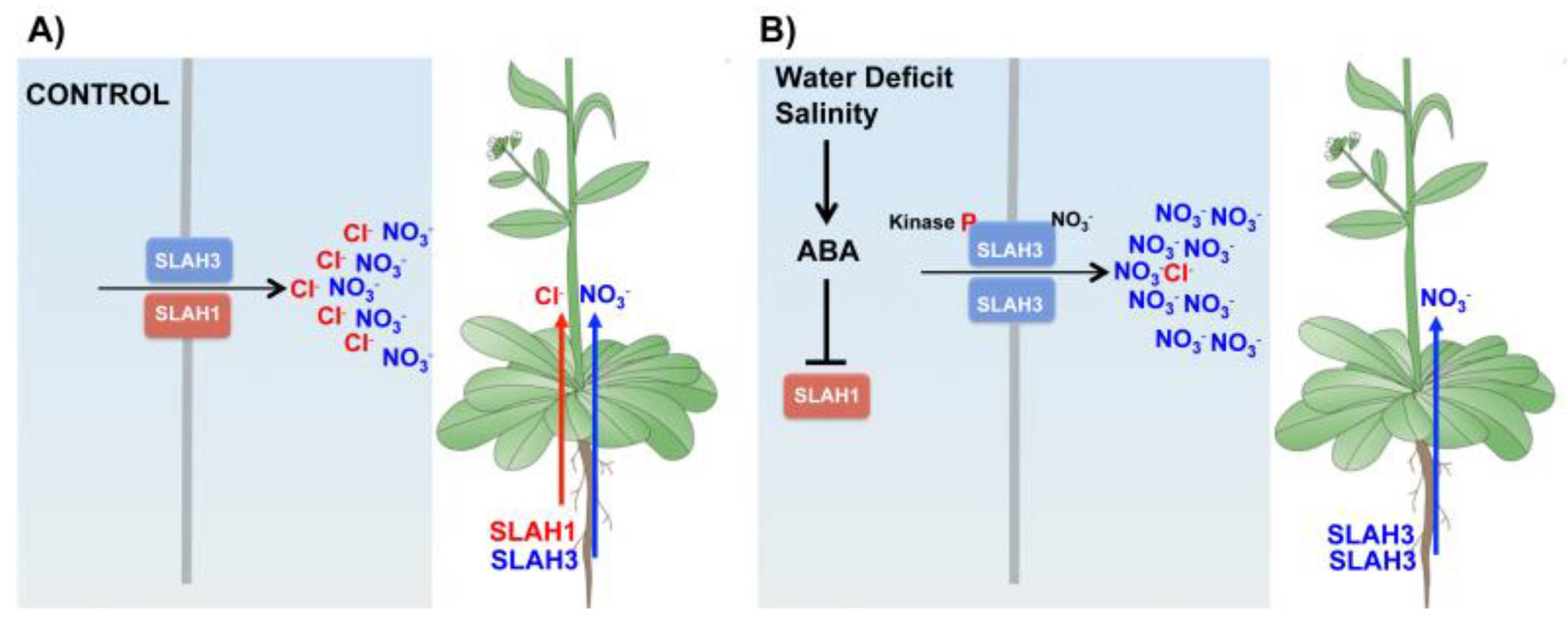
4.2. Root-to-Shoot Cl− Translocation
4.3. Cl− Compartmentalization and Subcellular Cl− Transport
4.3.1. Vacuolar Transporters
4.3.2. Golgi Transporters
4.3.3. Chloroplast Transporters
4.3.4. Endoplasmic Reticulum Transporters
4.4. Cl− Redistribution
5. Hormonal Regulation and Signal Transduction of Cl− Nutrition
6. Concluding Remarks and Future Prospects
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Broyer, T.C.; Carlton, A.B.; Johnson, C.M.; Stout, P.R. Chlorine—A micronutrient element for higher plants. Plant Physiol. 1954, 29, 526–532. [Google Scholar] [CrossRef] [PubMed]
- Franco-Navarro, J.D.; Brumos, J.; Rosales, M.A.; Cubero-Font, P.; Talon, M.; Colmenero-Flores, J.M. Chloride regulates leaf cell size and water relations in tobacco plants. J. Exp. Bot. 2016, 67, 873–891. [Google Scholar] [CrossRef] [PubMed]
- Raven, J.A. Chloride: Essential micronutrient and multifunctional beneficial ion. J. Exp. Bot. 2017, 68, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Wege, S.; Gilliham, M.; Henderson, S.W. Chloride: Not simply a ‘cheap osmoticum’, but a beneficial plant macronutrient. J. Exp. Bot. 2017, 68, 3057–3069. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.H.; Magen, H.; Tarchitzky, J.; Kafkafi, U. Advances in chloride nutrition of plants. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: San Diego, CA, USA, 2000; Volume 68, pp. 97–150. [Google Scholar]
- Flowers, T.J. Chloride as a nutrient and as an osmoticum B. In Advances in Plant Nutrition; Tinker, P.B., Läuchli, A., Eds.; Praeger: New York, NY, USA, 1988; pp. 55–78. [Google Scholar]
- White, P.J.; Broadley, M.R. Chloride in soils and its uptake and movement within the plant: A review. Ann. Bot. 2001, 88, 967–988. [Google Scholar] [CrossRef]
- Li, B.; Tester, M.; Gilliham, M. Chloride on the move. Trends Plant Sci. 2017, 22, 236–248. [Google Scholar] [CrossRef] [PubMed]
- Geilfus, C.M. Chloride in soil: From nutrient to soil pollutant. Environ. Exp. Bot. 2019, 157, 299–309. [Google Scholar] [CrossRef]
- Geilfus, C.M. Review on the significance of chlorine for crop yield and quality. Plant Sci. 2018, 270, 114–122. [Google Scholar] [CrossRef]
- Geilfus, C.M. Chloride: From Nutrient to Toxicant. Plant Cell Physiol. 2018, 59, 877–886. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: London, UK, 2012. [Google Scholar]
- Kawakami, K.; Umena, Y.; Kamiya, N.; Shen, J.R. Location of chloride and its possible functions in oxygen-evolving photosystem II revealed by X-ray crystallography. Proc. Natl. Acad. Sci. USA 2009, 106, 8567–8572. [Google Scholar] [CrossRef]
- Rognes, S.E. Anion regulation of lupin asparagine synthetase—Chloride activation of the glutamine-utilizing reactions. Phytochemistry 1980, 19, 2287–2293. [Google Scholar] [CrossRef]
- Churchill, K.A.; Sze, H. Anion-sensitive, h+-pumping atpase of oat roots—Direct effects of cl-, no3-, and a disulfonic stilbene. Plant Physiol. 1984, 76, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Metzler, D.E. Biochemistry: The Chemical Reactions of Living Cells; Academic Press: New York, NY, USA, 1979. [Google Scholar]
- Johnson, C.M.; Stout, P.R.; Broyer, T.C.; Carlton, A.B. Comparative chlorine requirements of different plant species. Plant Soil 1957, 8, 337–353. [Google Scholar] [CrossRef]
- Brumós, J.; TalÓN, M.; Bouhlal, R.Y.M.; Colmenero-Flores, J.M. Cl- homeostasis in includer and excluder citrus rootstocks: Transport mechanisms and identification of candidate genes. Plant Cell Environ. 2010, 33, 2012–2027. [Google Scholar] [CrossRef] [PubMed]
- Rosales, M.A.; Vázquez-Rodríguez, A.; Franco-Navarro, J.D.; Cubero-Font, P.; Colmenero-Flores, J.M. Chloride Nutrition Improves Water Use Eficiency and Drought Tolerance in Tomato Plants. In La Nutrición Mineral de las Plantas Como Base de Una Agricultura Sostenible; Bonilla, I., Hernández, L.E., Lucena, J.J., Eds.; Universidad Autónoma de Madrid: Madrid, Spain, 2012; pp. 314–320. [Google Scholar]
- Cubero-Font, P. Functional Characterization of Anion Channels of the SLAC/SLAH Family in Arabidopsis Thaliana, Escuela Internacional de Doctorado (EIDUS); Universidad de Sevilla: Seville, Spain, 2017. [Google Scholar]
- Felle, H.H. THE H+/CL- symporter in root-hair cells of sinapis-alba. Plant Physiol. 1994, 106, 1131–1136. [Google Scholar] [CrossRef]
- Britto, D.T.; Kronzucker, H.J. Futile cycling at the plasma membrane: A hallmark of low-affinity nutrient transport. Trends Plant Sci. 2006, 11, 529–534. [Google Scholar] [CrossRef]
- Franco-Navarro, J.D.; Rosales, M.A.; Álvarez, R.; Cubero-Font, P.; Calvo, P.; Díaz-Espejo, A.; Colmenero-Flores, J.M. Chloride as macronutrient increases water use efficiency by anatomically-driven reduced stomatal conductance and increased mesophyll diffusion to CO2. Plant J. 2019, 99, 815–831. [Google Scholar]
- Broadley, M.; Brown, P.; Cakmak, I.; Ma, J.F.; Rengel, Z.; Zhao, F. Chapter 8—Beneficial Elements. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 249–269. [Google Scholar]
- Downton, W.J.S. Growth and mineral-composition of the sultana grapevine as influenced by salinity and rootstock. Aust. J. Agric. Res. 1985, 36, 425–434. [Google Scholar] [CrossRef]
- Kafkafi, U. Plant nutrition under saline condition. Fertil. Agric. 1987, 95, 3–17. [Google Scholar]
- Yang, J.; Blanchar, R.W. Differentiating chloride susceptibility in soybean cultivars. Agron. J. 1993, 85, 880–885. [Google Scholar] [CrossRef]
- Bell, P.F.; Vaughn, J.A.; Bourgeois, W.J. Leaf analysis finds high levels of chloride and low levels of zinc and manganese in Louisiana citrus. J. Plant Nutr. 1997, 20, 733–743. [Google Scholar] [CrossRef]
- Bar, Y.; Apelbaum, A.; Kafkafi, U.; Goren, R. Relationship between chloride and nitrate and its effect on growth and mineral composition of avocado and citrus plants. J. Plant Nutr. 1997, 20, 715–731. [Google Scholar] [CrossRef]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Armstrong, C.M. The Na/K pump, Cl ion and osmotic stabilization of cells. Proc. Natl. Acad. Sci. USA 2003, 100, 6257–6262. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, A.V.; Dmitriev, A.A.; Linsenmeier, R.A. The logic of ionic homeostasis: Cations are for voltage, but not for volume. PLoS Comput. Biol. 2019, 15, e1006894. [Google Scholar] [CrossRef]
- Sze, H. H+-translocating atpases—Advances using membrane-vesicles. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1985, 36, 175–208. [Google Scholar] [CrossRef]
- Zonia, L.; Cordeiro, S.; Tupy, J.; Feijo, J.A. Oscillatory chloride efflux at the pollen tube apex has a role in growth and cell volume regulation and is targeted by inositol 3,4,5,6-tetrakisphosphate. Plant Cell 2002, 14, 2233–2249. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.I.; Hedrich, R. Involvement of ion channels and active-transport in osmoregulation and signaling of higher-plant cells. Trends Biochem. Sci. 1989, 14, 187–192. [Google Scholar] [CrossRef]
- Teodoro, A.E.; Zingarelli, L.; Lado, P. Early changes of Cl(-) efflux and H+ extrusion induced by osmotic stress in Arabidopsis thaliana cells. Physiol. Plant. 1998, 102, 29–37. [Google Scholar] [CrossRef]
- Shabala, S.; Babourina, O.; Newman, I. Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J. Exp. Bot. 2000, 51, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Sanders, D.; Bethke, P. Membrane transport. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruissem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 110–158. [Google Scholar]
- Fromm, J.; Eschrich, W. Transport processes in stimulated and non-stimulated leaves of Mimosa pudica III. Displacement of ions during seismonastic leaf movements. Trees Struct. Funct. 1988, 2, 65–72. [Google Scholar] [CrossRef]
- Iino, M.; Long, C.; Wang, X. Auxin- and Abscisic Acid-Dependent Osmoregulation in Protoplasts of Phaseolus vulgaris Pulvini. Plant Cell Physiol. 2001, 42, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Ishimaru, Y.; Takeuchi, Y.; Muraoka, Y. Plant nyctinasty—Who will decode the ‘Rosetta Stone’? New Phytol. 2019, 223, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Hedrich, R. Ion channels in plants. Physiol. Rev. 2012, 92, 1777–1811. [Google Scholar] [CrossRef] [PubMed]
- Munemasa, S.; Hauser, F.; Park, J.; Waadt, R.; Brandt, B.; Schroeder, J.I. Mechanisms of abscisic acid-mediated control of stomata! aperture. Curr. Opin. Plant Biol. 2015, 28, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Stolarz, M.; Dziubinska, H. Osmotic and Salt Stresses Modulate Spontaneous and Glutamate-Induced Action Potentials and Distinguish between Growth and Circumnutation in Helianthus annuus Seedlings. Front. Plant Sci. 2017, 8, 1766. [Google Scholar] [CrossRef] [PubMed]
- Geiger, D.; Maierhofer, T.; Al-Rasheid, K.A.S.; Scherzer, S.; Mumm, P.; Liese, A.; Ache, P.; Wellmann, C.; Marten, I.; Grill, E.; et al. Stomatal Closure by Fast Abscisic Acid Signaling Is Mediated by the Guard Cell Anion Channel SLAH3 and the Receptor RCAR1. Sci. Signal. 2011, 4. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, T.; Ishimaru, Y.; Munemasa, S.; Takeuchi, Y.; Washiyama, K.; Hamamoto, S.; Yoshikawa, N.; Mutara, Y.; Uozumi, N.; Ueda, M. Ion Channels Regulate Nyctinastic Leaf Opening in Samanea saman. Curr. Biol. 2018, 28, 2230. [Google Scholar] [CrossRef] [PubMed]
- Terry, N. Photosynthesis, growth, and role of chloride. Plant Physiol. 1977, 60, 69–75. [Google Scholar] [CrossRef]
- Yamagami, M.; Haga, K.; Napier, R.M.; Iino, M. Two Distinct Signaling Pathways Participate in Auxin-Induced Swelling of Pea Epidermal Protoplasts. Plant Physiol. 2004, 134, 735–747. [Google Scholar] [CrossRef]
- Babourina, O.; Shabala, S.; Newman, I. Auxin Stimulates Cl-Uptake by Oat Coleoptiles. Ann. Bot. 1998, 82, 331–336. [Google Scholar] [CrossRef][Green Version]
- Babourina, O.K.; Knowles, A.E.; Newman, I.A. Chloride uptake by oat coleoptile parenchyma described by combined influx and efflux transport systems. Aust. J. Plant Physiol. 1998, 25, 929–936. [Google Scholar] [CrossRef]
- Burdach, Z.; Kurtyka, R.; Siemieniuk, A.; Karcz, A. Role of chloride ions in the promotion of auxin-induced growth of maize coleoptile segments. Ann. Bot. 2014, 114, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Heslop-Harrison, J.S.; Reger, B.J. Chloride and potassium-ions and turgidity in the grass stigma. J. Plant Physiol. 1986, 124, 55–60. [Google Scholar] [CrossRef]
- Tavares, B.; Domingos, P.; Dias, P.N.; Feijo, J.A.; Bicho, A. The essential role of anionic transport in plant cells: The pollen tube as a case study. J. Exp. Bot. 2011, 62, 2273–2298. [Google Scholar] [CrossRef]
- Gutermuth, T.; Lassig, R.; Portes, M.T.; Maierhofer, T.; Romeis, T.; Borst, J.W.; Hedrich, R.; Feijo, J.A.; Konrad, K.R. Pollen Tube Growth Regulation by Free Anions Depends on the Interaction between the Anion Channel SLAH3 and Calcium-Dependent Protein Kinases CPK2 and CPK20. Plant Cell 2013, 25, 4525–4543. [Google Scholar] [CrossRef]
- Hepler, P.K.; Winship, L.J. The pollen tube clear zone: Clues to the mechanism of polarized growth. J. Integr. Plant Biol. 2015, 57, 79–92. [Google Scholar]
- Colmenero-Flores, J.M.; Martinez, G.; Gamba, G.; Vazquez, N.; Iglesias, D.J.; Brumos, J.; Talon, M. Identification and functional characterization of cation-chloride cotransporters in plants. Plant J. 2007, 50, 278–292. [Google Scholar] [CrossRef]
- Chen, Z.C.; Yamaji, N.; Fujii-Kashino, M.; Ma, J.F. A Cation-Chloride Cotransporter Gene Is Required for Cell Elongation and Osmoregulation in Rice. Plant Physiol. 2016, 171, 494–507. [Google Scholar] [CrossRef]
- Marcus, Y. Thermodynamics of solvation of ions.5. gibbs free-energy of hydration at 298.15-K. J. Chem. Soc. Faraday Trans. 1991, 87, 2995–2999. [Google Scholar] [CrossRef]
- Kropman, M.F.; Bakker, H.J. Dynamics of water molecules in aqueous solvation shells. Science 2001, 291, 2118–2120. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.M.; Gradmann, D. Impact of osmolytes on buoyancy of marine phytoplankton. Mar. Biol. 2002, 141, 605–618. [Google Scholar]
- Maron, L.G. From foe to friend: The role of chloride as a beneficial macronutrient. Plant J. 2019, 99, 813–814. [Google Scholar] [CrossRef] [PubMed]
- Wegner, L.H.; Zimmermann, U. Hydraulic conductance and K+ transport into the xylem depend on radial volume flow, rather than on xylem pressure, in roots of intact, transpiring maize seedlings. New Phytol. 2009, 181, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Gloser, V.; Zwieniecki, M.A.; Orians, C.M.; Holbrook, N.M. Dynamic changes in root hydraulic properties in response to nitrate availability. J. Exp. Bot. 2007, 58, 2409–2415. [Google Scholar] [CrossRef] [PubMed]
- Orieux, C.; Demarest, G.; Decau, M.L.; Beauclair, P.; Bataille, M.P.; le Deunff, E. Changes in (NO3-)-N-15 availability and transpiration rate are associated with a rapid diurnal adjustment of anion contents as well as N-15 and water fluxes between the roots and shoots. Front. Plant Sci. 2018, 9, 1751. [Google Scholar] [CrossRef]
- Moya, J.L.; Gomez-Cadenas, A.; Primo-Millo, E.; Talon, M. Chloride absorption in salt-sensitive Carrizo citrange and salt-tolerant Cleopatra mandarin citrus rootstocks is linked to water use. J. Exp. Bot. 2003, 54, 825–833. [Google Scholar] [CrossRef]
- Brumós, J.; Colmenero-Flores, J.M.; Conesa, A.; Izquierdo, P.; Sánchez, G.; Iglesias, D.J.; López-Climent, M.F.; Gómez-Cadenas, A.; Talón, M. Membrane transporters and carbon metabolism implicated in chloride homeostasis differentiate salt stress responses in tolerant and sensitive Citrus rootstocks. Funct. Integr. Genom. 2009, 9, 293–309. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Chapter 7—Function of Nutrients: Micronutrients, Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: San Diego, CA, USA, 2012; pp. 191–248. [Google Scholar]
- Fromm, J.; Eschrich, W. Correlation of ionic movements with phloem unloading and loading in barley leaves. Plant Physiol. Biochem. 1989, 27, 577–585. [Google Scholar]
- de Angeli, A.; Zhang, J.; Meyer, S.; Martinoia, E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013, 4, 1804. [Google Scholar] [CrossRef]
- Baetz, U.; Eisenach, C.; Tohge, T.; Martinoia, E.; de Angeli, A. Vacuolar Chloride Fluxes Impact Ion Content and Distribution during Early Salinity Stress. Plant Physiol. 2016, 172, 1167–1181. [Google Scholar] [CrossRef]
- de Angeli, A.; Monachello, D.; Ephritikhine, G.; Frachisse, J.M.; Thomine, S.; Gambale, F.; Barbier-Brygoo, H. The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 2006, 442, 939–942. [Google Scholar] [CrossRef]
- Neales, T.F.; Sharkey, P.J. Effect of salinity on growth and on mineral and organic-constituents of the halophyte disphyma-australe (soland.). Aust. J. Plant Physiol. 1981, 8, 165–179. [Google Scholar] [CrossRef]
- Yeo, A.R.; Flowers, T.J. Ion transport in Suaeda maritima: Its relation to growth and implications for the pathway of radial transport of ions across the root. J. Exp. Bot. 1986, 37, 143–159. [Google Scholar] [CrossRef]
- Flowers, T.J.; Hall, J.L. Salt Tolerance in the Halophyte, Suaeda maritima (L.) Dum.: The Influence of the Salinity of the Culture Solution on the Content of Various Organic Compounds. Ann. Bot. 1978, 42, 1057–1063. [Google Scholar] [CrossRef]
- Krapp, A.; David, L.C.; Chardin, C.; Girin, T.; Marmagne, A.; Leprince, A.S.; Chaillou, S.; Ferrario-Mery, S.; Meyer, C.; Daniel-Vedele, F. Nitrate transport and signalling in Arabidopsis. J. Exp. Bot. 2014, 65, 789–798. [Google Scholar] [CrossRef]
- Wang, Y.Y.; Hsu, P.K.; Tsay, Y.F. Uptake, allocation and signaling of nitrate. Trends Plant Sci. 2012, 17, 458–467. [Google Scholar] [CrossRef]
- Guan, P. Dancing with Hormones: A Current Perspective of Nitrate Signaling and Regulation in Arabidopsis. Front. Plant Sci. 2017, 8, 1697. [Google Scholar] [CrossRef]
- Glass, A.D.M.; Siddiqi, M.Y. Nitrate inhibition of chloride influx in barley—Implications for a proposed chloride homeostat. J. Exp. Bot. 1985, 36, 556–566. [Google Scholar] [CrossRef]
- Iglesias, D.J.; Levy, Y.; Gómez-Cadenas, A.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Nitrate improves growth in salt-stressed citrus seedlings through effects on photosynthetic activity and chloride accumulation. Tree Physiol. 2004, 24, 1027–1034. [Google Scholar] [CrossRef]
- Teakle, N.L.; Tyerman, S.D. Mechanisms of Cl- transport contributing to salt tolerance. Plant Cell Environ. 2010, 33, 566–589. [Google Scholar] [CrossRef]
- Han, Y.L.; Song, H.X.; Liao, Q.; Yu, Y.; Jian, S.F.; Lepo, J.E.; Liu, Q.; Rong, X.M.; Tian, C.; Zeng, J.; et al. Nitrogen Use Efficiency Is Mediated by Vacuolar Nitrate Sequestration Capacity in Roots of Brassica napus. Plant Physiol. 2016, 170, 1684–1698. [Google Scholar] [CrossRef]
- Fan, X.; Naz, M.; Fan, X.; Xuan, W.; Miller, A.J.; Xu, G. Plant nitrate transporters: From gene function to application. J. Exp. Bot. 2017, 68, 2463–2475. [Google Scholar] [CrossRef]
- Cerezo, M.; Garcia-Agustin, P.; Serna, M.D.; Primo-Millo, E. Kinetics of nitrate uptake by Citrus seedlings and inhibitory effects of salinity. Plant Sci. 1997, 126, 105–112. [Google Scholar] [CrossRef]
- Siddiqi, M.Y.; Glass, A.D.M.; Ruth, T.J.; Rufty, T.W. Studies of the uptake of nitrate in barley.1. kinetics of no-13(3)-influx. Plant Physiol. 1990, 93, 1426–1432. [Google Scholar] [CrossRef]
- Hawkesford, M.; Horst, W.; Kichey, T.; Lambers, H.; Schjoerring, J.; Møller, I.S.; White, P. Chapter 6—Functions of Macronutrients. In Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Marschner, P., Ed.; Academic Press: San Diego, CA, USA, 2012; pp. 135–189. [Google Scholar]
- Planes, M.D.; Ninoles, R.; Rubio, L.; Bissoli, G.; Bueso, E.; Garcia-Sanchez, M.J.; Alejandro, S.; Gonzalez-Guzman, M.; Hedrich, R.; Rodriguez, P.L.; et al. A mechanism of growth inhibition by abscisic acid in germinating seeds of Arabidopsis thaliana based on inhibition of plasma membrane H+-ATPase and decreased cytosolic pH, K+, and anions. J. Exp. Bot. 2015, 66, 813–825. [Google Scholar] [CrossRef]
- Krebs, M.; Beyhl, D.; Goerlich, E.; Al-Rasheid, K.A.S.; Marten, I.; Stierhof, Y.D.; Hedrich, R.; Schumacher, K. Arabidopsis V-ATPase activity at the tonoplast is required for efficient nutrient storage but not for sodium accumulation. Proc. Natl. Acad. Sci. USA 2010, 107, 3251–3256. [Google Scholar] [CrossRef]
- Siddiq, M.Y.; Glass, A.D.M.; Ruth, T.J. Studies of the Uptake of Nitrate in Barley: III. compartmentation of NO3‚àí. J. Exp. Bot. 1991, 42, 1455–1463. [Google Scholar] [CrossRef]
- Radcliffe, S.A.; Miller, A.J.; Ratcliffe, R.G. Microelectrode and 133Cs nuclear magnetic resonance evidence for variable cytosolic and cytoplasmic nitrate pools in maize root tips. Plant Cell Environ. 2005, 28, 1379–1387. [Google Scholar] [CrossRef]
- Wen, Z.; Kaiser, B.N. Unraveling the Functional Role of NPF6 Transporters. Front. Plant Sci. 2018, 9, 973. [Google Scholar] [CrossRef]
- Neuhaus, H.E.; Wagner, R. Solute pores, ion channels and metabolite transporters in the outer and inner envelope membranes of higher plant plastids. Biochim. Et Biophys. Acta Biomembr. 2000, 1465, 307–323. [Google Scholar] [CrossRef]
- Hind, G.; Nakatani, H.Y.; Izawa, S. Light-dependent redistribution of ions in suspensions of chloroplast thylakoid membranes. Proc. Natl. Acad. Sci. USA 1974, 71, 1484–1488. [Google Scholar] [CrossRef]
- Bose, J.; Munns, R.; Shabala, S.; Gilliham, M.; Pogson, B.; Tyerman, S.D. Chloroplast function and ion regulation in plants growing on saline soils: Lessons from halophytes. J. Exp. Bot. 2017, 68, 3129–3143. [Google Scholar] [CrossRef]
- Pyke, K.A. Plastid division. Aob Plants 2010, 10. [Google Scholar] [CrossRef]
- Enz, C.; Steinkamp, T.; Wagner, R. Ion channels in the thylakoid membrane (a patch-clamp study). Biochim. Et Biophys. Acta 1993, 1143, 67–76. [Google Scholar] [CrossRef]
- Kirchhoff, H. Structural changes of the thylakoid membrane network induced by high light stress in plant chloroplasts. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130225. [Google Scholar] [CrossRef]
- Herdean, A.; Nziengui, H.; Zsiros, O.; Solymosi, K.; Garab, G.; Lundin, B.; Spetea, C. The Arabidopsis Thylakoid Chloride Channel AtCLCe Functions in Chloride Homeostasis and Regulation of Photosynthetic Electron Transport. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef]
- Herdean, A.; Teardo, E.; Nilsson, A.K.; Pfeil, B.E.; Johansson, O.N.; Unnep, R.; Nagy, G.; Zsiros, O.; Dana, S.; Solymosi, K.; et al. A voltage-dependent chloride channel fine-tunes photosynthesis in plants. Nat. Commun. 2016, 7, 11654. [Google Scholar] [CrossRef]
- Duan, Z.; Kong, F.; Zhang, L.; Li, W.; Zhang, J.; Peng, L. A bestrophin-like protein modulates the proton motive force across the thylakoid membrane in Arabidopsis. J. Integr. Plant Biol. 2016, 58, 848–858. [Google Scholar] [CrossRef]
- Szabo, I.; Spetea, C. Impact of the ion transportome of chloroplasts on the optimization of photosynthesis. J. Exp. Bot. 2017, 68, 3115–3128. [Google Scholar] [CrossRef]
- Stauber, T.; Jentsch, T.J. Chloride in Vesicular Trafficking and Function. Annu. Rev. Physiol. 2013, 75, 453–477. [Google Scholar] [CrossRef]
- Zifarelli, G. A tale of two CLCs: Biophysical insights toward understanding ClC-5 and ClC-7 function in endosomes and lysosomes. J. Physiol. Lond. 2015, 593, 4139–4150. [Google Scholar] [CrossRef]
- Huber, A.E.; Bauerle, T.L. Long-distance plant signaling pathways in response to multiple stressors: The gap in knowledge. J. Exp. Bot. 2016, 67, 2063–2079. [Google Scholar] [CrossRef]
- Hedrich, R.; Salvador-Recatala, V.; Dreyer, I. Electrical Wiring and Long-Distance Plant Communication. Trends Plant Sci. 2016, 21, 376–387. [Google Scholar] [CrossRef]
- Szechynska-Hebda, M.; Lewandowska, M.; Karpinski, S. Electrical Signaling, Photosynthesis and Systemic Acquired Acclimation. Front. Physiol. 2017, 8, 684. [Google Scholar] [CrossRef]
- Michard, E.; Simon, A.A.; Tavares, B.; Wudick, M.M.; Feijo, J.A. Signaling with Ions: The Keystone for Apical Cell Growth and Morphogenesis in Pollen Tubes. Plant Physiol. 2017, 173, 91–111. [Google Scholar] [CrossRef]
- Colcombet., J.; Mathieu, Y.; Peyronnet, R.; Agier, N.; Lelievre, F.; Barbier-Brygoo, H.; Frachisse, J.-M. R-type anion channel activation is an essential step for ROS-dependent innate immune response in Arabidopsis suspension cells. Funct. Plant Biol. 2009, 36, 832–843. [Google Scholar] [CrossRef]
- Guo, W.; Zuo, Z.; Cheng, X.; Sun, J.; Li, H.; Li, L.; Qiu, J.L. The chloride channel family gene CLCd negatively regulates pathogen-associated molecular pattern (PAMP)-triggered immunity in Arabidopsis. J. Exp. Bot. 2014, 65, 1205–1215. [Google Scholar] [CrossRef]
- Noutoshi, Y.; Ikeda, M.; Shirasu, K. Diuretics Prime Plant Immunity in Arabidopsis thaliana. PLoS ONE 2012, 7. [Google Scholar] [CrossRef]
- Fixen, P.E. Chloride fertilization. Crop. Soils Manag. 1987, 39, 14–16. [Google Scholar]
- Chen, W.; He, Z.L.; Yang, X.E.; Mishra, S.; Stoffella, P.J. Chlorine nutrition of higher plants: Progress and perspectives. J. Plant Nutr. 2010, 33, 943–952. [Google Scholar] [CrossRef]
- Schwenke, G.D.; Simpfendorfer, S.R.; Collard, B.C.Y. Confirmation of chloride deficiency as the cause of leaf spotting in durum wheat grown in the Australian northern grains region. Crop Pasture Sci. 2015, 66, 122–134. [Google Scholar] [CrossRef]
- Smith, G.S.; Clark, C.J.; Holland, P.T. Chlorine Requirement of Kiwifruit (Actinidia deliciosa). New Phytol. 1987, 106, 71–80. [Google Scholar] [CrossRef]
- Braconnier, S.; Dauzac, J. Chloride and stomatal conductance in coconut. Plant Physiol. Biochem. 1990, 28, 105–111. [Google Scholar]
- Mueller, H.M.; Schaefer, N.; Bauer, H.; Geiger, D.; Lautner, S.; Fromm, J.; Riederer, M.; Bueno, A.; Nussbaumer, T.; Mayer, K.; et al. The desert plant Phoenix dactylifera closes stomata via nitrate-regulated SLAC1 anion channel. New Phytol. 2017, 216, 150–162. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef]
- Inal, A.; Gunes, A.; Alpaslan, M.; Demir, K. Nitrate versus chloride nutrition effects in a soil-plant system on the growth, nitrate accumulation and nitrogen, potassium, sodium, calcium and chloride content of carrot. J. Plant Nutr. 1998, 21, 2001–2011. [Google Scholar] [CrossRef]
- Maynard, D.N.; Barker, A.V.; Minotti, P.L.; Peck, N.H. Nitrate accumulation in vegetables. Adv. Agron. 1976, 28, 71–118. [Google Scholar]
- European Food Safety Authority. Opinion of the Scientific Panel on Contaminants in the Food chain on a request from the European Commission to perform a scientific risk assessment on nitrate in vegetables. EFSA J. 2008, 689, 1–79. [Google Scholar]
- Henderson, S.W.; Baumann, U.; Blackmore, D.H.; Walker, A.R.; Walker, R.R.; Gilliham, M. Shoot chloride exclusion and salt tolerance in grapevine is associated with differential ion transporter expression in roots. BMC Plant Biol. 2014, 14, 18. [Google Scholar] [CrossRef]
- Henderson, S.W.; Gilliham, M. The “Gatekeeper” Concept: Cell-Type Specific Molecular Mechanisms of Plant Adaptation to Abiotic Stress. Molecular Mechanisms in Plant Adaptation; Laitinen, R.A.E., Ed.; John wiley & Sons: Hoboken, NJ, USA, 2015; pp. 83–115. [Google Scholar]
- Henderson, S.W.; Wege, S.; Gilliham, M. Plant Cation-Chloride Cotransporters (CCC): Evolutionary Origins and Functional Insights. Int. J. Mol. Sci. 2018, 19, E492. [Google Scholar] [CrossRef]
- Warburg, O.; Luttgens, W. Photochemische Reduktion des Chinons in grünen Zellen und Granula. Biochimia 1946, 11, 303–322. [Google Scholar]
- Bove, J.M.; Arnon, D.I.; Bove, C.; Whatley, F.R. Chloride Requirement for Oxygen Evolution in Photosynthesis. Zeitschrift Für Naturforschung-B 1963, 18, 683–688. [Google Scholar] [CrossRef]
- Roberts, S.K. Plasma membrane anion channels in higher plants and their putative functions in roots. New Phytol. 2006, 169, 647–666. [Google Scholar] [CrossRef]
- Hedrich, R.; Geiger, D. Biology of SLAC1-type anion channels—From nutrient uptake to stomatal closure. New Phytol. 2017, 216, 46–61. [Google Scholar] [CrossRef]
- Konrad, K.R.; Maierhofer, T.; Hedrich, R. Spatio-temporal aspects of Ca2+ signalling: Lessons from guard cells and pollen tubes. J. Exp. Bot. 2018, 69, 4195–4214. [Google Scholar] [CrossRef]
- Saito, S.; Uozumi, N. Guard Cell Membrane Anion Transport Systems and Their Regulatory Components: An Elaborate Mechanism Controlling Stress-Induced Stomatal Closure. Plants 2019, 8, 9. [Google Scholar] [CrossRef]
- Hodges, T.K.; Vaadia, Y. Uptake and transport of radiochloride and titriated water by various zones of onion roots of different chloride status. Plant Physiol. 1964, 39, 104–108. [Google Scholar] [CrossRef]
- Shone, M.G.T. Electrochemical relations in the transfer of ions to the xylem sap of maize roots. J. Exp. Bot. 1968, 19, 468–485. [Google Scholar] [CrossRef]
- Dunlop, J.; Bowling, D.J.F. The movement of ions to the xylem exudate of maize roots. II. A comparison of the electrical potential and electrochemical potentials of ions in the exudate and in the root cells. J. Exp. Biol. 1971, 22, 445–452. [Google Scholar]
- Läuchli, A.; Epstein, E. Lateral transport of ions into the xylem of corn roots. I. Kinetics and energetics. Plant Physiol. 1971, 48, 111–117. [Google Scholar] [CrossRef][Green Version]
- Sanders, D. The mechanism of Cl- transport at the plasma membrane of Chara chorallina. I. Cotransport with H+. J. Membr. Biol. 1980, 53, 129–141. [Google Scholar] [CrossRef]
- Pitman, M.G. Transport across plant-roots. Q. Rev. Biophys. 1982, 15, 481–554. [Google Scholar] [CrossRef]
- Munns, R. Na+, K+ and Cl- in xylem sap flowing to shoots of NaCl-treated barley. J. Exp. Bot. 1985, 36, 1032–1042. [Google Scholar] [CrossRef]
- Gong, H.; Blackmore, D.; Clingeleffer, P.; Sykes, S.; Jha, D.; Tester, M.; Walker, R. Contrast in chloride exclusion between two grapevine genotypes and its variation in their hybrid progeny. J. Exp. Bot. 2011, 62, 989–999. [Google Scholar] [CrossRef]
- Lee, R.B. Selectivity and kinetics of ion uptake by barley plants following nutrient deficiency. Ann. Bot. 1982, 50, 429–449. [Google Scholar] [CrossRef]
- Pitman, M.G. Simulation of Cl- uptake by low-salt barley roots as a test of models of salt uptake. Plant Physiol. 1969, 44, 1417–1427. [Google Scholar] [CrossRef]
- Cram, W.J. Chloride Accumulation as a Homeostatic System: Set Points and Perturbations: The physiological significance of influx isotherms, temperature effects and the influence of plant growth substances. J. Exp. Bot. 1983, 34, 1484–1502. [Google Scholar] [CrossRef]
- Lorenzen, I.; Aberle, T.; Plieth, C. Salt stress-induced chloride flux: A study using transgenic Arabidopsis expressing a fluorescent anion probe. Plant J. 2004, 38, 539–544. [Google Scholar] [CrossRef]
- Saleh, L.; Plieth, C. A9C sensitive Cl−—Accumulation in A. thaliana root cells during salt stress is controlled by internal and external calcium. Plant Signal. Behav. 2013, 8, e24259. [Google Scholar] [CrossRef]
- Epstein, E. Mineral Nutrition of Plants: Principles and Perspectives; John Wiley & Sons: New York, NY, USA, 1972. [Google Scholar]
- Beilby, M.J.; Walker, N.A. Chloride transport in Chara. I. Kinetics and current-voltage curves for a probable proton symport. J. Exp. Bot. 1981, 32, 43–54. [Google Scholar] [CrossRef]
- Yamashita, K.; Yamamoto, Y.; Matsumoto, H. Characterization of an anion transporter in the plasma membrane of barley roots. Plant Cell Physiol. 1996, 37, 949–956. [Google Scholar] [CrossRef]
- Skerrett, M.; Tyerman, S.D. A channel that allows inwardly directed fluxes of anions in protoplasts derived from wheat roots. Planta 1994, 192, 295–305. [Google Scholar] [CrossRef]
- Bazihizina, N.; Colmer, T.D.; Cuin, T.A.; Mancuso, S.; Shabala, S. Friend or Foe? Chloride Patterning in Halophytes. Trends Plant Sci. 2019, 24, 142–151. [Google Scholar] [CrossRef]
- Wen, Z.; Tyerman, S.D.; Dechorgnat, J.; Ovchinnikova, E.; Dhugga, K.S.; Kaiser, B.N. Maize NPF6 Proteins Are Homologs of Arabidopsis CHL1 That Are Selective for Both Nitrate and Chloride. Plant Cell 2017, 29, 2581–2596. [Google Scholar] [CrossRef]
- Leran, S.; Varala, K.; Boyer, J.C.; Chiurazzi, M.; Crawford, N.; Daniel-Vedele, F.; David, L.; Dickstein, R.; Fernandez, E.; Forde, B.; et al. A unified nomenclature of NITRATE TRANSPORTER 1/PEPTIDE TRANSPORTER family members in plants. Trends Plant Sci. 2014, 19, 5–9. [Google Scholar] [CrossRef]
- Corratge-Faillie, C.; Lacombe, B. Substrate (un)specificity of Arabidopsis nrt1/ptr family (NPF) proteins. J. Exp. Bot. 2017, 68, 3107–3113. [Google Scholar] [CrossRef]
- Bouguyon, E.; Brun, F.; Meynard, D.; Kubes, M.; Pervent, M.; Leran, S.; Lacombe, B.; Krouk, G.; Guiderdoni, E.; Zazimalova, E.; et al. Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1. Nat. Plants 2015, 1, 15015. [Google Scholar] [CrossRef]
- Longo, A.; Miles, N.W.; Dickstein, R. Genome Mining of Plant NPFs Reveals Varying Conservation of Signature Motifs Associated with the Mechanism of Transport. Front. Plant Sci. 2018, 9, 1668. [Google Scholar] [CrossRef]
- Li, B.; Byrt, C.; Qiu, J.; Baumann, U.; Hrmova, M.; Evrard, A.; Johnson, A.A.T.; Birnbaum, K.D.; Mayo, G.M.; Jha, D.; et al. Identification of a Stelar-Localized Transport Protein That Facilitates Root-to-Shoot Transfer of Chloride in Arabidopsis. Plant Physiol. 2016, 170, 1014–1029. [Google Scholar] [CrossRef]
- Li, B.; Qiu, J.; Jayakannan, M.; Xu, B.; Li, Y.; Mayo, G.M.; Tester, M.; Gilliham, M.; Roy, S.J. AtNPF2.5 Modulates Chloride (Cl−) Efflux from Roots of Arabidopsis thaliana. Front. Plant Sci. 2017, 7, 2013. [Google Scholar] [CrossRef]
- Tsay, Y.F.; Schroeder, J.I.; Feldmann, K.A.; Crawford, N.M. The herbicide sensitivity gene chl1 of arabidopsis encodes a nitrate-inducible nitrate transporter. Cell 1993, 72, 705–713. [Google Scholar] [CrossRef]
- Liu, K.H.; Huang, C.Y.; Tsay, Y.F. CHL1 is a dual-affinity nitrate transporter of arabidopsis involved in multiple phases of nitrate uptake. Plant Cell 1999, 11, 865–874. [Google Scholar] [CrossRef]
- Liu, K.H.; Tsay, Y.F. Switching between the two action modes of the dual-affinity nitrate transporter CHL1 by phosphorylation. EMBO J. 2003, 22, 1005–1013. [Google Scholar] [CrossRef]
- Parker, J.L.; Newstead, S. Molecular basis of nitrate uptake by the plant nitrate transporter NRT1.1. Nature 2014, 507, 68. [Google Scholar] [CrossRef]
- Sun, J.; Bankston, J.R.; Payandeh, J.; Hinds, T.R.; Zagotta, W.N.; Zheng, N. Crystal structure of the plant dual-affinity nitrate transporter NRT1.1. Nature 2014, 507, 73. [Google Scholar] [CrossRef]
- Zhang, J.L.; Flowers, T.J.; Wang, S.M. Mechanisms of sodium uptake by roots of higher plants. Plant Soil 2010, 326, 45–60. [Google Scholar] [CrossRef]
- Kollist, H.; Jossier, M.; Laanemets, K.; Thomine, S. Anion channels in plant cells. FEBS J. 2011, 278, 4277–4292. [Google Scholar] [CrossRef]
- de Angeli, A.; Thomine, S.B.; Frachisse, J.M.; Ephritikhine, G.V.; Gambale, F.; Barbier-Brygoo, H.L.N. Anion channels and transporters in plant cell membranes. FEBS Lett. 2007, 581, 2367–2374. [Google Scholar] [CrossRef]
- Roelfsema, M.R.G.; Hedrich, R.; Geiger, D. Anion channels: Master switches of stress responses. Trends Plant Sci. 2012, 17, 221–229. [Google Scholar] [CrossRef]
- Kiegle, E.; Gilliham, M.; Haseloff, J.; Tester, M. Hyperpolarisation-activated calcium currents found only in cells from the elongation zone of Arabidopsis thaliana roots. Plant J. 2000, 21, 225–229. [Google Scholar] [CrossRef]
- Diatloff, E.; Roberts, M.; Sanders, D.; Roberts, S.K. Characterization of anion channels in the plasma membrane of Arabidopsis epidermal root cells and the identification of a citrate-permeable channel induced by phosphate starvation. Plant Physiol. 2004, 136, 4136–4149. [Google Scholar] [CrossRef]
- Hedrich, R.; Marten, I. Malate-induced feedback-regulation of plasma-membrane anion channels could provide a CO2 sensor to guard-cells. EMBO J. 1993, 12, 897–901. [Google Scholar] [CrossRef]
- Kolb, H.A.; Marten, I.; Hedrich, R. hodgkin-huxley analysis of a GCAC11 anion channel in the plasma-membrane of guard-cells. J. Membr. Biol. 1995, 146, 273–282. [Google Scholar] [CrossRef]
- Piñeros, M.A.; Cancado, G.M.A.; Maron, L.G.; Lyi, S.M.; Menossi, M.; Kochian, L.V. Not all ALMT1-type transporters mediate aluminum-activated organic acid responses: The case of ZmALMT1—An anion-selective transporter. Plant J. 2008, 53, 352–367. [Google Scholar] [CrossRef]
- Hedrich, R.; Becker, D. Green circuits—The potential of plant specific ion channels. Plant Mol. Biol. 1994, 26, 1637–1650. [Google Scholar] [CrossRef]
- Dietrich, P.; Hedrich, R. Anions permeate and gate GCAC1, a voltage-dependent guard cell anion channel. Plant J. 1998, 15, 479–487. [Google Scholar] [CrossRef]
- Negi, J.; Matsuda, O.; Nagasawa, T.; Oba, Y.; Takahashi, H.; Kawai-Yamada, M.; Uchimiya, H.; Hashimoto, M.; Iba, K. CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 2008, 452, 483–486. [Google Scholar] [CrossRef]
- Vahisalu, T.; Kollist, H.; Wang, Y.F.; Nishimura, N.; Chan, W.Y.; Valerio, G.; Lamminmaki, A.; Brosche, M.; Moldau, H.; Desikan, R.; et al. SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 2008, 452, 487–491. [Google Scholar] [CrossRef]
- Dauphin, A.; El-Maarouf, H.; Vienney, N.; Rona, J.P.; Bouteau, F. Effect of desiccation on potassium and anion currents from young root hairs: Implication on tip growth. Physiol. Plant. 2001, 113, 79–84. [Google Scholar] [CrossRef]
- Segonzac, C.; Boyer, J.C.; Ipotesi, E.; Szponarski, W.; Tillard, P.; Touraine, B.; Sommerer, N.; Rossignol, M.; Gibrat, R. Nitrate efflux at the root plasma membrane: Identification of an Arabidopsis excretion transporter. Plant Cell 2007, 19, 3760–3777. [Google Scholar] [CrossRef]
- Johannes, E.; Crofts, A.; Sanders, D. Control of Cl- efflux in chara corallina by cytosolic pH, free Ca2+, and phosphorylation indicates a role of plasma membrane anion channels in cytosolic pH regulation. Plant Physiol. 1998, 118, 173–181. [Google Scholar] [CrossRef]
- Gilliham, M.; Tester, M. The regulation of anion loading to the maize root xylem. Plant Physiol. 2005, 137, 819–828. [Google Scholar] [CrossRef]
- Cubero-Font, P.; Maierhofer, T.; Jaslan, J.; Rosales, M.A.; Espartero, J.; Díaz-Rueda, P.; Müller, H.M.; Hürter, A.L.; Al-Rasheid, K.A.S.; Marten, I.; et al. Silent S-Type Anion Channel Subunit SLAH1 Gates SLAH3 Open for Chloride Root-to-Shoot Translocation. Curr. Biol. 2016, 26, 2213–2220. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef]
- Tregeagle, J.M.; Tisdall, J.M.; Tester, M.; Walker, R.R. Cl- uptake, transport and accumulation in grapevine rootstocks of differing capacity for Cl--exclusion. Funct. Plant Biol. 2010, 37, 665–673. [Google Scholar] [CrossRef]
- Kohler, B.; Raschke, K. The delivery of salts to the xylem. Three types of anion conductance in the plasmalemma of the xylem parenchyma of roots of barley. Plant Physiol. 2000, 122, 243–254. [Google Scholar] [CrossRef]
- Kohler, B.; Wegner, L.H.; Osipov, V.; Raschke, K. Loading of nitrate into the xylem: Apoplastic nitrate controls the voltage dependence of X-QUAC, the main anion conductance in xylem-parenchyma cells of barley roots. Plant J. 2002, 30, 133–142. [Google Scholar] [CrossRef]
- Schroeder, J.I.; Schmidt, C.; Sheaffer, J. Identification of high-affinity slow anion channel blockers and evidence for stomatal regulation by slow anion channels in guard-cells. Plant Cell 1993, 5, 1831–1841. [Google Scholar] [CrossRef]
- Maierhofer, T.; Diekmann, M.; Offenborn, J.N.; Lind, C.; Bauer, H.; Hashimoto, K.; Al-Rasheid, K.A.S.; Luan, S.; Kudla, J.; Geiger, D.; et al. Site- and kinase-specific phosphorylation-mediated activation of SLAC1, a guard cell anion channel stimulated by abscisic acid. Sci. Signal. 2014, 7, ra86. [Google Scholar] [CrossRef]
- Scherzer, S.; Maierhofer, T.; Al-Rasheid, K.A.S.; Geiger, D.; Hedrich, R. Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol. Plant 2012, 5, 1409–1412. [Google Scholar] [CrossRef]
- Qiu, J.; Henderson, S.W.; Tester, M.; Roy, S.J.; Gilliham, M. SLAH1, a homologue of the slow type anion channel SLAC1, modulates shoot Cl- accumulation and salt tolerance in Arabidopsis thaliana. J. Exp. Bot. 2017, 68, 4495–4505. [Google Scholar]
- Meyer, S.; Mumm, P.; Imes, D.; Endler, A.; Weder, B.; Al-Rasheid, K.A.S.; Geiger, D.; Marten, I.; Martinoia, E.; Hedrich, R. AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J. 2010, 63, 1054–1062. [Google Scholar] [CrossRef]
- Sasaki, T.; Mori, I.C.; Furuichi, T.; Munemasa, S.; Toyooka, K.; Matsuoka, K.; Murata, Y.; Yamamoto, Y. Closing Plant Stomata Requires a Homolog of an Aluminum-Activated Malate Transporter. Plant Cell Physiol. 2010, 51, 354–365. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef]
- Henderson, S.W.; Wege, S.; Qiu, J.; Blackmore, D.H.; Walker, A.R.; Tyerman, S.D.; Walker, R.R.; Gilliham, M. Grapevine and Arabidopsis Cation-Chloride Cotransporters Localize to the Golgi and Trans-Golgi Network and Indirectly Influence Long-Distance Ion Transport and Plant Salt Tolerance. Plant Physiol. 2015, 169, 2215–2229. [Google Scholar]
- Zhu, M.; Zhou, M.; Shabala, L.; Shabala, S. Physiological and molecular mechanisms mediating xylem Na+ loading in barley in the context of salinity stress tolerance. Plant Cell Environ. 2017, 40, 1009–1020. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; Vinauger, M.; Colcombet, J.; Ephritikhine, G.; Frachisse, J.M.; Maurel, C. Anion channels in higher plants: Functional characterization, molecular structure and physiological role. Biochim. Biophys. Acta 2000, 1465, 199–218. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, F.G.; Tang, R.J.; Yu, Y.; Song, J.; Wang, Y.; Li, L.; Luan, S. Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E2036–E2045. [Google Scholar] [CrossRef]
- Wege, S.; de Angeli, A.; Droillard, M.J.; Kroniewicz, L.; Merlot, S.; Cornu, D.; Gambale, F.; Martinoia, E.; Barbier-Brygoo, H.; Thomine, S.; et al. Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal. 2014, 7. [Google Scholar] [CrossRef]
- von der Fecht-Bartenbach, J.; Bogner, M.; Dynowski, M.; Ludewig, U. CLC-b-Mediated NO3-/H+ Exchange Across the Tonoplast of Arabidopsis Vacuoles. Plant Cell Physiol. 2010, 51, 960–968. [Google Scholar] [CrossRef]
- Li, W.Y.F.; Wong, F.L.; Tsai, S.N.; Phang, T.H.; Shao, G.; Lam, H.M. Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ. 2006, 29, 1122–1137. [Google Scholar] [CrossRef]
- Wong, T.H.; Li, M.W.; Yao, X.Q.; Lam, H.M. The GmCLC1 protein from soybean functions as a chloride ion transporter. J. Plant Physiol. 2013, 170, 101–104. [Google Scholar] [CrossRef]
- Wei, P.P.; Wang, L.C.; Liu, A.L.; Yu, B.J.; Lam, H.M. GmCLC1 Confers Enhanced Salt Tolerance through Regulating Chloride Accumulation in Soybean. Front. Plant Sci. 2016, 7, 1082. [Google Scholar] [CrossRef]
- Barbier-Brygoo, H.; de Angeli, A.; Filleur, S.; Frachisse, J.M.; Gambale, F.; Thomine, S.; Wege, S. Anion Channels/Transporters in Plants: From Molecular Bases to Regulatory Networks. Annu. Rev. Plant Biol. 2011, 62, 25–51. [Google Scholar] [CrossRef]
- Hu, R.; Zhu, Y.; Wei, J.; Chen, J.; Shi, H.; Shen, G.; Zhang, H. Overexpression of PP2A-C5 that encodes the catalytic subunit 5 of protein phosphatase 2A in Arabidopsis confers better root and shoot development under salt conditions. Plant Cell Environ. 2017, 40, 150–164. [Google Scholar] [CrossRef]
- Jossier, M.; Kroniewicz, L.; Dalmas, F.; le Thiec, D.; Ephritikhine, G.; Thomine, S.; Barbier-Brygoo, H.; Vavasseur, A.; Filleur, S.; Leonhardt, N. The Arabidopsis vacuolar anion transporter, AtCLCc, is involved in the regulation of stomatal movements and contributes to salt tolerance. Plant J. 2010, 64, 563–576. [Google Scholar] [CrossRef]
- Wei, P.P.; Che, B.N.; Shen, L.K.; Cui, Y.Q.; Wu, S.Y.; Cheng, C.; Liu, F.; Li, M.W.; Yu, B.J.; Lam, H.M. Identification and functional characterization of the chloride channel gene, GsCLC-c2 from wild soybean. BMC Plant Biol. 2019, 19, 121. [Google Scholar] [CrossRef]
- Nguyen, C.T.; Agorio, A.; Jossier, M.; Depre, S.; Thomine, S.; Filleur, S. Characterization of the Chloride Channel-Like, AtCLCg, Involved in Chloride Tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 764–775. [Google Scholar] [CrossRef]
- Kovermann, P.; Meyer, S.; Hoertensteiner, S.; Picco, C.; Scholz-Starke, J.; Ravera, S.; Lee, Y.; Martinoia, E. The Arabidopsis vacuolar malate channel is a member of the ALMT family. Plant J. 2007, 52, 1169–1180. [Google Scholar] [CrossRef]
- Von der Fecht-Bartenbach, J.; Bogner, M.; Krebs, M.; Stierhof, Y.D.; Schumacher, K.; Ludewig, U. Function of the anion transporter AtCLC-d in the trans-Golgi network. Plant J. 2007, 50, 466–474. [Google Scholar] [CrossRef]
- Marmagne, A.; Vinauger-Douard, M.; Monachello, D.; de Longevialle, A.F.; Charon, C.; Allot, M.; Rappaport, F.; Wollman, F.A.; Barbier-Brygoo, H.; Ephritikhine, G. Two members of the Arabidopsis CLC (chloride channel) family, AtCLCe and AtCLCf, are associated with thylakoid and Golgi membranes, respectively. J. Exp. Bot. 2007, 58, 3385–3393. [Google Scholar] [CrossRef]
- Spetea, C.; Herdean, A.; Allorent, G.; Carraretto, L.; Finazzi, G.; Szabo, I. An update on the regulation of photosynthesis by thylakoid ion channels and transporters in Arabidopsis. Physiol. Plant. 2017, 161, 16–27. [Google Scholar] [CrossRef]
- Heber, U.; Heldt, H.W. The Chloroplast Envelope: Structure, Function, and Role in Leaf Metabolism. Annu. Rev. Plant Physiol. 1981, 32, 139–168. [Google Scholar] [CrossRef]
- Teardo, E.; Frare, E.; Segalla, A.; de Marco, V.; Giacometti, G.M.; Szabo, I. Localization of a putative ClC chloride channel in spinach chloroplasts. FEBS Lett. 2005, 579, 4991–4996. [Google Scholar] [CrossRef]
- Zifarelli, G.; Pusch, M. CLC transport proteins in plants. FEBS Lett. 2010, 584, 2122–2127. [Google Scholar] [CrossRef]
- Selga, T.; Selga, M. The synapse-like interaction between chloroplast, dictyosome, and other cell compartments during increased ethylene production in leaves of rye (Secale cereale L.). Photosynthetica 2000, 38, 433–441. [Google Scholar] [CrossRef]
- Guan, R.; Qu, Y.; Guo, Y.; Yu, L.; Liu, Y.; Jiang, J.; Chen, J.; Ren, Y.; Liu, G.; Tian, L.; et al. Salinity tolerance in soybean is modulated by natural variation in GmSALT3. Plant J. 2014, 80, 937–950. [Google Scholar] [CrossRef]
- Duc, D.T.; Chen, H.; Thu, V.H.T.; Hamwieh, A.; Yamada, T.; Sato, T.; Yan, Y.; Cong, H.; Shono, M.; Suenaga, K.; et al. Ncl Synchronously Regulates Na+, K+ and Cl- in Soybean and Greatly Increases the Grain Yield in Saline Field Conditions. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, L.; Qu, Y.; Chen, J.; Liu, X.; Hong, H.; Liu, Z.; Chang, R.; Gilliham, M.; Qiu, L.; et al. GmSALT3, Which Confers Improved Soybean Salt Tolerance in the Field, Increases Leaf Cl- Exclusion Prior to Na+ Exclusion But Does Not Improve Early Vigor under Salinity. Front. Plant Sci. 2016, 7, 1485. [Google Scholar] [CrossRef]
- van Bel, A.J.E. The phloem, a miracle of ingenuity. Plant Cell Environ. 2003, 26, 125–149. [Google Scholar] [CrossRef]
- Liesche, J.; Schulz, A. Symplasmic transport in phloem loading and unloading. Symplasmic Transport in Vascular Plants; Sokolowska, K., Sowinski, P., Eds.; Springer: New York, NY, USA, 2013; pp. 133–163. [Google Scholar]
- Lessani, H.; Marschner, H. Relation between salt tolerance and long-distance transport of sodium and chloride in various crop species. Aust. J. Plant Physiol. 1978, 5, 27–37. [Google Scholar] [CrossRef]
- Moran, N. Osmoregulation of leaf motor cells. FEBS Lett. 2007, 581, 2337–2347. [Google Scholar] [CrossRef]
- Bañuls, J.; Primo-Millo, E. Effects of Salinity on Some Citrus Scion-Rootstock Combinations. Ann. Bot. 1995, 76, 97–102. [Google Scholar] [CrossRef]
- Colmenero-Flores, J.M.; Arbona, V.; Morillon, R.; Gómez-Cadenas, A. Salinity and Water Deficit. The Genus Citrus; Talon, M., Gmitter, F.G., Caruso, M., Eds.; Woodhead Publishing, Elsevier: London, UK, 2019. [Google Scholar]
- Davenport, R.J.; Munoz-Mayor, A.; Jha, D.; Essah, P.A.; Rus, A.; Tester, M. The Na+ transporter AtHKT1;1 controls retrieval of Na+ from the xylem in Arabidopsis. Plant Cell Environ. 2007, 30, 497–507. [Google Scholar] [CrossRef]
- Herbell, S.; Gutermuth, T.; Konrad, K.R. An interconnection between tip-focused Ca2+ and anion homeostasis controls pollen tube growth. Plant Signal. Behav. 2018, 13, e1529521. [Google Scholar] [CrossRef]
- Hedrich, R.; Bregante, M.; Dreyer, I.; Gambale, F. The voltage-dependent potassium-uptake channel of corn coleoptiles has permeation properties different from other K+ channels. Planta 1995, 197, 193–199. [Google Scholar] [CrossRef]
- Shabala, S.N.; Lew, R.R. Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol. 2002, 129, 290–299. [Google Scholar] [CrossRef]
- Cram, W.J.; Pitman, M.G. The action of abscisic acid on ion uptake and water flow in plant roots. Aust. J. Biol. Sci. 1972, 25, 1125–1132. [Google Scholar] [CrossRef]
- Cram, W.J. Chloride fluxes in cells of the isolated root cortex of Zea mays. Aust. J. Biol. Sci. 1973, 26, 757. [Google Scholar] [CrossRef]
- Pitman, M.G.; Wellfare, D. Inhibition of ion-transport in excised barley roots by abscisic-acid—Relation to water permeability of roots. J. Exp. Bot. 1978, 29, 1125–1138. [Google Scholar] [CrossRef]
- Roberts, S.K. Regulation of K+ channels in maize roots by water stress and abscisic acid. Plant Physiol. 1998, 116, 145–153. [Google Scholar] [CrossRef]
- Roberts, S.K.; Snowman, B.N. The effects of ABA on channel-mediated K+ transport across higher plant roots. J. Exp. Bot. 2000, 51, 1585–1594. [Google Scholar] [CrossRef]
- Gaymard, F.; Pilot, G.; Lacombe, B.; Bouchez, D.; Bruneau, D.; Lytton, J.; Michaux-Ferrière, N.; Thibaud, J.; Sentenac, H. Identification and Disruption of a Plant Shaker-like Outward Channel Involved in K+ Release into the Xylem Sap. Cell 1998, 94, 647–655. [Google Scholar] [CrossRef]
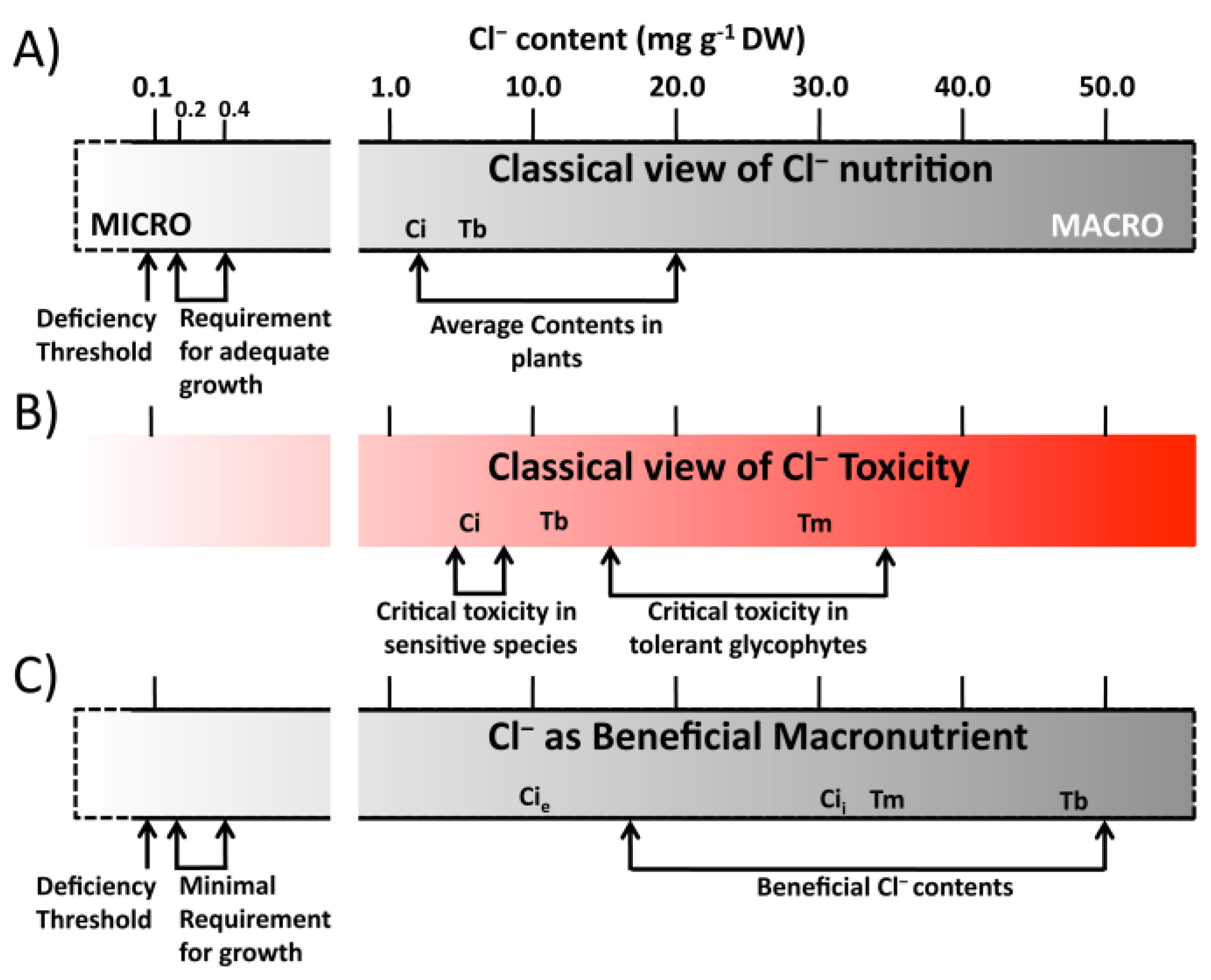
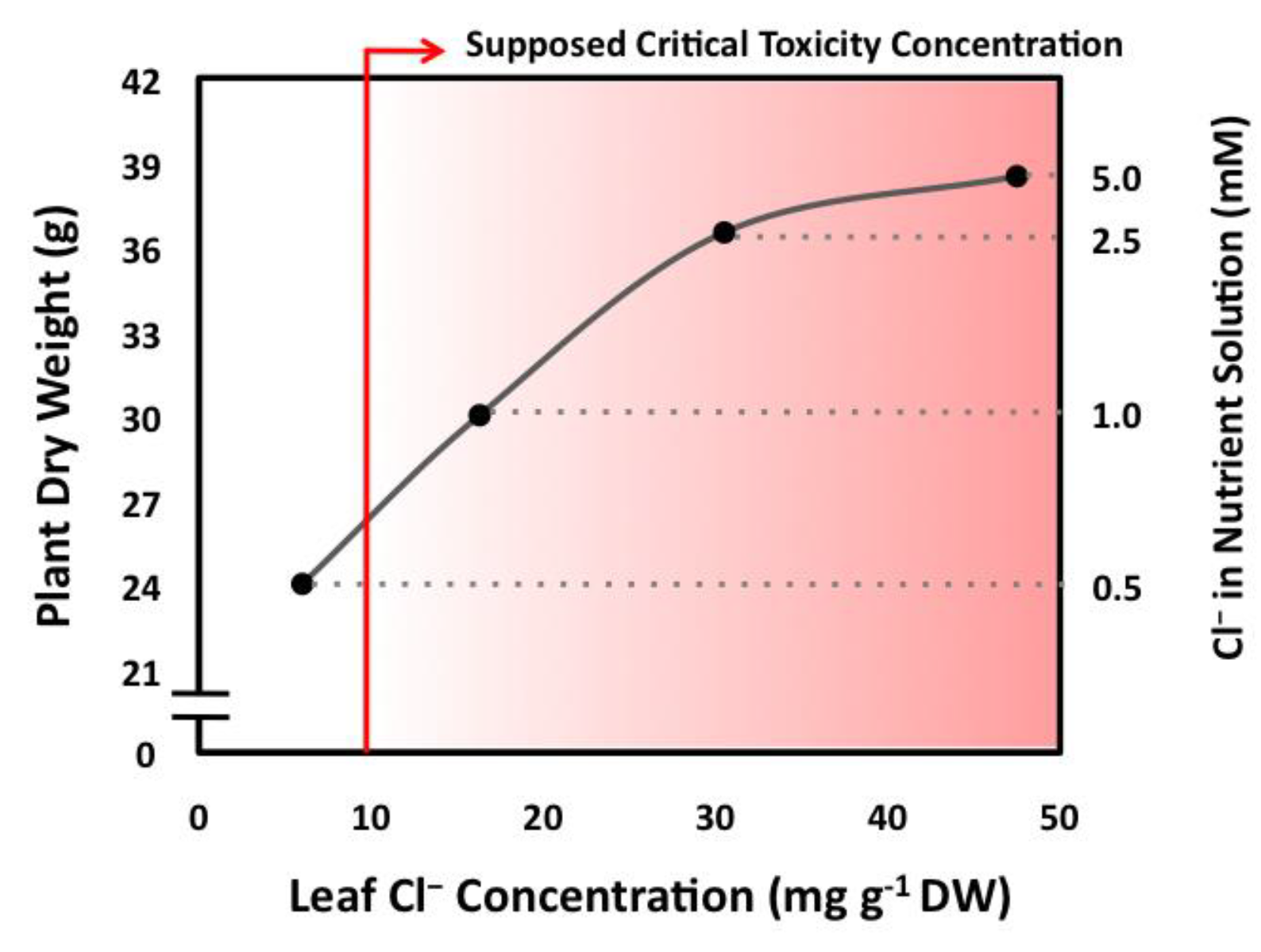
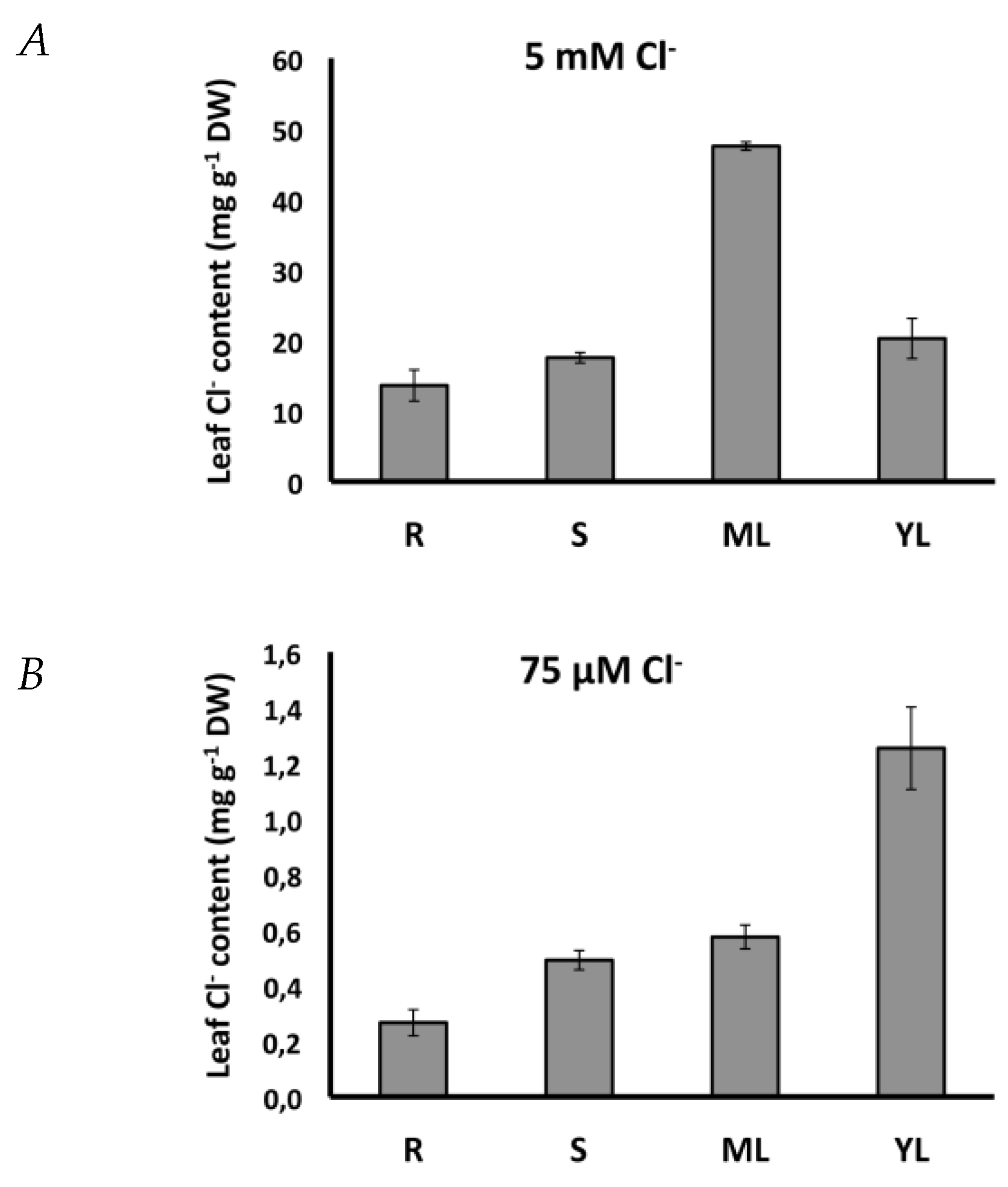
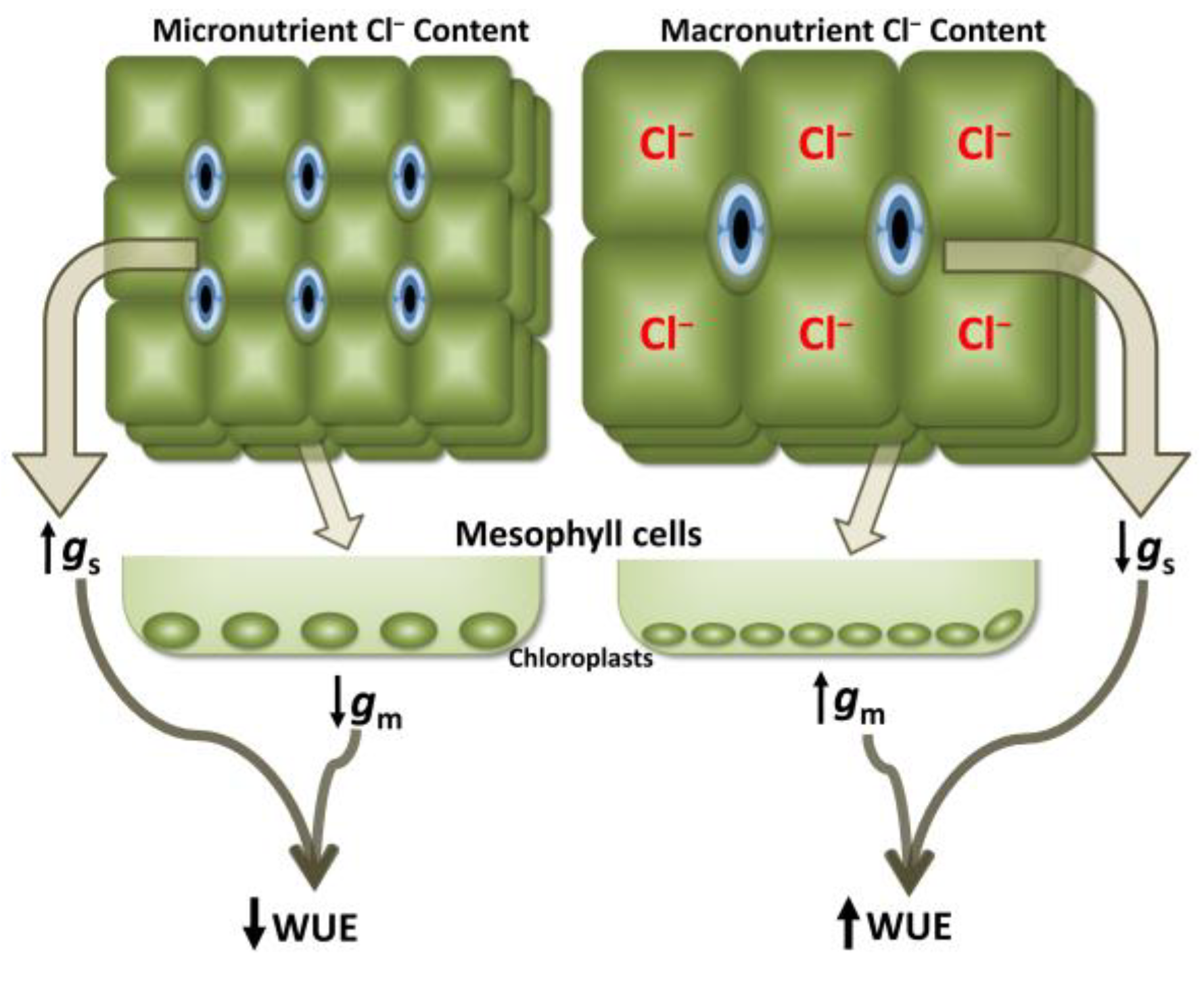
| Citrus Cl− Excluder 2 | Arabidopsis (Col0) 3 | Citrus Cl− Includer 4 | Tomato 5 | Tobacco 6 | |
|---|---|---|---|---|---|
| Leaf Cl− Concentration (mg·g−1 DW) | 5–13 | 25 | 30 | 32 | 50 |
| Cl− Excess (Respect to micronutrient requirement) 1 | 25–70 | 125 | 150 | 150 | 250 |
| Year | Cl− Function | Reference |
|---|---|---|
| 1946 | - Cl− is required for photochemical activity in washed chloroplasts - Cl− is proposed to be an essential micronutrient | [123] |
| 1954 | - Demonstration of Cl− as essential micronutrient in tomato plants | [1] |
| 1956 | - Demonstration of Cl− as essential micronutrient in other plant species | [17] |
| 1963 | - Cl− is required for Oxygen evolution in Photosystem-II | [124] |
| 1977 | - The requirement of Cl− is not limited to photosynthesis - Cl− is also required for adequate cell division rate in the leaves | [47] |
| 1980 | - Cl− regulates the activity of some proteins | [14,15] |
| 1987 | - Some plant species require Cl− beyond micronutrient levels | [112,113,114] |
| 1991 | - Cl− has a more tightly bound hydration shell | [58,59] |
| 2009 | - Localization and role of Cl− in oxygen-evolving Photosystem II | [13] |
| 2014 | - Cl− is required for adequate cell elongation | [2,51] |
| 2015 | - Occurence of Cl− deficiency in agricultural soils is demonstrated for a relevant crop species | [112] |
| 2016 | - Cl− specifically improves osmoregulation, water balance and turgor - Cl− is proposed as a beneficial macronutrient | [2] |
| 2016 | - Chloroplast Cl− homeostasis regulates photosynthetic electron transport and photoprotective mechanisms | [97,98] |
| 2019 | - As beneficial macronutrient Cl− improves water-use efficiency by reduced stomatal conductance and increased mesophyll diffusion to CO2 | [23] |
| Protein Name | Localization | Cell Function | Biological Role | References |
|---|---|---|---|---|
| ZmNPF6.4 |
|
|
| [147] |
| ZmALMT1 |
|
|
| [167] |
| AtNPF2.5 |
|
|
| [153] |
| AtSLAH3 |
|
|
| [45,176] |
| AtSLAH1 |
|
|
| [176,184] |
| AtNPF2.4 |
|
|
| [152] |
| AtALMT12 |
|
|
| [185,186] |
| AtCCC |
|
|
| [56,122] |
| OsCCC1 |
|
|
| [57] |
| AtALMT9 |
|
|
| [69,70] |
| AtCLCc |
|
|
| [198,199] |
| AtCLCg |
|
|
| [201] |
| GmCLC1 |
|
|
| [194,195,196] |
| GsCLC-c2 |
|
|
| [200] |
| GmSALT3/CHX1 |
|
|
| [210,211,212] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colmenero-Flores, J.M.; Franco-Navarro, J.D.; Cubero-Font, P.; Peinado-Torrubia, P.; Rosales, M.A. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. Int. J. Mol. Sci. 2019, 20, 4686. https://doi.org/10.3390/ijms20194686
Colmenero-Flores JM, Franco-Navarro JD, Cubero-Font P, Peinado-Torrubia P, Rosales MA. Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. International Journal of Molecular Sciences. 2019; 20(19):4686. https://doi.org/10.3390/ijms20194686
Chicago/Turabian StyleColmenero-Flores, José M., Juan D. Franco-Navarro, Paloma Cubero-Font, Procopio Peinado-Torrubia, and Miguel A. Rosales. 2019. "Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation" International Journal of Molecular Sciences 20, no. 19: 4686. https://doi.org/10.3390/ijms20194686
APA StyleColmenero-Flores, J. M., Franco-Navarro, J. D., Cubero-Font, P., Peinado-Torrubia, P., & Rosales, M. A. (2019). Chloride as a Beneficial Macronutrient in Higher Plants: New Roles and Regulation. International Journal of Molecular Sciences, 20(19), 4686. https://doi.org/10.3390/ijms20194686







