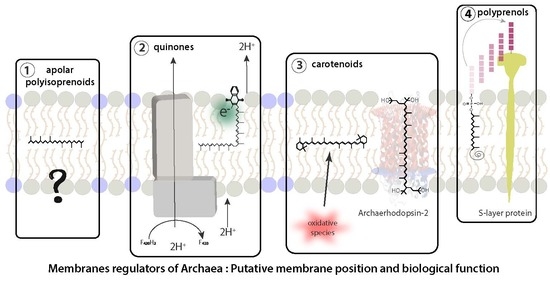
In Search for the Membrane Regulators of Archaea
Abstract
1. Introduction
2. Structure of Bacterial and Eukaryal Membrane Regulators
3. Mechanisms of Membrane Regulation in Eukarya and Bacteria
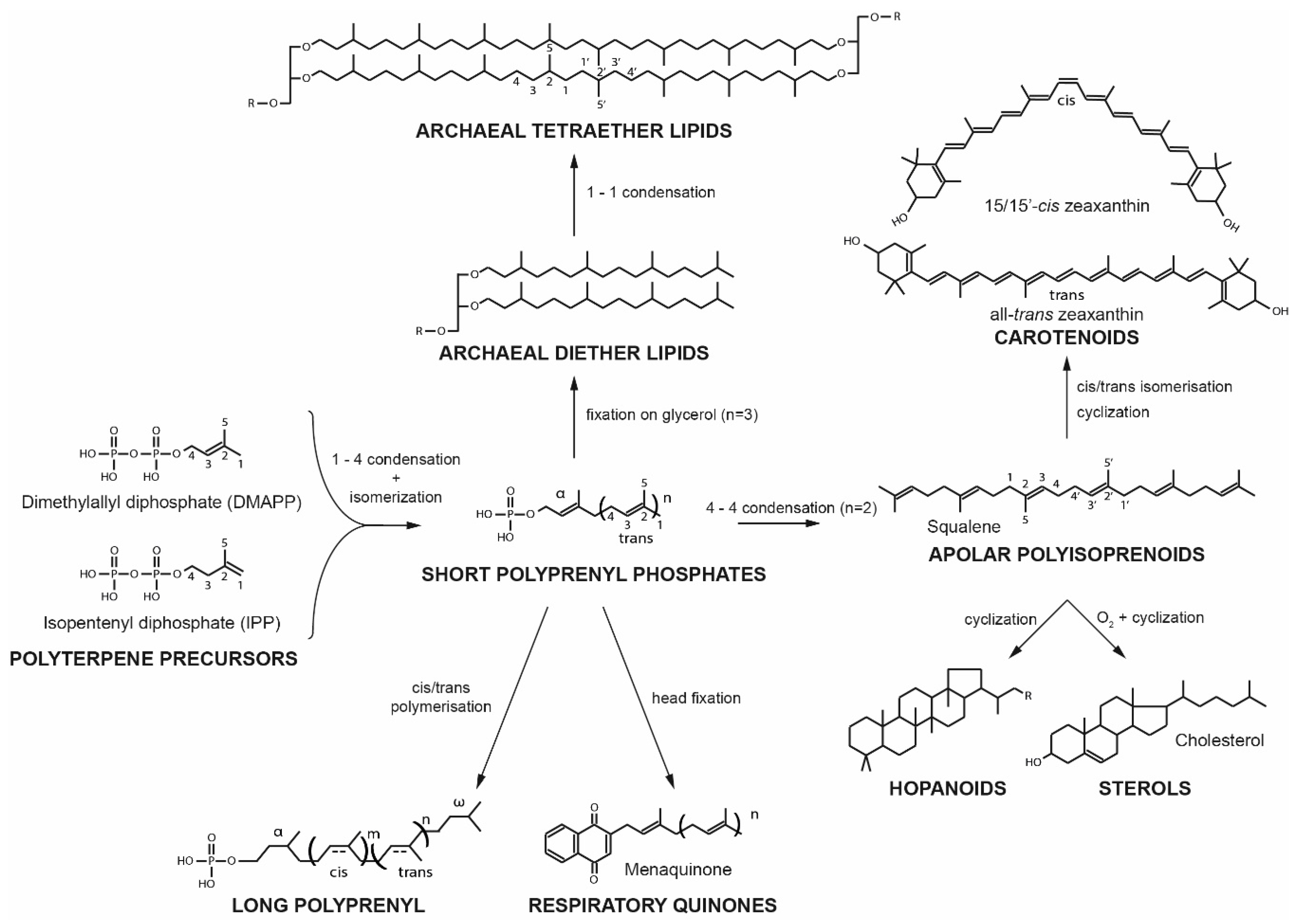
4. Candidate Surrogates for Sterol and Hopanoid Membrane Regulators in Archaea
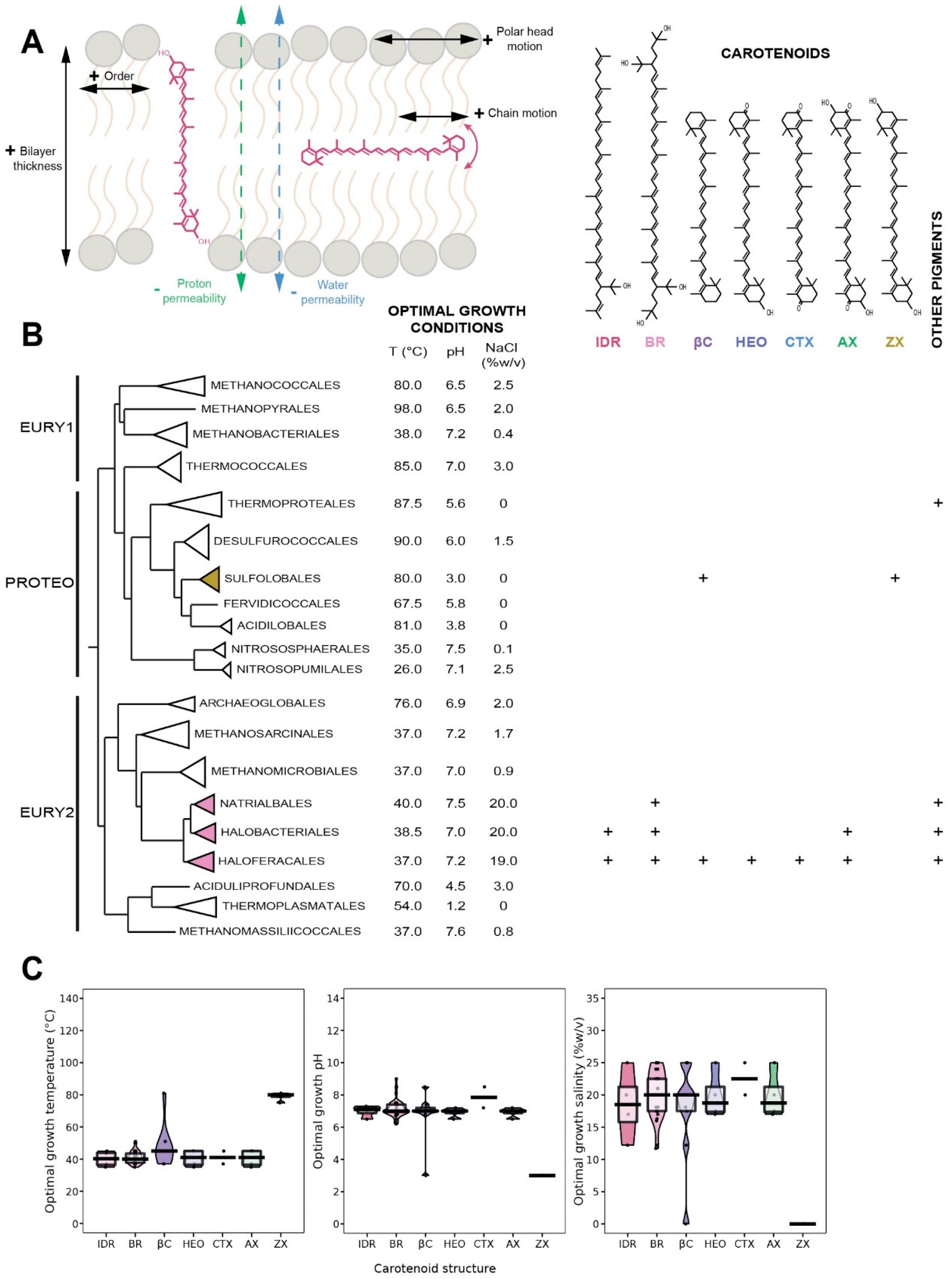
5. Carotenoids
5.1. Distribution in Archaea
5.2. Biological Function of Carotenoids
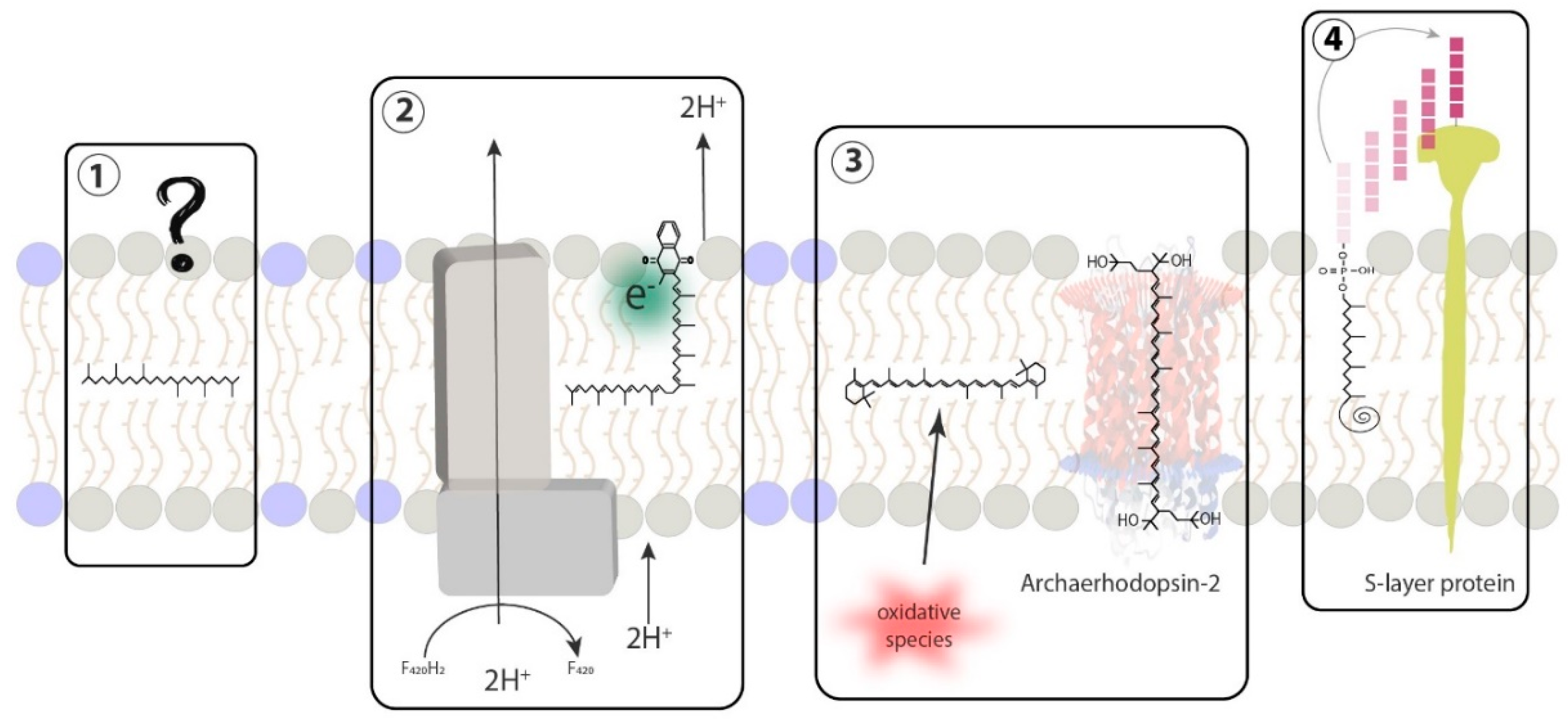
5.3. Insertion of Carotenoids in the Membrane
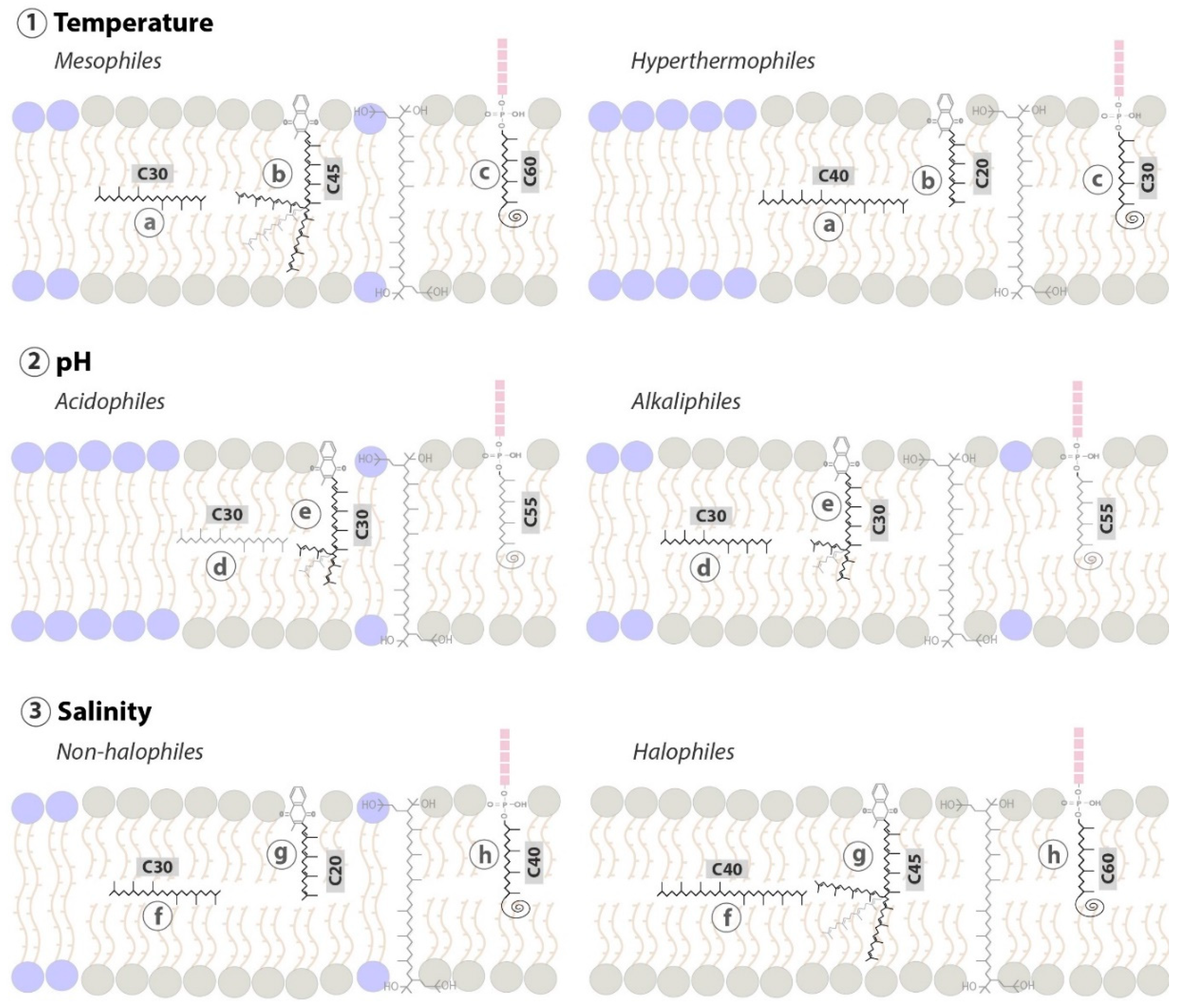
5.4. Carotenoids as Putative Membrane Regulators in Archaea
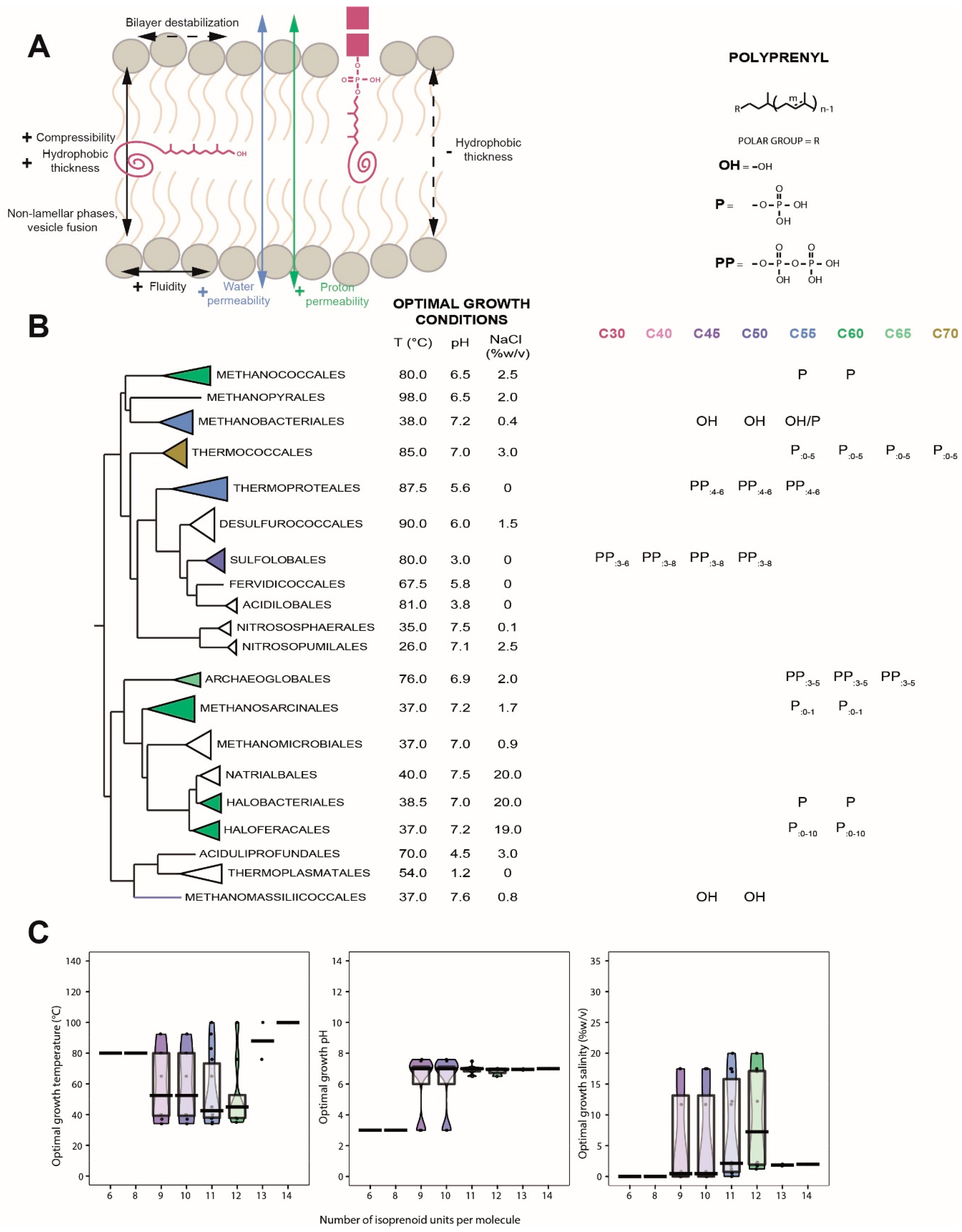
6. Polyprenols
6.1. Distribution in Archaea
6.2. Biological Function of Polyprenols
6.3. Insertion of Polyprenols in the Membrane
6.4. Polyprenols as Putative Membrane Regulators in Archaea
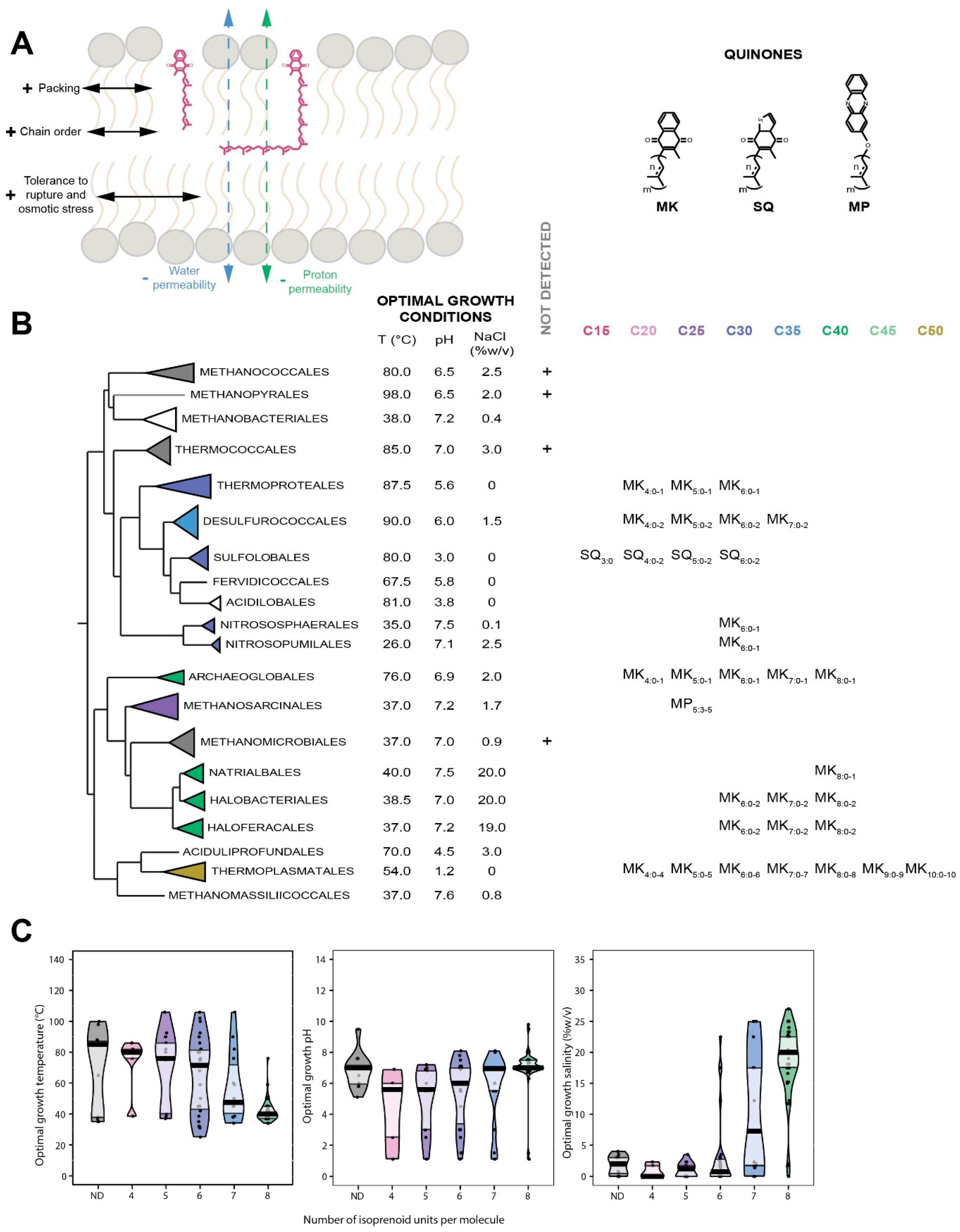
7. Quinones
7.1. Distribution in Archaea
7.2. Biological Function of Quinones
7.3. Insertion of Quinones in the Membrane
7.4. Quinones as Putative Membrane Regulators in Archaea
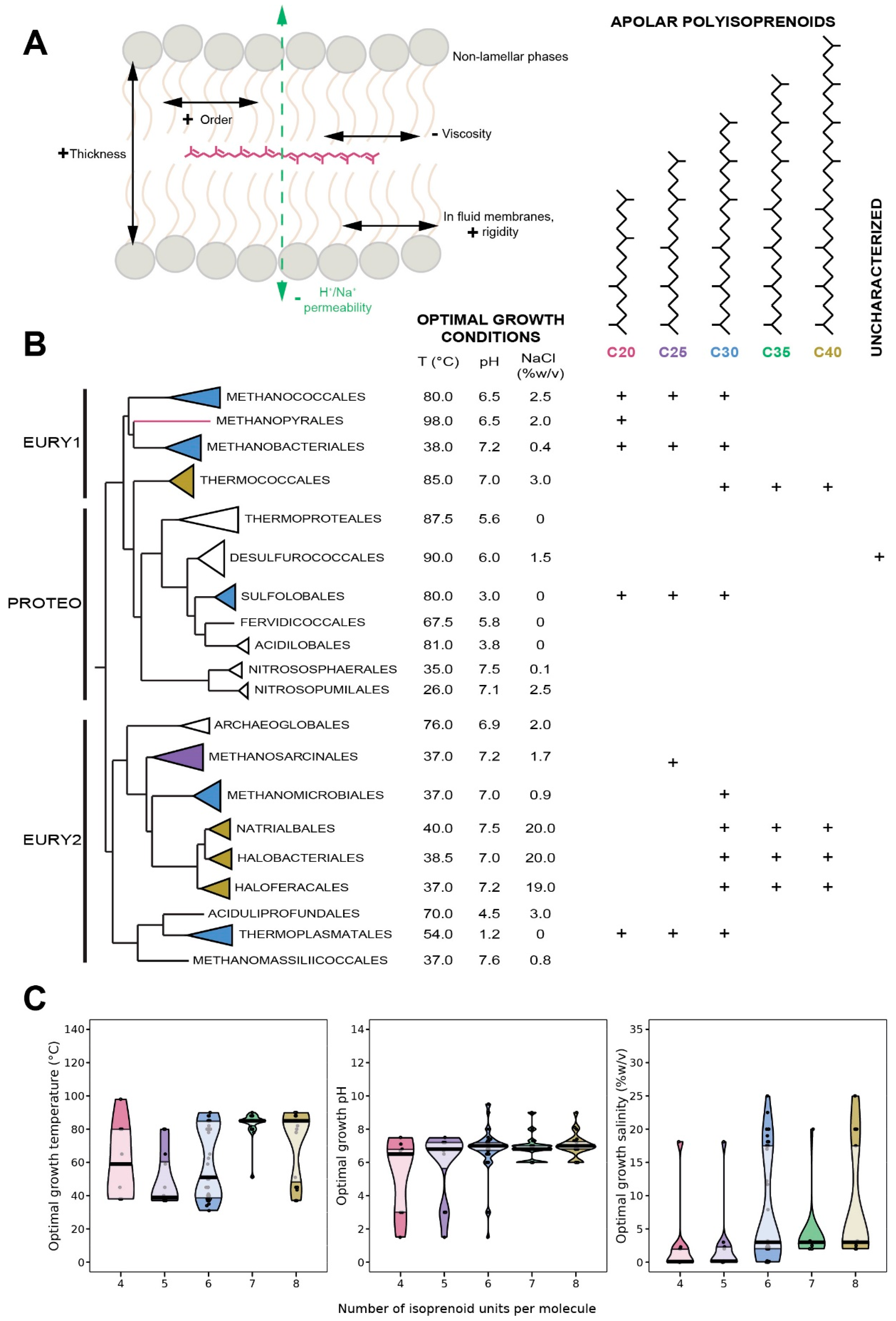
8. Apolar Polyisoprenoids
8.1. Distribution in Archaea
8.2. Biological Function of Apolar Polyisoprenoids
8.3. Insertion of Apolar Polyisoprenoids in the Membrane
8.4. Apolar Polyisoprenoids as Putative Membrane Regulators in Archaea
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IPP | Isopentenyl pyrophosphate |
| DMAP | Dimethylallyl diphosphate |
| Tm | Lipid phase transition temperature |
| IDR | Isopentenyldehydrorhodopsin |
| BR | Bacterioruberin |
| BC | β-carotene |
| HEO | 3-hydroechinenone |
| CTX | Canthaxanthin |
| AX | Astaxanthin |
| ZX | Zeaxanthin |
| EURY1 | Euryarchaeota cluster I |
| PROTEO | Proteoarchaeota |
| EURY2 | Euryarchaeota cluster II |
| Topt | Optimal growth temperature |
| Ubx | Ubiquinones and its number of isoprenoid units (x) |
| MK | Menaquinones |
| MMK | Monomethylmenaquinones |
| MTK | Methionaquinones |
| DMK | Demethylmenaquinones |
| DMMK | Dimethylmenaquinones |
| SQ | Sulfolobusquinones |
| CQ | Caldariellaquinones |
| BDTQ | Benzodithiophenoquinones |
| MP | Methanophenazines |
| Qm:n | Polar head group from quinone (Q), size of the isoprenoid side chain (m) and its number of unsaturations (n) |
| ATP | Adenosine triphosphate |
| NADH | Nicotinamide adenine dinucleotide |
References
- Singer, S.J.J.; Nicolson, G.L.L. The fluid mosaic model of the structure of cell membranes. Science 1972, 175, 720–731. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, G.L. The fluid-mosaic model of membrane structure: Still relevant to understanding the structure, function and dynamics of biological membranes after more than 40 years. Biochim. Biophys. Acta-Biomembr. 2014, 1838, 1451–1466. [Google Scholar] [CrossRef] [PubMed]
- Subczynski, W.K.; Pasenkiewicz-Gierula, M.; Widomska, J.; Mainali, L.; Raguz, M. High cholesterol/low cholesterol: Effects in biological membranes: A review. Cell Biochem. Biophys. 2017, 75, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Oldfield, E.; Chapman, D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972, 23, 285–297. [Google Scholar] [CrossRef]
- Demel, R.A.; De Kruyff, B. The function of sterols in membranes. Biochim. Biophys. Acta 1976, 457, 109–132. [Google Scholar] [CrossRef]
- Yeagle, P.L. Cholesterol and the cell membrane. Bba-Rev. Biomembr. 1985, 822, 267–287. [Google Scholar] [CrossRef]
- García-Arribas, A.B.; Alonso, A.; Goñi, F.M. Cholesterol interactions with ceramide and sphingomyelin. Chem. Phys. Lipids 2016, 199, 26–34. [Google Scholar] [CrossRef]
- Grouleff, J.; Irudayam, S.J.; Skeby, K.K.; Schiøtt, B. The influence of cholesterol on membrane protein structure, function, and dynamics studied by molecular dynamics simulations. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 1783–1795. [Google Scholar] [CrossRef]
- Marquardt, D.; Kučerka, N.; Wassall, S.R.; Harroun, T.A.; Katsaras, J. Cholesterol’s location in lipid bilayers. Chem. Phys. Lipids 2016, 199, 17–25. [Google Scholar] [CrossRef]
- Ourisson, G.; Rohmer, M.; Poralla, K. Prokaryotic hopanoids and other polyterpenoid sterol surrogates. Ann. Rev. Microbiol. 1987, 41, 301–333. [Google Scholar] [CrossRef] [PubMed]
- Sáenz, J.P.; Broda, M.; Lagny, T.J.; Grosser, D.; Lavrynenko, O.; Bradley, A.S.; Simons, K. Hopanoids as functional analogues of cholesterol in bacterial membranes. Proc. Natl. Acad. Sci. USA 2015, 112, 11971–11976. [Google Scholar] [CrossRef] [PubMed]
- Belin, B.J.; Busset, N.; Giraud, E.; Molinaro, A.; Silipo, A.; Newman, D.K. Hopanoid lipids: From membranes to plant-bacteria interactions. Nat. Rev. Microbiol. 2018, 16, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Lombard, J.; Moreira, D. Origins and early evolution of the mevalonate pathway of isoprenoid biosynthesis in the three domains of life. Mol. Biol. Evol. 2011, 28, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Paniagua-Michel, J.; Olmos-Soto, J.; Ruiz, M.A. Pathways of Carotenoid Biosynthesis in Bacteria and Microalgae. In Microbial Carotenoids from Bacteria and Microalgae: Methods and Protocols, Methods in Molecular Biology, 1st ed.; Barredo, J.-L., Ed.; Springer Science & Business Media: Heidelberg, Germany, 2012; Volume 892, pp. 1–12. [Google Scholar]
- Róg, T.; Pasenkiewicz-Gierula, M. Cholesterol Effects on the Phosphatidylcholine Bilayer Nonpolar Region: A Molecular Simulation Study. Biophys. J. 2001, 84, 1818–1826. [Google Scholar] [CrossRef]
- Bloch, K.E. Sterol, Structure and Membrane Function. Crit. Rev. Biochem. 1983, 14, 47–92. [Google Scholar] [CrossRef]
- Chen, Z.; Rand, R.P. The influence of cholesterol on phospholipid membrane curvature and bending elasticity. Biophys. J. 1997, 73, 267–276. [Google Scholar] [CrossRef]
- Baumgart, T.; Hess, S.; Webb, W. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature 2003, 425, 821–824. [Google Scholar] [CrossRef] [PubMed]
- Poger, D.; Mark, A.E. The relative effect of sterols and hopanoids on lipid bilayers: When comparable is not identical. J. Phys. Chem. B 2013, 117, 16129–16140. [Google Scholar] [CrossRef] [PubMed]
- Krajewski-Bertrand, M.A.; Hayer, M.; Wolff, G.; Milon, A.; Albrecht, A.-M.; Heissler, D.; Nakatanif, Y.; Ourisson, G. Tricyclohexaprenol and an octaprenediol, two of the “primitive” amphiphilic lipids do improve phospholipidic membranes. Tetrahedron 1989, 46, 3143–3154. [Google Scholar] [CrossRef]
- Coleman, G.A.; Pancost, R.D.; Williams, T.A. Investigating the origins of membrane phospholipid biosynthesis genes using outgroup-free rooting. Genome Biol. Evol. 2019, 11, 883–898. [Google Scholar] [CrossRef] [PubMed]
- Landrum, J.T. Carotenoids Biological Functions and Properties, 1st ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database 2017, 2017, 1–11. [Google Scholar] [CrossRef]
- Kull, D.R.; Pfander, H. Isolation and structure elucidation of carotenoid glycosides from the thermoacidophilic Archaea Sulfolobus shibatae. J. Nat. Prod. 1997, 60, 371–374. [Google Scholar] [CrossRef]
- Asker, D.; Awad, T.; Ohta, Y. Lipids of Haloferax alexandrinus strain TMT: An extremely halophilic canthaxanthin-producing archaeon. J. Biosci. Bioeng. 2002, 93, 37–43. [Google Scholar] [CrossRef]
- Leuko, S.; Coyle, C.M.; Neilan, B.A.; Walter, M.R.; Marshall, C.P.; Burns, B.P. Carotenoid analysis of halophilic archaea by resonance Raman spectroscopy. Astrobiology 2007, 7, 631–643. [Google Scholar]
- Mandelli, F.; Miranda, V.S.; Rodrigues, E.; Mercadante, A.Z. Identification of carotenoids with high antioxidant capacity produced by extremophile microorganisms. World J. Microbiol. Biotechnol. 2012, 28, 1781–1790. [Google Scholar] [CrossRef]
- Jehlička, J.; Edwards, H.G.M.; Oren, A. Bacterioruberin and salinixanthin carotenoids of extremely halophilic Archaea and Bacteria: A Raman spectroscopic study. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2013, 106, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Squillaci, G.; Parrella, R.; Carbone, V.; Minasi, P.; La Cara, F.; Morana, A. Carotenoids from the extreme halophilic archaeon Haloterrigena turkmenica: Identification and antioxidant activity. Extremophiles 2017, 21, 933–945. [Google Scholar] [CrossRef] [PubMed]
- Kushwaha, S.C.; Pugh, E.L.; Kramer, J.K.G.; Kates, M. Isolation and identification of dehydrosqualene and C40-carotenoid pigments in Halobacterium cutirubrum. Biochim. Biophys. Acta (Bba)/Lipids Lipid Metab. 1972, 260, 492–506. [Google Scholar] [CrossRef]
- Lobasso, S.; Lopalco, P.; Mascolo, G.; Corcelli, A. Lipids of the ultra-thin square halophilic archaeon Haloquadratum walsbyi. Archaea 2008, 2, 177–183. [Google Scholar] [CrossRef]
- Yatsunami, R.; Ando, A.; Yang, Y.; Takaichi, S.; Kohno, M.; Matsumura, Y.; Ikeda, H.; Fukui, T.; Nakasone, K.; Fujita, N.; et al. Identification of carotenoids from the extremely halophilic archaeon Haloarcula japonica. Front. Microbiol. 2014, 5, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Adam, P.S.; Borrel, G.; Brochier-Armanet, C.; Gribaldo, S. The growing tree of Archaea: New perspectives on their diversity, evolution and ecology. ISME J. 2017, 11, 2407–2425. [Google Scholar] [CrossRef] [PubMed]
- Krinsky, N.I. Carotenoid protection against oxidation. Pure Appl. Chem. 1979, 51, 649–660. [Google Scholar] [CrossRef]
- McNulty, H.P.; Byun, J.; Lockwood, S.F.; Jacob, R.F.; Mason, R.P. Differential effects of carotenoids on lipid peroxidation due to membrane interactions: X-ray diffraction analysis. Biochim. Biophys. Acta-Biomembr. 2007, 1768, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Johnson, Q.R.; Mostofian, B.; Fuente Gomez, G.; Smith, J.C.; Cheng, X. Effects of carotenoids on lipid bilayers. Phys. Chem. Chem. Phys. 2018, 20, 3795–3804. [Google Scholar] [CrossRef] [PubMed]
- Merhan, O. The biochemistry and antioxidant properties of carotenoids. In Carotenoids, 1st ed.; Cvetkovic, D., Nikolic, G., Eds.; InTech: Rijeka, Croatia, 2017; pp. 51–66. [Google Scholar]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant properties of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Albrecht, M.; Takaichi, S.; Steiger, S.; Wang, Z.-Y.; Sandmann, G. Novel hydroxycarotenoids with improved antioxidative properties produced by gene combination in Escherichia coli. Nat. Biotechnol. 2000, 18, 843–846. [Google Scholar] [CrossRef]
- Gruszecki, W.I.; Widomska, J.; Strzałka, K.; Kostecka-Gugała, A.; Latowski, D. Calorimetric studies of the effect of cis-carotenoids on the thermotropic phase behavior of phosphatidylcholine bilayers. Biophys. Chem. 2008, 140, 108–114. [Google Scholar]
- Gruszecki, W.I.; Strzałka, K. Carotenoids as modulators of lipid membrane physical properties. Biochim. Biophys. Acta-Mol. Basis. Dis. 2005, 1740, 108–115. [Google Scholar] [CrossRef]
- Yoshimura, K.; Kouyama, T. Structural role of bacterioruberin in the trimeric structure of Archaerhodopsin-2. J. Mol. Biol. 2008, 375, 1267–1281. [Google Scholar] [CrossRef]
- Kouyama, T.; Kanada, S.; Takeguchi, Y.; Narusawa, A.; Murakami, M.; Ihara, K. Crystal structure of the light-driven chloride pump halorhodopsin from Natronomonas pharaonis. J. Mol. Biol. 2010, 396, 564–579. [Google Scholar] [CrossRef] [PubMed]
- Lazrak, T.; Wolff, G.; Albrecht, A.M.; Nakatani, Y.; Ourisson, G.; Kates, M. Bacterioruberins reinforce reconstituted Halobacterium lipid membranes. Biochim. Biophys. Acta-Biomembr. 1988, 939, 160–162. [Google Scholar] [CrossRef]
- Socaciu, C.; Bojarski, P.; Aberle, L.; Diehl, H.A. Different ways to insert carotenoids into liposomes affect structure and dynamics of the bilayer differently. Biophys. Chem. 2002, 99, 1–15. [Google Scholar] [CrossRef]
- Shinoda, W.; Shinoda, K.; Baba, T.; Mikami, M. Molecular dynamics study of bipolar tetraether lipid membranes. Biophys. J. 2005, 89, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Gabrielska, J.; Gruszecki, W.I. Zeaxanthin (dihydroxy-β-carotene) but not β-carotene rigidities lipid membranes: A 1H-NMR study of carotenoid-egg phosphatidylcholine liposomes. Biochim. Biophys. Acta-Biomembr. 1996, 1285, 167–174. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Gruszecki, W.I.; Sielewiesiuk, J. Effects of polar carotenoids on dimyristoylphosphatidylcholine membranes: A spin-label study. Biochim. Biophys. Acta-Biomembr. 1992, 1105, 97–108. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Wisniewska, A.; Widomska, J. Can macular xanthophylls replace cholesterol in formation of the liquid-ordered phase in lipid-bilayer membranes? Acta Biochim. Pol. 2012, 59, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Strzałka, K.; Gruszecki, W.I. Effect of β-carotene on structural and dynamic properties of model phosphatidylcholine membranes. I. An EPR spin label study. Biochim. Biophys. Acta-Biomembr. 1994, 1194, 138–142. [Google Scholar] [CrossRef]
- Berglund, A.H.; Nilsson, R.; Liljenberg, C. Permeability of large unilamellar digalactosyldiacylglycerol vesicles for protons and glucose - Influence of α-tocopherol, β-carotene, zeaxanthin and cholesterol. Plant Physiol. Biochem. 1999, 37, 179–186. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Sielewiesiuk, J. Effect of polar carotenoids on the oxygen diffusion-concentration product in lipid bilayers. An EPR spin label study. Biochim. Biophys. Acta-Biomembr. 1991, 1068, 68–72. [Google Scholar] [CrossRef]
- Subczynski, W.K.; Markowska, E.; Sielewiesiuk, J. Spin-label studies on phosphatidylcholine-polar carotenoid membranes: Effects of alkyl-chain length and unsaturation. Biochim. Biophys. Acta-Biomembr. 1993, 1150, 173–181. [Google Scholar] [CrossRef]
- Suwalsky, M.; Hidalgo, P.; Strzalka, K.; Kostecka-Gugala, A. Comparative X-ray studies on the interaction of carotenoids with a model phosphatidylcholine membrane. Z. Fur. Nat-Sect. C. J. Biosci. 2002, 57, 129–134. [Google Scholar]
- Augustynska, D.; Jemioła-Rzemiå ska, M.; Burda, K.; Strzałka, K. Influence of polar and nonpolar carotenoids on structural and adhesive properties of model membranes. Chem. Biol. Interact. 2015, 239, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Tan, C.; Zhang, Y.; Abbas, S.; Feng, B.; Zhang, X.; Qin, F. Modulating effect of lipid bilayer-carotenoid interactions on the property of liposome encapsulation. Colloids Surf. B. Biointerfaces 2015, 128, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Holthuis, J. Regulating membrane curvature. In Regulatory Mechanisms of Intracellular Membrane Transport, 1st ed.; Keränen, S., Jäntti, J., Eds.; Springer: Heidelberg, Germany, 2004; pp. 39–64. [Google Scholar]
- Shahmohammadi, H.R.; Asgarani, E.; Terato, H.; Saito, T.; Ohyama, Y.; Gekko, K.; Yamamoto, O.; Ide, H. Protective roles of bacterioruberin and intracellular KCl in the resistance of Halobacterium salinarium against DNA-damaging agents. J. Radiat. Res. 1998, 39, 251–262. [Google Scholar] [CrossRef]
- Calegari-Santos, R.; Diogo, R.A.; Fontana, J.D.; Bonfim, T.M.B. Carotenoid production by halophilic archaea under different culture conditions. Curr. Microbiol. 2016, 72, 641–651. [Google Scholar] [CrossRef]
- D’Souza, S.E.; Altekar, W.; D’Souza, S.F. Adaptive response of Haloferax mediterranei to low concentrations. Arch. Microbiol. 1997, 168, 68–71. [Google Scholar] [CrossRef]
- Bidle, K.A.; Hanson, T.E.; Howell, K.; Nannen, J. HMG-CoA reductase is regulated by salinity at the level of transcription in Haloferax volcanii. Extremophiles 2007, 11, 49–55. [Google Scholar] [CrossRef]
- Asker, D.; Ohta, Y. Production of canthaxanthin by extremely halophilic bacteria. J. Biosci. Bioeng. 1999, 88, 617–621. [Google Scholar] [CrossRef]
- Kushwaha, S.C.; Juez-Pérez, G.; Rodriguez-Valera, F.; Kates, M.; Kushner, D.J. Survey of lipids of a new group of extremely halophilic bacteria from salt ponds in Spain. Can. J. Microbiol. 1982, 28, 1365–1372. [Google Scholar] [CrossRef]
- Gochnauer, M.B.; Kushwaha, S.C.; Kates, M.; Kushner, D.J. Nutritional control of pigment and isoprenoid compound formation in extremely halophilic bacteria. Arch. Mikrobiol. 1972, 84, 339–349. [Google Scholar] [CrossRef]
- Swiezewska, E.; Danikiewicz, W. Polyisoprenoids: Structure, biosynthesis and function. Prog. Lipid Res. 2005, 44, 235–258. [Google Scholar] [CrossRef] [PubMed]
- Skorupinska-Tudek, K.; Wojcik, J.; Swiezewska, E. Polyisoprenoid alcohols - Recent results of structural studies. Chem. Rec. 2008, 8, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Hartley, M.D.; Imperiali, B. At the membrane frontier: A prospectus on the remarkable evolutionary conservation of polyprenols and polyprenyl-phosphates. Arch. Biochem. Biophys. 2012, 517, 83–97. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Knudsen, M.J.; Troy, F.A. Nuclear magnetic resonance studies of polyisoprenols in model membranes. Chem. Phys. Lipids 1989, 51, 205–212. [Google Scholar] [CrossRef]
- Bauersachs, T.; Schouten, S.; Compaoré, J.; Stal, L.J.; Sinninghe Damsté, J.S. Occurrence of C35-C45 polyprenols in filamentous and unicellular cyanobacteria. Org. Geochem. 2010, 41, 867–870. [Google Scholar] [CrossRef]
- Szabo, E.I.; Amdur, B.H.; Socransky, S.S. Lipid composition of Streptococcus mutans. Caries Res. 1978, 12, 21–27. [Google Scholar] [CrossRef]
- Jones, M.B.; Rosenberg, J.N.; Betenbaugh, M.J.; Krag, S.S. Structure and synthesis of polyisoprenoids used in N-glycosylation across the three domains of life. Biochim. Biophys. Acta-Gen. Subj. 2009, 1790, 485–494. [Google Scholar] [CrossRef]
- Swiezewska, E.; Sasak, W.; Mankowski, T.; Jankowski, W.; Vogtman, T.; Krajewska, I.; Hertel, J.; Skoczylas, E.; Chojnacki, T. The search for plant polyprenols. Acta Biochim. Pol. 1994, 41, 221–260. [Google Scholar]
- Manat, G.; Roure, S.; Auger, R.; Bouhss, A.; Barreteau, H.; Mengin-Lecreulx, D.; Touzé, T. Deciphering the metabolism of undecaprenyl-phosphate: The bacterial cell-wall unit carrier at the membrane frontier. Microb. Drug Resist. 2014, 20, 199–214. [Google Scholar] [CrossRef]
- Guan, Z.; Eichler, J. Liquid chromatography/tandem mass spectrometry of dolichols and polyprenols, lipid sugar carriers accross evolution. Biochim. Biophys. Acta 2011, 1811, 800–806. [Google Scholar] [CrossRef] [PubMed]
- Guan, Z.; Meyer, B.H.; Albers, S.V.; Eichler, J. The thermoacidophilic archaeon Sulfolobus acidocaldarius contains an unsually short, highly reduced dolichyl phosphate. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2011, 1811, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.M.; Imperiali, B.; Eichler, J.; Guan, Z. N-linked glycans are assembled on highly reduced dolichol phosphate carriers in the hyperthermophilic archaea Pyrococcus furiosus. PLoS ONE 2015, 10, e0130482. [Google Scholar] [CrossRef] [PubMed]
- Yoshinaga, M.Y.; Gagen, E.J.; Wörmer, L.; Broda, N.K.; Meador, T.B.; Wendt, J.; Thomm, M.; Hinrichs, K.-U. Methanothermobacter thermautotrophicus modulates its membrane lipids in response to hydrogen and nutrient availability. Front. Microbiol. 2015, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.W.; Elling, F.J.; Yoshinaga, M.Y.; Söllinger, A.; Urich, T.; Hinrichs, K.U. Unusual butane- and pentanetriol-based tetraether lipids in Methanomassiliicoccus luminyensis, a representative of the seventh order of methanogens. Appl. Environ. Microbiol. 2016, 82, 4505–4516. [Google Scholar] [CrossRef] [PubMed]
- Larkin, A.; Chang, M.M.; Whitworth, G.E.; Imperiali, B. Biochemical evidence for and alternate patway in N-linked glycoprotein biosynthesis. Nat. Chem. Biol. 2013, 9, 367–373. [Google Scholar] [CrossRef][Green Version]
- Ogawa, T.; Emi, K.; Koga, K.; Yoshimura, T.; Hemmi, H. A cis -prenyltransferase from Methanosarcina acetivorans catalyzes both head-to-tail and nonhead-to-tail prenyl condensation. FEBS J. 2016, 283, 2369–2383. [Google Scholar] [CrossRef]
- Hartmann, E.; König, H. Isolation of lipid activated pseudomurein precursors from Methanobacterium thermoautotrophicum. Arch. Microbiol. 1990, 153, 444–447. [Google Scholar] [CrossRef]
- Hartmann, E.; König, H. Uridine and dolichyl diphosphate activated oligosaccharides are intermediates in the biosynthesis of the S-layer glycoprotein of Methanothermus fervidus. Arch. Microbiol. 1989, 151, 274–281. [Google Scholar] [CrossRef]
- Taguchi, Y.; Fujinami, D.; Kohda, D. Comparative analysis of archaeal lipid-linked oligosaccharides that serve as oligosaccharide donors for Asn glycosylation. J. Biol. Chem. 2016, 291, 11042–11054. [Google Scholar] [CrossRef]
- Guy, L.; Ettema, T.J.G. The archaeal “TACK” superphylum and the origin of eukaryotes. Trends Microbiol. 2011, 19, 580–587. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, B.; van Dam, V.; Breukink, E. Lipid II: A central component in bacterial cell wall synthesis and a target for antibiotics. Prostaglandins Leukot. Essent. Fat. Acids 2008, 79, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R. A link between polyprenols and outer spore wall formation in Saccharomyces cerevisiae. For the Degree of Doctor of Philosophy, Stony Brook University, Stony Brook, NY, USA, 2017. [Google Scholar]
- Troy, F.A. Polysialylation: From bacteria to brains. Glycobiology 1992, 2, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.P.; Troy, F.A. Characterization by NMR and molecular modeling of the binding of polyisoprenols and polyisoprenyl recognition sequence peptides: 3D structure of the complexes reveals sites of specific interactions. Glycobiology 2003, 13, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, C.; Zhang, Y.; Zhang, Q.; Xie, P.; Xu, F.; Ding, S. Synthesis and biological activity of polyprenols. Fitoterapia 2015, 106, 184–193. [Google Scholar]
- Vigo, C.; Grossman, S.H.; Drost-Hansen, W. Interaction of dolichol and dolichyl phosphate with phospholipid bilayers. Biochim. Biophys. Acta 1984, 774, 221–226. [Google Scholar] [CrossRef]
- Valtersson, C.; van Duyn, G.; Verkleij, A.J.; Chojnacki, T.; de Kruijff, B.; Dallner, G. The influence of dolichol, dolichol esters, and dolichyl phosphate on phospholipid polymorphism and fluidity in model membranes. J. Biol. Chem. 1985, 260, 2742–2751. [Google Scholar]
- De Ropp, J.S.; Troy, F.A. 2H NMR Investigation of the organization and dynamics of polyisoprenols in membranes. J. Biol. Chem. 1985, 260, 15669–15674. [Google Scholar]
- Arantes, P.R.; Pedebos, C.; Pol-fachin, L.; Poleto, M.D.; Verli, H. Dynamics of membrane-embedded lipid-linked oligosaccharides for the three domains of life. ChemRxiv 2019. Preprint. [Google Scholar]
- Zhou, G.P.; Troy, F.A. NMR study of the preferred membrane orientation of polyisoprenols (dolichol) and the impact of their complex with polyisoprenyl recognition sequence peptides on membrane structure. Glycobiology 2005, 15, 347–359. [Google Scholar] [CrossRef][Green Version]
- McCloskey, M.A.; Troy, F.A. Paramagnetic isoprenoid carrier Lipids. 2. Dispersion and dynamics in lipid membranes. Biochemistry 1980, 19, 2061–2066. [Google Scholar] [CrossRef]
- Chojnacki, T.; Dallner, G. The biological role of dolichol. Biochem. J. 1988, 251, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lamson, M.J.; Herbette, L.G.; Peters, K.R.; Carson, J.H.; Morgan, F.; Chester, D.C.; Kramer, P.A. Effects of hexagonal phase induction by dolichol on phospholipid membrane permeability and morphology. Int. J. Pharm. 1994, 105, 259–272. [Google Scholar] [CrossRef]
- Streiff, S.; Ribeiro, N.; Wu, Z.; Gumienna-Kontecka, E.; Elhabiri, M.; Albrecht-Gary, A.M.; Ourisson, G.; Nakatani, Y. “Primitive” Membrane from Polyprenyl Phosphates and Polyprenyl Alcohols. Chem. Biol. 2007, 14, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Van Duijn, G.; Valtersson, C.; Chojnacki, T.; Verkleij, A.J.; Dallner, G.; de Kruijff, B. Dolichyl phosphate induces non-bilayer structures, vesicle fusion and transbilayer movement of lipids: A model membrane study. Biochim. Biophys. Acta-Biomembr. 1986, 861, 211–223. [Google Scholar] [CrossRef]
- Walinska, K. Comparison of the influence of the polyprenol structure on model membranes. Desalination 2004, 163, 239–245. [Google Scholar] [CrossRef]
- Monti, J.A.; Christian, S.T.; Schutzbach, J.S. Effects of dolichol on membrane permeability. Biochim. Biophys. Acta-Biomembr. 1987, 905, 133–142. [Google Scholar] [CrossRef]
- Janas, T.; Walińska, K.; Chojnacki, T.; Świezewska, E.; Janas, T. Modulation of properties of phospholipid membranes by the long-chain polyprenol (C160). Chem. Phys. Lipids 2000, 106, 31–40. [Google Scholar] [CrossRef]
- Janas, T.; Walińska, K.; Janas, T. Electroporation of polyprenol-phosphatidylcholine bilayer lipid membranes. Bioelectrochem. Bioenerg. 1998, 45, 215–220. [Google Scholar] [CrossRef]
- Schutzbach, J.S.; Jensen, J.W. Bilayer membrane destabilization induced by dolichylphosphate. Chem. Phys. Lipids 1989, 51, 213–218. [Google Scholar] [CrossRef]
- Van Gelder, K.; Rea, K.A.; Virta, L.K.A.; Whitnell, K.L.; Osborn, M.; Vatta, M.; Khozin, A.; Skorupinska-Tudek, K.; Surmacz, L.; Akhtar, T.A. Medium-chain polyprenols influence chloroplast membrane dynamics in Solanum lycopersicum. Plant Cell Physiol. 2018, 59, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, T.A.; Surowiecki, P.; Siekierska, H.; Kania, M.; Van Gelder, K.; Rea, K.A.; Virta, L.K.A.; Vatta, M.; Gawarecka, K.; Wojcik, J.; et al. Polyprenols are synthesized by a plastidial cis-prenyltransferase and influence photosynthetic performance. Plant Cell 2017, 29, 1709–1725. [Google Scholar] [CrossRef] [PubMed]
- Baczewska, A.H.; Dmuchowski, W.; Jozwiak, A.; Gozdowski, D.; Brągoszewska, P.; Dąbrowski, P.; Swiezewska, E. Effect of salt stress on prenol lipids in the leaves of Tilia ‘Euchlora’. Dendrobiology 2014, 72, 177–186. [Google Scholar] [CrossRef]
- Lis, M.; Kuramitsu, H.K. Characterization of a suppressor mutation complementing an acid-sensitive mutation in Streptococcus mutans. FEMS Microbiol. Lett. 2003, 229, 179–182. [Google Scholar] [CrossRef]
- Kellermann, M.Y.; Yoshinaga, M.Y.; Valentine, R.C.; Wörmer, L.; Valentine, D.L. Important roles for membrane lipids in haloarchaeal bioenergetics. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2940–2956. [Google Scholar] [CrossRef] [PubMed]
- Calo, D.; Guan, Z.; Naparstek, S.; Eichler, J. Different routes to the same ending: Comparing the N-glycosylation processes of Haloferax volcanii and Haloarcula marismortui, two halophilic archaea from the Dead Sea. Mol. Microbiol. 2011, 81, 1166–1177. [Google Scholar] [CrossRef] [PubMed]
- Oger, P.M.; Cario, A. Adaptation of the membrane in Archaea. Biophys. Chem. 2013, 183, 42–56. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R.H. Naturally Occurring Quinones IV, 4th ed.; Springer: Dordrecht, The Netherlands, 1997. [Google Scholar]
- Nowicka, B.; Kruk, J. Occurrence, biosynthesis and function of isoprenoid quinones. Biochim. Biophys. Acta-Bioenerg. 2010, 1797, 1587–1605. [Google Scholar] [CrossRef]
- Elling, F.J.; Becker, K.W.; Könneke, M.; Schröder, J.M.; Kellermann, M.Y.; Thomm, M.; Hinrichs, K.U. Respiratory quinones in Archaea: Phylogenetic distribution and application as biomarkers in the marine environment. Env. Microbiol. 2016, 18, 692–707. [Google Scholar] [CrossRef]
- Abken, H.-J.; Tietze, M.; Brodersen, J.; Bäumer, S.; Beifuss, U.; Deppenmeier, U. Isolation and characterization of methanophenazine and function of phenazines in membrane-bound electron transport of Methanosarcina mazei Gö1. J. Bacteriol. 1998, 180, 2027–2032. [Google Scholar]
- Goodwin, T.W. The prenyllipids of the membranes of higher plants. In Lipids and Lipid Polymers in Higher Plants, 1st ed.; Tevini, M., Lichtenthaler, H.K., Eds.; Springer; Berlin/Heidelberg, Germany: New York, NY, USA, 1977; pp. 29–42. [Google Scholar]
- Collins, M.D.; Jones, D. Distribution of isoprenoid quinone structural types in bacteria and their taxonomic implication. Microbiol. Rev. 1981, 45, 316–354. [Google Scholar] [PubMed]
- Thurl, S.; Witke, W.; Buhrow, I.; Schäfer, W. Quinones from Archaebacteria, II. Different types of quinones from sulphur-Dependent Archaebacteria. Biol. Chem. Hoppe. Seyler. 1986, 367, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Elling, F.J. Factors controlling the lipid composition of marine planktonic Thaumarchaeota. For the Degree Doctor of Philosophy, Universität Bremen, Bremen, Germany, 2015. [Google Scholar]
- Golyshina, O.V.; Lünsdorf, H.; Kublanov, I.V.; Goldenstein, N.I.; Hinrichs, K.U.; Golyshin, P.N. The novel extremely acidophilic, cell-wall-deficient archaeon Cuniculiplasma divulgatum gen. nov., sp. nov. represents a new family, Cuniculiplasmataceae fam. nov., of the order Thermoplasmatales. Int. J. Syst. Evol. Microbiol. 2016, 66, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, E.L.; Girvin, M.E.; Cramer, W.A.; Markley, J.L. Location and mobility of ubiquinones of different chain lengths in artificial membrane vesicles. Biochemistry 1985, 24, 2501–2508. [Google Scholar] [CrossRef] [PubMed]
- Lenaz, G.; Samori, B.; Fato, R.; Battino, M.; Castelli, G.P.; Domini, I. Localization and preferred orientations of ubiquinone homologs in model bilayers. Biochem. Cell Biol. 1991, 70, 504–514. [Google Scholar] [CrossRef]
- Roche, Y.; Peretti, P.; Bernard, S. DSC and Raman studies of the side chain length effect of ubiquinones on the thermotropic phase behavior of liposomes. Thermochim. Acta 2006, 447, 81–88. [Google Scholar] [CrossRef]
- Teixeira, M.H.; Arantes, G.M. Effects of lipid composition on membrane distribution and permeability of natural quinones. RSC Adv. 2019, 9, 16892–16899. [Google Scholar] [CrossRef]
- Jemiola-Rzeminska, M.; Kruk, J.; Skowronek, M.; Strzalka, K. Location of ubiquinone homologues in liposome membranes studied by fluorescence anisotropy of diphenyl-hexatriene and trimethylammonium-diphenyl-hexatriene. Chem. Phys. Lipids 1996, 79, 55–63. [Google Scholar] [CrossRef]
- Stidham, M.A.; McIntosh, T.J.; Siedow, J.N. On the localization of ubiquinone in phosphatidylcholine bilayers. Biochim. Biophys. Acta-Bioenerg. 1984, 767, 423–431. [Google Scholar] [CrossRef]
- Cornell, B.A.; Keniry, M.A.; Post, A.; Robertson, R.N.; Weir, L.E.; Westerman, P.W. Location and activity of ubiquinone 10 and ubiquinone analogs in model and biological membranes. Biochemistry 1987, 26, 7702–7707. [Google Scholar] [CrossRef]
- Galassi, V.V.; Arantes, G.M. Partition, orientation and mobility of ubiquinones in a lipid bilayer. Biochim. Biophys. Acta-Bioenerg. 2015, 1847, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Hauß, T.; Dante, S.; Haines, T.H.; Dencher, N.A. Localization of coenzyme Q10 in the center of a deuterated lipid membrane by neutron diffraction. Biochim. Biophys. Acta-Bioenerg. 2005, 1710, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Söderhäll, J.A.; Laaksonen, A. Molecular dynamics simulations of ubiquinone inside a lipid bilayer. J. Phys. Chem. B 2001, 105, 9308–9315. [Google Scholar] [CrossRef]
- Kaurola, P.; Sharma, V.; Vonk, A.; Vattulainen, I.; Róg, T. Distribution and dynamics of quinones in the lipid bilayer mimicking the inner membrane of mitochondria. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2116–2122. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.F.; Rowat, A.C.; Gober, J.W. Is CoQ a membrane stabilizer? Nat. Chem. Biol. 2014, 10, 242–243. [Google Scholar] [CrossRef] [PubMed]
- Agmo Hernández, V.; Eriksson, E.K.; Edwards, K. Ubiquinone-10 alters mechanical properties and increases stability of phospholipid membranes. Biochim. Biophys. Acta-Biomembr. 2015, 1848, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Lucy, J.A.; Dingle, J.T. Fat-soluble vitamins and biological membranes. Nature 1964, 204, 156–160. [Google Scholar] [CrossRef]
- Shimada, H.; Shida, Y.; Nemoto, N.; Oshima, T.; Yamagishi, A. Quinone profiles of Thermoplasma acidophilum HO-62. J. Bacteriol. 2001, 183, 1462–1465. [Google Scholar] [CrossRef]
- Nicolaus, B.; Trincone, A.; Lama, L.; Palmieri, G.; Gambacorta, A. Quinone Composition in Sulfolobus solfataricus Grown under Different Conditions. Syst. Appl. Microbiol. 1992, 15, 18–20. [Google Scholar] [CrossRef]
- Trincone, A.; Lanzotti, V.; Nicolaus, B.; Zillig, W.; De Rosa, M.; Gambacorta, A. Comparative lipid composition of aerobically and anaerobically grown Desulfurolobus ambivalens, an autotrophic thermophilic Archaeobacterium. J. Gen. Microbiol. 1989, 135, 2751–2757. [Google Scholar]
- Sévin, D.C.; Sauer, U. Ubiquinone accumulation improves osmotic-stress tolerance in Escherichia coli. Nat. Chem. Biol. 2014, 10, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Seel, W.; Flegler, A.; Zunabovic-Pichler, M.; Lipski, A. Increased isoprenoid quinone concentration modulates membrane fluidity in Listeria monocytogenes at low growth temperatures. J. Bacteriol. 2018, 200, e00148-18. [Google Scholar] [CrossRef] [PubMed]
- Ourisson, G.; Nakatani, Y. The terpenoid theory of the origin of cellular life: The evolution of terpenoids to cholesterol. Chem. Biol. 1994, 1, 11–23. [Google Scholar] [CrossRef]
- Xu, R.; Fazio, G.C.; Matsuda, S.P.T. On the origins of triterpenoid skeletal diversity. Phytochemistry 2004, 65, 261–291. [Google Scholar] [CrossRef] [PubMed]
- Matsumi, R.; Atomi, H.; Driessen, A.J.M.; van der Oost, J. Isoprenoid biosynthesis in Archaea - Biochemical and evolutionary implications. Res. Microbiol. 2011, 162, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Córdova, P.; Baeza, M.; Cifuentes, V.; Alcaíno, J. Microbiological Synthesis of Carotenoids: Pathways and Regulation. In Progress in Carotenoid Research, 1st ed.; Queiroz Zepka, L., Jacob-Lopes, E., Rosso, V.V., Eds.; InTech, 2018; pp. 63–83. [Google Scholar]
- Langworthy, T.A.; Tornabene, T.G.; Holzer, G. Lipids of archaebacteria. Zent. Fur Bakteriol. Angew. Und Okol. Microbiol. Abt. L. Orig. C. Hyg. 1982, 3, 228–244. [Google Scholar] [CrossRef]
- Heller, J.H.; Heller, M.S.; Springer, S.; Clark, E. Squalene content of various shark livers. Nature 1957, 179, 919–920. [Google Scholar] [CrossRef]
- Agarwal, A.; Shen, H.; Agarwal, S.; Rao, A.V. Lycopene content of tomato products: Its stability, bioavailability and in vivo antioxidant properties. J. Med. Food 2003, 4, 9–15. [Google Scholar] [CrossRef]
- Clejan, S.; Krulwich, T.A.; Mondrus, K.R.; Seto-Young, D. Membrane lipid composition of obligatively and facultatively alkalophilic strains of Bacillus. J. Bacteriol. 1986, 168, 334–340. [Google Scholar] [CrossRef]
- Banciu, H.; Sorokin, D.Y.; Rijpstra, W.I.C.; Sinninghe Damsté, J.S.; Galinski, E.A.; Takaichi, S.; Muyzer, G.; Kuenen, J.G. Fatty acid, compatible solute and pigment composition of obligately chemolithoautotrophic alkaliphilic sulfur-oxidizing bacteria from soda lakes. Fems. Microbiol. Lett. 2005, 243, 181–187. [Google Scholar] [CrossRef]
- Cario, A.; Grossi, V.; Schaeffer, P.; Oger, P.M. Membrane homeoviscous adaptation in the piezo-hyperthermophilic archaeon Thermococcus barophilus. Front. Microbiol. 2015, 6, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lattuati, A.; Guezennec, J.; Metzger, P.; Largeau, C. Lipids of Thermococcus hydrothermalis, an archaea isolated from a deep- sea hydrothermal vent. Lipids 1998, 33, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Kohno, Y.; Egawa, Y.; Itoh, S.; Nagaoka, S.; Takahashi, M.; Mukai, K. Kinetic study of quenching reaction of singlet oxygen and scavenging reaction of free radical by squalene in n-butanol. Biochim. Biophys. Acta-Lipids Lipid Metab. 1995, 1256, 52–56. [Google Scholar] [CrossRef]
- Conforti, F.; Statti, G.; Loizzo, M.R.; Sacchetti, G.; Poli, F.; Menichini, F. In vitro antioxidant effect and inhibition of α-amylase of two varieties of Amaranthus caudatus seeds. Biol. Pharm. Bull. 2005, 28, 1098–1102. [Google Scholar] [CrossRef] [PubMed]
- Warleta, F.; Campos, M.; Allouche, Y.; Sánchez-Quesada, C.; Ruiz-Mora, J.; Beltrán, G.; Gaforio, J.J. Squalene protects against oxidative DNA damage in MCF10A human mammary epithelial cells but not in MCF7 and MDA-MB-231 human breast cancer cells. Food Chem. Toxicol. 2010, 48, 1092–1100. [Google Scholar] [CrossRef]
- De Rosa, M.; Gambacorta, A.; Gliozzi, A. Structure, biosynthesis, and physicochemical properties of archaebacterial lipids. Microbiol. Rev. 1986, 50, 70–80. [Google Scholar] [PubMed]
- Gambacorta, A.; Trincone, A.; Nicolaus, B.; Lama, L.; De Rosa, M. Unique features of lipids of Archaea. Syst. Appl. Microbiol. 1993, 16, 518–527. [Google Scholar] [CrossRef]
- Haines, T.H. Do sterols reduce proton and sodium leaks through lipid bilayers? Prog. Lipid Res. 2001, 40, 299–324. [Google Scholar] [CrossRef]
- Hauß, T.; Dante, S.; Dencher, N.A.; Haines, T.H. Squalane is in the midplane of the lipid bilayer: Implications for its function as a proton permeability barrier. Biochim. Biophys. Acta 2002, 1556, 149–154. [Google Scholar] [CrossRef]
- Lanyi, J.K.; Plachy, W.Z.; Kates, M. Lipid interactions in membranes of extremely halophilic bacteria. II. Modification of the bilayer structure by squalene. Biochemistry 1974, 13, 4914–4920. [Google Scholar] [CrossRef]
- Kowert, B.A.; Watson, M.B.; Dang, N.C. Diffusion of squalene in n-alkanes and squalane. J. Phys. Chem. B 2014, 118, 2157–2163. [Google Scholar] [CrossRef] [PubMed]
- Spanova, M.; Zweytick, D.; Lohner, K.; Klug, L.; Leitner, E.; Hermetter, A.; Daum, G. Influence of squalene on lipid particle/droplet and membrane organization in the yeast Saccharomyces cerevisiae. Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2012, 1821, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.P.; Banschbach, J.; Yeagle, P.L. Stabilization of H II phases by low leves of diglycerides and alkanes: An NMR, calorimetric, and X-ray diffraction study. Biochemistry 1989, 28, 5010–5019. [Google Scholar] [CrossRef]
- Lohner, K.; Degovics, G.; Laggner, P.; Gnamusch, E.; Paltauf, F. Squalene promotes the formation of nonbilayer stuctures in phospholipid model membranes. Biochim. Biophys. Acta 1993, 1152, 69–77. [Google Scholar] [CrossRef]
- Palanco, M.E.; Skovgaard, N.; Hansen, J.S.; Berg-Sørensen, K.; Hélix-Nielsen, C. Tuning biomimetic membrane barrier properties by hydrocarbon, cholesterol and polymeric additives. Bioinspir. Biomim. 2017, 13, 016005. [Google Scholar] [CrossRef] [PubMed]
- Horbach, S.; Neuss, B.; Sahm, H. Effect of azasqualene on hopanoid biosynthesis and ethanol tolerance of Zymomonas mobilis. FEMS Microbiol. Lett. 1991, 79, 347–350. [Google Scholar] [CrossRef]
- Russell, D.J. Lycopene carotenogenesis and function in the haloarchaeon Haloferax volcanii. For the Degree Doctor of Philosophy, University of Nottingham, Nottingham, UK, 2013. [Google Scholar]
- Manquin, B.P.; Morgan, J.A.; Ju, J.; Müller-Späth, T.; Clark, D.S. Production of C35 isoprenoids depends on H2 availability during cultivation of the hyperthermophile Methanococcus jannaschii. Extremophiles 2004, 8, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Upasani, V.N.; Desai, S.G.; Moldoveanu, N.; Kates, M. Lipids of extremely halophilic archaeobacteria from saline environments in India: A novel glycolipid in Natronobacterium strains. Microbiology 1994, 140, 1959–1966. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Nir, S.; Ohki, S. Permeability properties of phospholipid membranes: Effect of cholesterol and temperature. Biochim. Biophys. Acta-Biomembr. 1971, 266, 561–583. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvador-Castell, M.; Tourte, M.; Oger, P.M. In Search for the Membrane Regulators of Archaea. Int. J. Mol. Sci. 2019, 20, 4434. https://doi.org/10.3390/ijms20184434
Salvador-Castell M, Tourte M, Oger PM. In Search for the Membrane Regulators of Archaea. International Journal of Molecular Sciences. 2019; 20(18):4434. https://doi.org/10.3390/ijms20184434
Chicago/Turabian StyleSalvador-Castell, Marta, Maxime Tourte, and Philippe M. Oger. 2019. "In Search for the Membrane Regulators of Archaea" International Journal of Molecular Sciences 20, no. 18: 4434. https://doi.org/10.3390/ijms20184434
APA StyleSalvador-Castell, M., Tourte, M., & Oger, P. M. (2019). In Search for the Membrane Regulators of Archaea. International Journal of Molecular Sciences, 20(18), 4434. https://doi.org/10.3390/ijms20184434