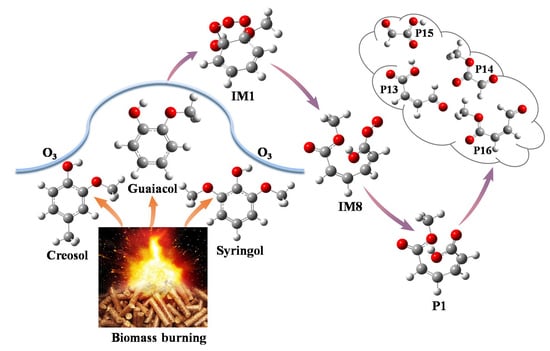
Mechanistic and Kinetic Investigations on the Ozonolysis of Biomass Burning Products: Guaiacol, Syringol and Creosol
Abstract
1. Introduction
2. Results and Discussion
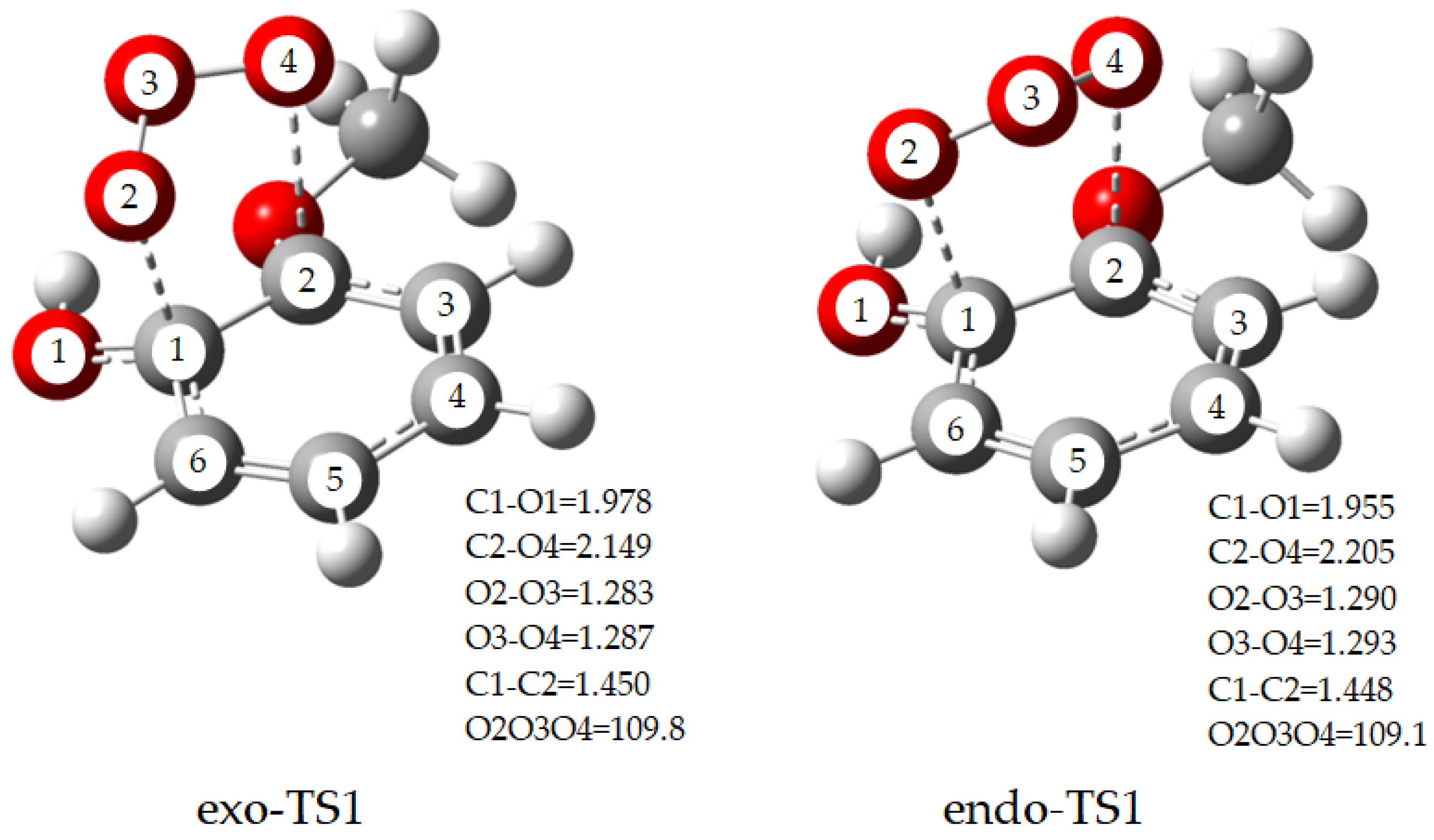
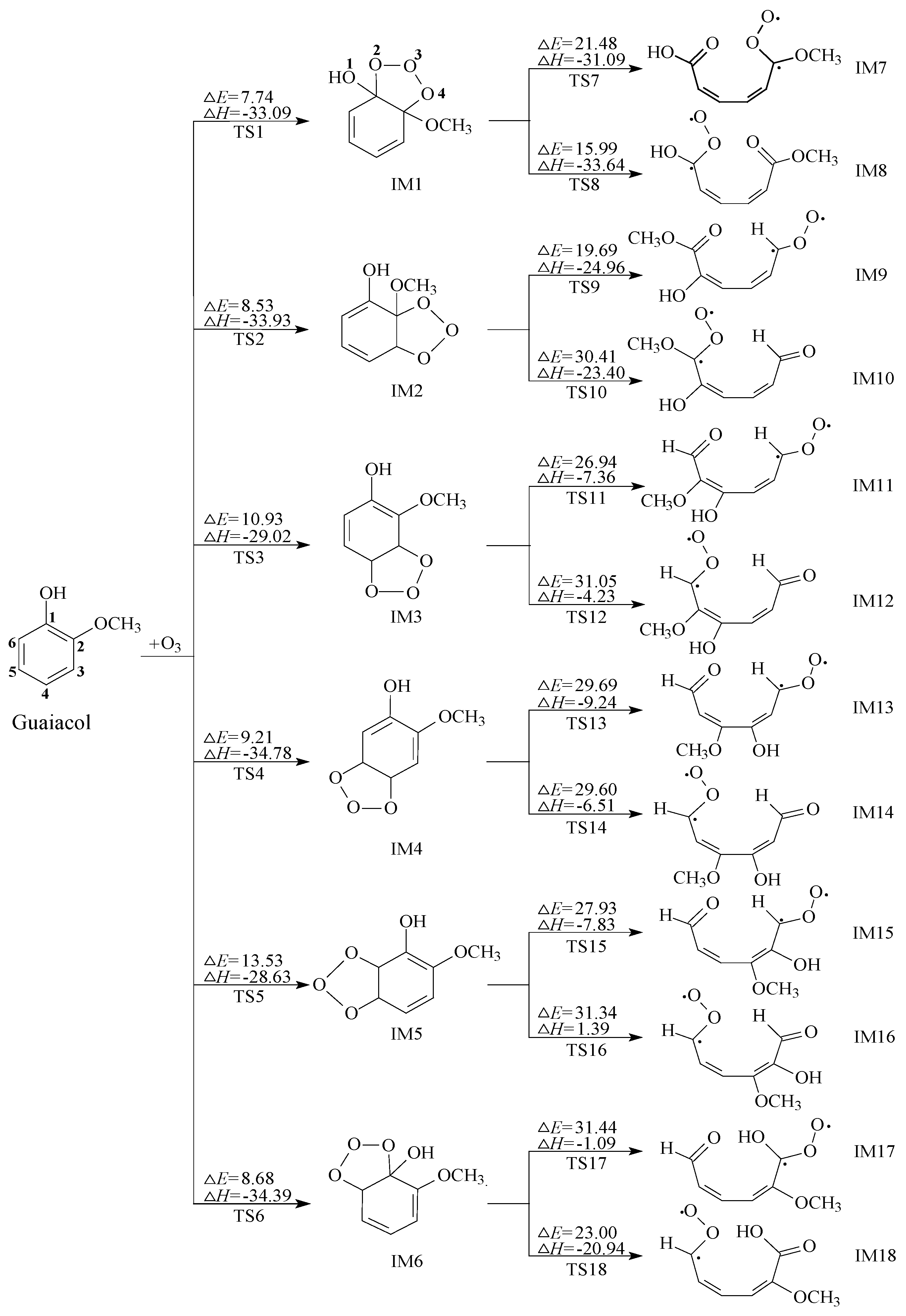
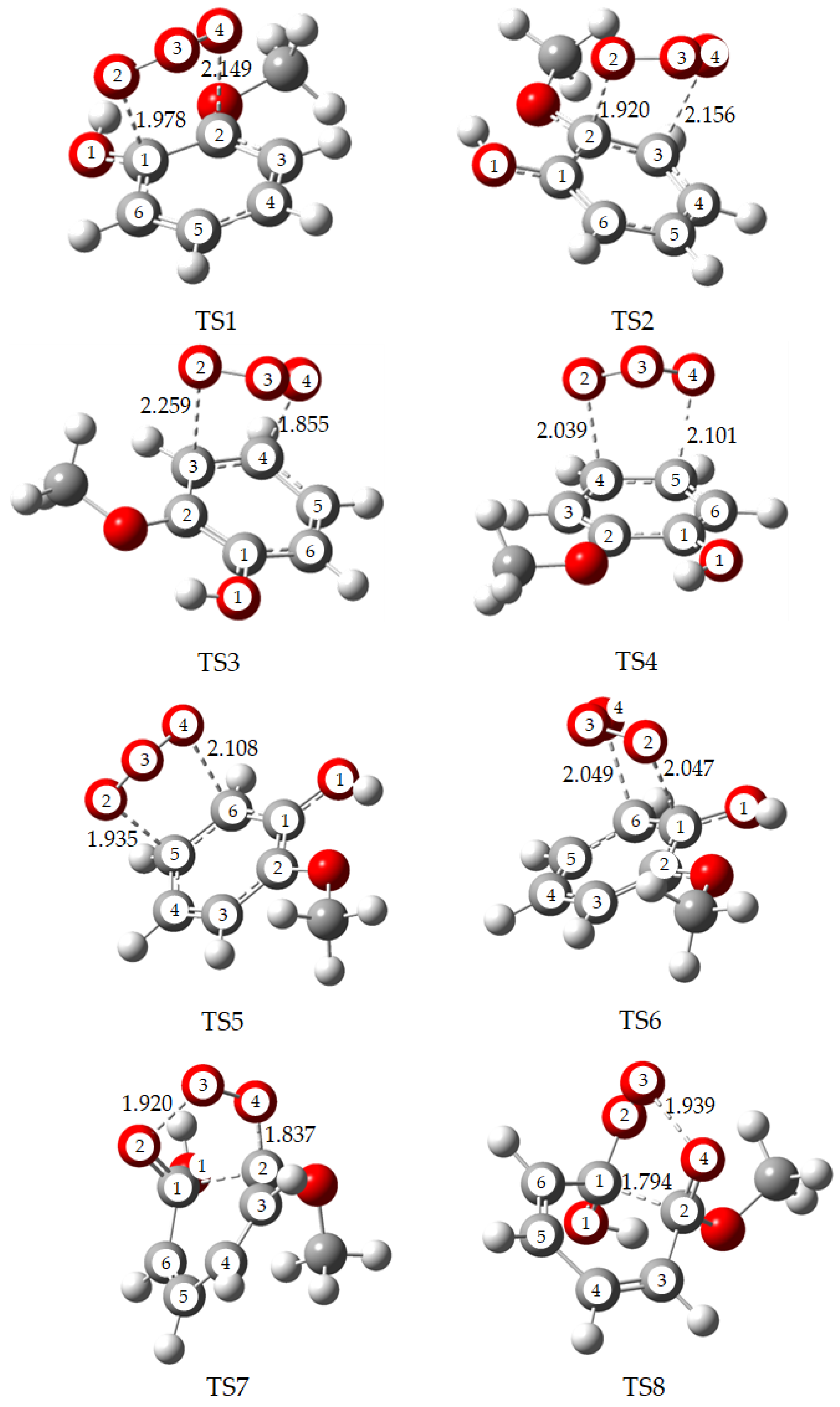
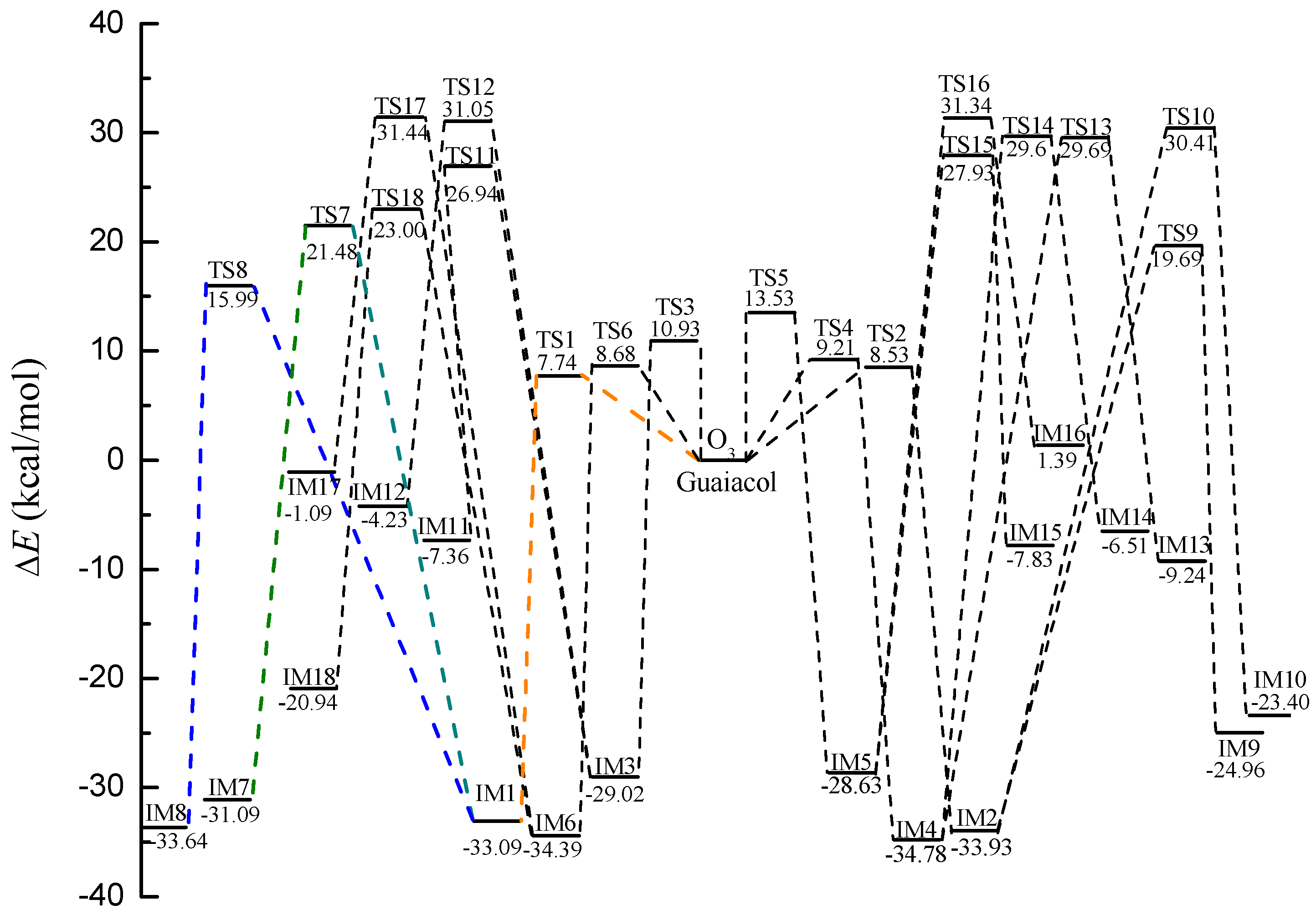
2.1. Initial Reaction of Guaiacol with Ozone
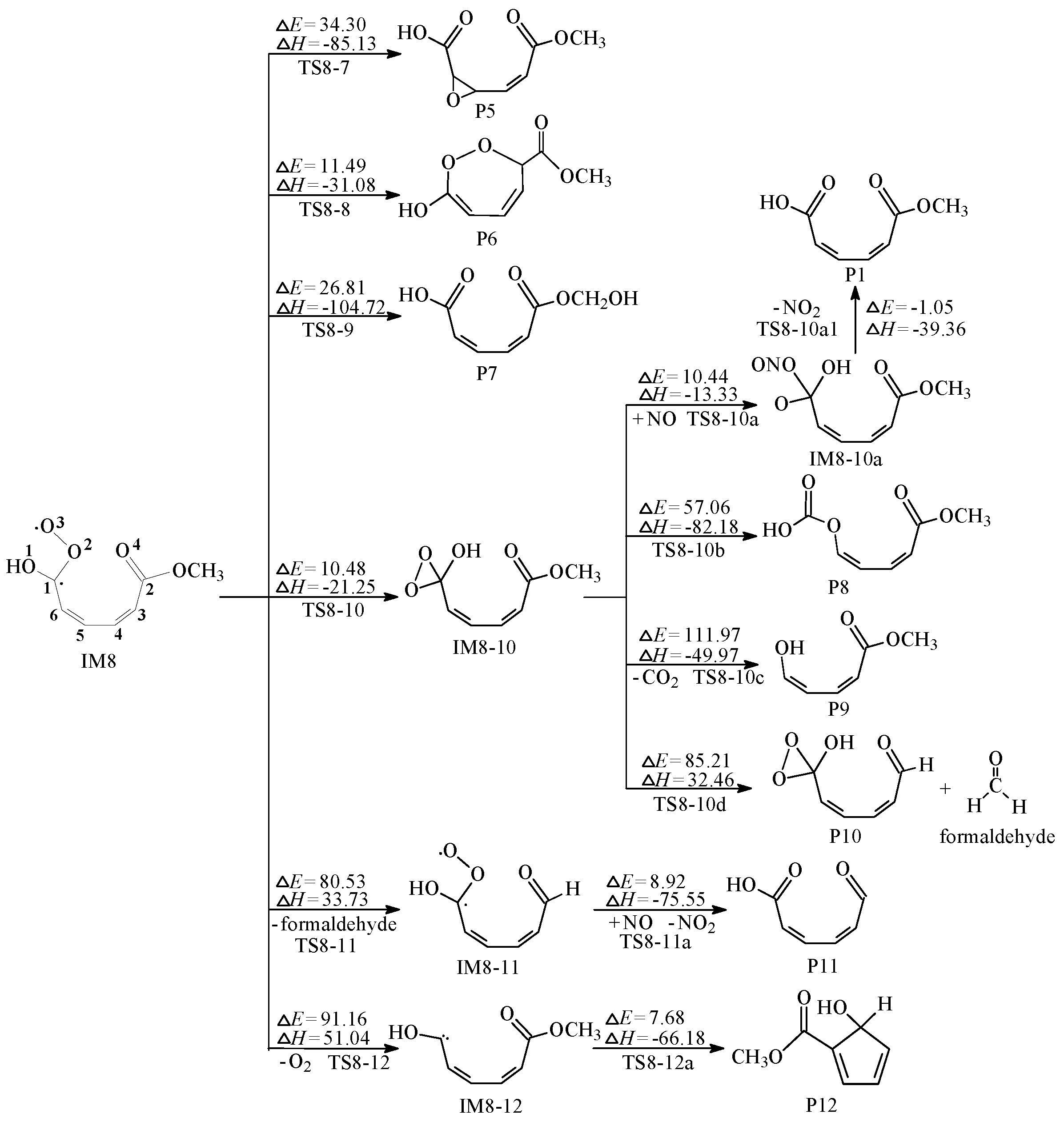
2.2. Secondary Reactions of IM8
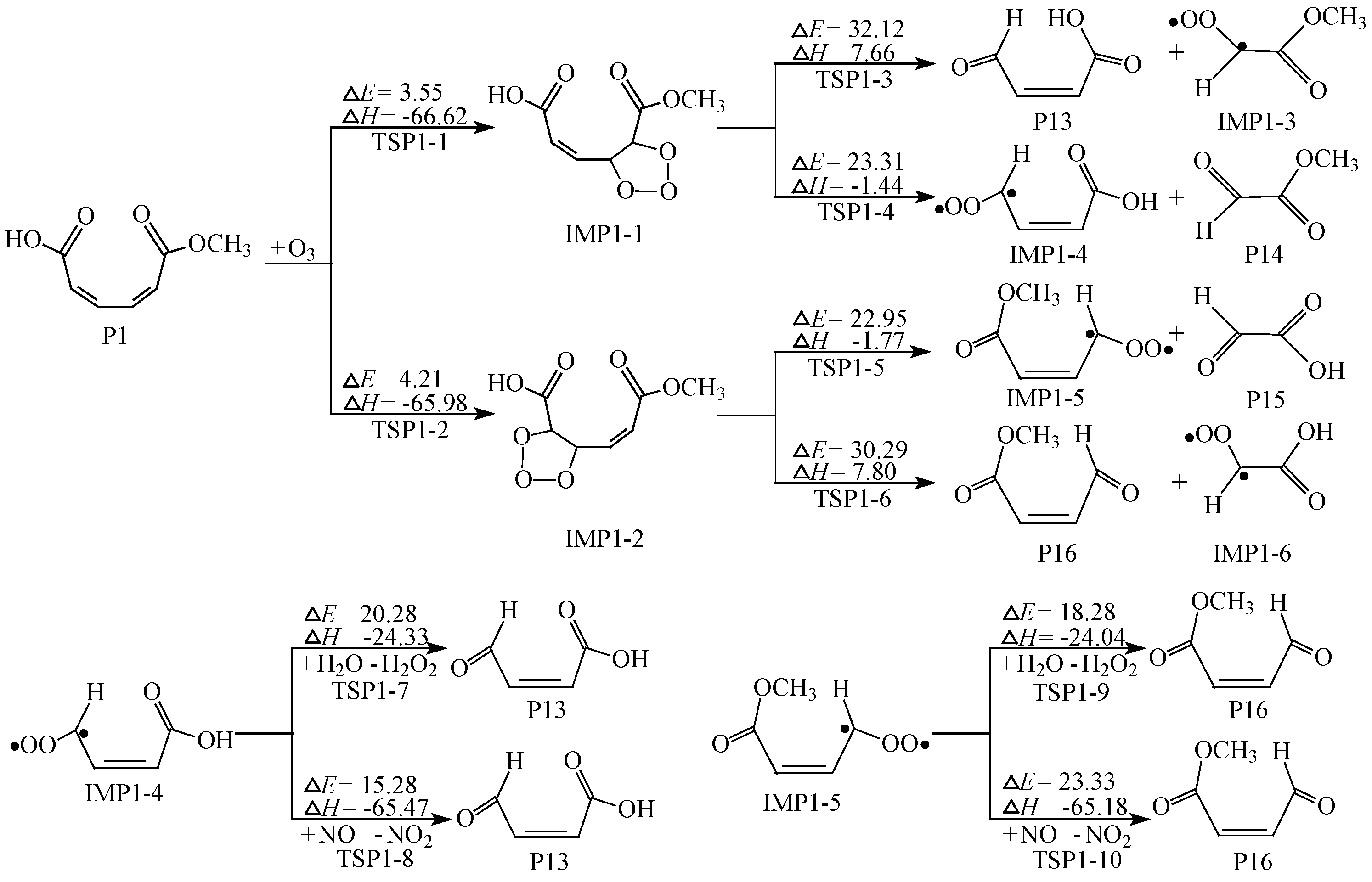
2.3. Further Reaction of P1
2.4. Kinetic Calculations
2.4.1. Rate-constant Calculations
2.4.2. Branching Ratio
2.4.3. Atmospheric Implications
2.5. Initial Reactions of Syringol and Creosol with Ozone
3. Computational Details
3.1. Electronic Structure Calculations
3.2. Rate Constant Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Akagi, S.K.; Yokelson, R.J.; Wiedinmyer, C.; Alvarado, M.J.; Reid, J.S.; Karl, T.; Crounse, J.D.; Wennberg, P.O. Emission factors for open and domestic biomass burning for use in atmospheric models. Atmos. Chem. Phys. 2011, 11, 4039–4072. [Google Scholar] [CrossRef]
- Bond, T.C. A technology-based global inventory of black and organic carbon emissions from combustion. J. Geophys. Res. 2004, 109, D14203–D14245. [Google Scholar] [CrossRef]
- Hennigan, C.J.; Miracolo, M.A.; Engelhart, G.J.; May, A.A.; Presto, A.A.; Lee, T.; Sullivan, A.P.; McMeeking, G.R.; Coe, H.; Wold, C.E.; et al. Chemical and physical transformations of organic aerosol from the photo-oxidation of open biomass burning emissions in an environmental chamber. Atmos. Chem. Phys. 2011, 11, 7669–7686. [Google Scholar] [CrossRef]
- Lelieveld, J.; Crutzen, P.J.; Ramanathan, V.; Andreae, M.O.; Brenninkmeijer, C.A.M.; Campos, T.; Cass, G.R.; Dickerson, R.R.; Fischer, H.; de Gouw, J.A.; et al. The Indian ocean experiment: Widespread air pollution from south and southeast Asia. Science 2001, 291, 1031–1036. [Google Scholar] [CrossRef] [PubMed]
- Lighty, J.S.; Veranth, J.M.; Sarofim, A.F. Combustion aerosols: Factors governing their size and composition and implications to human health. J. Air. Waste. Manage. 2011, 50, 1565–1618. [Google Scholar] [CrossRef]
- Rodríguez-Olalde, N.E.; Mendoza-Chávez, E.A.; Castro-Montoya, A.J.; Saucedo-Luna, J.; Maya-Yescas, R.; Rutiaga-Quiñones, J.G.; Ortega, J.M.P. Simulation of syngas production from lignin using guaiacol as a model compound. Energies 2015, 8, 6705–6714. [Google Scholar] [CrossRef]
- Wang, L.; Li, J.; Chen, Y.Q.; Yang, H.P.; Shao, J.G.; Zhang, X.Y.; Yu, H.B.; Chen, H.P. Investigation of the pyrolysis characteristics of guaiacol lignin using combined Py-GC × GC/TOF-MS and in-situ FTIR. Fuel 2019, 251, 496–505. [Google Scholar] [CrossRef]
- Cheng, F.; Brewer, C.E. Producing jet fuel from biomass lignin: Potential pathways to alkyl-benzenes and cycloalkanes. Renew. Sustain. Energy Rev. 2017, 72, 673–722. [Google Scholar] [CrossRef]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; Peinder, P.d.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green. Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, Y.; Liu, S.; Zhou, N.; Chen, P.; Cheng, Y.; Addy, M.; Lu, Q.; Omar, M.M.; Liu, Y.; et al. Bio-oil from fast pyrolysis of lignin: Effects of process and upgrading parameters. Bioresour. Technol. 2017, 241, 1118–1126. [Google Scholar] [CrossRef]
- Jiang, Z.C.; Hu, C.W. Selective extraction and conversion of lignin in actual biomass to monophenols: A review. J. Energy Chem. 2016, 25, 947–956. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J.; Barkley, R.M.; Krieger, M.S. Identification of methoxylated phenols as candidate tracers for atmospheric wood smoke pollution. Environ. Sci. Technol. 1988, 22, 1191–1196. [Google Scholar] [CrossRef] [PubMed]
- Schauer, J.J.; Kleeman, M.J.; Cass, G.R.; Simoneit, B.R.T. Measurement of emissions from air pollution sources. 2. C1 through C30 organic compounds from medium duty diesel trucks. Environ. Sci. Technol. 1999, 33, 1578–1587. [Google Scholar] [CrossRef]
- Sagebiel, J.C.; Seiber, J.N. Studies on the occurrence and distribution of wood smoke marker compounds in foggy atmospheres. Environ. Toxicol. Chem. 1993, 12, 813–822. [Google Scholar] [CrossRef]
- Lauraguais, A.; Coeur-Tourneur, C.; Cassez, A.; Seydi, A. Rate constant and secondary organic aerosol yields for the gas-phase reaction of hydroxyl radicals with syringol (2,6-dimethoxyphenol). Atmos. Environ. 2012, 55, 43–48. [Google Scholar] [CrossRef]
- Liu, C.G.; Liu, J.; Liu, Y.C.; Chen, T.Z.; He, H. Secondary organic aerosol formation from the OH-initiated oxidation of guaiacol under different experimental conditions. Atmos. Environ. 2019, 207, 30–37. [Google Scholar] [CrossRef]
- Yee, L.D.; Kautzman, K.E.; Loza, C.L.; Schilling, K.A.; Coggon, M.M.; Chhabra, P.S.; Chan, M.N.; Chan, A.W.H.; Hersey, S.P.; Crounse, J.D.; et al. Secondary organic aerosol formation from biomass burning intermediates: Phenol and methoxyphenols. Atmos. Chem. Phys. 2013, 13, 8019–8043. [Google Scholar] [CrossRef]
- Yang, B.; Zhang, H.X.; Wang, Y.F.; Zhang, P.; Shu, J.N.; Sun, W.Q.; Ma, P.K. Experimental and theoretical studies on gas-phase reactions of NO3 radicals with three methoxyphenols: Guaiacol, creosol, and syringol. Atmos. Environ. 2016, 125, 243–251. [Google Scholar] [CrossRef]
- Perring, A.E.; Pusede, S.E.; Cohen, R.C. An observational perspective on the atmospheric impacts of alkyl and multifunctional nitrates on ozone and secondary organic aerosol. Chem. Rev. 2013, 113, 5848–5870. [Google Scholar] [CrossRef]
- Kang, L.Y.; Zhang, C.X.; Sun, X.M. The mechanism and kinetic studies on oxidation reaction of acetofenate initiated by HOx, NO3, O3, and Cl radicals. Rsc. Adv. 2015, 5, 96518–96524. [Google Scholar] [CrossRef]
- Khudoshin, A.G.; Mitrofanova, A.N.; Lunin, V.V. Kinetics and mechanism of the reactions of ozone with guaiacol, veratrol, and veratrol derivatives. Russ. Chem. Bull. 2008, 57, 283–288. [Google Scholar] [CrossRef]
- Zein, A.E.; Coeur, C.; Obeid, E.; Lauraguais, A.; Fagniez, T. Reaction kinetics of catechol (1,2-benzenediol) and guaiacol (2-methoxyphenol) with ozone. J. Phys. Chem. A 2015, 119, 6759–6765. [Google Scholar] [CrossRef] [PubMed]
- Barnum, T.J.; Medeiros, N.; Hinrichs, R.Z. Condensed-phase versus gas-phase ozonolysis of catechol: A combined experimental and theoretical study. Atmos. Environ. 2012, 55, 98–106. [Google Scholar] [CrossRef]
- Jiang, X.Y.; Lu, Q.; Hu, B.; Liu, J.; Dong, C.Q.; Yang, Y.P. A Comprehensive study on pyrolysis mechanism of substituted beta-O-4 type lignin dimers. Int. J. Mol. Sci. 2017, 18, 2364–2377. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.F.; Wei, B.; Mei, Q.; An, Z.X.; Wang, X.Y.; He, M.X. Ozonation of 3-methylcatechol and 4-methylcatechol in the atmosphere and aqueous particles: Mechanism, kinetics and ecotoxicity assessment. Chem. Eng. J. 2019, 358, 456–466. [Google Scholar] [CrossRef]
- Johnson, D.; Marston, G. The gas-phase ozonolysis of unsaturated volatile organic compounds in the troposphere. Chem. Soc. Rev. 2008, 37, 699–716. [Google Scholar] [CrossRef] [PubMed]
- Lightfoot, P.D.; Cox, R.A.; Crowley, J.N.; Destriau, M.; Hayman, G.D.; Jenkin, M.E.; Moortgat, G.K.; Zabel, F. Organic peroxy radicals: Kinetics, spectroscopy and tropospheric chemistry. Atmos. Environ. 1992, 26, 1805–1961. [Google Scholar] [CrossRef]
- Sun, J.F.; Cao, H.J.; Zhang, S.Q.; Li, X.; He, M.X. Theoretical study on the mechanism of the gas phase reaction of methoxybenzene with ozone. Rsc. Adv. 2016, 6, 113561–113569. [Google Scholar] [CrossRef]
- Zhang, Q.Z.; Zhang, R.Q.; Gu, Y.S. Kinetics and mechanism of O (3P) reaction with CH3CHF2: A theoretical study. J. Phys. Chem. A 2004, 108, 1064–1068. [Google Scholar] [CrossRef]
- Sun, T.L.; Wang, Y.D.; Zhang, C.X.; Sun, X.M.; Wang, W.X. The chemical mechanism of the limonene ozonolysis reaction in the SOA formation: A quantum chemistry and direct dynamic study. Atmos. Environ. 2011, 45, 1725–1731. [Google Scholar] [CrossRef]
- Huang, Z.X.; Zhang, Q.Z.; Wang, W.X. Mechanical and kinetic study on gas-phase formation of dinitronaphthalene from 1- and 2-nitronaphthalene. Chemosphere 2016, 156, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.M.; Bai, J.; Zhao, Y.Y.; Zhang, C.X.; Wang, Y.D.; Hu, J.T. Chemical mechanism and kinetics study on the ocimene ozonolysis reaction in atmosphere. Atmos. Environ. 2011, 45, 6197–6203. [Google Scholar] [CrossRef]
- Xu, F.; Shi, X.L.; Zhang, Q.Z.; Wang, W.X. Formation of chlorotriophenoxy radicals from complete series reactions of chlorotriophenols with H and OH radicals. Int. J. Mol. Sci. 2015, 16, 18714–18731. [Google Scholar] [CrossRef] [PubMed]
- Logan, J.A. Tropospheric ozone: Seasonal behavior, trends, and anthropogenic influence. J. Geophys. Res.-Atmos. 1985, 90, 10463–10482. [Google Scholar] [CrossRef]
- Lin, C.Y.C.; Jacob, D.J.; Fiore, A.M. Trends in Exceedances of the Ozone Air Quality Standard in the Continental United Stated, 1980–1998. Atmos. Environ. 2001, 35, 3217–3228. [Google Scholar] [CrossRef]
- An, Z.X.; Sun, J.F.; Han, D.D.; Mei, Q.; Wei, B.; Wang, X.Y.; He, M.X. Theoretical study on the mechanisms, kinetics and ecotoxicity assessment of OH-initiated reactions of guaiacol in atmosphere and wastewater. Sci. Total Environ. 2019, 685, 729–740. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision, A.02; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Fukui, K. The path of chemical reactions—The IRC approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Corchado, J.C.; Chuang, Y.Y.; Fast, P.L.; Villa, J.; Hu, W.P.; Liu, Y.P.; Lynch, G.C.; Nguyen, K.A.; Jackels, C.F.; Melissas, V.S.; et al. POLYRATE Version 9.7; University of Minnesota: Minneapolis, MN, USA, 2007. [Google Scholar]

| T/K | k1 | k2 | k3 | k4 | k5 | k6 | ktot |
|---|---|---|---|---|---|---|---|
| 170 | 4.25 × 10−24 | 2.20 × 10−25 | 7.22 × 10−28 | 7.58 × 10−28 | 7.46 × 10−32 | 1.26 × 10−25 | 4.60 × 10−24 |
| 195 | 8.01 × 10−23 | 5.57 × 10−24 | 2.50 × 10−26 | 5.27 × 10−26 | 1.33 × 10−29 | 3.60 × 10−24 | 8.93 × 10−23 |
| 220 | 7.97 × 10−22 | 7.30 × 10−23 | 3.98 × 10−25 | 1.43 × 10−24 | 7.56 × 10−28 | 4.77 × 10−23 | 9.20 × 10−22 |
| 245 | 5.10 × 10−21 | 5.67 × 10−22 | 3.68 × 10−24 | 2.04 × 10−23 | 1.93 × 10−26 | 3.91 × 10−22 | 6.08 × 10−21 |
| 270 | 2.36 × 10−20 | 3.08 × 10−21 | 2.30 × 10−23 | 1.80 × 10−22 | 2.76 × 10−25 | 2.21 × 10−21 | 2.91 × 10−20 |
| 295 | 8.62 × 10−20 | 1.28 × 10−20 | 1.08 × 10−22 | 1.12 × 10−21 | 2.57 × 10−24 | 9.50 × 10−21 | 1.10 × 10−19 |
| 320 | 2.61 × 10−19 | 4.34 × 10−20 | 4.01 × 10−22 | 5.34 × 10−21 | 1.71 × 10−23 | 3.23 × 10−20 | 3.42 × 10−19 |
| 345 | 6.85 × 10−19 | 1.25 × 10−19 | 1.25 × 10−21 | 2.05 × 10−20 | 8.82 × 10−23 | 9.55 × 10−20 | 9.27 × 10−19 |
| 370 | 1.60 × 10−18 | 3.16 × 10−19 | 3.40 × 10−21 | 6.67 × 10−20 | 3.69 × 10−22 | 2.47 × 10−19 | 2.23 × 10−18 |
| T/K | r1 | r2 | r3 | r4 | r5 | r6 |
|---|---|---|---|---|---|---|
| 170 | 0.9244 | 0.0479 | 0.0002 | 0.0002 | 0.0000 | 0.0274 |
| 195 | 0.8965 | 0.0623 | 0.0003 | 0.0006 | 0.0000 | 0.0403 |
| 220 | 0.8667 | 0.0794 | 0.0004 | 0.0016 | 0.0000 | 0.0519 |
| 245 | 0.8385 | 0.0932 | 0.0006 | 0.0034 | 0.0000 | 0.0643 |
| 270 | 0.8112 | 0.1059 | 0.0008 | 0.0062 | 0.0000 | 0.0760 |
| 295 | 0.7856 | 0.1166 | 0.0010 | 0.0102 | 0.0000 | 0.0866 |
| 320 | 0.7621 | 0.1267 | 0.0012 | 0.0156 | 0.0000 | 0.0943 |
| 345 | 0.7387 | 0.1348 | 0.0013 | 0.0221 | 0.0001 | 0.1030 |
| 370 | 0.7164 | 0.1415 | 0.0015 | 0.0299 | 0.0002 | 0.1106 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Sun, Y.; Qi, Y.; Liu, L.; Xu, F.; Zhao, Y. Mechanistic and Kinetic Investigations on the Ozonolysis of Biomass Burning Products: Guaiacol, Syringol and Creosol. Int. J. Mol. Sci. 2019, 20, 4492. https://doi.org/10.3390/ijms20184492
Chen X, Sun Y, Qi Y, Liu L, Xu F, Zhao Y. Mechanistic and Kinetic Investigations on the Ozonolysis of Biomass Burning Products: Guaiacol, Syringol and Creosol. International Journal of Molecular Sciences. 2019; 20(18):4492. https://doi.org/10.3390/ijms20184492
Chicago/Turabian StyleChen, Xiaoxiao, Yanhui Sun, Youxiao Qi, Lin Liu, Fei Xu, and Yan Zhao. 2019. "Mechanistic and Kinetic Investigations on the Ozonolysis of Biomass Burning Products: Guaiacol, Syringol and Creosol" International Journal of Molecular Sciences 20, no. 18: 4492. https://doi.org/10.3390/ijms20184492
APA StyleChen, X., Sun, Y., Qi, Y., Liu, L., Xu, F., & Zhao, Y. (2019). Mechanistic and Kinetic Investigations on the Ozonolysis of Biomass Burning Products: Guaiacol, Syringol and Creosol. International Journal of Molecular Sciences, 20(18), 4492. https://doi.org/10.3390/ijms20184492