Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats
Abstract
:1. Introduction
2. Results
2.1. Effect of Tempol and Eplerenone on Systolic BP
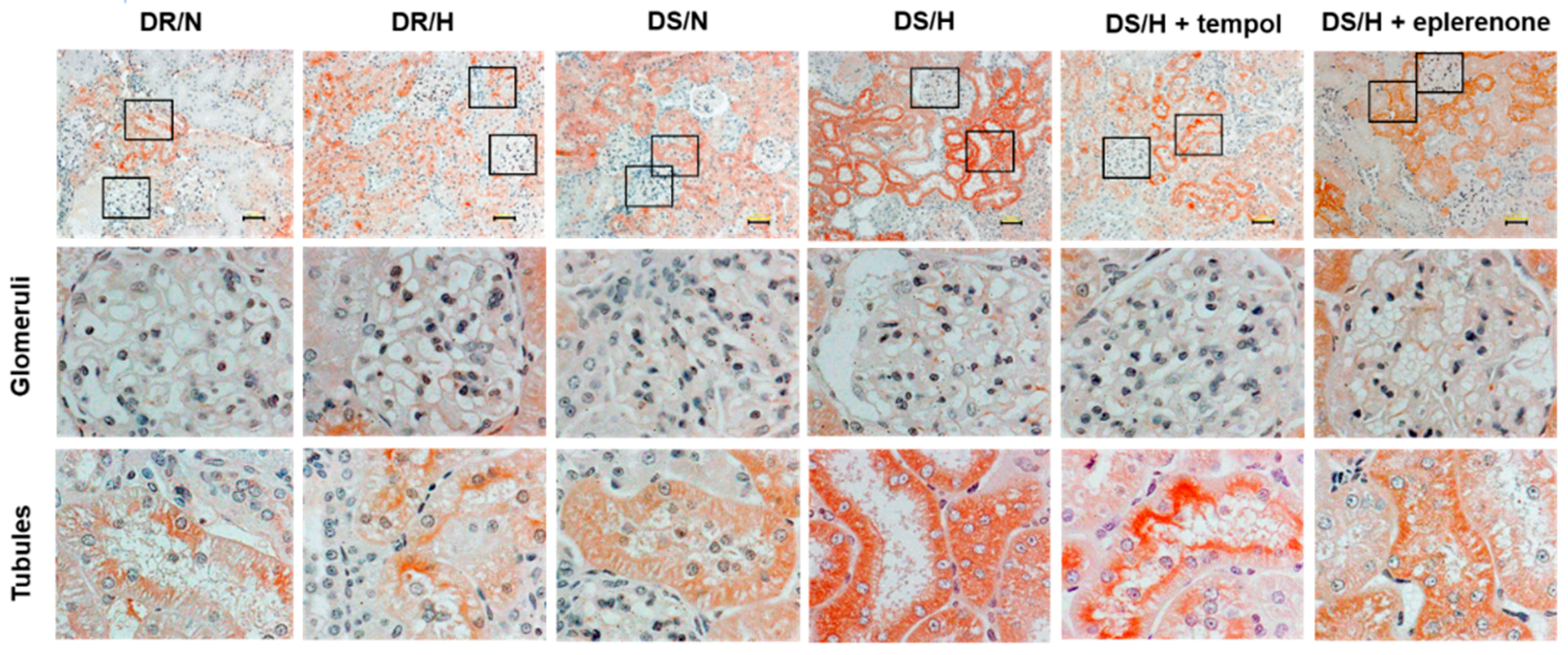
2.2. Effect of Tempol and Eplerenone on Renal Damage
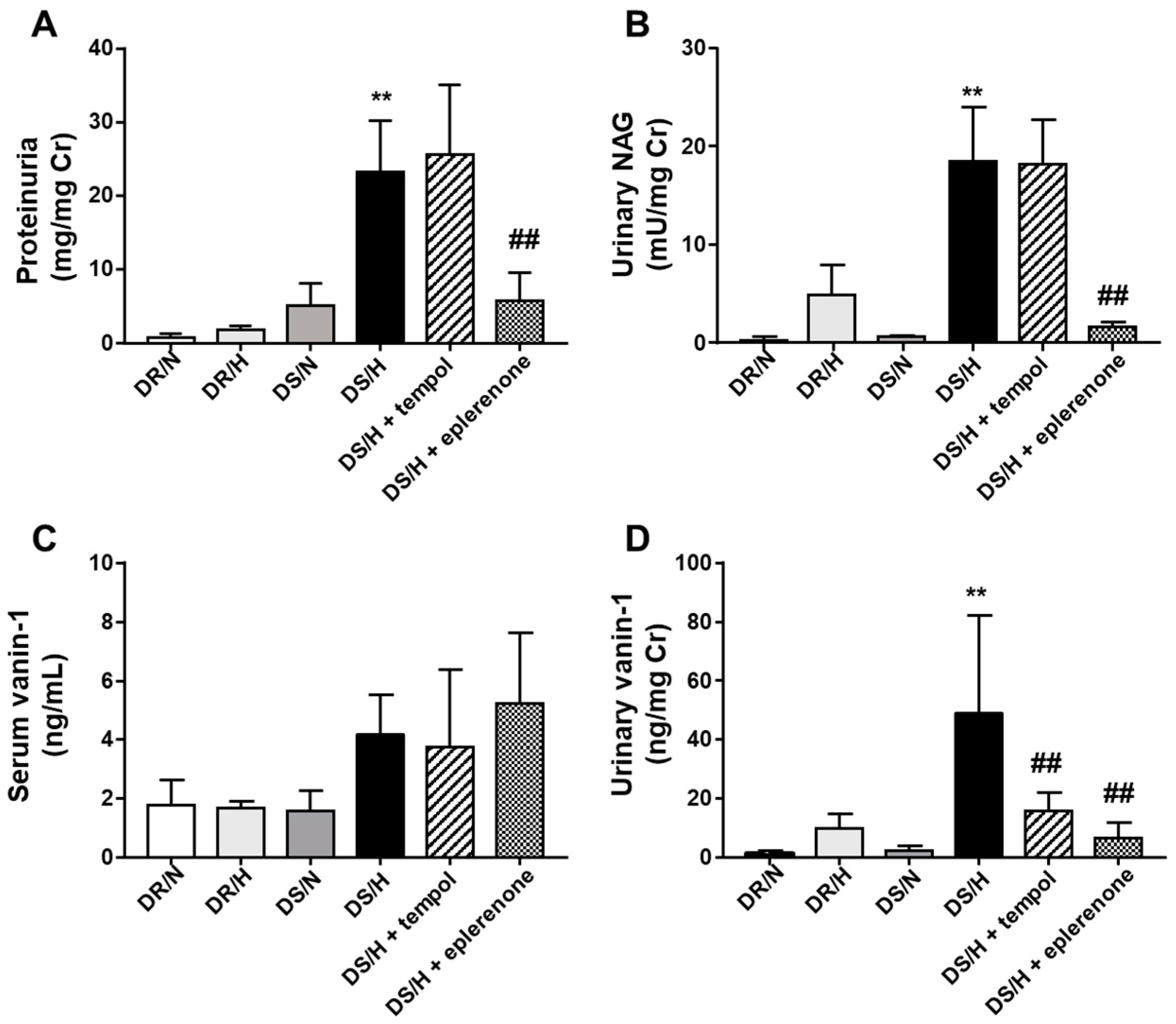
2.3. Evaluation of Renal Tubular Injury by Traditional and Newly Developed Biomarkers
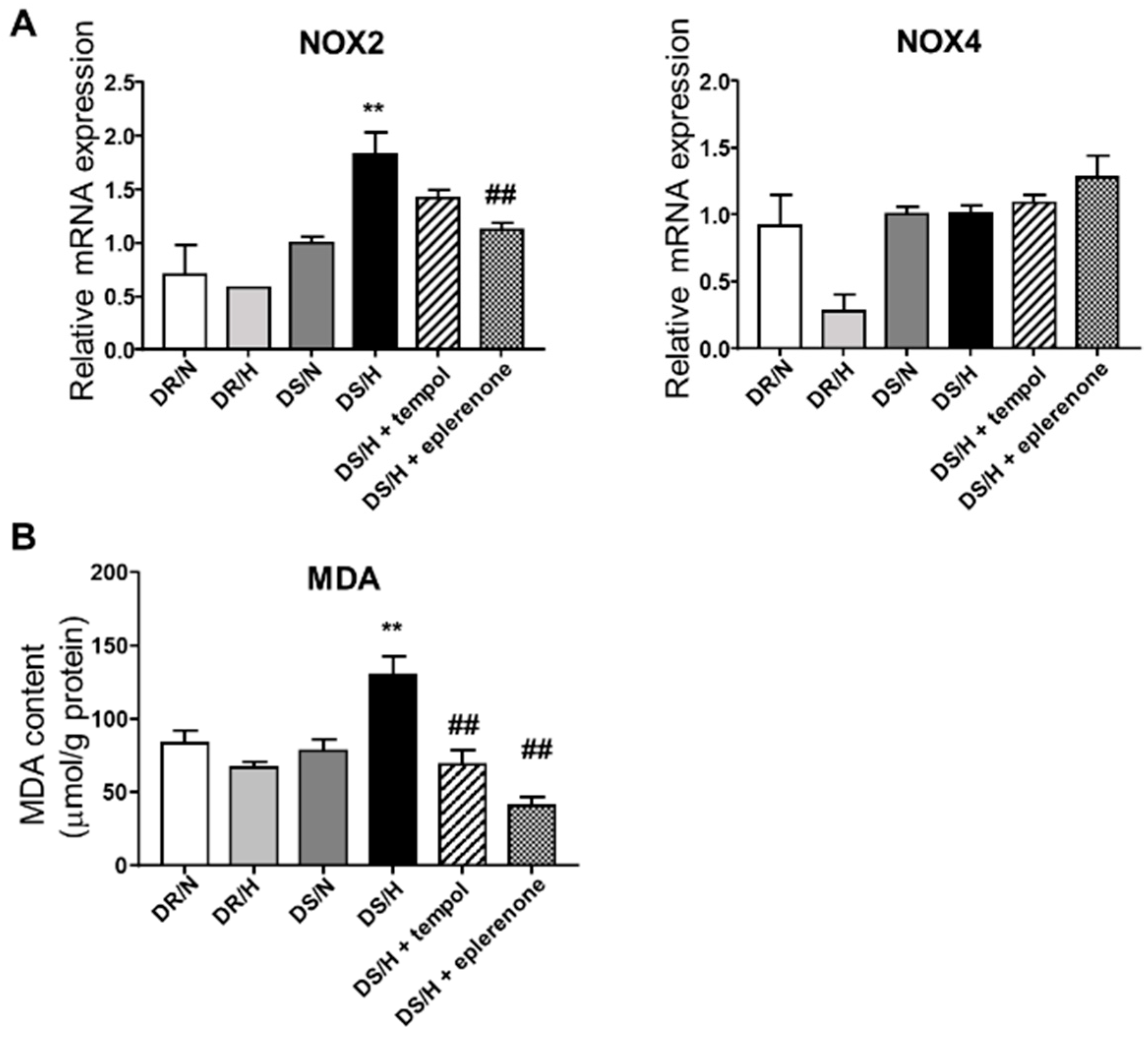
2.4. Effect of Eplerenone and Tempol on Oxidative Stress
3. Discussion
4. Materials and Methods
4.1. Experimental Protocol
4.2. Histological Analysis
4.3. Immunohistochemical Analysis
4.4. Laboratory Measurements
4.5. Measurement of Malondialdehyde (MDA)
4.6. RNA Extraction and Real-Time Quantitative PCR
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| DR rats | Dahl salt-resistant rats |
| DS rats | Dahl salt-sensitive rats |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GSH | glutathione |
| γGCS | γ-glutamylcysteine synthetase |
| 4-HNE | 4-hydroxy-2-nonenal |
| MAPK | mitogen-activated protein kinase |
| MR | mineralocorticoid receptor |
| MDA | malondialdehyde |
| NAG | N-acetyl-β-D-glucosaminidase |
| NOX | NADPH oxidase |
| O2− | superoxide |
| ROS | reactive oxygen species |
| RAAS | renin-angiotensin-aldosterone system |
References
- Chobanian, A.V.; Bakris, G.L.; Black, H.R.; Cushman, W.C.; Green, L.A.; Izzo, J.L., Jr.; Jones, D.W.; Materson, B.J.; Oparil, S.; Wright, J.T.; et al. The seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure: The JNC 7 report. JAMA 2003, 289, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.H. Salt sensitivity of blood pressure in humans. Hypertension 1996, 27, 481–490. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Delea, C.S.; Bartter, F.C.; Smith, H. The effect of high-sodium and low-sodium intakes on blood pressure and other related variables in human subjects with idiopathic hypertension. Am. J. Med. Sci. 1978, 64, 193–198. [Google Scholar] [CrossRef]
- Kotchen, T.A.; Cowley, A.W., Jr.; Frohlich, E.D. Salt in health and disease—A delicate balance. N. Engl. J. Med. 2013, 368, 1229–1237. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, A.; Uzu, T.; Fujii, T.; Nishimura, M.; Kuroda, S.; Nakamura, S.; Inenaga, T.; Kimura, G. Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet 1997, 350, 1734–1737. [Google Scholar] [CrossRef]
- Grim, C.E.; Wilson, T.W.; Nicholson, G.D.; Hassell, T.A.; Fraser, H.S.; Grim, C.M.; Wilson, D.M. Blood pressure in blacks. Twin studies in Barbados. Hypertension 1990, 15, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, M.H.; Fineberg, N.S.; Fineberg, S.E.; Weinberger, M. Salt sensitivity, pulse pressure, and death in normal and hypertensive humans. Hypertension 2001, 37, 429–432. [Google Scholar] [CrossRef]
- Fujita, T. Aldosterone in salt-sensitive hypertension and metabolic syndrome. J. Mol. Med. 2008, 86, 729–734. [Google Scholar] [CrossRef]
- Cao, W.; Li, A.; Wang, L.; Zhou, Z.; Su, Z.; Bin, W.; Wilcox, C.S.; Hou, F.F. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. JASN 2015, 26, 1619–1633. [Google Scholar] [CrossRef] [PubMed]
- Lai, E.Y.; Luo, Z.; Onozato, M.L.; Rudolph, E.H.; Solis, G.; Jose, P.A.; Wellstein, A.; Aslam, S.; Quinn, M.T.; Griendling, K.; et al. Effects of the antioxidant drug tempol on renal oxygenation in mice with reduced renal mass. Am. J. Physiol. Reg. I. 2012, 303, F64–F74. [Google Scholar] [CrossRef] [Green Version]
- Lipkowitz, M.S.; Wilcox, C.S. What level of sodium intake worsens renal outcomes? Am. J. Hypertens. 2014, 27, 1243–1244. [Google Scholar] [CrossRef] [PubMed]
- Swei, A.; Lacy, F.; DeLano, F.A.; Schmid-Schonbein, G.W. Oxidative stress in the Dahl hypertensive rat. Hypertension 1997, 30, 1628–1633. [Google Scholar] [CrossRef] [PubMed]
- Trolliet, M.R.; Rudd, M.A.; Loscalzo, J. Oxidative stress and renal dysfunction in salt-sensitive hypertension. Kidney Blood Press. Res. 2001, 24, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Kitada, K.; Nakano, D.; Liu, Y.; Fujisawa, Y.; Hitomi, H.; Shibayama, Y.; Shibata, H.; Nagai, Y.; Mori, H.; Masaki, T.; et al. Oxidative stress-induced glomerular mineralocorticoid receptor activation limits the benefit of salt reduction in Dahl salt-sensitive rats. PLoS ONE 2012, 7, e41896. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Ando, H.; Fujiwara, Y.; Fujimura, A. Vanin-1: A potential biomarker for nephrotoxicant-induced renal injury. Toxicology 2011, 290, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Ando, H.; Fujimura, A. Urinary vanin-1 as a novel biomarker for early detection of drug-induced acute kidney injury. J. Pharmacol. Exp. Ther. 2012, 341, 656–662. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Washino, S.; Kubo, T.; Natsui, S.; Fujisaki, A.; Kurokawa, S.; Ando, H.; Fujimura, A.; Morita, T. Early prediction of cisplatin-induced nephrotoxicity by urinary vanin-1 in patients with urothelial carcinoma. Toxicology 2016, 359, 71–75. [Google Scholar] [CrossRef]
- Aurrand-Lions, M.; Galland, F.; Bazin, H.; Zakharyev, V.M.; Imhof, B.A.; Naquet, P. Vanin-1, a novel GPI-linked perivascular molecule involved in thymus homing. Immunity 1996, 5, 391–405. [Google Scholar] [CrossRef]
- Pitari, G.; Malergue, F.; Martin, F.; Philippe, J.M.; Massucci, M.T.; Chabret, C.; Maras, B.; Dupre, S.; Naquet, P.; Galland, F. Pantetheinase activity of membrane-bound Vanin-1: Lack of free cysteamine in tissues of Vanin-1 deficient mice. FEBS Lett. 2000, 483, 149–154. [Google Scholar] [CrossRef]
- Dupre, S.; Graziani, M.T.; Rosei, M.A.; Fabi, A.; Del. Grosso, E. The enzymatic breakdown of pantethine to pantothenic acid and cystamine. Eur. J. Biochem. 1970, 16, 571–578. [Google Scholar] [CrossRef]
- Berruyer, C.; Martin, F.M.; Castellano, R.; Macone, A.; Malergue, F.; Garrido-Urbani, S.; Millet, V.; Imbert, J.; Dupre, S.; Pitari, G.; et al. Vanin-1−/−mice exhibit a glutathione-mediated tissue resistance to oxidative stress. Mol. Cell. Biol. 2004, 24, 7214–7224. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lin, L.; Lu, Y.; Liu, H.; Duan, Y.; Zhu, X.; Zou, C.; Manning, R.D., Jr.; Liu, R. Interaction between nitric oxide and superoxide in the macula densa in aldosterone-induced alterations of tubuloglomerular feedback. Am. J. Physiol. Renal Physiol. 2013, 304, F326–F332. [Google Scholar] [CrossRef] [PubMed]
- Shavit, L.; Lifschitz, M.D.; Epstein, M. Aldosterone blockade and the mineralocorticoid receptor in the management of chronic kidney disease: Current concepts and emerging treatment paradigms. Kidney Int. 2012, 81, 955–968. [Google Scholar] [CrossRef] [PubMed]
- Oshima, N.; Onimaru, H.; Takechi, H.; Yamamoto, K.; Watanabe, A.; Uchida, T.; Nishida, Y.; Oda, T.; Kumagai, H. Aldosterone is synthesized in and activates bulbospinal neurons through mineralocorticoid receptors and ENaCs in the RVLM. Hypertens Res. 2013, 36, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Brown, N.J. Contribution of aldosterone to cardiovascular and renal inflammation and fibrosis. Nat. Rev. Nephrol. 2013, 9, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Guichard, J.L.; Clark, D.; Calhoun, D.A.; Ahmed, M.I. Aldosterone receptor antagonists: Current perspectives and therapies. Vasc. Health. Risk Manag. 2013, 9, 321–331. [Google Scholar] [PubMed]
- Keidar, S.; Kaplan, M.; Pavlotzky, E.; Coleman, R.; Hayek, T.; Hamoud, S.; Aviram, M. Aldosterone administration to mice stimulates macrophage NADPH oxidase and increases atherosclerosis development: A possible role for angiotensin-converting enzyme and the receptors for angiotensin II and aldosterone. Circulation 2004, 109, 2213–2220. [Google Scholar] [CrossRef]
- Gill, P.S.; Wilcox, C.S. NADPH oxidases in the kidney. Antioxid. Redox. Sign. 2006, 8, 1597–1607. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Wilcox, C.S. Oxidative stress in hypertension: Role of the kidney. Antioxid. Redox. Sign. 2014, 20, 74–101. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.H. The NOX family of ROS-generating NADPH oxidases: Physiology and pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Geiszt, M.; Kopp, J.B.; Varnai, P.; Leto, T.L. Identification of renox, an NAD(P)H oxidase in kidney. Proc. Natl. Acad. Sci. USA 2000, 97, 8010–8014. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.J.; Garvin, J.L. NADPH oxidase 4 mediates flow-induced superoxide production in thick ascending limbs. Am. J. Physiol. Renal 2012, 303, F1151–F1156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huling, J.C.; Pisitkun, T.; Song, J.H.; Yu, M.J.; Hoffert, J.D.; Knepper, M.A. Gene expression databases for kidney epithelial cells. Am. J. Physiol. Renal 2012, 302, F401–F407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Lai, E.Y.; Luo, Z.; Solis, G.; Griendling, K.K.; Taylor, W.R.; Jose, P.A.; Wellstein, A.; Welch, W.J.; Wilcox, C.S. Superoxide and hydrogen peroxide counterregulate myogenic contractions in renal afferent arterioles from a mouse model of chronic kidney disease. Kidney Int. 2017, 92, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.I.; Lassegue, B.; Lee, M.; Zafari, R.; Long, J.S.; Saavedra, H.I.; Griendling, K.K. Poldip2 knockout results in perinatal lethality, reduced cellular growth and increased autophagy of mouse embryonic fibroblasts. PLoS ONE 2014, 9, e96657. [Google Scholar] [CrossRef] [PubMed]
- Kitiyakara, C.; Chabrashvili, T.; Chen, Y.; Blau, J.; Karber, A.; Aslam, S.; Welch, W.J.; Wilcox, C.S. Salt intake, oxidative stress, and renal expression of NADPH oxidase and superoxide dismutase. JASN 2003, 14, 2775–2782. [Google Scholar] [CrossRef]
- Chan, S.H.; Wu, K.L.; Chang, A.Y.; Tai, M.H.; Chan, J.Y. Oxidative impairment of mitochondrial electron transport chain complexes in rostral ventrolateral medulla contributes to neurogenic hypertension. Hypertension 2009, 53, 217–227. [Google Scholar] [CrossRef]
- Nagae, A.; Fujita, M.; Kawarazaki, H.; Matsui, H.; Ando, K.; Fujita, T. Sympathoexcitation by oxidative stress in the brain mediates arterial pressure elevation in obesity-induced hypertension. Circulation 2009, 119, 978–986. [Google Scholar] [CrossRef]
- Nagasu, H.; Satoh, M.; Kuwabara, A.; Yorimitsu, D.; Sakuta, T.; Tomita, N.; Kashihara, N. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol. Dial. Transplant. 2010, 25, 2889–2898. [Google Scholar] [CrossRef] [Green Version]
- Gloire, G.; Legrand-Poels, S.; Piette, J. NF-kappaB activation by reactive oxygen species: Fifteen years later. Biochem. Pharmacol. 2006, 72, 1493–1505. [Google Scholar] [CrossRef] [PubMed]
- Matoba, K.; Kawanami, D.; Ishizawa, S.; Kanazawa, Y.; Yokota, T.; Utsunomiya, K. Rho-kinase mediates TNF-alpha-induced MCP-1 expression via p38 MAPK signaling pathway in mesangial cells. Biochem. Biophys. Res. Commun. 2010, 402, 725–730. [Google Scholar] [CrossRef] [PubMed]
- Bao, W.; Behm, D.J.; Nerurkar, S.S.; Ao, Z.; Bentley, R.; Mirabile, R.C.; Johns, D.G.; Woods, T.N.; Doe, C.P.; Coatney, R.W.; et al. Effects of p38 MAPK Inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc. Pharmacol. 2007, 49, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Bonizzi, G.; Piette, J.; Merville, M.P.; Bours, V. Cell type-specific role for reactive oxygen species in nuclear factor-kappaB activation by interleukin-1. Biochem. Pharmacol. 2000, 59, 7–11. [Google Scholar] [CrossRef]
- Kuwabara, N.; Tamada, S.; Iwai, T.; Teramoto, K.; Kaneda, N.; Yukimura, T.; Nakatani, T.; Miura, K. Attenuation of renal fibrosis by curcumin in rat obstructive nephropathy. J. Urol. 2006, 67, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhong, J.; Yang, H.C.; Fogo, A.B. Cross Talk from Tubules to Glomeruli. Toxicol. Pathol. 2018, 46, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Hosohata, K.; Jin, D.; Takai, S.; Iwanaga, K. Vanin-1 in Renal Pelvic Urine Reflects Kidney Injury in a Rat Model of Hydronephrosis. Int. J. Mol. Sci. 2018, 19, 3186. [Google Scholar] [CrossRef]
- Gross, M.L.; Adamczak, M.; Rabe, T.; Harbi, N.A.; Krtil, J.; Koch, A.; Hamar, P.; Amann, K.; Ritz, E. Beneficial Effects of Estrogens on Indices of Renal Damage in Uninephrectomized SHRsp. JASN 2004, 15, 348–358. [Google Scholar] [CrossRef]


| Parameters | DR Rats | DS Rats | ||||
|---|---|---|---|---|---|---|
| Normal Salt (DR/N) | High Salt (DR/H) | Normal Salt (DS/N) | High Salt (DS/H) | High Salt + Tempol (DS/H + Tempol) | High Salt + Eplerenone (DS/H + Eplerenone) | |
| SBP, mmHg | 101.7 ± 4.5 | 110 ± 2.6 | 124.5 ± 2.4 | 160.8 ± 9.2 aa | 121.2 ± 7.4 bb | 132.2 ± 3.4 b |
| BW, g | 364.8 ± 4.4 | 354.5 ± 3.6 | 336.5 ± 3.3 | 316 ± 5.4 aa | 308.2 ± 6.9 | 296 ± 7.4 bb |
| Left KW, mg/g BW | 3.6 ± 0.1 | 3.3 ± 0.06 | 3.9 ± 0.09 | 5.2 ± 0.13 aa | 5.4 ± 0.16 | 4.2 ± 0.06 bb |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosohata, K.; Jin, D.; Takai, S.; Iwanaga, K. Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats. Int. J. Mol. Sci. 2019, 20, 4481. https://doi.org/10.3390/ijms20184481
Hosohata K, Jin D, Takai S, Iwanaga K. Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats. International Journal of Molecular Sciences. 2019; 20(18):4481. https://doi.org/10.3390/ijms20184481
Chicago/Turabian StyleHosohata, Keiko, Denan Jin, Shinji Takai, and Kazunori Iwanaga. 2019. "Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats" International Journal of Molecular Sciences 20, no. 18: 4481. https://doi.org/10.3390/ijms20184481
APA StyleHosohata, K., Jin, D., Takai, S., & Iwanaga, K. (2019). Involvement of Vanin-1 in Ameliorating Effect of Oxidative Renal Tubular Injury in Dahl-Salt Sensitive Rats. International Journal of Molecular Sciences, 20(18), 4481. https://doi.org/10.3390/ijms20184481







