The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases
Abstract
1. Introduction
2. Disorders of Altered Bone Resorption
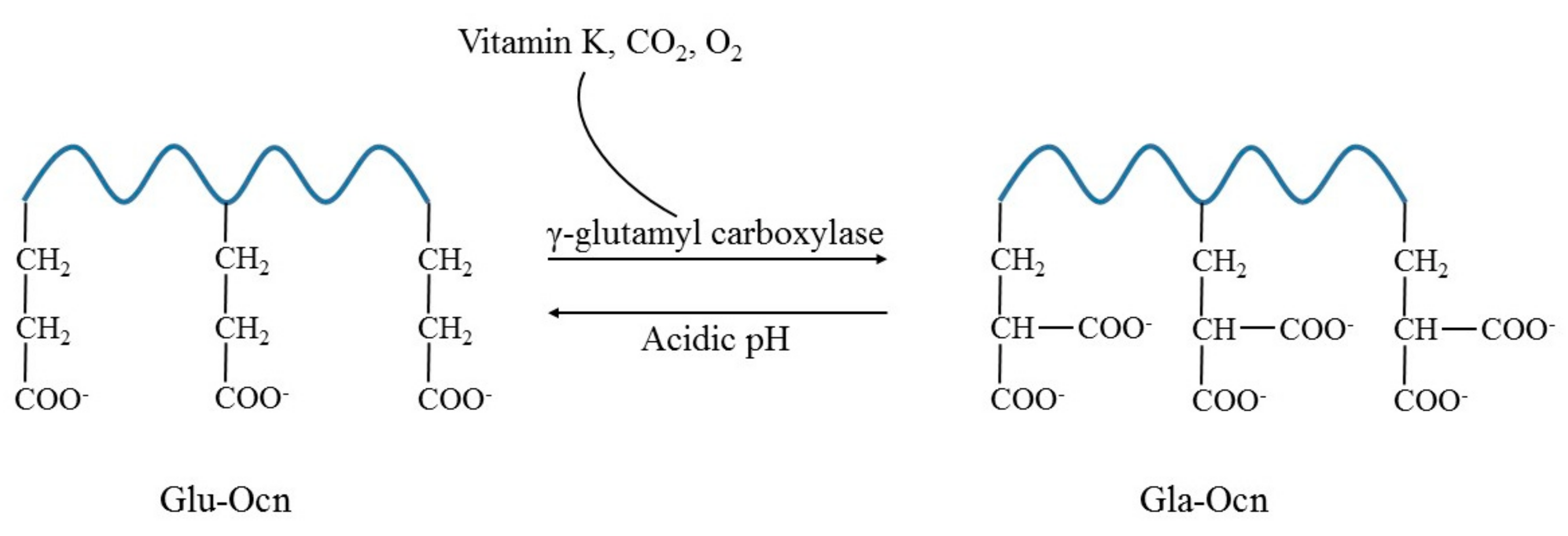
3. Osteocalcin
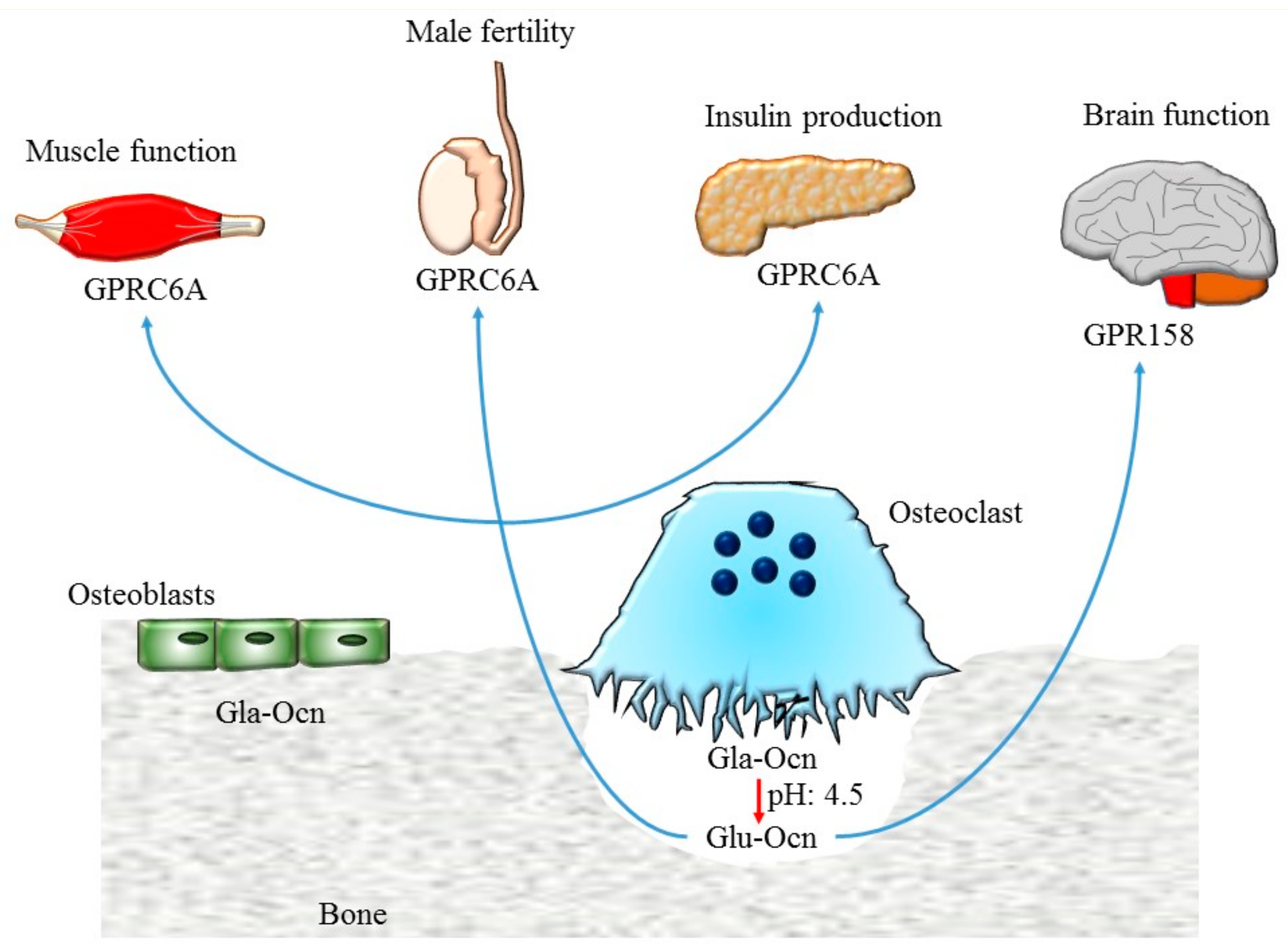
4. Glucose Metabolism
5. Male Fertility
6. Brain Functions
7. Conclusions
Funding
Conflicts of Interest
References
- Lee, N.K.; Sowa, H.; Hinoi, E.; Ferron, M.; Ahn, J.D.; Confavreux, C.; Dacquin, R.; Mee, P.J.; McKee, M.D.; Jung, D.Y.; et al. Endocrine regulation of energy metabolism by the skeleton. Cell 2007, 130, 456–469. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Hinoi, E.; Karsenty, G.; Ducy, P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc. Natl. Acad. Sci. USA 2008, 105, 5266–5270. [Google Scholar] [CrossRef] [PubMed]
- Ferron, M.; Wei, J.; Yoshizawa, T.; Del Fattore, A.; DePinho, R.A.; Teti, A.; Ducy, P.; Karsenty, G. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Sumara, G.; Sumara, O.; Ferron, M.; Chang, H.; Smith, C.E.; Hermo, L.; Suarez, S.; Roth, B.L.; Ducy, P.; et al. Endocrine regulation of male fertility by the skeleton. Cell 2011, 144, 796–809. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Ferron, M.; Confavreux, C.; Wei, J.; Galan-Diez, M.; Lacampagne, A.; Mitchell, S.J.; Mattison, J.A.; Chen, Y.; et al. Osteocalcin Signaling in Myofibers Is Necessary and Sufficient for Optimum Adaptation to Exercise. Cell Metab. 2016, 23, 1078–1092. [Google Scholar] [CrossRef] [PubMed]
- Mera, P.; Laue, K.; Wei, J.; Berger, J.M.; Karsenty, G. Osteocalcin is necessary and sufficient to maintain muscle mass in older mice. Mol. Metab. 2016, 5, 1042–1047. [Google Scholar] [CrossRef]
- Oury, F.; Khrimian, L.; Denny, C.A.; Gardin, A.; Chamouni, A.; Goeden, N.; Huang, Y.Y.; Lee, H.; Srinivas, P.; Gao, X.B.; et al. Maternal and offspring pools of osteocalcin influence brain development and functions. Cell 2013, 155, 228–241. [Google Scholar] [CrossRef]
- Vaananen, H.K.; Laitala-Leinonen, T. Osteoclast lineage and function. Arch. Biochem. Biophys. 2008, 473, 132–138. [Google Scholar] [CrossRef]
- Hadjidakis, D.J.; Androulakis, II. Bone remodeling. Ann. N. Y. Acad. Sci. 2006, 1092, 385–396. [Google Scholar] [CrossRef]
- Hill, P.A. Bone remodelling. Br. J. Orthod. 1998, 25, 101–107. [Google Scholar] [CrossRef]
- Matsuo, K.; Irie, N. Osteoclast-osteoblast communication. Arch. Biochem. Biophys. 2008, 473, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Rousselle, A.V.; Heymann, D. Osteoclastic acidification pathways during bone resorption. Bone 2002, 30, 533–540. [Google Scholar] [CrossRef]
- Graves, A.R.; Curran, P.K.; Smith, C.L.; Mindell, J.A. The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 2008, 453, 788–792. [Google Scholar] [CrossRef]
- Kornak, U.; Kasper, D.; Bosl, M.R.; Kaiser, E.; Schweizer, M.; Schulz, A.; Friedrich, W.; Delling, G.; Jentsch, T.J. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 2001, 104, 205–215. [Google Scholar] [CrossRef]
- Everts, V.; Korper, W.; Hoeben, K.A.; Jansen, I.D.; Bromme, D.; Cleutjens, K.B.; Heeneman, S.; Peters, C.; Reinheckel, T.; Saftig, P.; et al. Osteoclastic bone degradation and the role of different cysteine proteinases and matrix metalloproteinases: Differences between calvaria and long bone. J. Bone Miner. Res. 2006, 21, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Teti, A.; Rucci, N. Bone cells and the mechanisms of bone remodelling. Front. Biosci. (Elite Ed.) 2012, 4, 2302–2321. [Google Scholar] [CrossRef]
- Consensus development conference: Diagnosis, prophylaxis, and treatment of osteoporosis. Am. J. Med. 1993, 94, 646–650. [CrossRef]
- Colangelo, L.; Biamonte, F.; Pepe, J.; Cipriani, C.; Minisola, S. Understanding and managing secondary osteoporosis. Expert Rev. Endocrinol. Metab. 2019, 14, 111–122. [Google Scholar] [CrossRef]
- Garnero, P.; Sornay-Rendu, E.; Chapuy, M.C.; Delmas, P.D. Increased bone turnover in late postmenopausal women is a major determinant of osteoporosis. J. Bone Miner. Res. 1996, 11, 337–349. [Google Scholar] [CrossRef]
- Compston, J.E.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Imaz, I.; Zegarra, P.; Gonzalez-Enriquez, J.; Rubio, B.; Alcazar, R.; Amate, J.M. Poor bisphosphonate adherence for treatment of osteoporosis increases fracture risk: Systematic review and meta-analysis. Osteoporos. Int. 2010, 21, 1943–1951. [Google Scholar] [CrossRef] [PubMed]
- Siris, E.S.; Selby, P.L.; Saag, K.G.; Borgstrom, F.; Herings, R.M.; Silverman, S.L. Impact of osteoporosis treatment adherence on fracture rates in North America and Europe. Am. J. Med. 2009, 122, S3–S13. [Google Scholar] [CrossRef] [PubMed]
- Albers-Schonberg. Rontgernbilder einer seltenen Knockenerkrankung. Munc. Med. Wochenschr. 1904, 5, 365–368. [Google Scholar]
- Del Fattore, A.; Cappariello, A.; Teti, A. Genetics, pathogenesis and complications of osteopetrosis. Bone 2008, 42, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Del Fattore, A.; Peruzzi, B.; Rucci, N.; Recchia, I.; Cappariello, A.; Longo, M.; Fortunati, D.; Ballanti, P.; Iacobini, M.; Luciani, M.; et al. Clinical, genetic, and cellular analysis of 49 osteopetrotic patients: Implications for diagnosis and treatment. J. Med. Genet. 2006, 43, 315–325. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pangrazio, A.; Cassani, B.; Guerrini, M.M.; Crockett, J.C.; Marrella, V.; Zammataro, L.; Strina, D.; Schulz, A.; Schlack, C.; Kornak, U.; et al. RANK-dependent autosomal recessive osteopetrosis: Characterization of five new cases with novel mutations. J. Bone Miner. Res. 2012, 27, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Sobacchi, C.; Frattini, A.; Guerrini, M.M.; Abinun, M.; Pangrazio, A.; Susani, L.; Bredius, R.; Mancini, G.; Cant, A.; Bishop, N.; et al. Osteoclast-poor human osteopetrosis due to mutations in the gene encoding RANKL. Nat. Genet. 2007, 39, 960–962. [Google Scholar] [CrossRef]
- Villa, A.; Guerrini, M.M.; Cassani, B.; Pangrazio, A.; Sobacchi, C. Infantile malignant, autosomal recessive osteopetrosis: The rich and the poor. Calcif. Tissue Int. 2009, 84, 1–12. [Google Scholar] [CrossRef]
- Benichou, O.D.; Laredo, J.D.; de Vernejoul, M.C. Type II autosomal dominant osteopetrosis (Albers-Schonberg disease): Clinical and radiological manifestations in 42 patients. Bone 2000, 26, 87–93. [Google Scholar] [CrossRef]
- Cleiren, E.; Benichou, O.; Van Hul, E.; Gram, J.; Bollerslev, J.; Singer, F.R.; Beaverson, K.; Aledo, A.; Whyte, M.P.; Yoneyama, T.; et al. Albers-Schonberg disease (autosomal dominant osteopetrosis, type II) results from mutations in the ClCN7 chloride channel gene. Hum. Mol. Genet. 2001, 10, 2861–2867. [Google Scholar] [CrossRef]
- De Ridder, R.; Boudin, E.; Mortier, G.; Van Hul, W. Human Genetics of Sclerosing Bone Disorders. Curr. Osteoporos. Rep. 2018, 16, 256–268. [Google Scholar] [CrossRef] [PubMed]
- Markatos, K.; Mavrogenis, A.F.; Karamanou, M.; Androutsos, G. Pycnodysostosis: The disease of Henri de Toulouse-Lautrec. Eur. J. Orthop. Surg. Traumatol. 2018, 28, 1569–1572. [Google Scholar] [CrossRef] [PubMed]
- Motyckova, G.; Fisher, D.E. Pycnodysostosis: Role and regulation of cathepsin K in osteoclast function and human disease. Curr. Mol. Med. 2002, 2, 407–421. [Google Scholar] [CrossRef] [PubMed]
- Hauschka, P.V.; Lian, J.B.; Cole, D.E.; Gundberg, C.M. Osteocalcin and matrix Gla protein: Vitamin K-dependent proteins in bone. Physiol. Rev. 1989, 69, 990–1047. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A. Gla-containing proteins of bone. Connect. Tissue Res. 1989, 21, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Otsuka, A.A.; Poser, J.W.; Kristaponis, J.; Raman, N. Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc. Natl. Acad. Sci. USA 1976, 73, 1447–1451. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Poser, J.W.; Raman, N. Primary structure of the gamma-carboxyglutamic acid-containing protein from bovine bone. Proc. Natl. Acad. Sci. USA 1976, 73, 3374–3375. [Google Scholar] [CrossRef]
- Gundberg, C.M.; Clough, M.E. The osteocalcin propeptide is not secreted in vivo or in vitro. J. Bone Miner. Res. 1992, 7, 73–80. [Google Scholar] [CrossRef]
- Gundberg, C.M.; Markowitz, M.E.; Mizruchi, M.; Rosen, J.F. Osteocalcin in human serum: A circadian rhythm. J. Clin. Endocrinol. Metab. 1985, 60, 736–739. [Google Scholar] [CrossRef]
- Morris, D.P.; Stevens, R.D.; Wright, D.J.; Stafford, D.W. Processive post-translational modification. Vitamin K-dependent carboxylation of a peptide substrate. J. Biol. Chem. 1995, 270, 30491–30498. [Google Scholar] [CrossRef]
- Gundberg, C.M.; Weinstein, R.S. Multiple immunoreactive forms of osteocalcin in uremic serum. J. Clin. Investig. 1986, 77, 1762–1767. [Google Scholar] [CrossRef] [PubMed]
- Razzaque, M.S. Osteocalcin: A pivotal mediator or an innocent bystander in energy metabolism? Nephrol. Dial. Transplant. 2011, 26, 42–45. [Google Scholar] [CrossRef] [PubMed]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell. Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Ivaska, K.K.; Hentunen, T.A.; Vaaraniemi, J.; Ylipahkala, H.; Pettersson, K.; Vaananen, H.K. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J. Biol. Chem. 2004, 279, 18361–18369. [Google Scholar] [CrossRef] [PubMed]
- Price, P.A.; Williamson, M.K.; Epstein, D.J. Specific tritium incorporation into gamma-carboxyglutamic acid in proteins. The pH dependence of gamma-proton exchange. J. Biol. Chem. 1981, 256, 1172–1176. [Google Scholar] [PubMed]
- Ducy, P. The role of osteocalcin in the endocrine cross-talk between bone remodelling and energy metabolism. Diabetologia 2011, 54, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Franco, M.C.; Franco-Diaz de Leon, R.; Villafan-Bernal, J.R. OsteocalcinGPRC6A: An update of its clinical and biological multiorganic interactions (Review). Mol. Med. Rep. 2019, 19, 15–22. [Google Scholar] [PubMed]
- Oldknow, K.J.; MacRae, V.E.; Farquharson, C. Endocrine role of bone: Recent and emerging perspectives beyond osteocalcin. J. Endocrinol. 2015, 225, R1–R19. [Google Scholar] [CrossRef]
- Arnold, K.A.; Eichelbaum, M.; Burk, O. Alternative splicing affects the function and tissue-specific expression of the human constitutive androstane receptor. Nucl. Recept. 2004, 2, 1. [Google Scholar] [CrossRef]
- Rauner, M.; Coudert, A.; Sobacchi, C.; Del Fattore, A. The endocrine role of the skeleton. Int. J. Endocrinol. 2015, 2015, 265151. [Google Scholar] [CrossRef]
- Khrimian, L.; Obri, A.; Ramos-Brossier, M.; Rousseaud, A.; Moriceau, S.; Nicot, A.S.; Mera, P.; Kosmidis, S.; Karnavas, T.; Saudou, F.; et al. Gpr158 mediates osteocalcin’s regulation of cognition. J. Exp. Med. 2017, 214, 2859–2873. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, S.; Polyzos, A.; Harvey, L.; Youssef, M.; Denny, C.A.; Dranovsky, A.; Kandel, E.R. RbAp48 Protein Is a Critical Component of GPR158/OCN Signaling and Ameliorates Age-Related Memory Loss. Cell Rep. 2018, 25, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Pavlopoulos, E.; Jones, S.; Kosmidis, S.; Close, M.; Kim, C.; Kovalerchik, O.; Small, S.A.; Kandel, E.R. Molecular mechanism for age-related memory loss: The histone-binding protein RbAp48. Sci. Transl. Med. 2013, 5, 200ra115. [Google Scholar] [CrossRef] [PubMed]
- Kaji, H.; Kuroki, Y.; Murakawa, Y.; Funakawa, I.; Funasaka, Y.; Kanda, F.; Sugimoto, T. Effect of alendronate on bone metabolic indices and bone mineral density in patients treated with high-dose glucocorticoid: A prospective study. Osteoporos. Int. 2010, 21, 1565–1571. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yamauchi, M.; Yamaguchi, T.; Nawata, K.; Takaoka, S.; Sugimoto, T. Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin. Nutr. 2010, 29, 761–765. [Google Scholar] [CrossRef] [PubMed]
- Karimi Fard, M.; Aminorroaya, A.; Kachuei, A.; Salamat, M.R.; Hadi Alijanvand, M.; Aminorroaya Yamini, S.; Karimifar, M.; Feizi, A.; Amini, M. Alendronate improves fasting plasma glucose and insulin sensitivity, and decreases insulin resistance in prediabetic osteopenic postmenopausal women: A randomized triple-blind clinical trial. J. Diabetes. Investig. 2019, 10, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, A.V.; Schafer, A.L.; Grey, A.; Vittinghoff, E.; Palermo, L.; Lui, L.Y.; Wallace, R.B.; Cummings, S.R.; Black, D.M.; Bauer, D.C.; et al. Effects of antiresorptive therapies on glucose metabolism: Results from the FIT, HORIZON-PFT, and FREEDOM trials. J. Bone Miner. Res. 2013, 28, 1348–1354. [Google Scholar] [CrossRef]
- Toulis, K.A.; Nirantharakumar, K.; Ryan, R.; Marshall, T.; Hemming, K. Bisphosphonates and glucose homeostasis: A population-based, retrospective cohort study. J. Clin. Endocrinol. Metab. 2015, 100, 1933–1940. [Google Scholar] [CrossRef]
- Vestergaard, P.; Rejnmark, L.; Mosekilde, L. Are antiresorptive drugs effective against fractures in patients with diabetes? Calcif. Tissue Int. 2011, 88, 209–214. [Google Scholar] [CrossRef]
- Xuan, Y.; Sun, L.H.; Liu, D.M.; Zhao, L.; Tao, B.; Wang, W.Q.; Zhao, H.Y.; Liu, J.M.; Ning, G. Positive association between serum levels of bone resorption marker CTX and HbA1c in women with normal glucose tolerance. J. Clin. Endocrinol. Metab. 2015, 100, 274–281. [Google Scholar] [CrossRef]
- Yang, S.; Leslie, W.D.; Morin, S.N.; Majumdar, S.R.; Lix, L.M. Antiresorptive therapy and newly diagnosed diabetes in women: A historical cohort study. Diabetes Obes. Metab. 2016, 18, 875–881. [Google Scholar] [CrossRef] [PubMed]
- Urano, T.; Shiraki, M.; Kuroda, T.; Tanaka, S.; Urano, F.; Uenishi, K.; Inoue, S. Low serum osteocalcin concentration is associated with incident type 2 diabetes mellitus in Japanese women. J. Bone Miner. Metab 2018, 36, 470–477. [Google Scholar] [CrossRef] [PubMed]
- Oury, F.; Ferron, M.; Huizhen, W.; Confavreux, C.; Xu, L.; Lacombe, J.; Srinivas, P.; Chamouni, A.; Lugani, F.; Lejeune, H.; et al. Osteocalcin regulates murine and human fertility through a pancreas-bone-testis axis. J. Clin. Investig. 2013, 123, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, A.; Breer, S.; Wallaschofski, H.; Nauck, M.; Baumeister, S.E.; Barvencik, F.; Amling, M.; Schinke, T.; Haring, R.; Keller, J. Osteocalcin is associated with testosterone in the general population and selected patients with bone disorders. Andrology 2013, 1, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, I.; Tanaka, K.; Ogawa, N.; Yamauchi, M.; Yamaguchi, T.; Sugimoto, T. Undercarboxylated osteocalcin is positively associated with free testosterone in male patients with type 2 diabetes mellitus. Osteoporos. Int. 2013, 24, 1115–1119. [Google Scholar] [CrossRef] [PubMed]
- Legrand, E.; Hedde, C.; Gallois, Y.; Degasne, I.; Boux de Casson, F.; Mathieu, E.; Basle, M.F.; Chappard, D.; Audran, M. Osteoporosis in men: A potential role for the sex hormone binding globulin. Bone 2001, 29, 90–95. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Yang, Y.; Wen, C.Y.; Rong, L.M. Serum Osteocalcin and Testosterone Concentrations in Adult Males with or without Primary Osteoporosis: A Meta-Analysis. Biomed. Res. Int 2017, 2017, 9892048. [Google Scholar] [CrossRef]
- Lui, L.Y.; Stone, K.; Cauley, J.A.; Hillier, T.; Yaffe, K. Bone loss predicts subsequent cognitive decline in older women: The study of osteoporotic fractures. J. Am. Geriatr. Soc. 2003, 51, 38–43. [Google Scholar] [CrossRef]
- Rothman, M.S.; Arciniegas, D.B.; Filley, C.M.; Wierman, M.E. The neuroendocrine effects of traumatic brain injury. J. Neuropsychiatry Clin. Neurosci. 2007, 19, 363–372. [Google Scholar] [CrossRef]
- Tan, Z.S.; Seshadri, S.; Beiser, A.; Zhang, Y.; Felson, D.; Hannan, M.T.; Au, R.; Wolf, P.A.; Kiel, D.P. Bone mineral density and the risk of Alzheimer disease. Arch. Neurol. 2005, 62, 107–111. [Google Scholar] [CrossRef]
- Sohrabi, H.R.; Bates, K.A.; Weinborn, M.; Bucks, R.S.; Rainey-Smith, S.R.; Rodrigues, M.A.; Bird, S.M.; Brown, B.M.; Beilby, J.; Howard, M.; et al. Bone mineral density, adiposity, and cognitive functions. Front. Aging Neurosci. 2015, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Deng, J.; Zhang, M.; Zhou, H.D.; Wang, Y.J. Association between bone mineral density and the risk of Alzheimer’s disease. J. Alzheimers Dis. 2011, 24, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Puig, J.; Blasco, G.; Daunis-i-Estadella, J.; Moreno, M.; Molina, X.; Alberich-Bayarri, A.; Xifra, G.; Pedraza, S.; Ricart, W.; Fernandez-Aranda, F.; et al. Lower serum osteocalcin concentrations are associated with brain microstructural changes and worse cognitive performance. Clin. Endocrinol. (Oxf) 2016, 84, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Bradburn, S.; McPhee, J.S.; Bagley, L.; Sipila, S.; Stenroth, L.; Narici, M.V.; Paasuke, M.; Gapeyeva, H.; Osborne, G.; Sassano, L.; et al. Association between osteocalcin and cognitive performance in healthy older adults. Age Ageing 2016, 45, 844–849. [Google Scholar] [CrossRef] [PubMed]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nature Rev. Endocrinol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Merle, B.; Delmas, P.D. Normal carboxylation of circulating osteocalcin (bone Gla-protein) in Paget’s disease of bone. Bone Miner. 1990, 11, 237–245. [Google Scholar] [CrossRef]
- Poser, J.W.; Price, P.A. A method for decarboxylation of gamma-carboxyglutamic acid in proteins. Properties of the decarboxylated gamma-carboxyglutamic acid protein from calf bone. J. Biol. Chem. 1979, 254, 431–436. [Google Scholar]
- Heshmati, H.M.; Riggs, B.L.; Burritt, M.F.; McAlister, C.A.; Wollan, P.C.; Khosla, S. Effects of the circadian variation in serum cortisol on markers of bone turnover and calcium homeostasis in normal postmenopausal women. J. Clin. Endocrinol. Metab. 1998, 83, 751–756. [Google Scholar] [CrossRef]
- Gong, S.; Miao, Y.L.; Jiao, G.Z.; Sun, M.J.; Li, H.; Lin, J.; Luo, M.J.; Tan, J.H. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS ONE 2015, 10, e0117503. [Google Scholar] [CrossRef]
| Osteopetrosis | Genetic Transmission | Gene Mutation | Protein |
|---|---|---|---|
| ARO | Autosomal recessive osteopetrosis | TCIRG1 | α3 subunit V-H+ATPase |
| CLCN7 | Chloride channel 7 | ||
| OSTM1 | Osteopetrosis associated transmembrane protein | ||
| PLEKHM1 | Pleckstrin homology domain containing family M, member I | ||
| SNX10 | Sorting nexin 10 | ||
| TNFSF11 | Receptor activator for nuclear factor κB ligand | ||
| TNFRSF11A | Receptor activator for nuclear factor κB | ||
| IRO | Autosomal recessive osteopetrosis | CAII | Carbonic anhydrase |
| ADO | Autosomal dominant osteopetrosis | CLCN7 | Chloride channel 7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, M.; Battafarano, G.; Pepe, J.; Minisola, S.; Del Fattore, A. The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases. Int. J. Mol. Sci. 2019, 20, 4502. https://doi.org/10.3390/ijms20184502
Rossi M, Battafarano G, Pepe J, Minisola S, Del Fattore A. The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases. International Journal of Molecular Sciences. 2019; 20(18):4502. https://doi.org/10.3390/ijms20184502
Chicago/Turabian StyleRossi, Michela, Giulia Battafarano, Jessica Pepe, Salvatore Minisola, and Andrea Del Fattore. 2019. "The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases" International Journal of Molecular Sciences 20, no. 18: 4502. https://doi.org/10.3390/ijms20184502
APA StyleRossi, M., Battafarano, G., Pepe, J., Minisola, S., & Del Fattore, A. (2019). The Endocrine Function of Osteocalcin Regulated by Bone Resorption: A Lesson from Reduced and Increased Bone Mass Diseases. International Journal of Molecular Sciences, 20(18), 4502. https://doi.org/10.3390/ijms20184502








