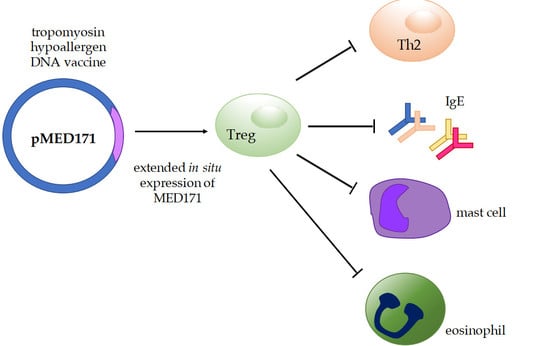
Modulating Shrimp Tropomyosin-Mediated Allergy: Hypoallergen DNA Vaccines Induce Regulatory T Cells to Reduce Hypersensitivity in Mouse Model
Abstract
1. Introduction
2. Results
2.1. pMEM49 and pMED171 Treatment Groups have Marked Reduction in Anaphylactic Symptoms and Intestinal Inflammation Upon Oral Shrimp Tropomyosin Challenge
2.2. pMEM49 and pMED171 Therapy Reduces Shrimp Tropomyosin-Specific Serum IgE Level and Intestinal Th2-gene Expression
2.3. pMED171 Markedly Enhances Intestinal Gut-Homing Treg Cell Responses
2.4. Restoration of Intestinal Inflammatory Responses but not Th2 Cell Activity upon Treg Cell Depletion
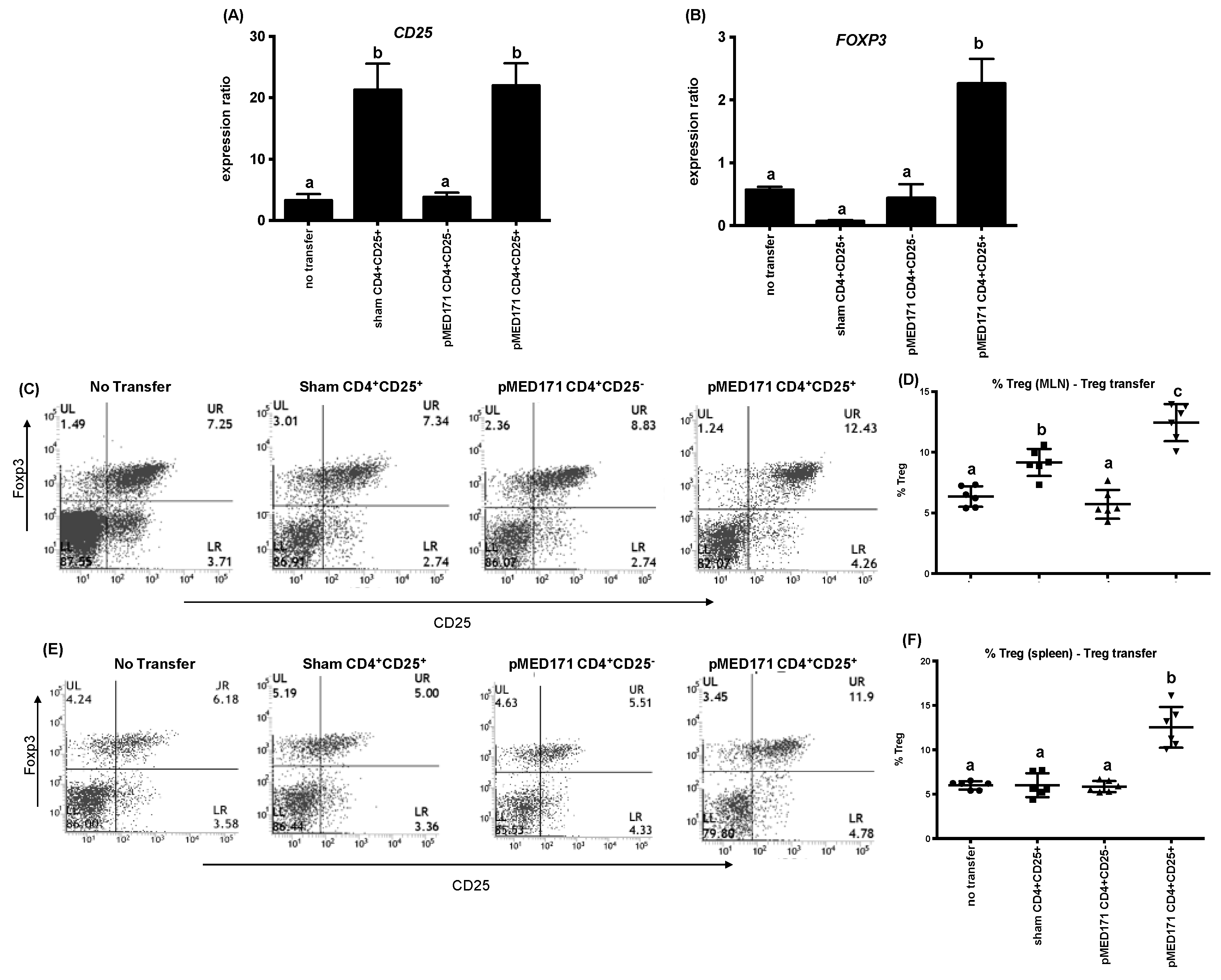
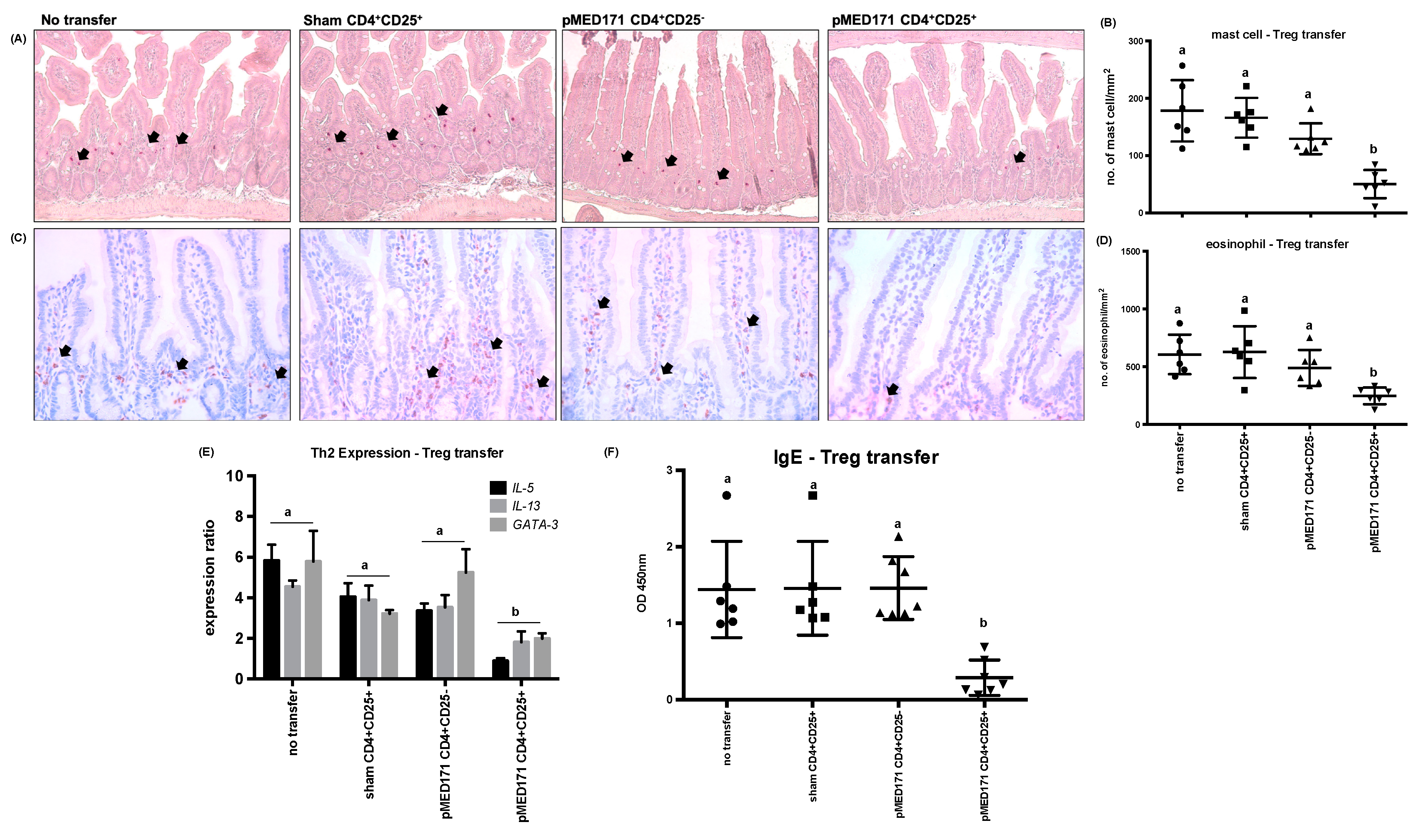
2.5. The Effect of pMED171 on Limiting Intestinal Inflammation Resides on CD4+CD25+Foxp3+Treg Cells
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. DNA Vaccine Preparation
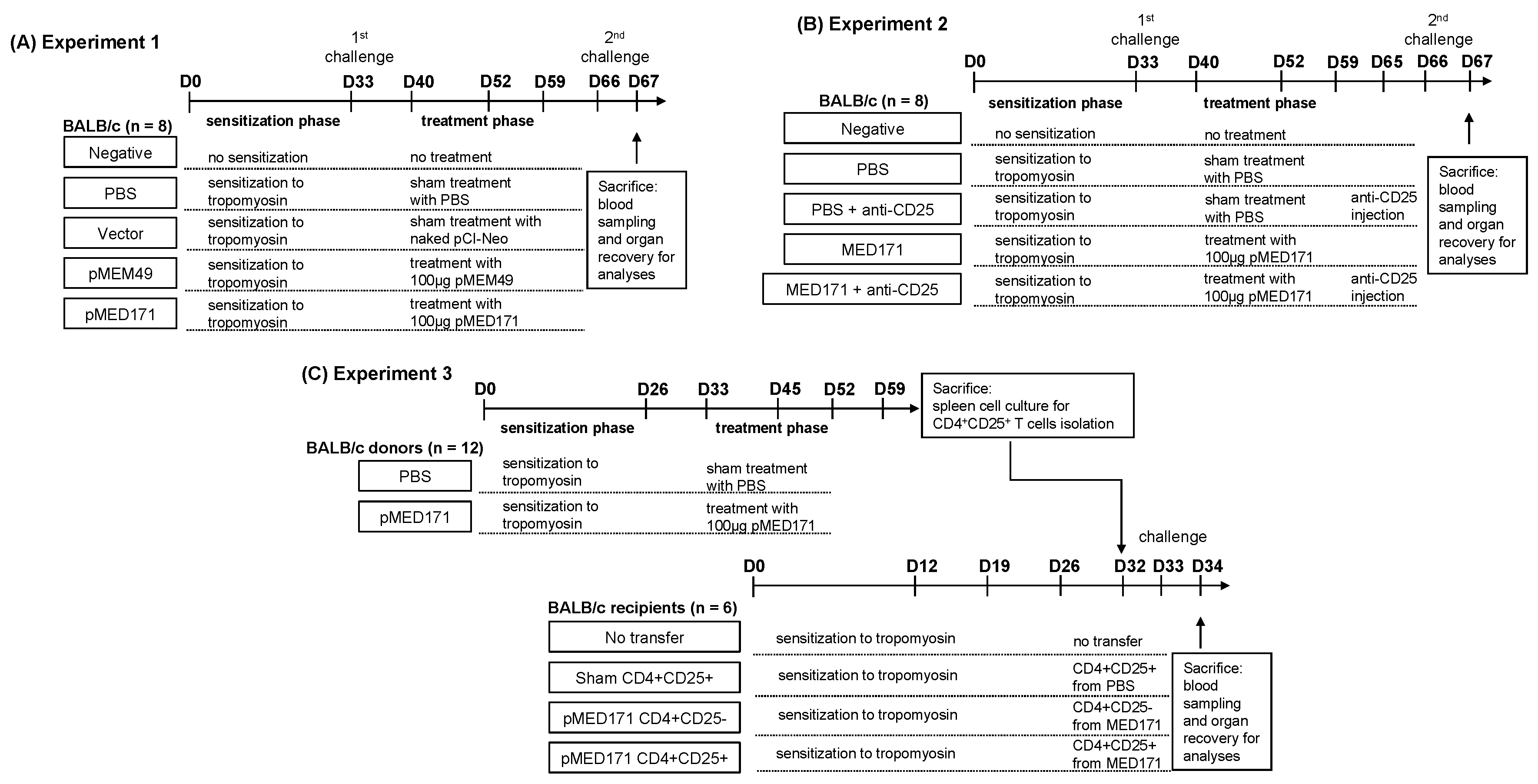
4.3. Study Design
4.4. Assessment of Hypersensitivity Responses and Condition of Feces
4.5. Measurement of Antibody, mMCP-1, and Cytokine Levels
4.6. Measurement of Regulatory T Cells
4.7. Intestinal Gene Expression
4.8. Immunohistochemistry
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Patents
References
- Moonesinghe, H.; Mackenzie, H.; Venter, C.; Kilburn, S.; Turner, P.; Weir, K.; Dean, T. Prevalence of fish and shellfish allergy: A systematic review. Annu. Allergy Asthma Immunol. 2016, 117, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.S.; Warren, C.M.; Smith, B.M.; Jiang, J.L.; Blumenstock, J.A.; Davis, M.M.; Schleimer, R.P.; Nadeau, K.C. Prevalence and severity of food allergies among US adults. JAMA Netw. Open 2019, 2, e185630. [Google Scholar] [CrossRef] [PubMed]
- Shanti, K.N.; Martin, B.M.; Nagpal, S.; Metcalfe, D.D.; Rao, P.V.S. Identification of tropomyosin as the major shrimp allergen and characterization of its IgE-binding epitopes. J. Immunol. 1993, 151, 5354–5363. [Google Scholar] [PubMed]
- Leung, P.S.C.; Chu, K.H.; Chow, W.K.; Ansari, A.; Bandea, C.I.; Kwan, H.S.; Nagy, S.M.; Gershwin, M.E. Cloning, expression, and primary structure of Metapenaeus ensis tropomyosin, the major heat-stable shrimp allergen. J. Allergy Clin. Immunol. 1994, 94, 882–890. [Google Scholar] [CrossRef]
- Feuille, E.; Nowak-Wegrzyn, A. Allergen-specific immunotherapies for food allergy. Allergy Asthma Immunol. Res. 2018, 10, 189–206. [Google Scholar] [CrossRef]
- Jones, S.M.; Burks, A.W.; Dupont, C. State of the art on food allergen immunotherapy: Oral, sublingual, and epicutaneous. J. Allergy Clin. Immunol. 2014, 133, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Keet, C.A.; Wood, R.A. Emerging therapies for food allergy. J. Clin. Investig. 2014, 124, 1880–1886. [Google Scholar] [CrossRef][Green Version]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, P.S.C.; Chu, K.H. Immunotherapy of food allergy: A comprehensive review. Clin. Rev. Allergy Immunol. 2019, 57, 55–73. [Google Scholar] [CrossRef]
- Ye, Y.L.; Chuang, Y.H.; Chiang, B.L. Strategies of mucosal immunotherapy for allergic diseases. Cell Mol. Immunol. 2011, 8, 453–461. [Google Scholar] [CrossRef][Green Version]
- Leung, A.S.Y.; Leung, N.Y.H.; Wai, C.Y.Y.; Leung, T.F.; Wong, G.W.K. Allergen immunotherapy for food allergy from the Asian perspective: Key challenges and opportunities. Expert Rev. Clin. Immunol. 2019, 15, 153–164. [Google Scholar] [CrossRef]
- Hobernik, D.; Bros, M. DNA vaccines-how far from clinical use? Int. J. Mol. Sci. 2018, 19, 3605. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.F.; Song, P.P.; Lin, T.M.; Chiu, Y.T.; Chen, Y.H. For t 2 DNA vaccine prevents Forcipomyia taiwana (biting midge) allergy in a mouse model. Allergy 2016, 71, 522–531. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Connolly, M.; Marketon, A.; Heiland, T. CryJ-LAMP DNA vaccines for Japanese red cedar allergy induce robust Th1-type immune responses in murine model. J. Immunol. Res. 2016, 2016, 4857869. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.K.; Cha, S.C.; Smith, D.L.; Kim, K.H.; Parshottam, S.R.; Rao, S.; Popescu, M.; Lee, V.Y.; Neelapu, S.S.; Kwak, L.W. Phase I study of an active immunotherapy for asymptomatic phase Lymphoplasmacytic lymphoma with DNA vaccines encoding antigen-chemokine fusion: Study protocol. BMC Cancer 2018, 18, 187. [Google Scholar] [CrossRef] [PubMed]
- Son, H.Y.; Apostolopoulos, V.; Chung, J.K.; Kim, C.W.; Park, J.U. Protective efficacy of a plasmid DNA vaccine against transgene-specific tumors by Th1 cellular immune responses after intradermal injection. Cell. Immunol. 2018, 329, 17–26. [Google Scholar] [CrossRef]
- Hannaman, D.; Dupuy, L.C.; Ellefsen, B.; Schmaljohn, C.S. A Phase 1 clinical trial of a DNA vaccine for Venezuelan equine encephalitis delivered by intramuscular or intradermal electroporation. Vaccine 2016, 34, 3607–3612. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Kanda, Y.; Takenaka, K.; Okamoto, S.; Kato, J.; Kanda, J.; Yoshimoto, G.; Gondo, H.; Doi, S.; Inaba, M.; et al. Safety of ASP0113, a cytomegalovirus DNA vaccine, in recipients undergoing allogeneic hematopoietic cell transplantation: An open-label phase 2 trial. Int. J. Hematol. 2017, 105, 206–212. [Google Scholar] [CrossRef]
- Su, Y.; Romeu-Bonilla, E.; Anagnostou, A.; Fitz-Patrick, D.; Hearl, W.; Heiland, T. Safety and long-term immunological effects of CryJ2-LAMP plasmid vaccine in Japanese red cedar atopic subjects: A phase I study. Hum. Vaccines Immunother. 2017, 13, 2804–2813. [Google Scholar] [CrossRef]
- Li, X.M.; Song, Y.; Su, Y.; Heiland, T.; Sampson, H.A. Immunization with ARA h1,2,3-Lamp-Vax peanut vaccine blocked IgE mediated-anaphylaxis in a peanut allergic murine model. J. Allergy Clin. Immunol. 2015, 135, Ab167. [Google Scholar] [CrossRef]
- Leung, N.Y.H.; Wai, C.Y.Y.; Shu, S.A.; Chang, C.C.; Chu, K.H.; Leung, P.S.C. Low-dose allergen-specific immunotherapy induces tolerance in a murine model of shrimp allergy. Int. Arch. Allergy Immunol. 2017, 174, 86–96. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Ho, M.H.; Gershwin, L.J.; Shu, S.A.; Leung, P.S.C.; Chu, K.H. Immunization with hypoallergens of shrimp allergen tropomyosin inhibits shrimp tropomyosin specific IgE reactivity. PLoS ONE 2014, 9, e111649. [Google Scholar] [CrossRef] [PubMed]
- Lam, Y.F.; Tong, K.K.; Kwan, K.M.; Tsuneyama, K.; Shu, S.A.; Leung, P.S.; Chu, K.H. Gastrointestinal immune response to the shrimp allergen tropomyosin: Histological and immunological analysis in an animal model of shrimp tropomyosin hypersensitivity. Int. Arch. Allergy Immunol. 2015, 167, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S.C.; Lee, Y.S.; Tang, C.Y.; Kung, W.Y.; Chuang, Y.H.; Chiang, B.L.; Fung, M.C.; Chu, K.H. Induction of shrimp tropomyosin-specific hypersensitivity in mice. Int. Arch. Allergy Immunol. 2008, 147, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, D.M.; Greenhawt, M.; Sussman, G.; Begin, P.; Nowak-Wegrzyn, A.; Petroni, D.; Beyer, K.; Brown-Whitehorn, T.; Hebert, J.; Hourihane, J.O.; et al. Effect of epicutaneous immunotherapy vs. placebo on reaction to peanut protein ingestion among children with peanut allergy the PEPITES randomized clinical trial. Jama 2019, 321, 946–955. [Google Scholar] [CrossRef] [PubMed]
- Sampson, H.A.; Shreffler, W.G.; Yang, W.H.; Sussman, G.L.; Brown-Whitehorn, T.F.; Nadeau, K.C.; Cheema, A.S.; Leonard, S.A.; Pongracic, J.A.; Sauvage-Delebarre, C.; et al. Effect of varying doses of epicutaneous immunotherapy vs. placebo on reaction to peanut protein exposure among patients with peanut sensitivity a randomized clinical trial. Jama 2017, 318, 1798–1809. [Google Scholar] [CrossRef] [PubMed]
- Narisety, S.D.; Frischmeyer-Guerrerio, P.A.; Keet, C.A.; Gorelik, M.; Schroeder, J.; Hamilton, R.G.; Wood, R.A. A randomized, double-blind, placebo-controlled pilot study of sublingual versus oral immunotherapy for the treatment of peanut allergy. J. Allergy Clin. Immunol. 2015, 135, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Gorelik, M.; Narisety, S.D.; Guerrerio, A.L.; Chichester, K.L.; Keet, C.A.; Bieneman, A.P.; Hamilton, R.G.; Wood, R.A.; Schroeder, J.T.; Frischmeyer-Guerrerio, P.A. Suppression of the immunologic response to peanut during immunotherapy is often transient. J Allergy Clin Immunol 2015, 135, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Begin, P.; Dominguez, T.; Wilson, S.P.; Bacal, L.; Mehrotra, A.; Kausch, B.; Trela, A.; Tavassoli, M.; Hoyte, E.; O’Riordan, G.; et al. Phase 1 results of safety and tolerability in a rush oral immunotherapy protocol to multiple foods using Omalizumab. Allergy Asthma Clin. Immunol. 2014, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- MacGinnitie, A.J.; Rachid, R.; Gragg, H.; Little, S.V.; Lakin, P.; Cianferoni, A.; Heimall, J.; Makhija, M.; Robison, R.; Chinthrajah, R.S.; et al. Omalizumab facilitates rapid oral desensitization for peanut allergy. J. Allergy Clin. Immunol. 2017, 139, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Yee, C.S.K.; Albuhairi, S.; Noh, E.; El-Khoury, K.; Rezaei, S.; Abdel-Gadir, A.; Umetsu, D.T.; Burke-Roberts, E.; LeBovidge, J.; Schneider, L.; et al. Long-term outcome of peanut oral immunotherapy facilitated initially by omalizumab. J. Allergy Clin. Immunol. Pract. 2019, 7, 451–461. [Google Scholar] [CrossRef]
- Palomares, F.; Gomez, F.; Bogas, G.; Campo, P.; Perkins, J.R.; Diaz-Perales, A.; Rodriguez, M.J.; Prieto, A.; Barber, D.; Torres, M.J.; et al. Immunological changes induced in peach allergy patients with systemic reactions by Pru p 3 sublingual immunotherapy. Mol. Nutr. Food Res. 2018, 62, 1700669. [Google Scholar] [CrossRef] [PubMed]
- Tordesillas, L.; Mondoulet, L.; Blazquez, A.B.; Benhamou, P.H.; Sampson, H.A.; Berin, M.C. Epicutaneous immunotherapy induces gastrointestinal LAP(+) regulatory T cells and prevents food-induced anaphylaxis. J. Allergy Clin. Immunol. 2017, 139, 189–201. [Google Scholar] [CrossRef] [PubMed]
- Scheiblhofer, S.; Thalhamer, J.; Weiss, R. DNA and mRNA vaccination against allergies. Pediatric Allergy Immunol. 2018, 29, 679–688. [Google Scholar] [CrossRef] [PubMed]
- Globinska, A.; Boonpiyathad, T.; Satitsuksanoa, P.; Kleuskens, M.; van de Veen, W.; Sokolowska, M.; Akdis, M. Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens. Ann. Allergy Asthma Immunol. 2018, 121, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Martin-Orozco, E.; Norte-Munoz, M.; Martinez-Garcia, J. Regulatory T cells in allergy and asthma. Front. Pediatrics 2017, 5, 117. [Google Scholar] [CrossRef] [PubMed]
- Boonpiyathad, T.; Sokolowska, M.; Morita, H.; Ruckert, B.; Kast, J.I.; Wawrzyniak, M.; Sangasapaviliya, A.; Pradubpongsa, P.; Fuengthong, R.; Thantiworasit, P.; et al. Der p 1-specific regulatory T-cell response during house dust mite allergen immunotherapy. Allergy 2019, 74, 976–985. [Google Scholar] [CrossRef] [PubMed]
- Qamar, N.; Fishbein, A.B.; Erickson, K.A.; Cai, M.; Szychlinski, C.; Bryce, P.J.; Schleimer, R.P.; Fuleihan, R.L.; Singh, A.M. Naturally occurring tolerance acquisition to foods in previously allergic children is characterized by antigen specificity and associated with increased subsets of regulatory T cells. Clin. Exp. Allergy 2015, 45, 1663–1672. [Google Scholar] [CrossRef] [PubMed]
- Renand, A.; Shamji, M.H.; Harris, K.M.; Qin, T.; Wambre, E.; Scadding, G.W.; Wurtzen, P.A.; Till, S.J.; Togias, A.; Nepom, G.T.; et al. Synchronous immune alterations mirror clinical response during allergen immunotherapy. J. Allergy Clin. Immunol. 2018, 141, 1750–1760. [Google Scholar] [CrossRef]
- Pei, Y.C.; Geng, S.; Liu, L.; Yan, F.X.; Guan, H.; Hou, J.; Chen, Y.F.; Wang, B.; An, X.R. Fel d 1-airway inflammation prevention and treatment by co-immunization vaccine via induction of CD4+CD25-Foxp3+ Treg cells. Hum. Vaccines Immunother. 2013, 9, 1019–1031. [Google Scholar] [CrossRef][Green Version]
- Van Wijk, F.; Wehrens, E.J.M.; Nierkens, S.; Boon, L.; Kasran, A.; Pieters, R.; Knippels, L.M.J. CD4+CD25+ T cells regulate the intensity of hypersensitivity responses to peanut, but are not decisive in the induction of oral sensitization. Clin. Exp. Allergy 2007, 37, 572–581. [Google Scholar] [CrossRef]
- Kanjarawi, R.; Dercamp, C.; Etchart, N.; Adel-Patient, K.; Nicolas, J.F.; Dubois, B.; Kaiserlian, D. Regulatory T cells control type I food allergy to beta-lactoglobulin in mice. Int. Arch. Allergy Immunol. 2011, 156, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Dioszeghy, V.; Mondoulet, L.; Puteaux, E.; Dhelft, V.; Ligouis, M.; Plaquet, C.; Dupont, C.; Benhamou, P.H. Differences in phenotype, homing properties and suppressive activities of regulatory T cells induced by epicutaneous, oral or sublingual immunotherapy in mice sensitized to peanut. Cell. Mol. Immunol. 2017, 14, 770–782. [Google Scholar] [CrossRef] [PubMed]
- Tozuka, M.; Oka, T.; Jounai, N.; Egawa, G.; Ishii, K.J.; Kabashima, K.; Takeshita, F. Efficient antigen delivery to the draining lymph nodes is a key component in the immunogenic pathway of the intradermal vaccine. J. Dermatol. Sci. 2016, 82, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Hadis, U.; Wahl, B.; Schulz, O.; Hardtke-Wolenski, M.; Schippers, A.; Wagner, N.; Muller, W.; Sparwasser, T.; Forster, R.; Pabst, O. Intestinal tolerance requires gut homing and expansion of FoxP3+ regulatory T cells in the lamina propria. Immunity 2011, 34, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Nitschke, M.; Halin, C. Dendritic cell interactions with lymphatic endothelium. Lymphat. Res. Biol. 2013, 11, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Guttman-Yassky, E.; Zhou, L.; Krueger, J.G. The skin as an immune organ: Tolerance versus effector responses and applications to food allergy and hypersensitivity reactions. J. Allergy Clin. Immunol. 2019. [Google Scholar] [CrossRef]
- Jilek, S.; Barbey, C.; Spertini, F.; Corthesy, B. Antigen-independent suppression of the allergic immune response to bee venom phospholipase A2 by DNA vaccination in CBA/J mice. J. Immunol. 2001, 166, 3612–3621. [Google Scholar] [CrossRef]
- Giuffrida, M.G.; Villalta, D.; Mistrello, G.; Amato, S.; Asero, R. Shrimp allergy beyond tropomyosin in Italy: Clinical relevance of arginine kinase, sarcoplasmic calcium binding protein and hemocyanin. Eur. Ann. Allergy Clin. Immunol. 2014, 46, 172–177. [Google Scholar]
- DeWitt, A.M.; Mattsson, L.; Lauer, I.; Reese, G.; Lidholm, J. Recombinant tropomyosin from Penaeus aztecus (rPen a 1) for measurement of specific immunoglobulin E antibodies relevant in food allergy to crustaceans and other invertebrates. Mol. Nutr. Food Res. 2004, 48, 370–379. [Google Scholar] [CrossRef]
- Gamez, C.; Sanchez-Garcia, S.; Ibanez, M.D.; Lopez, R.; Aguado, E.; Lopez, E.; Sastre, B.; Sastre, J.; del Pozo, V. Tropomyosin IgE-positive results are a good predictor of shrimp allergy. Allergy 2011, 66, 1375–1383. [Google Scholar] [CrossRef]
- Pascal, M.; Grishina, G.; Yang, A.C.; Sanchez-Garcia, S.; Lin, J.; Towle, D.; Ibanez, M.D.; Sastre, J.; Sampson, H.A.; Ayuso, R. Molecular diagnosis of shrimp allergy: Efficiency of several allergens to predict clinical reactivity. J. Allergy Clin. Immunol. Pract. 2015, 3, 521–529. [Google Scholar] [CrossRef] [PubMed]
- Leung, P.S.C.; Chow, W.K.; Duffey, S.; Kwan, H.S.; Gershwin, M.E.; Chu, K.H. IgE reactivity against a cross-reactive allergen in crustacea and mollusca: Evidence for tropomyosin as the common allergen. J. Allergy Clin. Immunol. 1996, 98, 954–961. [Google Scholar] [CrossRef]
- Wai, C.Y.Y.; Leung, N.Y.H.; Leung, P.S.C.; Chu, K.H. T cell epitope immunotherapy ameliorates allergic responses in a murine model of shrimp allergy. Clin. Exp. Allergy 2016, 46, 491–503. [Google Scholar] [CrossRef] [PubMed]
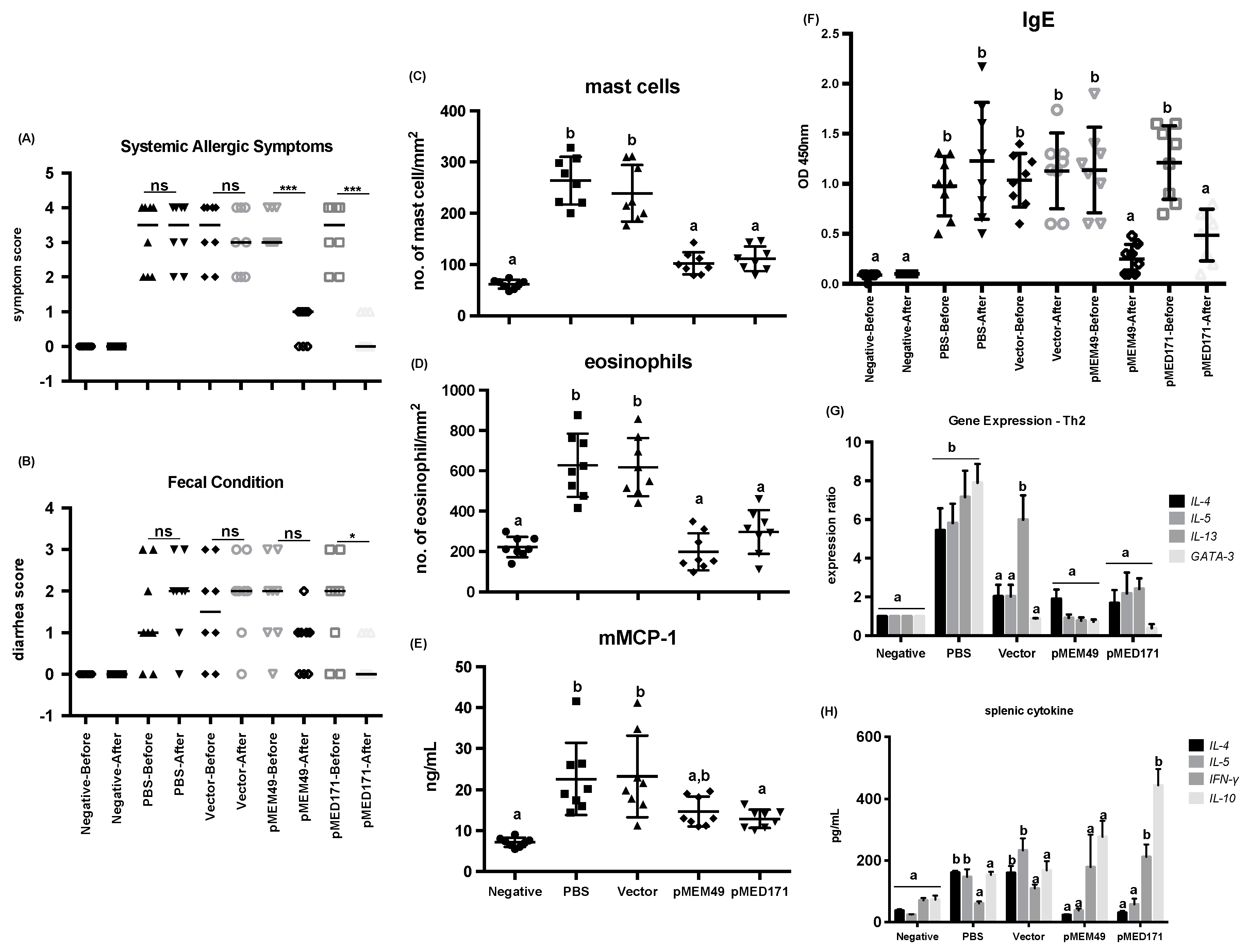
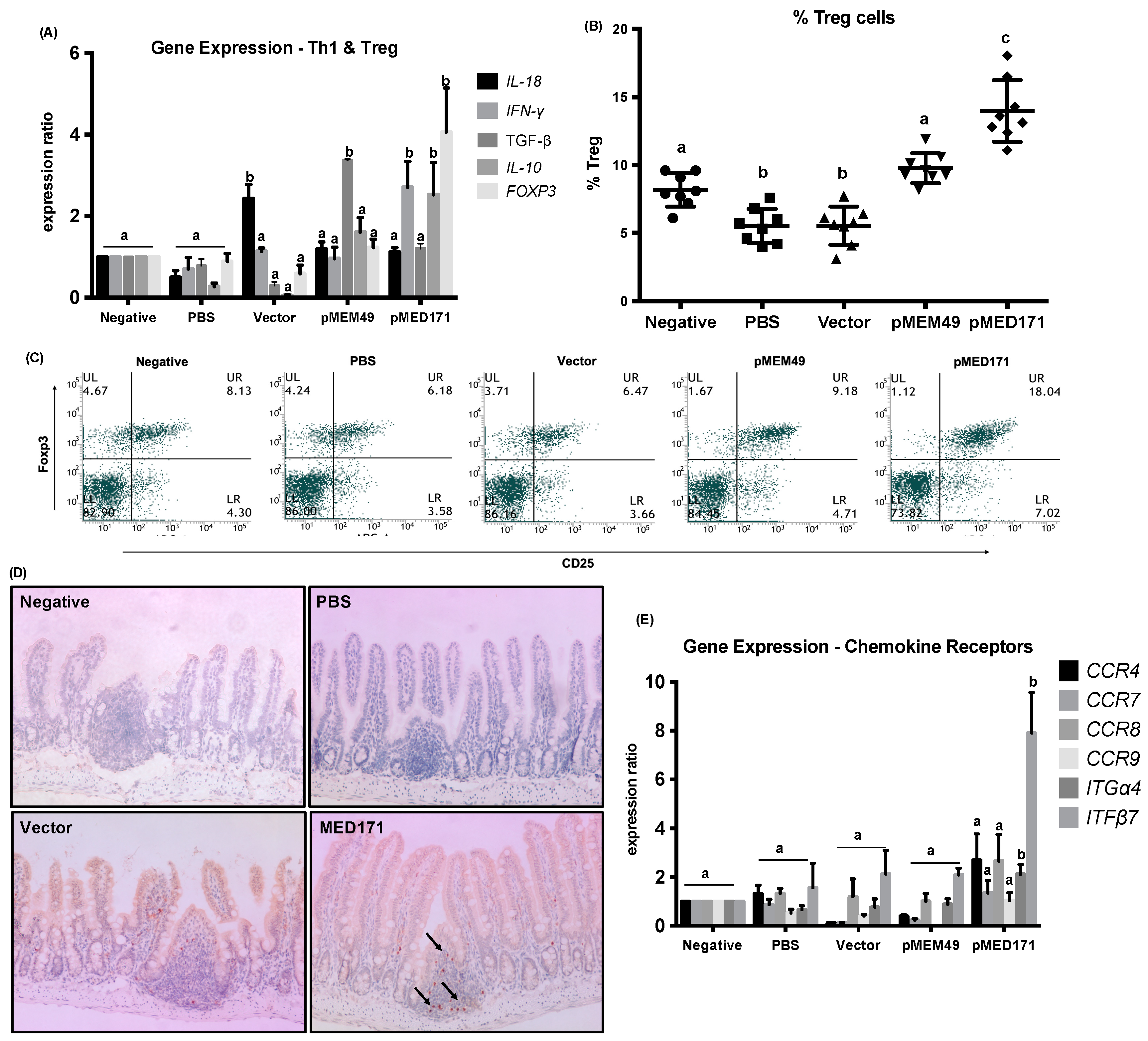
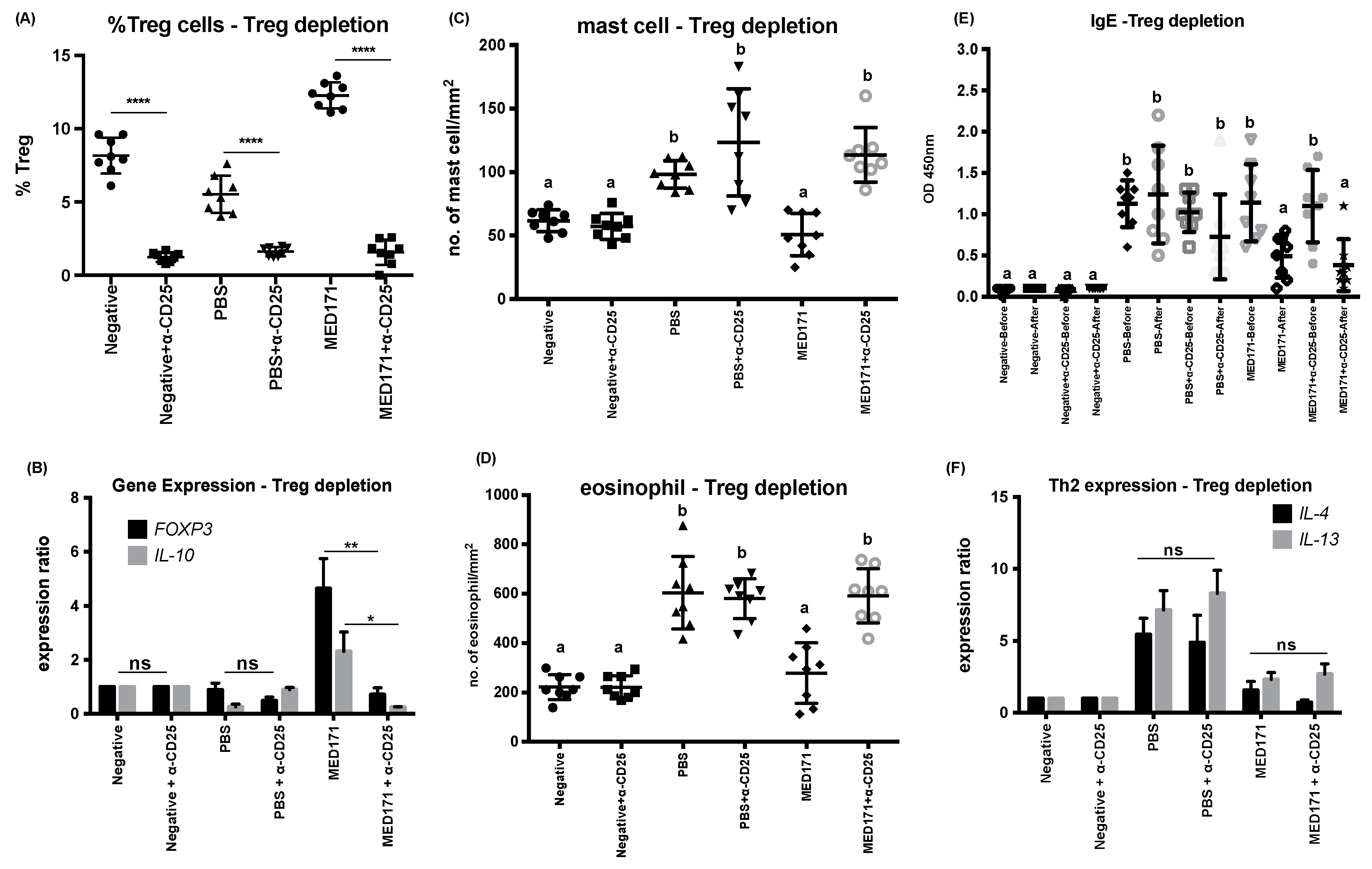
| Group | Treatment | Symptom Score | Diarrhea Score | Mast Cell Count (cells/mm2) | Eosinophil Count (cells/mm2) | mMCP-1 (ng/mL) | rMet e 1-Specific IgE (OD 450 nm) | Splenic IL-4 (pg/mL) | Splenic IL-5 (pg/mL) |
|---|---|---|---|---|---|---|---|---|---|
| Negative | Before | 0.0 | 0.0 | 62 ± 9 a | 221 ± 51 a | 7.1 ± 1.1 a | 0.09 ± 0.04 a | 38 ± 4 a | 25 ± 1 a |
| After | 0.0 | 0.0 | 0.10 ± 0.00 a | ||||||
| PBS | Before | 3.5 | 1.0 | 294 ± 46 b | 627 ± 157 b | 22.6 ± 8.8 b | 0.98 ± 0.30 b | 161 ± 6 b | 147 ± 25 b |
| After | 3.5 | 2.0 | 1.23 ± 0.58 b | ||||||
| Vector | Before | 3.5 | 1.5 | 239 ± 55 b | 618 ± 145 b | 23.2 ± 9.9 b | 1.04 ± 0.27 b | 160 ± 22 b | 233 ± 39 b |
| After | 3.0 | 2.0 | 1.13 ± 0.38 b | ||||||
| pMEM49 | Before | 3.0 | 2.0 | 102 ± 22 a | 198 ± 92 a | 14.6 ± 3.6 a,b | 1.14 ± 0.43 b | 24 ± 2 a | 38 ± 7 a |
| After | 1.0 *** | 1.0 | 0.25 ± 0.15 a | ||||||
| pMED171 | Before | 3.5 | 2.0 | 111 ± 24 a | 296 ± 109 a | 12.9 ± 2.2 a | 1.21 ± 0.37 b | 31 ± 5 a | 58 ± 18 a |
| After | 0.0 *** | 1.0 * | 0.49 ± 0.26 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wai, C.Y.Y.; Leung, N.Y.H.; Leung, P.S.C.; Chu, K.H. Modulating Shrimp Tropomyosin-Mediated Allergy: Hypoallergen DNA Vaccines Induce Regulatory T Cells to Reduce Hypersensitivity in Mouse Model. Int. J. Mol. Sci. 2019, 20, 4656. https://doi.org/10.3390/ijms20184656
Wai CYY, Leung NYH, Leung PSC, Chu KH. Modulating Shrimp Tropomyosin-Mediated Allergy: Hypoallergen DNA Vaccines Induce Regulatory T Cells to Reduce Hypersensitivity in Mouse Model. International Journal of Molecular Sciences. 2019; 20(18):4656. https://doi.org/10.3390/ijms20184656
Chicago/Turabian StyleWai, Christine Y.Y., Nicki Y.H. Leung, Patrick S.C. Leung, and Ka Hou Chu. 2019. "Modulating Shrimp Tropomyosin-Mediated Allergy: Hypoallergen DNA Vaccines Induce Regulatory T Cells to Reduce Hypersensitivity in Mouse Model" International Journal of Molecular Sciences 20, no. 18: 4656. https://doi.org/10.3390/ijms20184656
APA StyleWai, C. Y. Y., Leung, N. Y. H., Leung, P. S. C., & Chu, K. H. (2019). Modulating Shrimp Tropomyosin-Mediated Allergy: Hypoallergen DNA Vaccines Induce Regulatory T Cells to Reduce Hypersensitivity in Mouse Model. International Journal of Molecular Sciences, 20(18), 4656. https://doi.org/10.3390/ijms20184656