Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells
Abstract
1. Introduction
2. Results
2.1. Antitumor Effect of Dau–GnRH-[4Ser/4Lys(Bu)] Conjugates
2.2. Cellular Uptake of Dau–GnRH–[4Ser/4Lys(Bu)] Conjugates by HT-29 Cells
2.3. Apoptotic Effect of Dau–GnRH–[4Ser/4Lys(Bu)] Conjugates Detected by Flow Cytometry
2.4. Effect of Dau–GnRH–[4Ser/4Lys(Bu)] Conjugates on the Expression of Human Apoptosis-Related Genes
3. Discussion
4. Materials and Methods
4.1. Material
4.2. Synthesis of GnRH-Based Conjugates
4.3. Analytical Characterization of Conjugates
4.3.1. RP-HPLC
4.3.2. ESI-MS
4.4. Cell Culture
4.5. Cell Viability Assay
4.6. Flow Cytometric Analysis of Cellular Uptake
4.7. Apoptosis Assay
4.8. Molecular Biological Analysis of Apoptosis-Related Genes
4.8.1. RNA Isolation and cDNA Synthesis
4.8.2. Human Apoptosis Gene PCR Array and qRT-PCR
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GnRH | Gonadotropin-releasing hormone |
| GnRH-R | Gonadotropin-releasing hormone receptor |
| Dau | Daunorubicin |
| ROS | Reactive oxygen species |
| qRT-PCR | Quantitative, reverse transcription polymerase chain reaction |
| Lys(Bu) | Butyrated Lys |
| Lys(Ac) | Acetylated Lys |
| IC50 | Half maximal inhibitory concentration |
| GeoMean | Geometric mean channel |
| FITC | Fluorescein isothiocyanate |
| SPPS | Solid-phase peptide synthesis |
| ESI-MS | Electrospray ionization mass spectrometry |
| CI | Cell index |
| ANOVA | Analysis of variance |
| FASL | Fas ligand |
References
- Mező, G.; Manea, M. Receptor-mediated tumor targeting based on peptide hormones. Expert Opin. Drug Deliv. 2010, 7, 79–96. [Google Scholar] [CrossRef] [PubMed]
- Engel, J.B.; Tinneberg, H.R.; Rick, F.G.; Berkes, E.; Schally, A.V. Targeting of Peptide Cytotoxins to LHRH Receptors For Treatment of Cancer. Curr. Drug Targets 2016, 17, 488–494. [Google Scholar] [CrossRef] [PubMed]
- Szabó, I.; Manea, M.; Orbán, E.; Csampai, A.; Bősze, S.; Szabó, R.; Tejeda, M.; Gaál, D.; Kapuvári, B.; Przybylski, M.; et al. Development of an oxime bond containing daunorubicin-gonadotropin-releasing hormone-III conjugate as a potential anticancer drug. Bioconjug. Chem. 2009, 20, 656–665. [Google Scholar] [CrossRef] [PubMed]
- Millar, R.P.; Lu, Z.L.; Pawson, A.J.; Flanagan, C.A.; Morgan, K.; Maudsley, S.R. Gonadotropin-releasing hormone receptors. Endocr. Rev. 2004, 25, 235–275. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.K.; Leung, P.C.K. Molecular biology of gonadotropin-releasing hormone (GnRH)-I, GnRH-1I, and their receptors in humans. Endocr. Rev. 2005, 26, 283–306. [Google Scholar] [CrossRef]
- Manea, M.; Mezo, G. lGnRH-III—A promising candidate for anticancer drug development. Protein Pept. Lett. 2013, 20, 439–449. [Google Scholar]
- Millar, R.P.; Pawson, A.J.; Morgan, K.; Rissman, E.F.; Lu, Z.L. Diversity of actions of GnRHs mediated by ligand-induced selective signaling. Front. Neuroendocrinol. 2008, 29, 17–35. [Google Scholar] [CrossRef]
- Aguilar-Rojas, A.; Huerta-Reyes, M. Human gonadotropin-releasing hormone receptor-activated cellular functions and signaling pathways in extra-pituitary tissues and cancer cells (Review). Oncol. Rep. 2009, 22, 981–990. [Google Scholar] [CrossRef]
- Herédi-Szabó, K.; Murphy, R.F.; Lovas, S. Is lGnRH-III the most potent GnRH analog containing only natural amino acids that specifically inhibits the growth of human breast cancer cells? J. Pept. Sci. 2006, 12, 714–720. [Google Scholar] [CrossRef]
- Kovács, M.; Vincze, B.; Horváth, J.E.; Seprődi, J. Structure-activity study on the LH- and FSH-releasing and anticancer effects of gonadotropin-releasing hormone (GnRH)-III analogs. Peptides 2007, 28, 821–829. [Google Scholar] [CrossRef]
- Leung, P.C.; Cheng, C.K.; Zhu, X.M. Multi-factorial role of GnRH-I and GnRH-II in the human ovary. Mol. Cell. Endocrinol. 2003, 202, 145–153. [Google Scholar] [CrossRef]
- Szabo, I.; Bosze, S.; Orban, E.; Sipos, E.; Halmos, G.; Kovacs, M.; Mezo, G. Comparative in vitro biological evaluation of daunorubicin containing GnRH-I and GnRH-II conjugates developed for tumor targeting. J. Pept. Sci. 2015, 21, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Manea, M.; Leurs, U.; Orbán, E.; Baranyai, Z.; Ohlschlager, P.; Marquardt, A.; Schulcz, A.; Tejeda, M.; Kapuvári, B.; Továri, J.; et al. Enhanced enzymatic stability and antitumor activity of daunorubicin-GnRH-III bioconjugates modified in position 4. Bioconjug. Chem. 2011, 22, 1320–1329. [Google Scholar] [CrossRef] [PubMed]
- Sealfon, S.C.; Weinstein, H.; Millar, R.P. Molecular mechanisms of ligand interaction with the gonadotropin-releasing hormone receptor. Endocr. Rev. 1997, 18, 180–205. [Google Scholar] [CrossRef] [PubMed]
- Bajusz, S.; Janáky, T.; Csernus, V.J.; Bokser, L.; Fekete, M.; Srkalovic, G.; Redding, T.W.; Schally, A.V. Highly potent metallopeptide analogues of luteinizing hormone-releasing hormone. Proc. Natl. Acad. Sci. USA 1989, 86, 6313–6317. [Google Scholar] [CrossRef] [PubMed]
- Szepesházi, K.; Schally, A.V.; Juhasz, A.; Nagy, A.; Janaky, T. Effect of luteinizing hormone-releasing hormone analogs containing cytotoxic radicals on growth of estrogen-independent MXT mouse mammary carcinoma in vivo. Anticancer Drugs 1992, 3, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Nagy, A.; Schally, A.V. Targeting cytotoxic conjugates of somatostatin, luteinizing hormone-releasing hormone and bombesin to cancers expressing their receptors: A “smarter” chemotherapy. Curr. Pharm. Des. 2005, 11, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Mező, G.; Czajlik, A.; Jakab, A.; Bodor, A.; Farkas, V.; Vass, E.; Majer, Z.; Kapuvári, B.; Vincze, B.; Csuka, O.; et al. Structure-biological activity relationship of GnRH-III and its dimer derivatives. FEBS J. 2005, 272, 526. [Google Scholar]
- Mező, G.; Manea, M.; Szabó, I.; Vincze, B.; Kovács, M. New derivatives of GnRH as potential anticancer therapeutic agents. Curr. Med. Chem. 2008, 15, 2366–2379. [Google Scholar] [CrossRef]
- Herédi-Szabó, K.; Lubke, J.; Tóth, G.; Murphy, R.F.; Lovas, S. Importance of the central region of lamprey gonadotropin-releasing hormone III in the inhibition of breast cancer cell growth. Peptides 2005, 26, 419–422. [Google Scholar] [CrossRef]
- Hegedus, R.; Manea, M.; Orban, E.; Szabo, I.; Kiss, E.; Sipos, E.; Halmos, G.; Mezo, G. Enhanced cellular uptake and in vitro antitumor activity of short-chain fatty acid acylated daunorubicin-GnRH-III bioconjugates. Eur. J. Med. Chem. 2012, 56, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Lajkó, E.; Spring, S.; Hegedüs, R.; Biri-Kovács, B.; Ingebrandt, S.; Mező, G.; Kőhidai, L. Comparative cell biological study of in vitro antitumor and antimetastatic activity on melanoma cells of GnRH-III-containing conjugates modified with short-chain fatty acids. Beilstein J. Org. Chem. 2018, 14, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Schuster, S.; Biri-Kovacs, B.; Szeder, B.; Farkas, V.; Buday, L.; Szabo, Z.; Halmos, G.; Mezo, G. Synthesis and in vitro biochemical evaluation of oxime bond-linked daunorubicin-GnRH-III conjugates developed for targeted drug delivery. Beilstein J. Org. Chem. 2018, 14, 756–771. [Google Scholar] [CrossRef] [PubMed]
- Orbán, E.; Mező, G.; Schlage, P.; Csík, G.; Kulic, Z.; Ansorge, P.; Fellinger, E.; Moller, H.M.; Manea, M. In vitro degradation and antitumor activity of oxime bond-linked daunorubicin-GnRH-III bioconjugates and DNA-binding properties of daunorubicin-amino acid metabolites. Amino Acids 2011, 41, 469–483. [Google Scholar] [CrossRef] [PubMed]
- Zoptarelin Doxorubicin. Available online: http://adisinsight.springer.com/drugs/800009722 (accessed on 22 July 2019).
- Popovics, P.; Schally, A.V.; Szalontay, L.; Block, N.L.; Rick, F.G. Targeted cytotoxic analog of luteinizing hormone-releasing hormone (LHRH), AEZS-108 (AN-152), inhibits the growth of DU-145 human castration-resistant prostate cancer in vivo and in vitro through elevating p21 and ROS levels. Oncotarget 2014, 5, 4567–4578. [Google Scholar] [CrossRef] [PubMed]
- Laurent, G.; Jaffrezou, J.P. Signaling pathways activated by daunorubicin. Blood 2001, 98, 913–924. [Google Scholar] [CrossRef] [PubMed]
- Moretti, R.M.; Montagnani Marelli, M.; Taylor, D.M.; Martini, P.G.; Marzagalli, M.; Limonta, P. Gonadotropin-releasing hormone agonists sensitize, and resensitize, prostate cancer cells to docetaxel in a p53-dependent manner. PLoS ONE 2014, 9, e93713. [Google Scholar] [CrossRef]
- Zhang, N.; Qiu, J.; Zheng, T.; Zhang, X.; Hua, K.; Zhang, Y. Goserelin promotes the apoptosis of epithelial ovarian cancer cells by upregulating forkhead box O1 through the PI3K/AKT signaling pathway. Oncol. Rep. 2018, 39, 1034–1042. [Google Scholar]
- Aguilar-Rojas, A.; Perez-Solis, M.A.; Maya-Nunez, G. The gonadotropin-releasing hormone system: Perspectives from reproduction to cancer (Review). Int. J. Oncol. 2016, 48, 861–868. [Google Scholar] [CrossRef]
- Doroszko, M.; Chrusciel, M.; Stelmaszewska, J.; Slezak, T.; Anisimowicz, S.; Plockinger, U.; Quinkler, M.; Bonomi, M.; Wolczynski, S.; Huhtaniemi, I.; et al. GnRH antagonist treatment of malignant adrenocortical tumors. Endocr. Relat. Cancer 2019, 26, 103–117. [Google Scholar] [CrossRef]
- Park, S.; Han, J.M.; Cheon, J.; Hwang, J.I.; Seong, J.Y. Apoptotic death of prostate cancer cells by a gonadotropin-releasing hormone-II antagonist. PLoS ONE 2014, 9, e99723. [Google Scholar] [CrossRef] [PubMed]
- Fister, S.; Gunthert, A.R.; Aicher, B.; Paulini, K.W.; Emons, G.; Grundker, C. GnRH-II antagonists induce apoptosis in human endometrial, ovarian, and breast cancer cells via activation of stress-induced MAPKs p38 and JNK and proapoptotic protein Bax. Cancer Res. 2009, 69, 6473–6481. [Google Scholar] [CrossRef] [PubMed]
- Stangelberger, A.; Schally, A.V.; Rick, F.G.; Varga, J.L.; Baker, B.; Zarandi, M.; Halmos, G. Inhibitory effects of antagonists of growth hormone releasing hormone on experimental prostate cancers are associated with upregulation of wild-type p53 and decrease in p21 and mutant p53 proteins. Prostate 2012, 72, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Gunthert, A.R.; Grundker, C.; Bongertz, T.; Schlott, T.; Nagy, A.; Schally, A.V.; Emons, G. Internalization of cytotoxic analog AN-152 of luteinizing hormone-releasing hormone induces apoptosis in human endometrial and ovarian cancer cell lines independent of multidrug resistance-1 (MDR-1) system. Am. J. Obstet. Gynecol. 2004, 191, 1164–1172. [Google Scholar] [CrossRef] [PubMed]
- Szepeshazi, K.; Schally, A.V.; Block, N.L.; Halmos, G.; Nadji, M.; Szalontay, L.; Vidaurre, I.; Abi-Chaker, A.; Rick, F.G. Powerful inhibition of experimental human pancreatic cancers by receptor targeted cytotoxic LH-RH analog AEZS-108. Oncotarget 2013, 4, 751–760. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kőhidai, L.; Lajkó, E.; Szabó, I.; Polgár, L.; Manea, M.; Mező, G. GnRH based drug targeting—Cell adhesion and migration modulator effects of GnRH derivatives in tumour cells. In Gonadotropin-Releasing Hormone (GnRH): Production, Structure, and Function.; Sills, E.S., Ed.; Nova Scientific Press Inc.: New York, NY, USA, 2013; pp. 254–272. [Google Scholar]
- Schlage, P.; Mezo, G.; Orban, E.; Bosze, S.; Manea, M. Anthracycline-GnRH derivative bioconjugates with different linkages: Synthesis, in vitro drug release and cytostatic effect. J. Control Release 2011, 156, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Szepeshazi, K.; Schally, A.V.; Halmos, G. LH-RH receptors in human colorectal cancers: Unexpected molecular targets for experimental therapy. Int. J. Oncol. 2007, 30, 1485–1492. [Google Scholar] [CrossRef][Green Version]
- Eaveri, R.; Ben-Yehudah, A.; Lorberboum-Galski, H. Surface antigens/receptors for targeted cancer treatment: The GnRH receptor/binding site for targeted adenocarcinoma therapy. Curr. Cancer Drug Targets 2004, 4, 673–687. [Google Scholar] [CrossRef]
- Manea, M.; Tovari, J.; Tejeda, M.; Schulcz, A.; Kapuvari, B.; Vincze, B.; Mezo, G. In-vivo antitumour effect of daunorubicin-GnRH-III derivative conjugates on colon carcinoma-bearing mice. Anticancer Drugs 2012, 23, 90–97. [Google Scholar] [CrossRef]
- Schuster, S.; Biri-Kovacs, B.; Szeder, B.; Buday, L.; Gardi, J.; Szabo, Z.; Halmos, G.; Mezo, G. Enhanced In Vitro Antitumor Activity of GnRH-III-Daunorubicin Bioconjugates Influenced by Sequence Modification. Pharmaceutics 2018, 10, 223. [Google Scholar] [CrossRef]
- Kapuvari, B.; Hegedus, R.; Schulcz, A.; Manea, M.; Tovari, J.; Gacs, A.; Vincze, B.; Mezo, G. Improved in vivo antitumor effect of a daunorubicin—GnRH-III bioconjugate modified by apoptosis inducing agent butyric acid on colorectal carcinoma bearing mice. Invest. New Drugs 2016, 34, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Leontieva, O.V.; Blagosklonny, M.V. DNA damaging agents and p53 do not cause senescence in quiescent cells, while consecutive re-activation of mTOR is associated with conversion to senescence. Aging (Albany NY) 2010, 2, 924–935. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed]
- Van Deursen, J.M. The role of senescent cells in ageing. Nature 2014, 509, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hohla, F.; Winder, T.; Greil, R.; Rick, F.G.; Block, N.L.; Schally, A.V. Targeted therapy in advanced metastatic colorectal cancer: Current concepts and perspectives. World J. Gastroenterol. 2014, 20, 6102–6112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schally, A.V.; Engel, J.B.; Emons, G.; Block, N.L.; Pinski, J. Use of analogs of peptide hormones conjugated to cytotoxic radicals for chemotherapy targeted to receptors on tumors. Curr. Drug Deliv. 2011, 8, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Ben-Yehudah, A.; Aqeilan, R.; Robashkevich, D.; Lorberboum-Galski, H. Using apoptosis for targeted cancer therapy by a new gonadotropin releasing hormone-DNA fragmentation factor 40 chimeric protein. Clin. Cancer Res. 2003, 9, 1179–1190. [Google Scholar] [PubMed]
- Carlsson, E.; Ranki, A.; Sipila, L.; Karenko, L.; Abdel-Rahman, W.M.; Ovaska, K.; Siggberg, L.; Aapola, U.; Assamaki, R.; Hayry, V.; et al. Potential role of a navigator gene NAV3 in colorectal cancer. Br. J. Cancer 2012, 106, 517–524. [Google Scholar] [CrossRef]
- Ben-Yehudah, A.; Prus, D.; Lorberboum-Galski, H. IV Administration of L-GNRH-PE66 efficiently inhibits growth of colon adenocarcinoma xenografts in nude mice. Int. J. Cancer 2001, 92, 263–268. [Google Scholar] [CrossRef]
- Wang, C.; Ma, Y.; Feng, S.; Liu, K.; Zhou, N. Gonadotropin-releasing hormone receptor-targeted paclitaxel-degarelix conjugate: Synthesis and in vitro evaluation. J. Pept. Sci. 2015, 21, 569–576. [Google Scholar] [CrossRef]
- Emons, G.; Gorchev, G.; Sehouli, J.; Wimberger, P.; Stahle, A.; Hanker, L.; Hilpert, F.; Sindermann, H.; Grundker, C.; Harter, P. Efficacy and safety of AEZS-108 (INN: zoptarelin doxorubicin acetate) an LHRH agonist linked to doxorubicin in women with platinum refractory or resistant ovarian cancer expressing LHRH receptors: A multicenter phase II trial of the ago-study group (AGO GYN 5). Gynecol. Oncol. 2014, 133, 427–432. [Google Scholar] [PubMed]
- Zoptarelin Doxorubicin Fails to Improve Survival in Phase III Endometrial Cancer Trial. Available online: https://www.targetedonc.com/news/zoptarelin-doxorubicin-fails-to-improve-survival-in-phase-iii-endometrial-cancer-trial (accessed on 13 July 2019).
- So, W.K.; Cheng, J.C.; Poon, S.L.; Leung, P.C. Gonadotropin-releasing hormone and ovarian cancer: A functional and mechanistic overview. FEBS J. 2008, 275, 5496–5511. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.M.; Wang, H.S.; Huang, H.Y.; Soong, Y.K.; MacCalman, C.D.; Leung, P.C. GnRH signaling in intrauterine tissues. Reproduction 2009, 137, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Schreier, V.N.; Petho, L.; Orban, E.; Marquardt, A.; Petre, B.A.; Mezo, G.; Manea, M. Protein expression profile of HT-29 human colon cancer cells after treatment with a cytotoxic daunorubicin-GnRH-III derivative bioconjugate. PLoS ONE 2014, 9, e94041. [Google Scholar] [CrossRef] [PubMed]
- Kasioumi, P.; Vrazeli, P.; Vezyraki, P.; Zerikiotis, S.; Katsouras, C.; Damalas, A.; Angelidis, C. Hsp70 (HSP70A1A) downregulation enhances the metastatic ability of cancer cells. Int. J. Oncol. 2019, 54, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Grek, C.; Townsend, D.M. Protein Disulfide Isomerase Superfamily in Disease and the Regulation of Apoptosis. Endoplasmic Reticulum Stress Dis. 2014, 1, 4–17. [Google Scholar] [CrossRef]
- Leurs, U.; Lajko, E.; Mezo, G.; Orban, E.; Ohlschlager, P.; Marquardt, A.; Kohidai, L.; Manea, M. GnRH-III based multifunctional drug delivery systems containing daunorubicin and methotrexate. Eur. J. Med. Chem. 2012, 52, 173–183. [Google Scholar] [CrossRef]
- Lajko, E.; Szabo, I.; Andody, K.; Pungor, A.; Mezo, G.; Kohidai, L. Investigation on chemotactic drug targeting (chemotaxis and adhesion) inducer effect of GnRH-III derivatives in Tetrahymena and human leukemia cell line. J. Pept. Sci. 2013, 19, 46–58. [Google Scholar] [CrossRef]
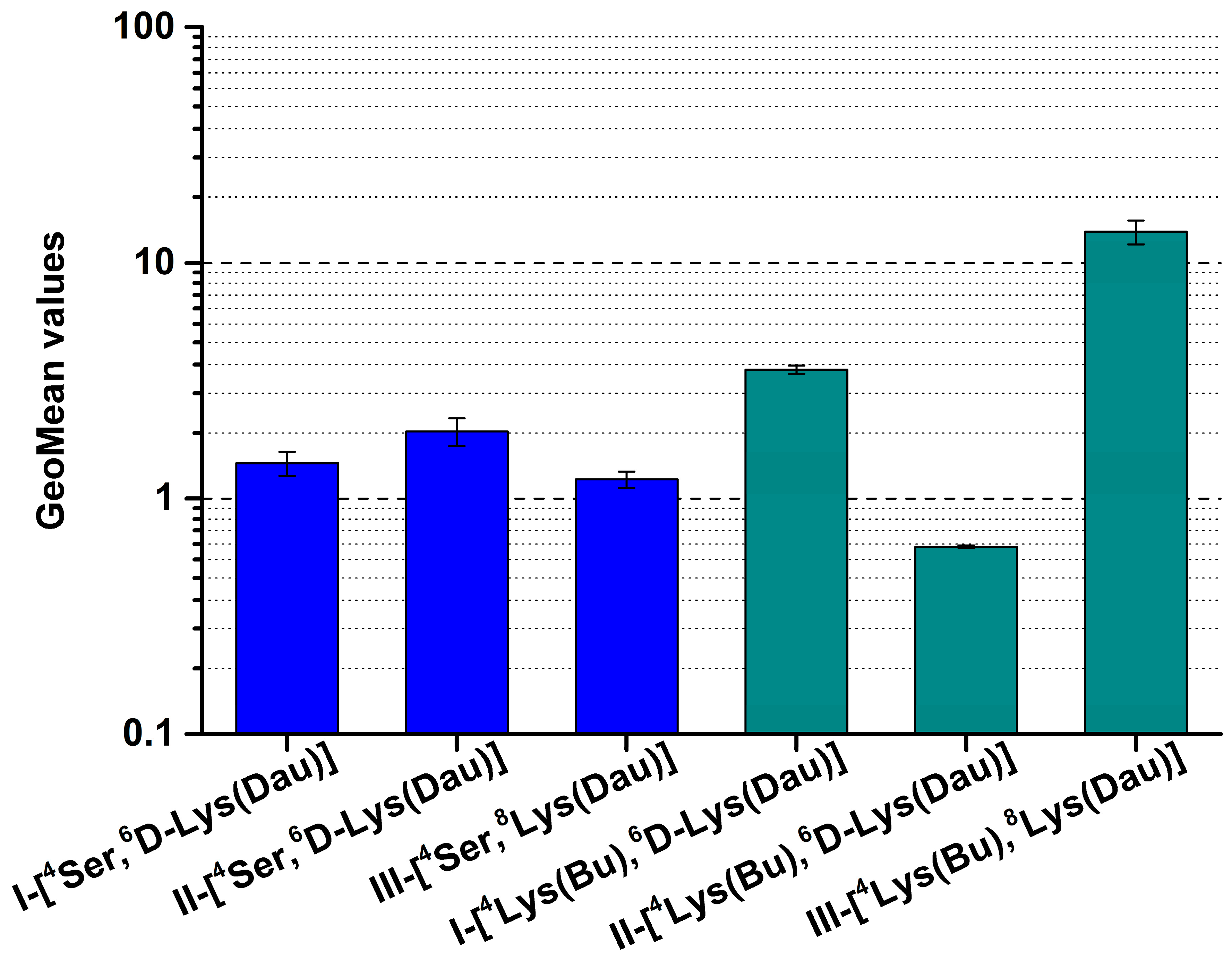
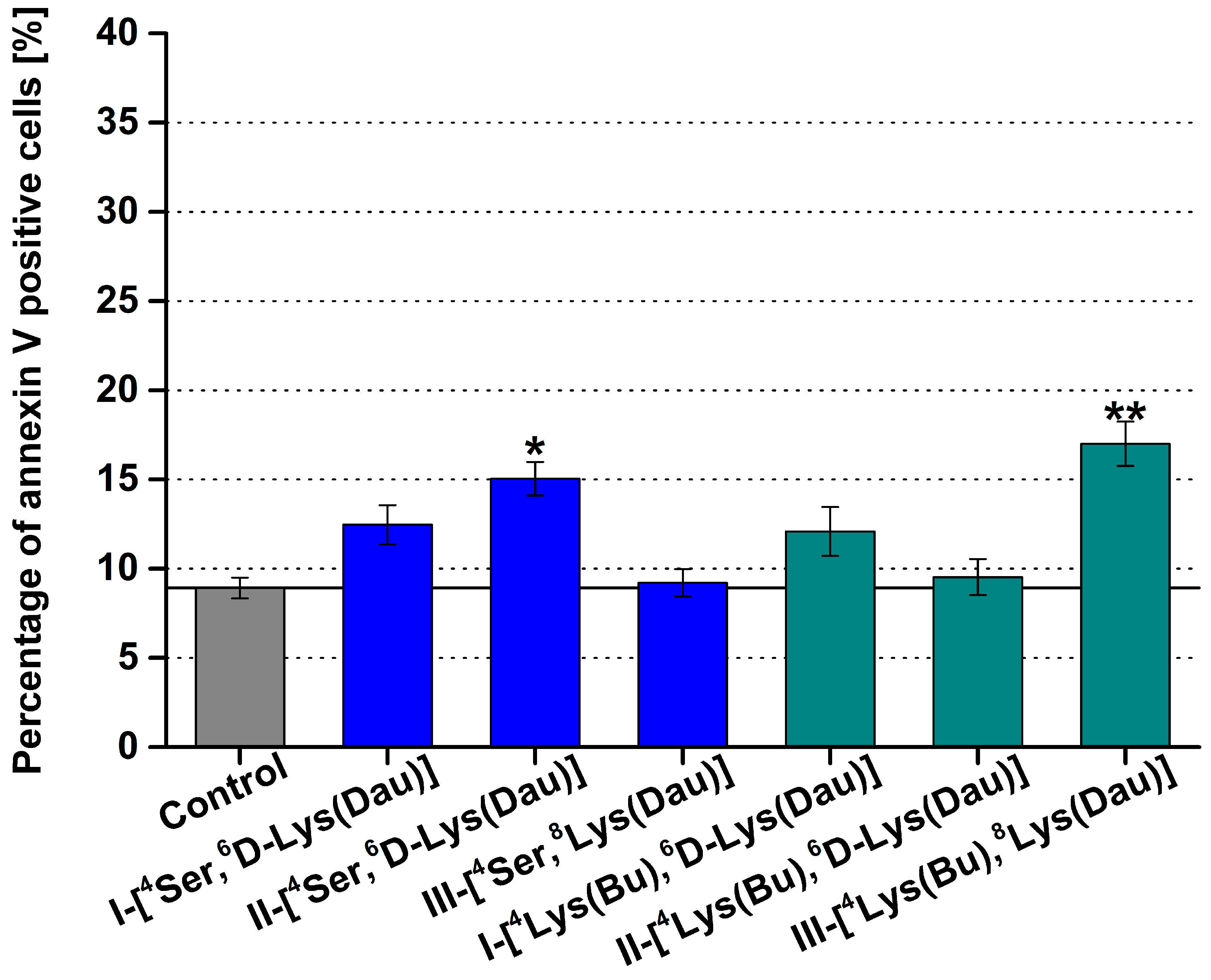

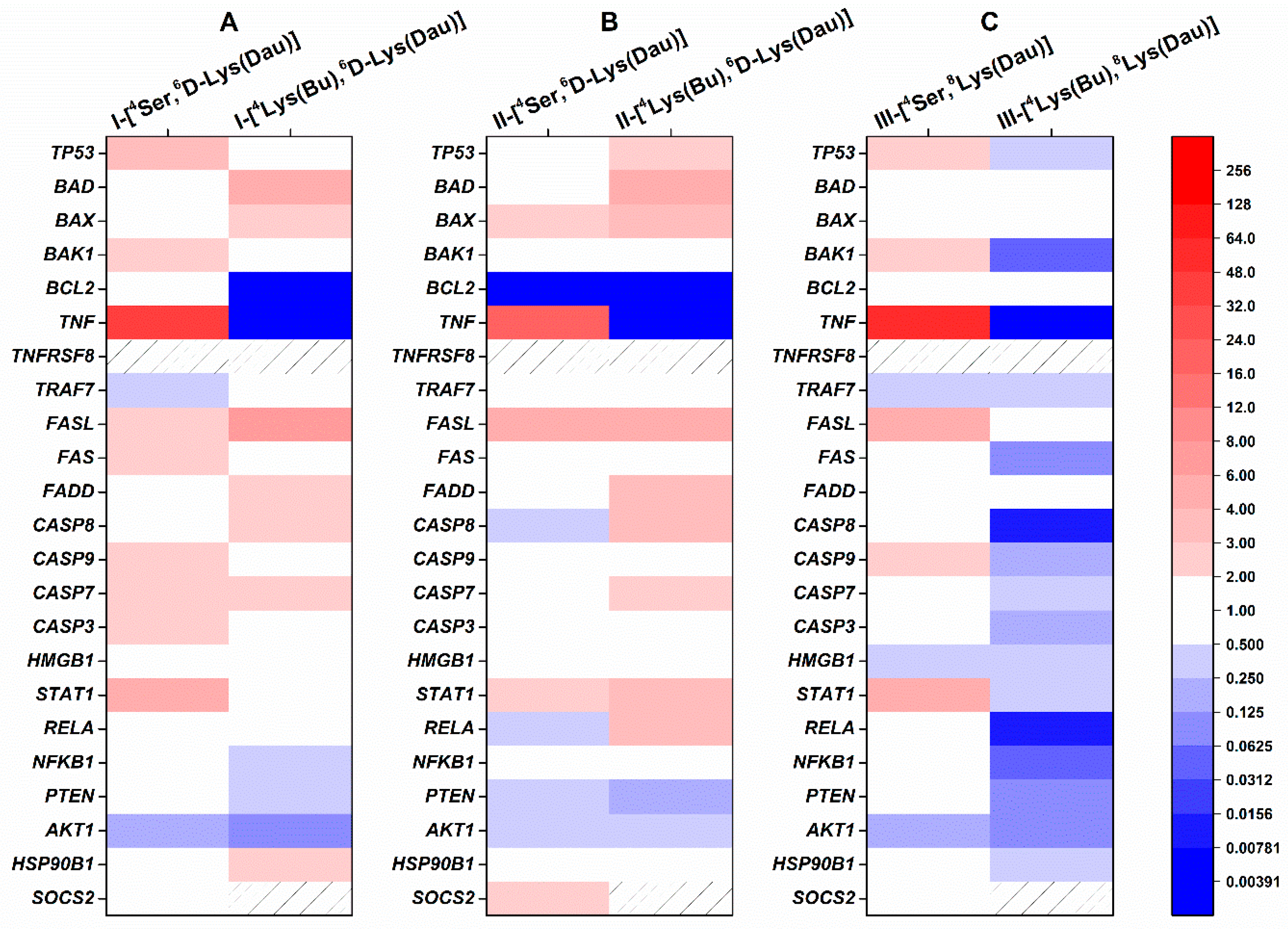
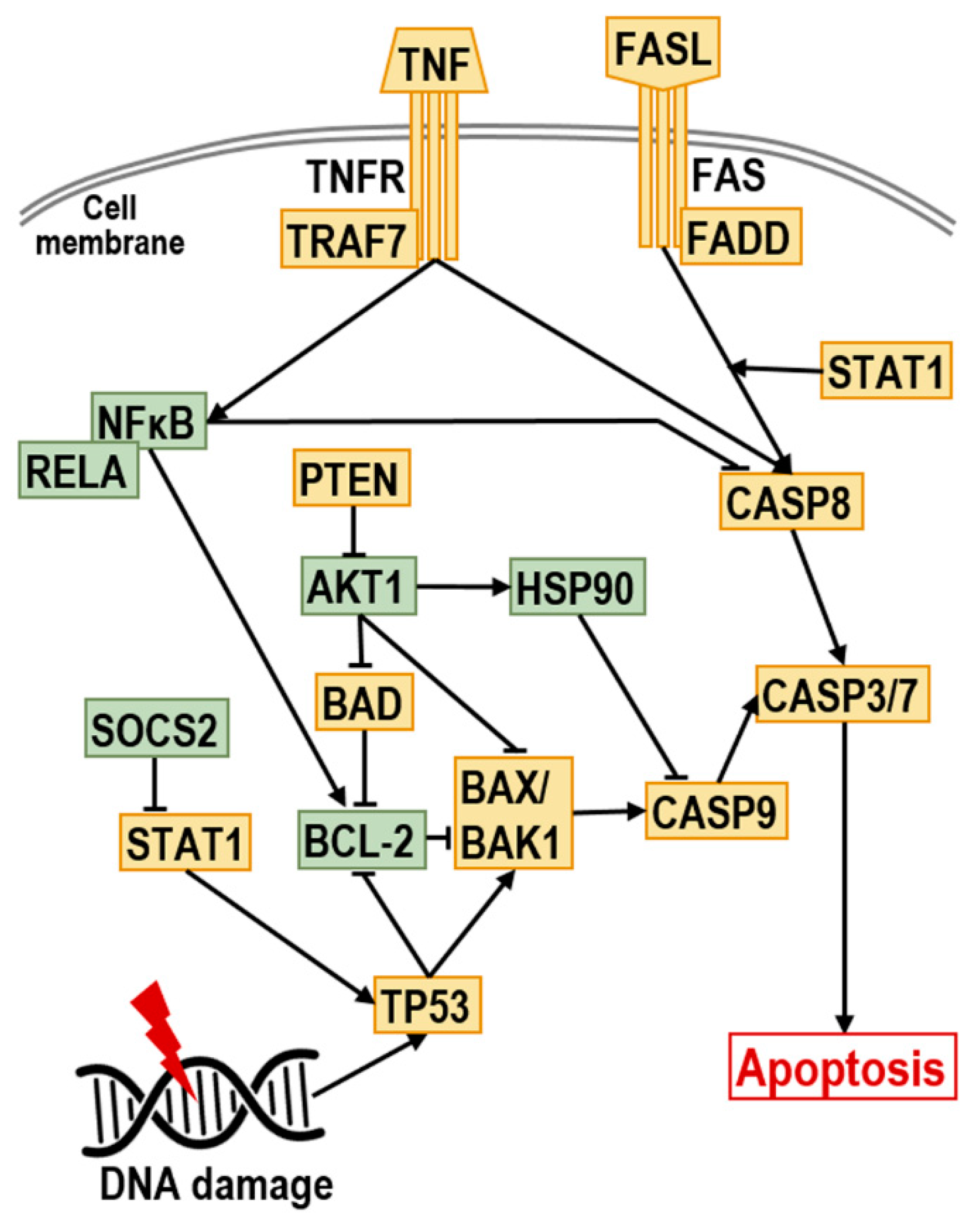
| Conjugate | Code |
|---|---|
| GnRH-I-[4Ser,6D-Lys(Dau=Aoa)] | I-[4Ser,6D-Lys(Dau)] |
| GnRH-II-[4Ser,6D-Lys(Dau=Aoa)] | II-[4Ser,6D-Lys(Dau)] |
| GnRH-III-[4Ser,8Lys(Dau=Aoa)] | III-[4Ser,8Lys(Dau)] |
| GnRH-I-[4Lys(Bu),6D-Lys(Dau=Aoa)] | I-[4Lys(Bu),6D-Lys(Dau)] |
| GnRH-II-[4Lys(Bu),6D-Lys(Dau=Aoa)] | II-[4Lys(Bu),6D-Lys(Dau)] |
| GnRH-III-[4Lys(Bu),8Lys(Dau=Aoa)] | III-[4Lys(Bu),8Lys(Dau)] |
| Conjugates | IC50 1 (µM) | ||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| I-[4Ser,6D-Lys(Dau)] | >100 | 60.25 ± 3.50 | 21.94 ± 1.54 |
| II-[4Ser,6D-Lys(Dau)] | 58.88 ± 10.04 | 24.20 ± 0.86 | 19.73 ± 2.51 |
| III-[4Ser,8Lys(Dau)] | >100 | >100 | 56.82 ± 5.44 |
| I-[4Lys(Bu),6D-Lys(Dau)] | >100 | 18.71 ±1.19 | 16.18 ± 1.75 |
| II-[4Lys(Bu),6D-Lys(Dau)] | 65.78 ± 3.89 | 69.70 ± 4.12 | 48.08 ± 6.89 |
| III-[4Lys(Bu),8Lys(Dau)] | 10.25 ± 1.66 | 4.26 ± 0.89 | 4.56 ± 0.27 |
| Gene | Gene Name | Forward and Reverse Primers | |
|---|---|---|---|
| Intrinsic Apoptotic Pathway | TP53 | Tumor protein p53 data | AGGCCTTGGAACTCAAGGAT, CCCTTTTTGGACTTCAGGTG |
| BAD | BCL2-associated agonist of cell death | CTACGGTGGGAGAGGAAGC, TGTTACGTAGTCAAGGCACAGC | |
| BAX | BCL2-associated X protein | GGACGTGGGCATTTTTCTTA, GTTTATTACCCCCTCAAGACCA | |
| BAK1 | BCL2-antagonist/killer 1 | AGACCTGAAAAATGGCTTCG, CGGAAAACCTCCTCTGTGTC | |
| BCL2 | B-cell CLL/lymphoma 2 | GCACCTGCACACCTGGAT, AGCCAGGAGAAATCAAACAGAG | |
| Extrinsic Apoptotic Pathway | TNF | TNF-alpha, tumor necrosis factor ligand superfamily member 2 | CGGTGCTTGTTCCTCAGC, GCCAGAGGGCTGATTAGAGA |
| TNFRSF8 | Tumor necrosis factor receptor superfamily, member 8 | GCTGTCAGGAGGTGCTGTTAC, GTAGGCCTCTGTGGGCACT | |
| TRAF7 | TNF receptor-associated factor 7 | ATGTCTCTGCGCTCCACATT, AGCTGACAGCACAGCTTCAC | |
| FASL | Fas ligand, TNF superfamily, member 6 | CAGTTCTTCCCTGTCCAACC, GTGGTGGTGGCCTCCTTT | |
| FAS | TNF receptor superfamily, member 6 | TGAGGAAGACTGTTACTACAGTTGAGA, GCAGTCCCTAGCTTTCCTTTC | |
| FADD | Fas (TNFRSF6)-associated via death domain | AGGTAGCCCAGCACTGTGAA, AGGTGGTCTGTGGCTCACTC | |
| Effector Apoptotic Proteins | CASP7 | Caspase 7 | CCCGCAAAGCAACGTCTA, CCCCTGCTCTTCAATACAGC |
| CASP3 | Caspase 3 | CTGGTTTTCGGTGGGTGT, CAGTGTTCTCCATGGATACCTTTATT | |
| CASP9 | Caspase 9 | CCCAAGCTCTTTTTCATCCA, AGTGGAGGCCACCTCAAAC | |
| CASP8 | Caspase 8 | GAAAGGGTGGAGCGGATT, GATTTCTGCTGAAGTCCATCTTTT | |
| HMGB1 | High-mobility group box 1 | GAGTGAGGAGGCTGCGTCT, TGCCCATGTTTAGTTATTTTTCC | |
| Cytokine/Growth-Factor Signaling Pathway | NFKB1 | Nuclear factor of kappa light polypeptide gene enhancer in B-cells 1 | CTGGCAGCTCTTCTCAAAGC, TCCAGGTCATAGAGAGGCTCA |
| RELA | v-rel reticuloendotheliosis viral oncogene homolog A | ACCGCTGCATCCACAGTT, GGATGCGCTGACTGATAGC | |
| AKT1 | v-akt murine thymoma viral oncogene homolog 1 | GCAGCACGTGTACGAGAAGA, GGTGTCAGTCTCCGACGTG | |
| PTEN | Phosphatase and tensin homolog | GCTACCTGTTAAAGAATCATCTGGA, CTGGCAGACCACAAACTGAG | |
| STAT1 | Signal transducer and activator of transcription 1 | GGATTGAAAGCATCCTAGAACTCA, GATGAAGCCCATGATGCAC | |
| Anti-Apoptotic Proteins | SOCS2 | Suppressor of cytokine signaling 2 | AGGCCTCACTGCAATTTGAT, TGCAAAATATAAAATGCCCAAG |
| HSP90B1 | Heat shock protein 90 kDa beta (Grp94), member 1 | CTGGAAATGAGGAACTAACAGTCA, TCTTCTCTGGTCATTCCTACACC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lajkó, E.; Hegedüs, R.; Mező, G.; Kőhidai, L. Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells. Int. J. Mol. Sci. 2019, 20, 4421. https://doi.org/10.3390/ijms20184421
Lajkó E, Hegedüs R, Mező G, Kőhidai L. Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells. International Journal of Molecular Sciences. 2019; 20(18):4421. https://doi.org/10.3390/ijms20184421
Chicago/Turabian StyleLajkó, Eszter, Rózsa Hegedüs, Gábor Mező, and László Kőhidai. 2019. "Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells" International Journal of Molecular Sciences 20, no. 18: 4421. https://doi.org/10.3390/ijms20184421
APA StyleLajkó, E., Hegedüs, R., Mező, G., & Kőhidai, L. (2019). Apoptotic Effects of Drug Targeting Conjugates Containing Different GnRH Analogs on Colon Carcinoma Cells. International Journal of Molecular Sciences, 20(18), 4421. https://doi.org/10.3390/ijms20184421







