Numerical Model for Magnetic Fluid Hyperthermia in a Realistic Breast Phantom: Calorimetric Calibration and Treatment Planning
Abstract
1. Introduction
2. Results and Discussion
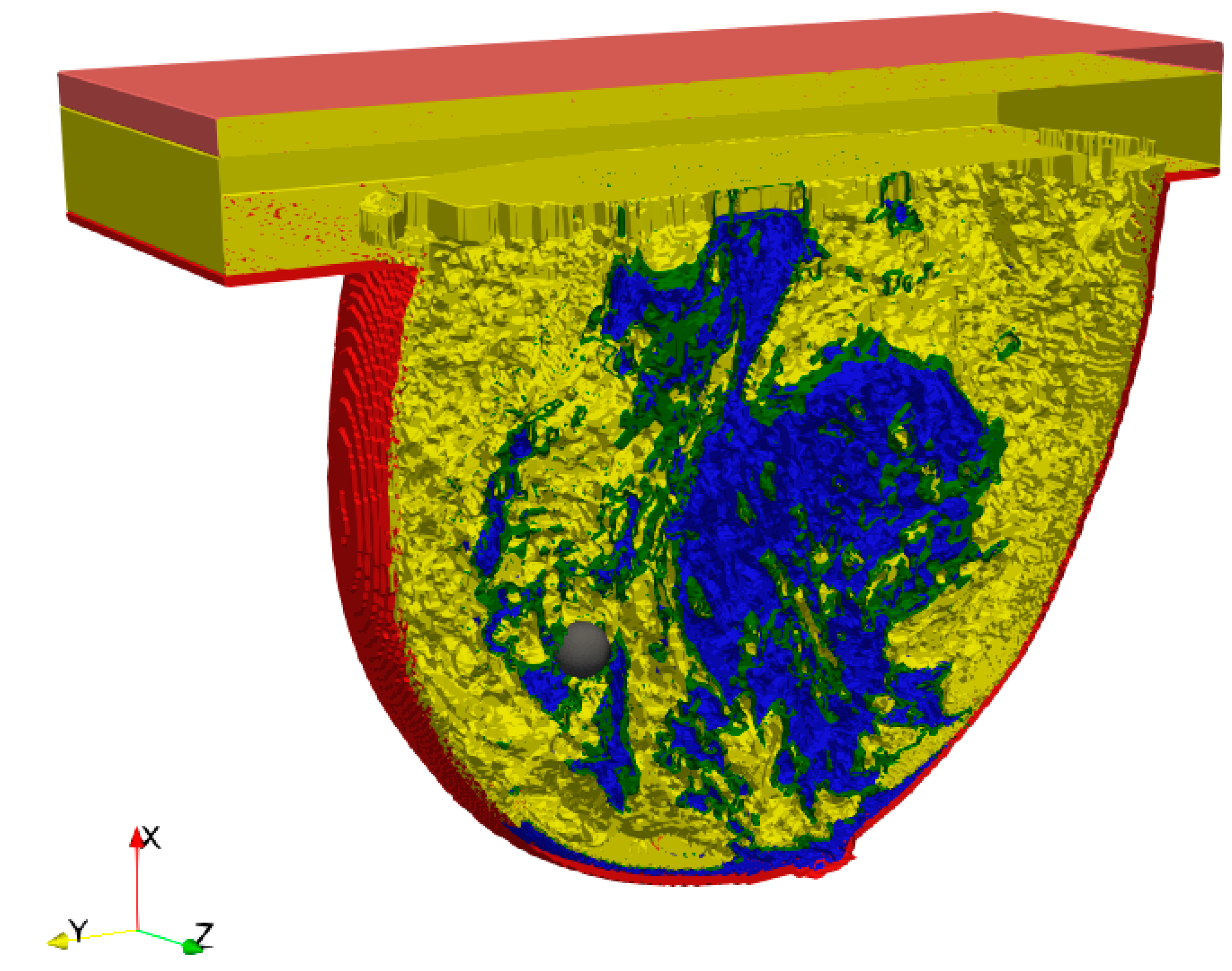
2.1. Breast Phantom Model
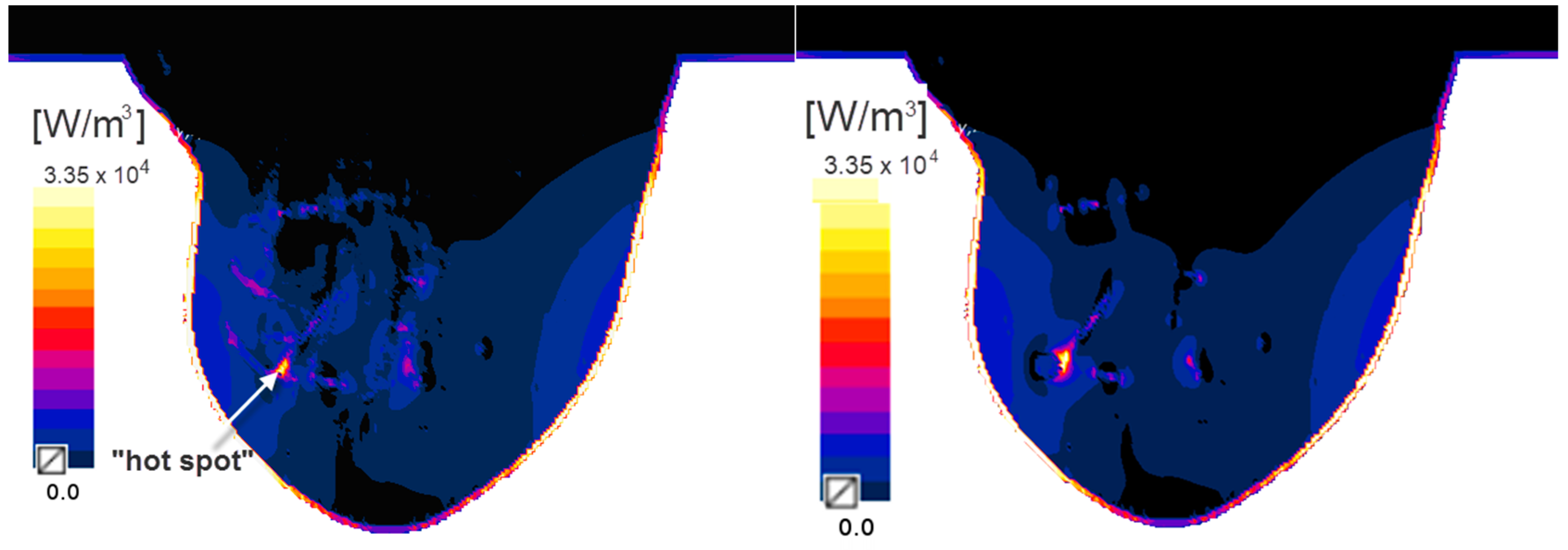
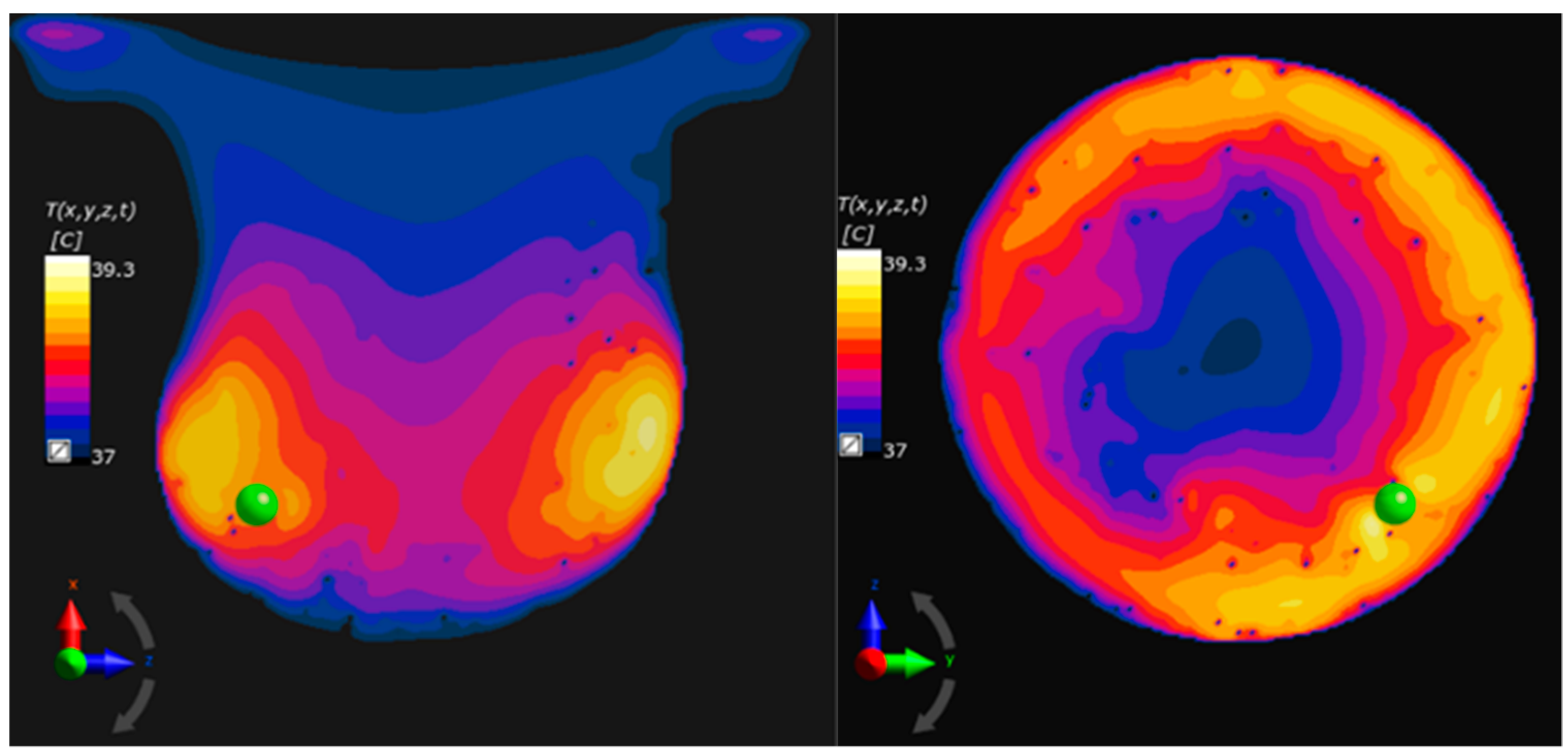
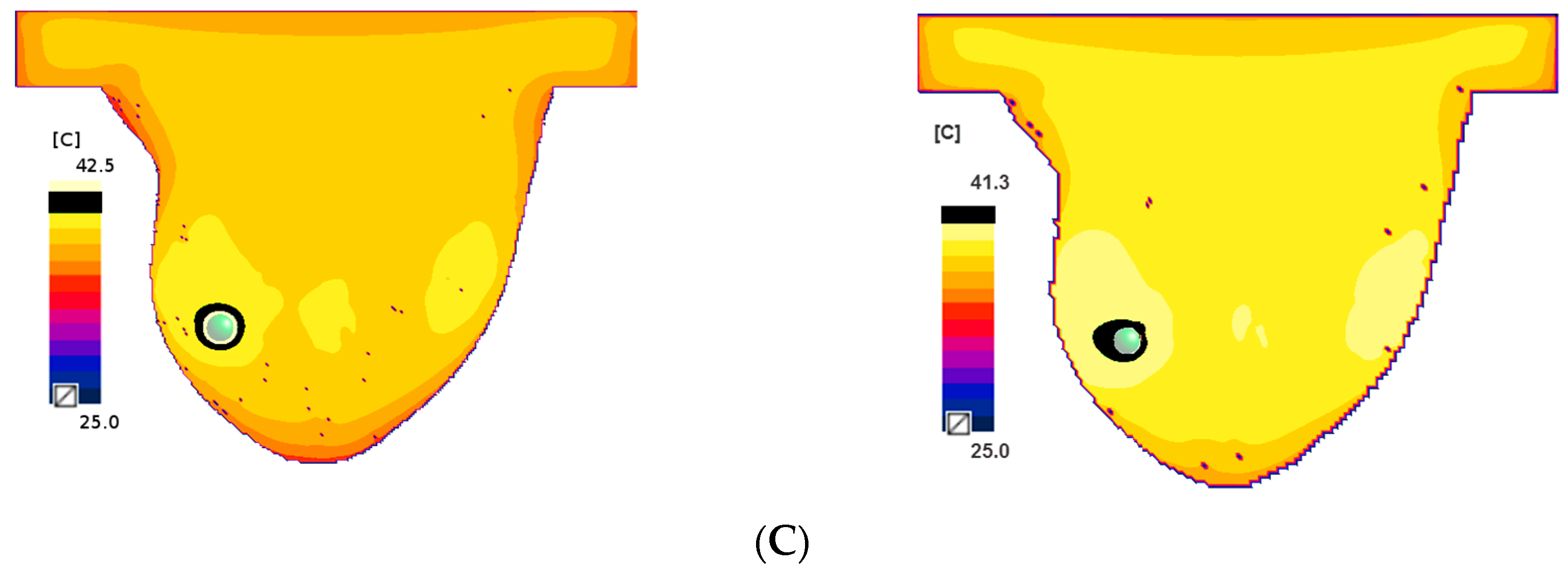
2.2. Eddy Currents Effect on Temperature Distribution
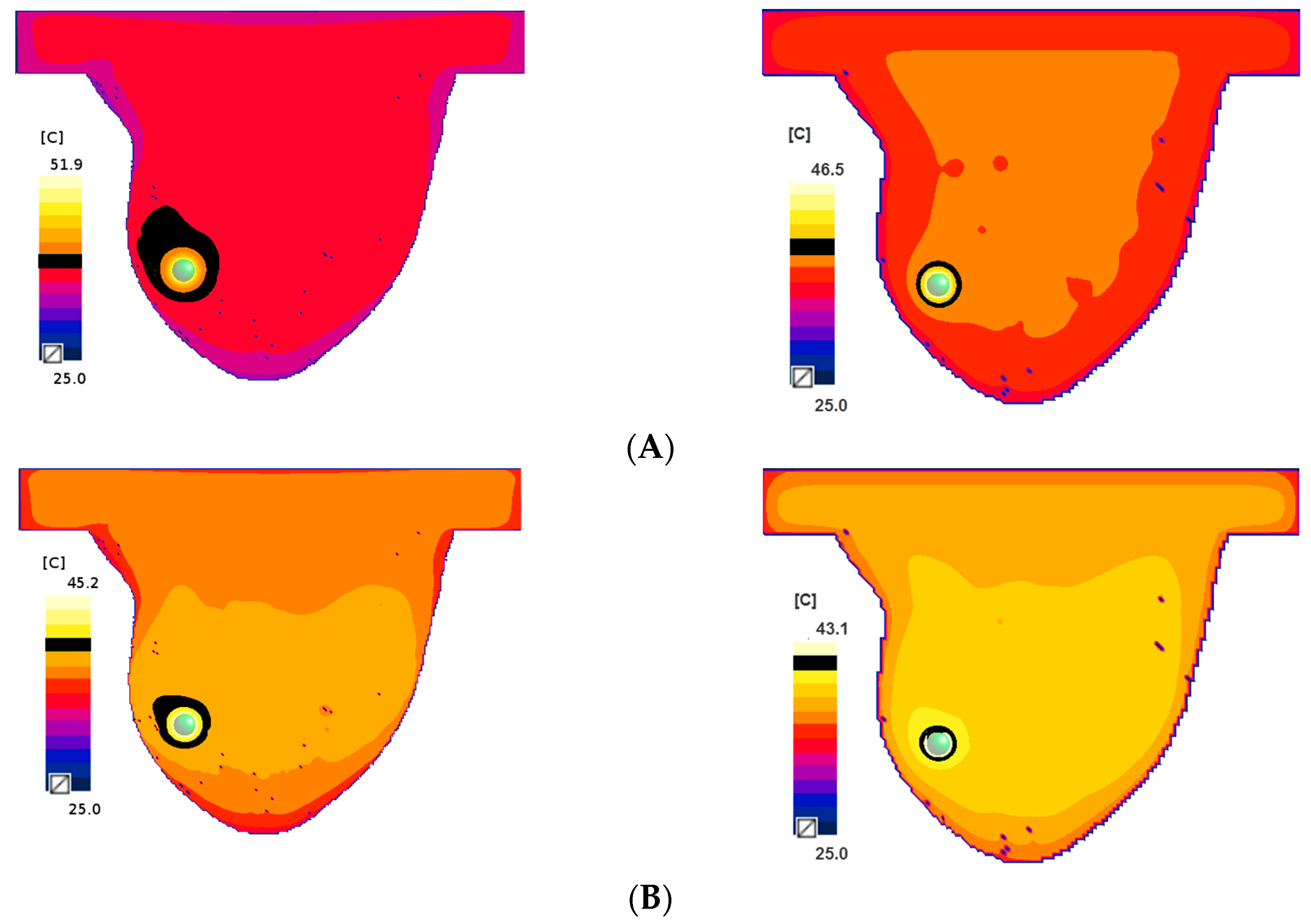
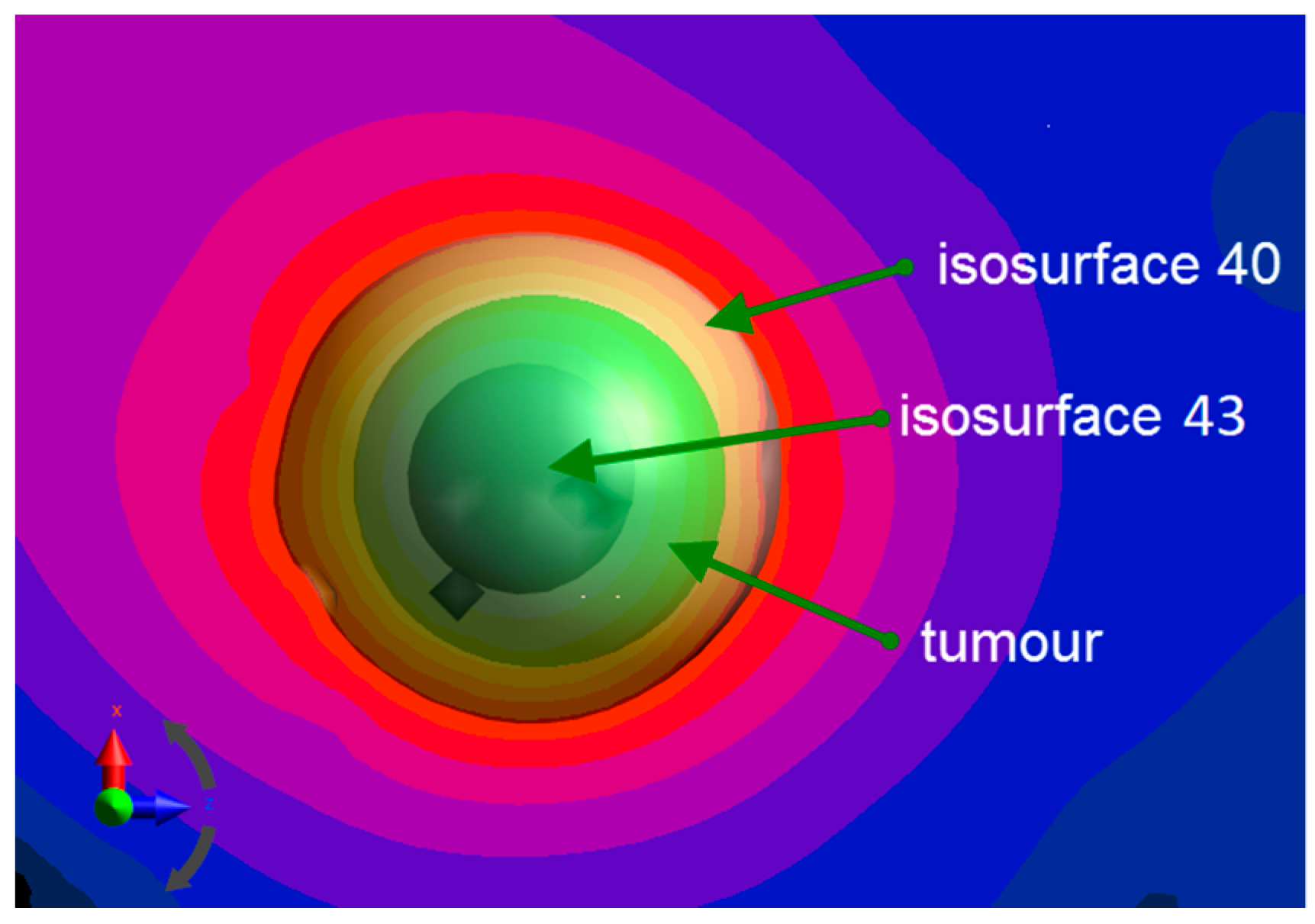
2.3. Single-Domain Magnetic Power Loss Analysis
3. Materials and Methods
3.1. Single-Domain Magnetic Power Losses Model
3.2. Eddy Current Effect Model
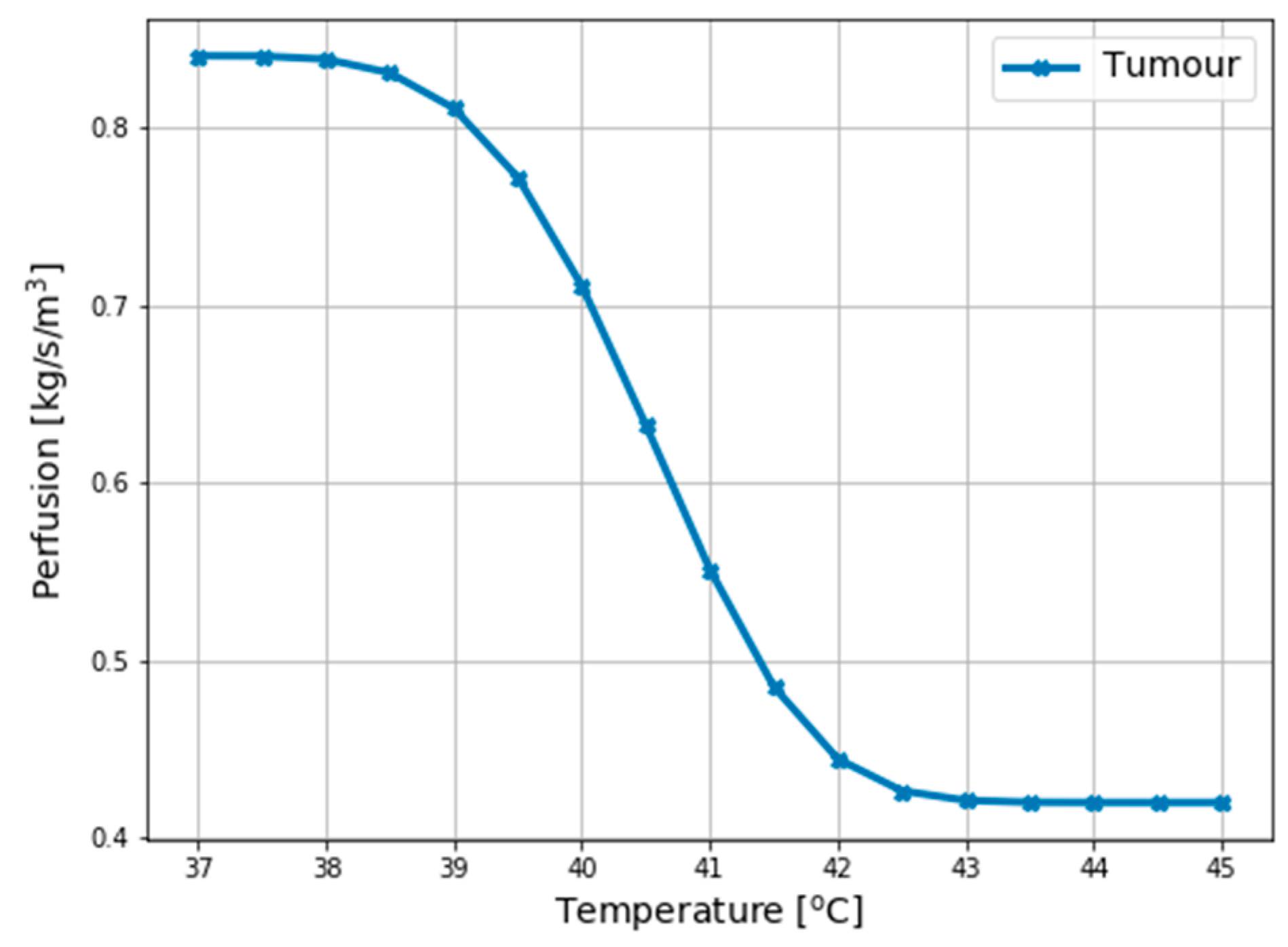
3.3. Pennes Equation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Subramanian, M.; Pearce, G.; Guldu, O.K.; Tekin, V.; Miaskowski, A.; Aras, O.; Unak, P. A pilot study into the use of FDG-mNP as an alternative approach in neuroblastoma cell hyperthermia. IEEE Trans. Nanobiosci. 2016, 15, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, M.; Miaskowski, A.; Pearce, G.; Dobson, J. A coil system for real-time magnetic fluid hyperthermia microscopy studies. Int. J. Hyperth. 2016, 32, 112–120. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Miaskowski, A.; Sawicki, B.; Subramanian, M. Identification of diffusion coefficients in heat equation on the base of non-adiabatic measurements of ferrofluids. In Proceedings of the 17th International Conference in Computational Problems of Electrical Engineering (CPEE), Sandomierz, Poland, 14 September 2016; IEEE: Piscataway, NJ, USA, 2016; pp. 1–4. [Google Scholar]
- Subramanian, M.; Miaskowski, A.; Mahapatro, A.K.; Dobson, J. Practical bioinstrumentation developments for AC magnetic field-mediated magnetic nanoparticle heating applications. Appl. Phys. A 2019, 125, 194. [Google Scholar] [CrossRef]
- Miaskowski, M.; Balakrishnan, P.; Subramanian, M.; Hovorka, O. An in vivo coil setup for AC magnetic field-mediated magnetic nanoparticle heating experiments. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Berlin, Germany, 2019. in press. [Google Scholar]
- Creixell, M.; Bohorquez, A.C.; Torres-Lugo, M.; Rinaldi, C. EGFR-targeted magnetic nanoparticle heaters kill cancer cells without a perceptible temperature rise. ACS Nano 2011, 5, 7124–7129. [Google Scholar] [CrossRef] [PubMed]
- Ivkov, R. Magnetic nanoparticle hyperthermia: A new frontier in biomedicine? Int. J. Hyperth. 2013, 29, 703–705. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Scholz, R.; Wust, P.; Fähling, H.; Felix, R. Magnetic fluid hyperthermia (MFH): Cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J. Magn. Magn. Mater. 1999, 201, 413–419. [Google Scholar] [CrossRef]
- Kozissnik, B.; Bohorquez, A.C.; Dobson, J.; Rinaldi, C. Magnetic fluid hyperthermia: Advances, challenges, and opportunity. Int. J. Hyperth. 2013, 29, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Use of magnetic nanoparticle heating in the treatment of breast cancer. In IEE Proceedings-Nanobiotechnology; IET Digital Library: Stevenage, UK, 2005. [Google Scholar]
- Hilger, I.; Hergt, R.; Kaiser, W.A. Towards breast cancer treatment by magnetic heating. J. Magn. Magn. Mater. 2005, 293, 314–319. [Google Scholar] [CrossRef]
- Wang, G.; Xu, D.; Chai, Q.; Tan, X.; Zhang, Y.; Gu, N.; Tang, J. Magnetic fluid hyperthermia inhibits the growth of breast carcinoma and downregulates vascular endothelial growth factor expression. Oncol. Lett. 2014, 7, 1370–1374. [Google Scholar] [CrossRef] [PubMed]
- Kossatz, S.; Grandke, J.; Couleaud, P.; Latorre, A.; Aires, A.; Crosbie-Staunton, K.; Ludwig, R.; Dähring, H.; Ettelt, V.; Lazaro-Carrillo, A.; et al. Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery. Breast Cancer Res. 2015, 17, 66. [Google Scholar] [CrossRef] [PubMed]
- Petryk, A.A.; Giustini, A.J.; Gottesman, R.E.; Kaufman, P.A.; Hoopes, P.J. Magnetic nanoparticle hyperthermia enhancement of cisplatin chemotherapy cancer treatment. Int. J. Hyperth. 2013, 29, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Kan-Dapaah, K.; Rahbar, N.; Soboyejo, W. Implantable magnetic nanocomposites for the localized treatment of breast cancer. J. Appl. Phys. 2014, 116. [Google Scholar] [CrossRef]
- Reynoso, F.J.; Lee, C.D.; Cheong, S.K.; Cho, S.H. Implementation of a multisource model for gold nanoparticle-mediated plasmonic heating with near-infrared laser by the finite element method. Med. Phys. 2013, 40, 073301. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, A.; Sawicki, B. Magnetic fluid hyperthermia modeling based on phantom measurements and realistic breast model. IEEE Trans. Biomed. Eng. 2013, 60, 1806–1813. [Google Scholar] [CrossRef] [PubMed]
- Burfeindt, M.J.; Colgan, T.J.; Mays, R.O.; Shea, J.D.; Behdad, N.; Van Veen, B.D.; Hagness, S.C. MRI-derived 3-D-printed breast phantom for microwave breast imaging validation. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Miaskowski, A.; Subramanian, M. Specific absorption rate parameter model in magnetic hyperthermia. In Proceedings of the 2017 18th International Conference on Computational Problems of Electrical Engineering (CPEE), Kutna Hora, Czech Republic, 11–13 September 2017; IEEE: Piscataway, NJ, USA, 2017; pp. 1–4. [Google Scholar]
- Miaskowski, A.; Sawicki, B.; Subramanian, M. Single-domain nanoparticle magnetic power losses calibrated with calorimetric measurements. Bulletin of the Polish Academy of Sciences. Tech. Sci. 2018, 66, 509–516. [Google Scholar]
- Hasgall, P.A.; Di Gennaro, F.; Baumgartner, C.; Neufeld, E.; Gosselin, M.C.; Payne, D.; Klingenböck, A.; Kuster, N. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues. Version 2.6, 13 January 2015. Available online: www.itis.ethz.ch/database (accessed on 10 May 2016).
- Miaskowski, A. Magnetic Fluid Hyperthermia Treatment Planning Correlated with Calorimetic Measurements under Non-Adiabatic Conditions; Science Publishing Society Libropolis: Lublin, Poland, 2018; ISBN 978-83-63761-34-9. [Google Scholar]
- Mamiya, H. Recent Advances in Understanding Magnetic Nanoparticles in AC Magnetic Fields and Optimal Design for Targeted Hyperthermia. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Pennes, H.H. Analysis of tissue and arterial blood temperatures in the resting human forearm. J. Appl. Physiol. 1948, 1, 93–122. [Google Scholar] [CrossRef] [PubMed]
- Rossmanna, C.; Haemmerich, D. Review of temperature dependence of thermal properties, dielectric properties, and perfusion of biological tissues at hyperthermic and ablation temperatures. Crit. Rev. Biomed. Eng. 2014, 42, 467–492. [Google Scholar] [CrossRef] [PubMed]
- Sawicki, B.; Miaskowski, A. Nonlinear higher-order transient solver for magnetic fluid hyperthermia. J. Comput. Appl. Math. 2014, 270, 143–151. [Google Scholar] [CrossRef]



| Tissue | c | HGR* | HGR** | |||
|---|---|---|---|---|---|---|
| [kg/m3] | [S/m] | [J/kg/C] | [W/m/C] | [W/kg] | [W/m3/C] | |
| Skin | 1109.0 | 0.17 | 3390.5 | 0.3722 | 1.647 | 7969.16 |
| Muscle (tumour) | 1090.4 | 0.355 | 3421.2 | 0.4949 | 0.9061 | 2705.95 |
| Breast gland | 1040.5 | 0.5412 | 2960.0 | 0.3345 | 2.323 | 10542.6 |
| Fat | 911.0 | 0.057 | 2348.3 | 0.21145 | 0.5 | 2012.82 |
| Breast fat | 911.0 | 0.0222 | 2348.33 | 0.209 | 0.7278 | 2892.22 |
| Hmax | K | kB | T | η | δ | Log-normal |
|---|---|---|---|---|---|---|
| kA/m | kJ/m3 | J/K | K | kg/m/s | nm | g(µ, s, VM) |
| 92.0 | 30.0 | 1.38−23 | 298.0 | 8.94−4 | 2.0 | g(15.2, 0.19, VM) |
| S-D Magnetic Power (Mobilised) | S-D Magnetic Power (Immobilised) | |
|---|---|---|
| kW/m3 | kW/m3 | |
| 1.0 | 243.0 | 170.0 |
| 0.71 | 174.0 | 121.0 |
| 0.5 | 121.0 | 84.2 |
| 0.35 | 84.2 | 59.3 |
| 0.3 | 72.8 | 51.2 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miaskowski, A.; Subramanian, M. Numerical Model for Magnetic Fluid Hyperthermia in a Realistic Breast Phantom: Calorimetric Calibration and Treatment Planning. Int. J. Mol. Sci. 2019, 20, 4644. https://doi.org/10.3390/ijms20184644
Miaskowski A, Subramanian M. Numerical Model for Magnetic Fluid Hyperthermia in a Realistic Breast Phantom: Calorimetric Calibration and Treatment Planning. International Journal of Molecular Sciences. 2019; 20(18):4644. https://doi.org/10.3390/ijms20184644
Chicago/Turabian StyleMiaskowski, Arkadiusz, and Mahendran Subramanian. 2019. "Numerical Model for Magnetic Fluid Hyperthermia in a Realistic Breast Phantom: Calorimetric Calibration and Treatment Planning" International Journal of Molecular Sciences 20, no. 18: 4644. https://doi.org/10.3390/ijms20184644
APA StyleMiaskowski, A., & Subramanian, M. (2019). Numerical Model for Magnetic Fluid Hyperthermia in a Realistic Breast Phantom: Calorimetric Calibration and Treatment Planning. International Journal of Molecular Sciences, 20(18), 4644. https://doi.org/10.3390/ijms20184644





