Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma
Abstract
1. Introduction
2. Pathogenesis of MCL
2.1. Cyclin D1
2.2. Recurrent Molecular Cytogenetic Aberrations
3. Recurrent Molecular/Cytogenetic Lesions
3.1. Genotoxic Stress Pathways
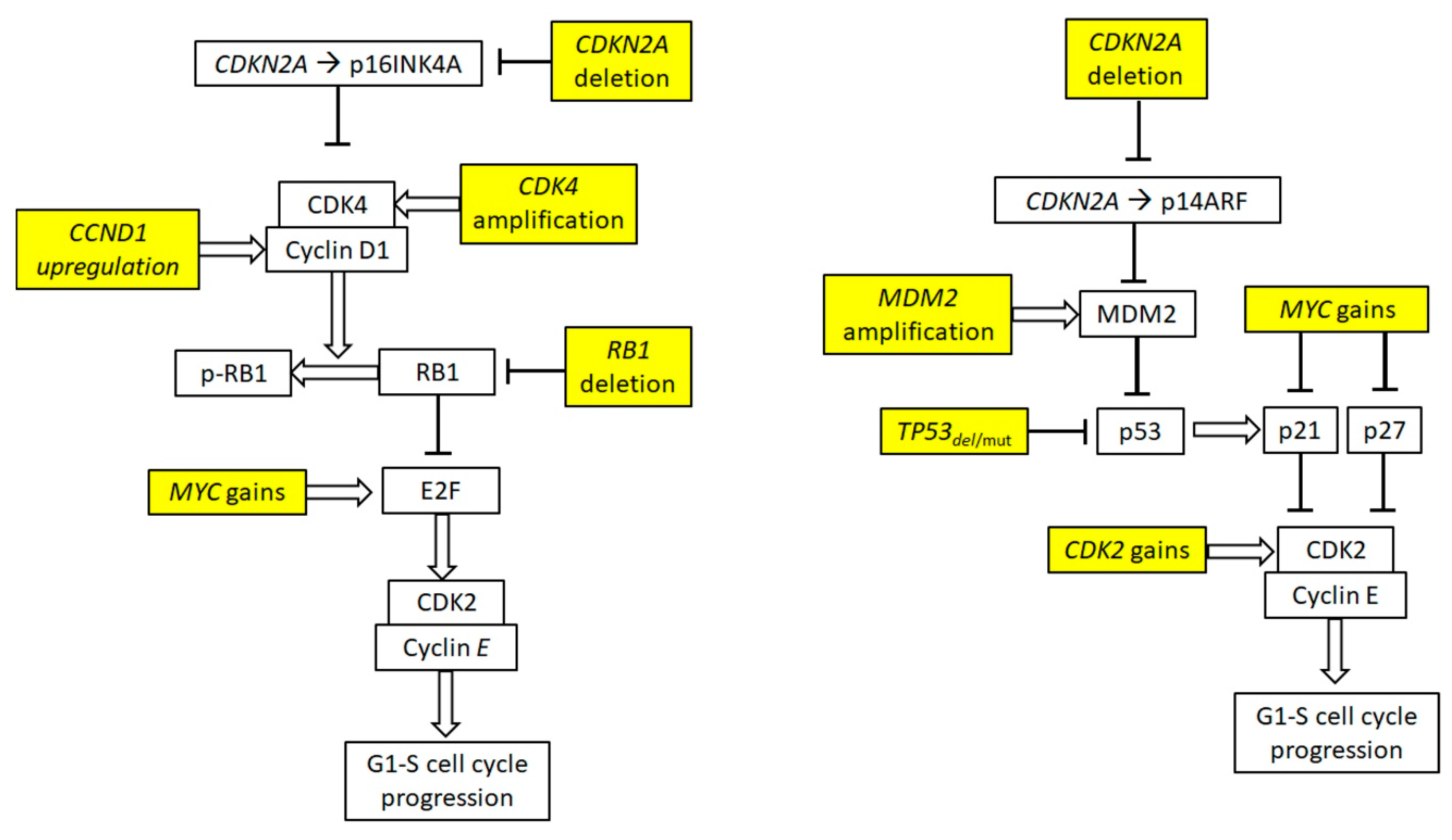
3.2. Cell Cycle Deregulation
3.3. Deregulation of Apoptosis
3.4. Prosurvival Signaling Cascades in MCL
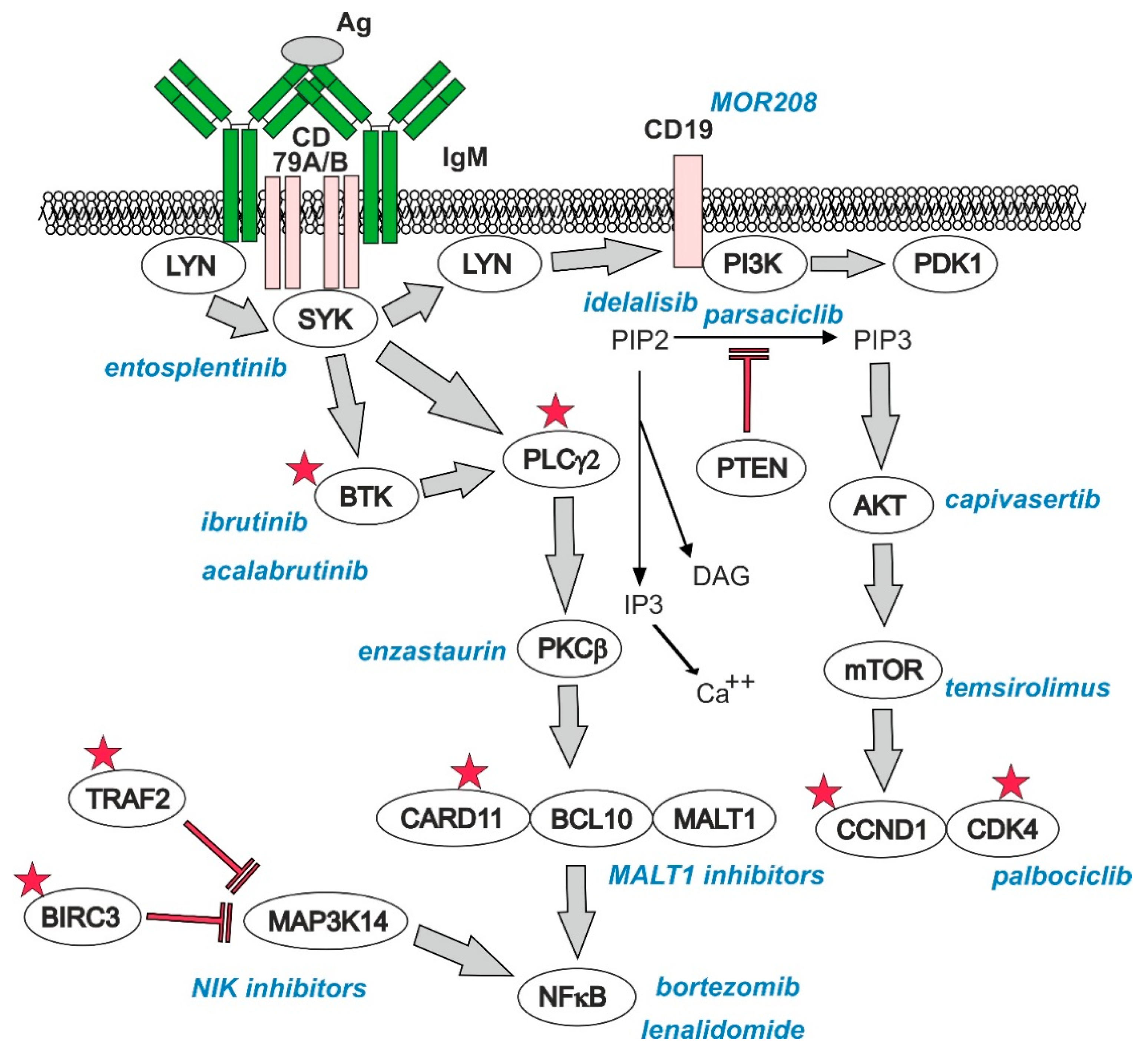
3.4.1. B-Cell Receptor (BCR) Signaling
3.4.2. PI3K–AKT–mTOR Pathway
3.4.3. Nuclear Factor kappa B (NFκB) Pathway
3.4.4. Notch Pathway
3.5. Epigenetic Modifiers in Pathogenesis of MCL
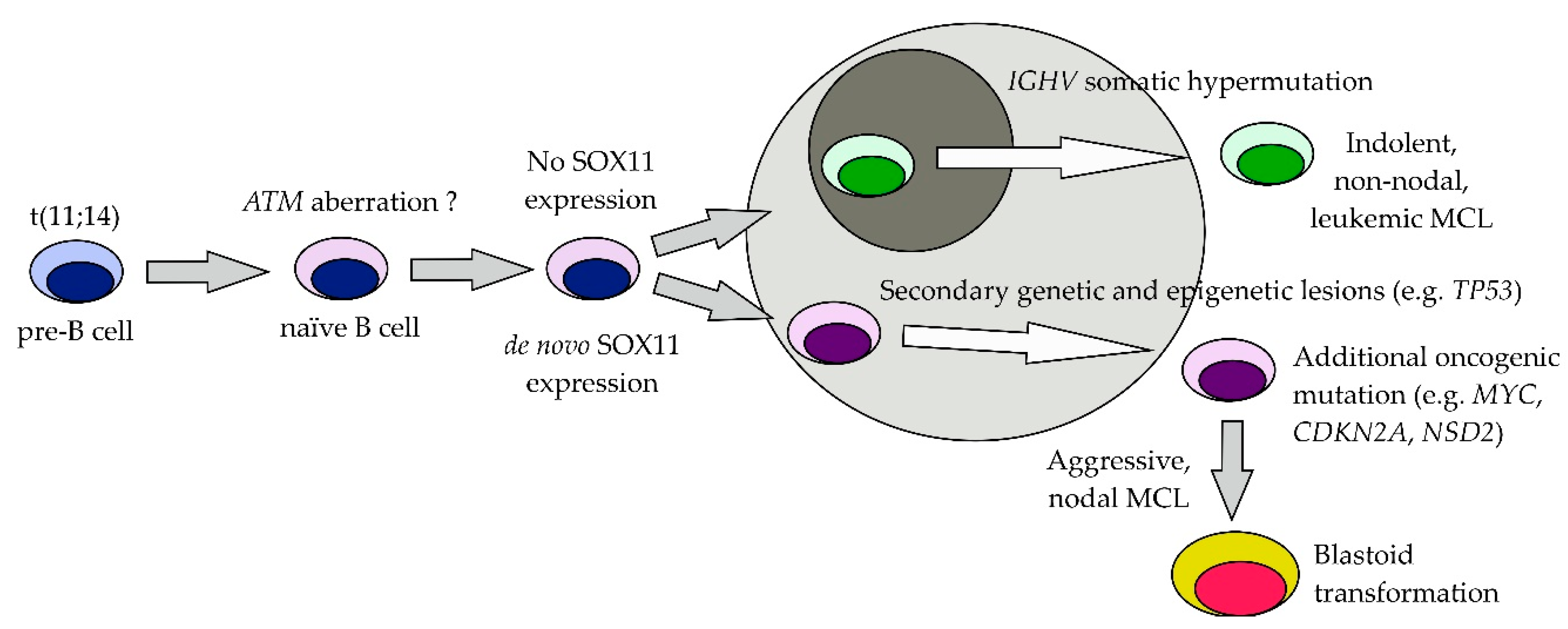
3.6. SOX11
4. Prognostic Factors before Therapy
5. Current Treatment Approaches and Outcomes of Patients after First-Line Treatment Approaches
6. Prognostic Factors during and after Induction
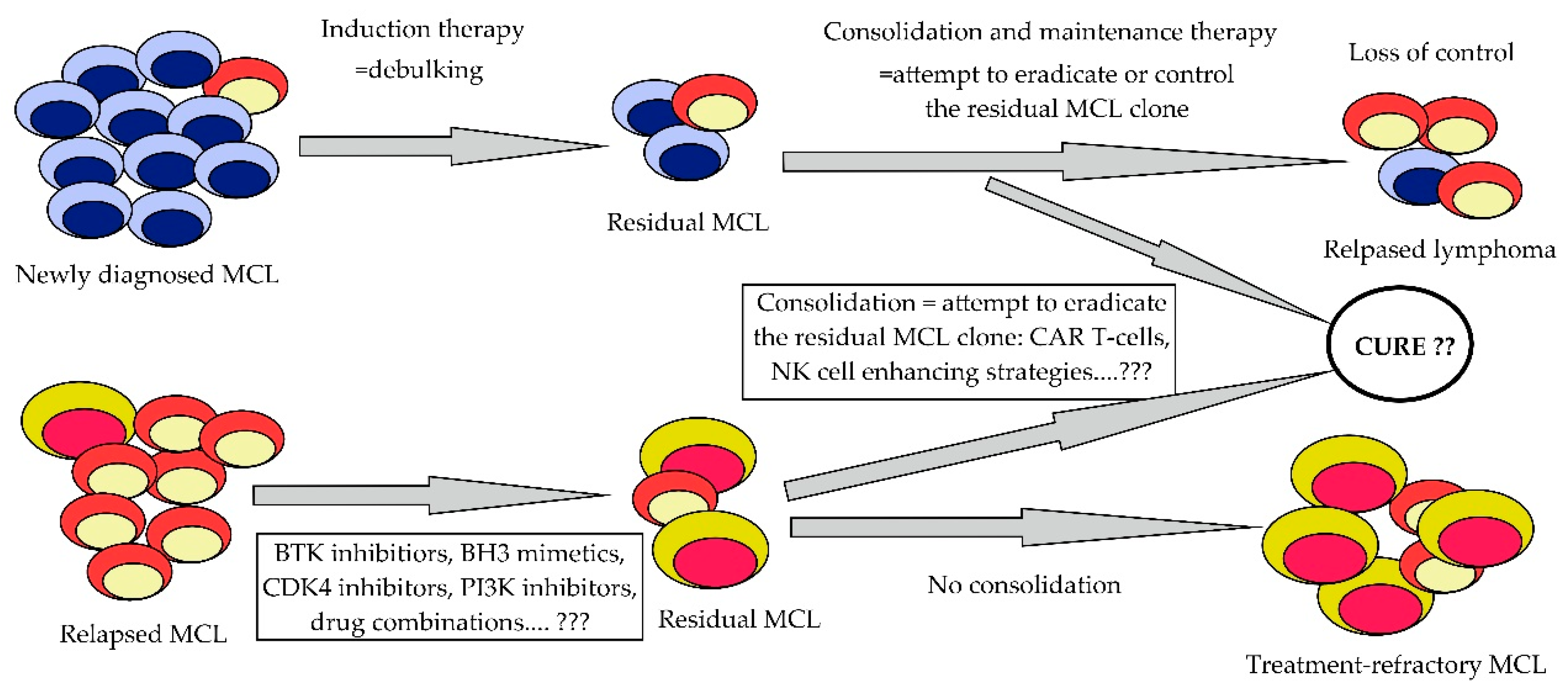
7. Salvage Therapy of Relapsed/Refractory MCL and Clonal Evolution of the Disease
7.1. Bruton’s Tyrosine Kinase (BTK) Inhibitors
7.2. Bortezomib, Lenalidomide, Temsirolimus, and Bendamustine
7.3. BCL2 Inhibitors
7.4. PI3K Inhibitors and Inhibitors of Other Prosurvival Pathways
7.5. Immunotherapy Approaches in Experimental Therapy of R/R MCL
8. Conclusions
Funding
Conflicts of Interest
Abbreviations
| BCR | B-cell receptor |
| BTK | Bruton’s tyrosine kinase |
| CAR | Chimeric antigen receptor |
| CDK | Cyclin-dependent kinase |
| CR | Complete remission |
| HDT-ASCT | High-dose therapy–Autologous stem cell transplantation |
| MCL | Mantle cell lymphoma |
| MIPI | MCL international prognostic index |
| MRD | Minimal residual disease |
| NK | Natural killer (cells) |
| ORR | Overall response rate (= complete and partial remissions) |
| OS | Overall survival |
| PD | Programmed cell death |
| PFS | Progression-free survival |
| PI3K | Phosphoinositide-3 kinase |
| PD-L1/2 | PD ligand 1/2 |
| PR | Partial remission |
| R-CHOP | Rituximab + cyclophosphamide + doxorubicin + vincristine + prednisone |
| R-DHAP | Rituximab + dexamethasone + high-dose cytarabine + cisplatin |
| RM | Rituximab maintenance |
| R/R-MCL | Relapsed/refractory MCL |
References
- Cheah, C.Y.; Seymour, J.F.; Wang, M.L. Mantle Cell Lymphoma. J. Clin. Oncol. 2016, 34, 1256–1269. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Klener, P.; Fronkova, E.; Berkova, A.; Jaksa, R.; Lhotska, H.; Forsterova, K.; Soukup, J.; Kulvait, V.; Vargova, J.; Fiser, K.; et al. Mantle cell lymphoma-variant Richter syndrome: Detailed molecular-cytogenetic and backtracking analysis reveals slow evolution of a pre-MCL clone in parallel with CLL over several years. Int. J. Cancer 2016, 139, 2252–2260. [Google Scholar] [CrossRef] [PubMed]
- Klener, P.; Salek, D.; Pytlik, R.; Mocikova, H.; Forsterova, K.; Blahovcova, P.; Campr, V.; Prochazka, V.; Obr, A.; Jaksa, R.; et al. Rituximab maintenance significantly prolongs progression-free survival of patients with newly diagnosed mantle cell lymphoma treated with the Nordic MCL2 protocol and autologous stem cell transplantation. Am. J. Hematol. 2019, 94, E50–E53. [Google Scholar] [CrossRef]
- Klener, P.; Fronkova, E.; Belada, D.; Forsterova, K.; Pytlik, R.; Kalinova, M.; Simkovic, M.; Salek, D.; Mocikova, H.; Prochazka, V.; et al. Alternating R-CHOP and R-cytarabine is a safe and effective regimen for transplant-ineligible patients with a newly diagnosed mantle cell lymphoma. Hematol. Oncol. 2018, 36, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; George, A.; Gine, E.; Chiappella, A.; Kluin-Nelemans, H.C.; Jurczak, W.; Krawczyk, K.; Mocikova, H.; Klener, P.; Salek, D.; et al. Central nervous system involvement in mantle cell lymphoma: Clinical features, prognostic factors and outcomes from the European Mantle Cell Lymphoma Network. Ann. Oncol. 2013, 24, 2119–2123. [Google Scholar] [CrossRef] [PubMed]
- Hadzidimitriou, A.; Agathangelidis, A.; Darzentas, N.; Murray, F.; Delfau-Larue, M.H.; Pedersen, L.B.; Lopez, A.N.; Dagklis, A.; Rombout, P.; Beldjord, K.; et al. Is there a role for antigen selection in mantle cell lymphoma? Immunogenetic support from a series of 807 cases. Blood 2011, 118, 3088–3095. [Google Scholar] [CrossRef] [PubMed]
- Xochelli, A.; Sutton, L.A.; Agathangelidis, A.; Stalika, E.; Karypidou, M.; Marantidou, F.; Lopez, A.N.; Papadopoulos, G.; Supikova, J.; Groenen, P.; et al. Molecular evidence for antigen drive in the natural history of mantle cell lymphoma. Am. J. Pathol. 2015, 185, 1740–1748. [Google Scholar] [CrossRef] [PubMed]
- Wiestner, A.; Tehrani, M.; Chiorazzi, M.; Wright, G.; Gibellini, F.; Nakayama, K.; Liu, H.; Rosenwald, A.; Muller-Hermelink, H.K.; Ott, G.; et al. Point mutations and genomic deletions in CCND1 create stable truncated cyclin D1 mRNAs that are associated with increased proliferation rate and shorter survival. Blood 2007, 109, 4599–4606. [Google Scholar] [CrossRef]
- Dal Col, J.; Dolcetti, R. GSK-3beta inhibition: At the crossroad between Akt and mTOR constitutive activation to enhance cyclin D1 protein stability in mantle cell lymphoma. Cell Cycle 2008, 7, 2813–2816. [Google Scholar] [CrossRef]
- Salaverria, I.; Royo, C.; Carvajal-Cuenca, A.; Clot, G.; Navarro, A.; Valera, A.; Song, J.Y.; Woroniecka, R.; Rymkiewicz, G.; Klapper, W.; et al. CCND2 rearrangements are the most frequent genetic events in cyclin D1− mantle cell lymphoma. Blood 2013, 121, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Martin-Garcia, D.; Navarro, A.; Valdes-Mas, R.; Clot, G.; Gutierrez-Abril, J.; Prieto, M.; Ribera-Cortada, I.; Woroniecka, R.; Rymkiewicz, G.; Bens, S.; et al. CCND2 and CCND3 hijack immunoglobulin light-chain enhancers in cyclin D1− mantle cell lymphoma. Blood 2019, 133, 940–951. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jima, D.; Moffitt, A.B.; Liu, Q.; Czader, M.; Hsi, E.D.; Fedoriw, Y.; Dunphy, C.H.; Richards, K.L.; Gill, J.I.; et al. The genomic landscape of mantle cell lymphoma is related to the epigenetically determined chromatin state of normal B cells. Blood 2014, 123, 2988–2996. [Google Scholar] [CrossRef] [PubMed]
- Bea, S.; Valdes-Mas, R.; Navarro, A.; Salaverria, I.; Martin-Garcia, D.; Jares, P.; Gine, E.; Pinyol, M.; Royo, C.; Nadeu, F.; et al. Landscape of somatic mutations and clonal evolution in mantle cell lymphoma. Proc. Natl. Acad. Sci. USA 2013, 110, 18250–18255. [Google Scholar] [CrossRef] [PubMed]
- Lovec, H.; Grzeschiczek, A.; Kowalski, M.B.; Moroy, T. Cyclin D1/bcl-1 cooperates with myc genes in the generation of B-cell lymphoma in transgenic mice. EMBO J. 1994, 13, 3487–3495. [Google Scholar] [CrossRef] [PubMed]
- Watson, I.R.; Takahashi, K.; Futreal, P.A.; Chin, L. Emerging patterns of somatic mutations in cancer. Nat. Rev. Genet. 2013, 14, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Kridel, R.; Meissner, B.; Rogic, S.; Boyle, M.; Telenius, A.; Woolcock, B.; Gunawardana, J.; Jenkins, C.; Cochrane, C.; Ben-Neriah, S.; et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood 2012, 119, 1963–1971. [Google Scholar] [CrossRef]
- Rahal, R.; Frick, M.; Romero, R.; Korn, J.M.; Kridel, R.; Chan, F.C.; Meissner, B.; Bhang, H.E.; Ruddy, D.; Kauffmann, A.; et al. Pharmacological and genomic profiling identifies NF-kappaB-targeted treatment strategies for mantle cell lymphoma. Nat. Med. 2014, 20, 87–92. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, W.; Wang, J.; Liu, Y.; An, R.; Jing, H. Genomic landscape and prognostic analysis of mantle cell lymphoma. Cancer Gene Ther. 2018, 25, 129–140. [Google Scholar] [CrossRef]
- Wu, C.; de Miranda, N.F.; Chen, L.; Wasik, A.M.; Mansouri, L.; Jurczak, W.; Galazka, K.; Dlugosz-Danecka, M.; Machaczka, M.; Zhang, H.; et al. Genetic heterogeneity in primary and relapsed mantle cell lymphomas: Impact of recurrent CARD11 mutations. Oncotarget 2016, 7, 38180–38190. [Google Scholar] [CrossRef]
- Onaindia, A.; Medeiros, L.J.; Patel, K.P. Clinical utility of recently identified diagnostic, prognostic, and predictive molecular biomarkers in mature B-cell neoplasms. Mod. Pathol. 2017, 30, 1338–1366. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Zhang, L.; Nomie, K.; Lam, L.; Wang, M. Gene mutations and actionable genetic lesions in mantle cell lymphoma. Oncotarget 2016, 7, 58638–58648. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Delfau-Larue, M.H.; Klapper, W.; Berger, F.; Jardin, F.; Briere, J.; Salles, G.; Casasnovas, O.; Feugier, P.; Haioun, C.; Ribrag, V.; et al. High-dose cytarabine does not overcome the adverse prognostic value of CDKN2A and TP53 deletions in mantle cell lymphoma. Blood 2015, 126, 604–611. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.C.; Moynihan, M.J.; Chan, W.C.; Lytle, D.M.; Pedersen, A.; Anderson, J.R.; Weisenburger, D.D. p53 mutations in mantle cell lymphoma are associated with variant cytology and predict a poor prognosis. Blood 1996, 87, 4302–4310. [Google Scholar] [PubMed]
- Eskelund, C.W.; Dahl, C.; Hansen, J.W.; Westman, M.; Kolstad, A.; Pedersen, L.B.; Montano-Almendras, C.P.; Husby, S.; Freiburghaus, C.; Ek, S.; et al. TP53 mutations identify younger mantle cell lymphoma patients who do not benefit from intensive chemoimmunotherapy. Blood 2017, 130, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Aukema, S.M.; Hoster, E.; Rosenwald, A.; Canoni, D.; Delfau-Larue, M.H.; Rymkiewicz, G.; Thorns, C.; Hartmann, S.; Kluin-Nelemans, H.; Hermine, O.; et al. Expression of TP53 is associated with the outcome of MCL independent of MIPI and Ki-67 in trials of the European MCL Network. Blood 2018, 131, 417–420. [Google Scholar] [CrossRef] [PubMed]
- Greiner, T.C.; Dasgupta, C.; Ho, V.V.; Weisenburger, D.D.; Smith, L.M.; Lynch, J.C.; Vose, J.M.; Fu, K.; Armitage, J.O.; Braziel, R.M.; et al. Mutation and genomic deletion status of ataxia telangiectasia mutated (ATM) and p53 confer specific gene expression profiles in mantle cell lymphoma. Proc. Natl. Acad. Sci. USA 2006, 103, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Williamson, C.T.; Kubota, E.; Hamill, J.D.; Klimowicz, A.; Ye, R.; Muzik, H.; Dean, M.; Tu, L.; Gilley, D.; Magliocco, A.M.; et al. Enhanced cytotoxicity of PARP inhibition in mantle cell lymphoma harbouring mutations in both ATM and p53. EMBO Mol. Med. 2012, 4, 515–527. [Google Scholar] [CrossRef]
- Golla, R.M.; Li, M.; Shen, Y.; Ji, M.; Yan, Y.; Fu, K.; Greiner, T.C.; McKeithan, T.W.; Chan, W.C. Inhibition of poly(ADP-ribose) polymerase (PARP) and ataxia telangiectasia mutated (ATM) on the chemosensitivity of mantle cell lymphoma to agents that induce DNA strand breaks. Hematol. Oncol. 2012, 30, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Yang, Y.; Yang, W. Inhibition of SKP2 Activity Impaired ATM-Mediated DNA Repair and Enhanced Sensitivity of Cisplatin-Resistant Mantle Cell Lymphoma Cells. Cancer Biother. Radiopharm. 2019. [Google Scholar] [CrossRef]
- Jares, P.; Colomer, D.; Campo, E. Genetic and molecular pathogenesis of mantle cell lymphoma: Perspectives for new targeted therapeutics. Nat. Rev. Cancer 2007, 7, 750–762. [Google Scholar] [CrossRef] [PubMed]
- Vincent-Fabert, C.; Fiancette, R.; Rouaud, P.; Baudet, C.; Truffinet, V.; Magnone, V.; Guillaudeau, A.; Cogne, M.; Dubus, P.; Denizot, Y. A defect of the INK4-Cdk4 checkpoint and Myc collaborate in blastoid mantle cell lymphoma-like lymphoma formation in mice. Am. J. Pathol. 2012, 180, 1688–1701. [Google Scholar] [CrossRef] [PubMed]
- Choe, J.Y.; Yun, J.Y.; Na, H.Y.; Huh, J.; Shin, S.J.; Kim, H.J.; Paik, J.H.; Kim, Y.A.; Nam, S.J.; Jeon, Y.K.; et al. MYC overexpression correlates with MYC amplification or translocation, and is associated with poor prognosis in mantle cell lymphoma. Histopathology 2016, 68, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Bouchlaka, M.N.; Wolff, J.; Grindle, K.M.; Lu, L.; Qian, S.; Zhong, X.; Pflum, N.; Jobin, P.; Kahl, B.S.; et al. FBXO10 deficiency and BTK activation upregulate BCL2 expression in mantle cell lymphoma. Oncogene 2016, 35, 6223–6234. [Google Scholar] [CrossRef] [PubMed]
- Yi, S.; Zou, D.; Li, C.; Zhong, S.; Chen, W.; Li, Z.; Xiong, W.; Liu, W.; Liu, E.; Cui, R.; et al. High incidence of MYC and BCL2 abnormalities in mantle cell lymphoma, although only MYC abnormality predicts poor survival. Oncotarget 2015, 6, 42362–42371. [Google Scholar] [CrossRef]
- Bea, S.; Salaverria, I.; Armengol, L.; Pinyol, M.; Fernandez, V.; Hartmann, E.M.; Jares, P.; Amador, V.; Hernandez, L.; Navarro, A.; et al. Uniparental disomies, homozygous deletions, amplifications, and target genes in mantle cell lymphoma revealed by integrative high-resolution whole-genome profiling. Blood 2009, 113, 3059–3069. [Google Scholar] [CrossRef] [PubMed]
- Pekarsky, Y.; Balatti, V.; Croce, C.M. BCL2 and miR-15/16: From gene discovery to treatment. Cell Death Differ. 2018, 25, 21–26. [Google Scholar] [CrossRef]
- Khoury, J.D.; Medeiros, L.J.; Rassidakis, G.Z.; McDonnell, T.J.; Abruzzo, L.V.; Lai, R. Expression of Mcl-1 in mantle cell lymphoma is associated with high-grade morphology, a high proliferative state, and p53 overexpression. J. Pathol. 2003, 199, 90–97. [Google Scholar] [CrossRef]
- Tagawa, H.; Karnan, S.; Suzuki, R.; Matsuo, K.; Zhang, X.; Ota, A.; Morishima, Y.; Nakamura, S.; Seto, M. Genome-wide array-based CGH for mantle cell lymphoma: Identification of homozygous deletions of the proapoptotic gene BIM. Oncogene 2005, 24, 1348–1358. [Google Scholar] [CrossRef]
- Mestre-Escorihuela, C.; Rubio-Moscardo, F.; Richter, J.A.; Siebert, R.; Climent, J.; Fresquet, V.; Beltran, E.; Agirre, X.; Marugan, I.; Marin, M.; et al. Homozygous deletions localize novel tumor suppressor genes in B-cell lymphomas. Blood 2007, 109, 271–280. [Google Scholar] [CrossRef]
- Katz, S.G.; Labelle, J.L.; Meng, H.; Valeriano, R.P.; Fisher, J.K.; Sun, H.; Rodig, S.J.; Kleinstein, S.H.; Walensky, L.D. Mantle cell lymphoma in cyclin D1 transgenic mice with Bim-deficient B cells. Blood 2014, 123, 884–893. [Google Scholar] [CrossRef] [PubMed]
- Prukova, D.; Andera, L.; Nahacka, Z.; Karolova, J.; Svaton, M.; Klanova, M.; Havranek, O.; Soukup, J.; Svobodova, K.; Zemanova, Z.; et al. Co-targeting of BCL2 with venetoclax and MCL1 with S63845 is synthetically lethal in vivo in relapsed mantle cell lymphoma. Clin. Cancer Res. 2019, 3275. [Google Scholar] [CrossRef]
- Merolle, M.I.; Ahmed, M.; Nomie, K.; Wang, M.L. The B cell receptor signaling pathway in mantle cell lymphoma. Oncotarget 2018, 9, 25332–25341. [Google Scholar] [CrossRef] [PubMed]
- Rao, E.; Jiang, C.; Ji, M.; Huang, X.; Iqbal, J.; Lenz, G.; Wright, G.; Staudt, L.M.; Zhao, Y.; McKeithan, T.W.; et al. The miRNA-17 approximately 92 cluster mediates chemoresistance and enhances tumor growth in mantle cell lymphoma via PI3K/AKT pathway activation. Leukemia 2012, 26, 1064–1072. [Google Scholar] [CrossRef] [PubMed]
- Kahl, B.S.; Spurgeon, S.E.; Furman, R.R.; Flinn, I.W.; Coutre, S.E.; Brown, J.R.; Benson, D.M.; Byrd, J.C.; Peterman, S.; Cho, Y.; et al. A phase 1 study of the PI3Kdelta inhibitor idelalisib in patients with relapsed/refractory mantle cell lymphoma (MCL). Blood 2014, 123, 3398–3405. [Google Scholar] [CrossRef] [PubMed]
- Thome, M.; Charton, J.E.; Pelzer, C.; Hailfinger, S. Antigen receptor signaling to NF-kappaB via CARMA1, BCL10, and MALT1. Cold Spring Harb. Perspect. Biol. 2010, 2, a003004. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lenardo, M.J.; Baltimore, D. 30 Years of NF-κB: A Blossoming of Relevance to Human Pathobiology. Cell 2017, 168, 37–57. [Google Scholar] [CrossRef]
- Yang, Y.; Kelly, P.; Shaffer, A.L., 3rd; Schmitz, R.; Yoo, H.M.; Liu, X.; Huang, D.W.; Webster, D.; Young, R.M.; Nakagawa, M.; et al. Targeting Non-proteolytic Protein Ubiquitination for the Treatment of Diffuse Large B Cell Lymphoma. Cancer Cell 2016, 29, 494–507. [Google Scholar] [CrossRef]
- Keats, J.J.; Fonseca, R.; Chesi, M.; Schop, R.; Baker, A.; Chng, W.J.; Van Wier, S.; Tiedemann, R.; Shi, C.X.; Sebag, M.; et al. Promiscuous mutations activate the noncanonical NF-κB pathway in multiple myeloma. Cancer Cell 2007, 12, 131–144. [Google Scholar] [CrossRef]
- Rossi, D.; Fangazio, M.; Rasi, S.; Vaisitti, T.; Monti, S.; Cresta, S.; Chiaretti, S.; Del Giudice, I.; Fabbri, G.; Bruscaggin, A.; et al. Disruption of BIRC3 associates with fludarabine chemorefractoriness in TP53 wild-type chronic lymphocytic leukemia. Blood 2012, 119, 2854–2862. [Google Scholar] [CrossRef]
- Diop, F.; Moia, R.; Favini, C.; Spaccarotella, E.; De Paoli, L.; Bruscaggin, A.; Spina, V.; Terzi-di-Bergamo, L.; Arruga, F.; Tarantelli, C.; et al. Biological and clinical implications of BIRC3 mutations in chronic lymphocytic leukemia. Haematologica 2019, 249550. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Grau, M.; Juilland, M.; Klener, P.; Horing, E.; Molinsky, J.; Schimmack, G.; Aukema, S.M.; Hoster, E.; Vogt, N.; et al. B-cell receptor-driven MALT1 activity regulates MYC signaling in mantle cell lymphoma. Blood 2017, 129, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.A.; Goy, A.; Ayoub, N.M.; Attia, C.; Oton, L.; Taruvai, V.; Costales, M.; Lin, Y.T.; Pecora, A.; Suh, K.S. Mantle cell lymphoma in the era of precision medicine-diagnosis, biomarkers and therapeutic agents. Oncotarget 2016, 7, 48692–48731. [Google Scholar] [CrossRef] [PubMed]
- Swaroop, A.; Oyer, J.A.; Will, C.M.; Huang, X.; Yu, W.; Troche, C.; Bulic, M.; Durham, B.H.; Wen, Q.J.; Crispino, J.D.; et al. An activating mutation of the NSD2 histone methyltransferase drives oncogenic reprogramming in acute lymphocytic leukemia. Oncogene 2019, 38, 671–686. [Google Scholar] [CrossRef]
- Zhang, J.; Lee, Y.R.; Dang, F.; Gan, W.; Menon, A.V.; Katon, J.M.; Hsu, C.H.; Asara, J.M.; Tibarewal, P.; Leslie, N.R.; et al. PTEN Methylation by NSD2 Controls Cellular Sensitivity to DNA Damage. Cancer Discov. 2019, CD-18-0083. [Google Scholar] [CrossRef]
- Jain, P.; Kanagal-Shamanna, R.; Zhang, S.; Ahmed, M.; Ghorab, A.; Zhang, L.; Ok, C.Y.; Li, S.; Hagemeister, F.; Zeng, D.; et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br. J. Haematol. 2018, 183, 578–587. [Google Scholar] [CrossRef]
- Shen, Y.; Morishita, M.; Lee, D.; Kim, S.; Lee, T.; Mevius, D.; Roh, Y.; di Luccio, E. Identification of LEM-14 inhibitor of the oncoprotein NSD2. Biochem. Biophys. Res. Commun. 2019, 508, 102–108. [Google Scholar] [CrossRef]
- Pasqualucci, L.; Trifonov, V.; Fabbri, G.; Ma, J.; Rossi, D.; Chiarenza, A.; Wells, V.A.; Grunn, A.; Messina, M.; Elliot, O.; et al. Analysis of the coding genome of diffuse large B-cell lymphoma. Nat. Genet. 2011, 43, 830–837. [Google Scholar] [CrossRef]
- Chen, R.; Frankel, P.; Popplewell, L.; Siddiqi, T.; Ruel, N.; Rotter, A.; Thomas, S.H.; Mott, M.; Nathwani, N.; Htut, M.; et al. A phase II study of vorinostat and rituximab for treatment of newly diagnosed and relapsed/refractory indolent non-Hodgkin lymphoma. Haematologica 2015, 100, 357–362. [Google Scholar] [CrossRef]
- Oki, Y.; Buglio, D.; Fanale, M.; Fayad, L.; Copeland, A.; Romaguera, J.; Kwak, L.W.; Pro, B.; de Castro Faria, S.; Neelapu, S.; et al. Phase I study of panobinostat plus everolimus in patients with relapsed or refractory lymphoma. Clin. Cancer Res. 2013, 19, 6882–6890. [Google Scholar] [CrossRef] [PubMed]
- Evens, A.M.; Balasubramanian, S.; Vose, J.M.; Harb, W.; Gordon, L.I.; Langdon, R.; Sprague, J.; Sirisawad, M.; Mani, C.; Yue, J.; et al. A Phase I/II Multicenter, Open-Label Study of the Oral Histone Deacetylase Inhibitor Abexinostat in Relapsed/Refractory Lymphoma. Clin. Cancer Res. 2016, 22, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Yazbeck, V.; Shafer, D.; Perkins, E.B.; Coppola, D.; Sokol, L.; Richards, K.L.; Shea, T.; Ruan, J.; Parekh, S.; Strair, R.; et al. A Phase II Trial of Bortezomib and Vorinostat in Mantle Cell Lymphoma and Diffuse Large B-cell Lymphoma. Clin. LymphomaMyeloma Leuk. 2018, 18, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.Y.; Kim, S.J.; Yoon, D.H.; Park, Y.; Kong, J.H.; Kim, J.A.; Kim, B.S.; Kim, H.J.; Won, J.H.; Park, S.K.; et al. Results of a phase II study of vorinostat in combination with intravenous fludarabine, mitoxantrone, and dexamethasone in patients with relapsed or refractory mantle cell lymphoma: An interim analysis. Cancer Chemother. Pharmacol. 2016, 77, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Chan, Y.-C.; Tam, C.S.; Hunter, T.; Vassiliadis, D.; Teh, C.E.; Thijssen, R.; Yeh, P.; Wong, S.Q.; Ftouni, S.; et al. Dynamic molecular monitoring reveals that SWI–SNF mutations mediate resistance to ibrutinib plus venetoclax in mantle cell lymphoma. Nat. Med. 2019, 25, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Royo, C.; Navarro, A.; Clot, G.; Salaverria, I.; Gine, E.; Jares, P.; Colomer, D.; Wiestner, A.; Wilson, W.H.; Vegliante, M.C.; et al. Non-nodal type of mantle cell lymphoma is a specific biological and clinical subgroup of the disease. Leukemia 2012, 26, 1895–1898. [Google Scholar] [CrossRef]
- Queiros, A.C.; Beekman, R.; Vilarrasa-Blasi, R.; Duran-Ferrer, M.; Clot, G.; Merkel, A.; Raineri, E.; Russinol, N.; Castellano, G.; Bea, S.; et al. Decoding the DNA Methylome of Mantle Cell Lymphoma in the Light of the Entire B Cell Lineage. Cancer Cell 2016, 30, 806–821. [Google Scholar] [CrossRef]
- Vegliante, M.C.; Palomero, J.; Perez-Galan, P.; Roue, G.; Castellano, G.; Navarro, A.; Clot, G.; Moros, A.; Suarez-Cisneros, H.; Bea, S.; et al. SOX11 regulates PAX5 expression and blocks terminal B-cell differentiation in aggressive mantle cell lymphoma. Blood 2013, 121, 2175–2185. [Google Scholar] [CrossRef]
- Ribera-Cortada, I.; Martinez, D.; Amador, V.; Royo, C.; Navarro, A.; Bea, S.; Gine, E.; de Leval, L.; Serrano, S.; Wotherspoon, A.; et al. Plasma cell and terminal B-cell differentiation in mantle cell lymphoma mainly occur in the SOX11-negative subtype. Mod. Pathol. 2015, 28, 1435–1447. [Google Scholar] [CrossRef]
- Kuo, P.Y.; Jatiani, S.S.; Rahman, A.H.; Edwards, D.; Jiang, Z.; Ahr, K.; Perumal, D.; Leshchenko, V.V.; Brody, J.; Shaknovich, R.; et al. SOX11 augments BCR signaling to drive MCL-like tumor development. Blood 2018, 131, 2247–2255. [Google Scholar] [CrossRef]
- Palomero, J.; Vegliante, M.C.; Rodriguez, M.L.; Eguileor, A.; Castellano, G.; Planas-Rigol, E.; Jares, P.; Ribera-Cortada, I.; Cid, M.C.; Campo, E.; et al. SOX11 promotes tumor angiogenesis through transcriptional regulation of PDGFA in mantle cell lymphoma. Blood 2014, 124, 2235–2247. [Google Scholar] [CrossRef] [PubMed]
- Balsas, P.; Palomero, J.; Eguileor, A.; Rodriguez, M.L.; Vegliante, M.C.; Planas-Rigol, E.; Sureda-Gomez, M.; Cid, M.C.; Campo, E.; Amador, V. SOX11 promotes tumor protective microenvironment interactions through CXCR4 and FAK regulation in mantle cell lymphoma. Blood 2017, 130, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Hoster, E.; Dreyling, M.; Klapper, W.; Gisselbrecht, C.; van Hoof, A.; Kluin-Nelemans, H.C.; Pfreundschuh, M.; Reiser, M.; Metzner, B.; Einsele, H.; et al. A new prognostic index (MIPI) for patients with advanced-stage mantle cell lymphoma. Blood 2008, 111, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Hoster, E.; Klapper, W.; Hermine, O.; Kluin-Nelemans, H.C.; Walewski, J.; van Hoof, A.; Trneny, M.; Geisler, C.H.; Di Raimondo, F.; Szymczyk, M.; et al. Confirmation of the mantle-cell lymphoma International Prognostic Index in randomized trials of the European Mantle-Cell Lymphoma Network. J. Clin. Oncol. 2014, 32, 1338–1346. [Google Scholar] [CrossRef] [PubMed]
- Greenwell, I.B.; Staton, A.D.; Lee, M.J.; Switchenko, J.M.; Saxe, D.F.; Maly, J.J.; Blum, K.A.; Grover, N.S.; Mathews, S.P.; Gordon, M.J.; et al. Complex karyotype in patients with mantle cell lymphoma predicts inferior survival and poor response to intensive induction therapy. Cancer 2018, 124, 2306–2315. [Google Scholar] [CrossRef] [PubMed]
- Sarkozy, C.; Terre, C.; Jardin, F.; Radford, I.; Roche-Lestienne, C.; Penther, D.; Bastard, C.; Rigaudeau, S.; Pilorge, S.; Morschhauser, F.; et al. Complex karyotype in mantle cell lymphoma is a strong prognostic factor for the time to treatment and overall survival, independent of the MCL international prognostic index. Genes Chromosom. Cancer 2014, 53, 106–116. [Google Scholar] [CrossRef]
- Determann, O.; Hoster, E.; Ott, G.; Wolfram Bernd, H.; Loddenkemper, C.; Leo Hansmann, M.; Barth, T.E.; Unterhalt, M.; Hiddemann, W.; Dreyling, M.; et al. Ki-67 predicts outcome in advanced-stage mantle cell lymphoma patients treated with anti-CD20 immunochemotherapy: Results from randomized trials of the European MCL Network and the German Low Grade Lymphoma Study Group. Blood 2008, 111, 2385–2387. [Google Scholar] [CrossRef]
- Hoster, E.; Rosenwald, A.; Berger, F.; Bernd, H.W.; Hartmann, S.; Loddenkemper, C.; Barth, T.F.; Brousse, N.; Pileri, S.; Rymkiewicz, G.; et al. Prognostic Value of Ki-67 Index, Cytology, and Growth Pattern in Mantle-Cell Lymphoma: Results From Randomized Trials of the European Mantle Cell Lymphoma Network. J. Clin. Oncol. 2016, 34, 1386–1394. [Google Scholar] [CrossRef]
- Dreyling, M.; Klapper, W.; Rule, S. Blastoid and pleomorphic mantle cell lymphoma: Still a diagnostic and therapeutic challenge! Blood 2018, 132, 2722–2729. [Google Scholar] [CrossRef]
- Ruan, J.; Martin, P.; Shah, B.; Schuster, S.J.; Smith, S.M.; Furman, R.R.; Christos, P.; Rodriguez, A.; Svoboda, J.; Lewis, J.; et al. Lenalidomide plus Rituximab as Initial Treatment for Mantle-Cell Lymphoma. N. Engl. J. Med. 2015, 373, 1835–1844. [Google Scholar] [CrossRef]
- Kumar, A.; Ying, Z.; Alperovich, A.; Dogan, A.; Hamlin, P.; Moskowitz, C.; Pichardo, J.; Portlock, C.; Sha, F.; Zelenetz, A.D.; et al. Clinical presentation determines selection of patients for initial observation in mantle cell lymphoma. Haematologica 2019, 104, e163–e166. [Google Scholar] [CrossRef] [PubMed]
- Hermine, O.; Hoster, E.; Walewski, J.; Bosly, A.; Stilgenbauer, S.; Thieblemont, C.; Szymczyk, M.; Bouabdallah, R.; Kneba, M.; Hallek, M.; et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): A randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet 2016, 388, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.H.; Kolstad, A.; Laurell, A.; Andersen, N.S.; Pedersen, L.B.; Jerkeman, M.; Eriksson, M.; Nordstrom, M.; Kimby, E.; Boesen, A.M.; et al. Long-term progression-free survival of mantle cell lymphoma after intensive front-line immunochemotherapy with in vivo-purged stem cell rescue: A nonrandomized phase 2 multicenter study by the Nordic Lymphoma Group. Blood 2008, 112, 2687–2693. [Google Scholar] [CrossRef] [PubMed]
- Geisler, C.H.; Kolstad, A.; Laurell, A.; Jerkeman, M.; Raty, R.; Andersen, N.S.; Pedersen, L.B.; Eriksson, M.; Nordstrom, M.; Kimby, E.; et al. Nordic MCL2 trial update: Six-year follow-up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem-cell support: Still very long survival but late relapses do occur. Br. J. Haematol. 2012, 158, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Lenz, G.; Dreyling, M.; Hoster, E.; Wormann, B.; Duhrsen, U.; Metzner, B.; Eimermacher, H.; Neubauer, A.; Wandt, H.; Steinhauer, H.; et al. Immunochemotherapy with rituximab and cyclophosphamide, doxorubicin, vincristine, and prednisone significantly improves response and time to treatment failure, but not long-term outcome in patients with previously untreated mantle cell lymphoma: Results of a prospective randomized trial of the German Low Grade Lymphoma Study Group (GLSG). J. Clin. Oncol. 2005, 23, 1984–1992. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.N.; Barta, S.K. Mantle Cell Lymphoma: Which Patients Should We Transplant? Curr. Hematol. Malig. Rep. 2019, 14, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Le Gouill, S.; Thieblemont, C.; Oberic, L.; Moreau, A.; Bouabdallah, K.; Dartigeas, C.; Damaj, G.; Gastinne, T.; Ribrag, V.; Feugier, P.; et al. Rituximab after Autologous Stem-Cell Transplantation in Mantle-Cell Lymphoma. N. Engl. J. Med. 2017, 377, 1250–1260. [Google Scholar] [CrossRef]
- Dietrich, S.; Weidle, J.; Rieger, M.; Meissner, J.; Radujkovic, A.; Ho, A.D.; Dreger, P.; Witzens-Harig, M. Rituximab maintenance therapy after autologous stem cell transplantation prolongs progression-free survival in patients with mantle cell lymphoma. Leukemia 2014, 28, 708–709. [Google Scholar] [CrossRef]
- Graf, S.A.; Stevenson, P.A.; Holmberg, L.A.; Till, B.G.; Press, O.W.; Chauncey, T.R.; Smith, S.D.; Philip, M.; Orozco, J.J.; Shustov, A.R.; et al. Maintenance rituximab after autologous stem cell transplantation in patients with mantle cell lymphoma. Ann. Oncol. 2015, 26, 2323–2328. [Google Scholar] [CrossRef]
- Mei, M.G.; Cao, T.M.; Chen, L.; Song, J.Y.; Siddiqi, T.; Cai, J.L.; Farol, L.T.; Al Malki, M.M.; Salhotra, A.; Aldoss, I.; et al. Long-Term Results of High-Dose Therapy and Autologous Stem Cell Transplantation for Mantle Cell Lymphoma: Effectiveness of Maintenance Rituximab. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 1861–1869. [Google Scholar] [CrossRef]
- Kumar, A.; Sha, F.; Toure, A.; Dogan, A.; Ni, A.; Batlevi, C.L.; Palomba, M.L.M.; Portlock, C.; Straus, D.J.; Noy, A.; et al. Patterns of survival in patients with recurrent mantle cell lymphoma in the modern era: Progressive shortening in response duration and survival after each relapse. Blood Cancer J. 2019, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Gerson, J.N.; Handorf, E.; Villa, D.; Gerrie, A.S.; Chapani, P.; Li, S.; Medeiros, L.J.; Wang, M.I.; Cohen, J.B.; Calzada, O.; et al. Survival Outcomes of Younger Patients With Mantle Cell Lymphoma Treated in the Rituximab Era. J. Clin. Oncol. 2019, 37, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Finotto, S.; Zambello, R.; Paolini, R.; Menin, A.; Zanotti, R.; Zaja, F.; Semenzato, G.; Pizzolo, G.; D’Amore, E.S.; et al. Combination of rituximab, bendamustine, and cytarabine for patients with mantle-cell non-Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. J. Clin. Oncol. 2013, 31, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Rummel, M.J.; Niederle, N.; Maschmeyer, G.; Banat, G.A.; von Grunhagen, U.; Losem, C.; Kofahl-Krause, D.; Heil, G.; Welslau, M.; Balser, C.; et al. Bendamustine plus rituximab versus CHOP plus rituximab as first-line treatment for patients with indolent and mantle-cell lymphomas: An open-label, multicentre, randomised, phase 3 non-inferiority trial. Lancet 2013, 381, 1203–1210. [Google Scholar] [CrossRef]
- Robak, T.; Jin, J.; Pylypenko, H.; Verhoef, G.; Siritanaratkul, N.; Drach, J.; Raderer, M.; Mayer, J.; Pereira, J.; Tumyan, G.; et al. Frontline bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisone (VR-CAP) versus rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) in transplantation-ineligible patients with newly diagnosed mantle cell lymphoma: Final overall survival results of a randomised, open-label, phase 3 study. Lancet. Oncol. 2018, 19, 1449–1458. [Google Scholar] [CrossRef]
- Kluin-Nelemans, H.C.; Hoster, E.; Hermine, O.; Walewski, J.; Trneny, M.; Geisler, C.H.; Stilgenbauer, S.; Thieblemont, C.; Vehling-Kaiser, U.; Doorduijn, J.K.; et al. Treatment of older patients with mantle-cell lymphoma. N. Engl. J. Med. 2012, 367, 520–531. [Google Scholar] [CrossRef]
- Visco, C.; Chiappella, A.; Nassi, L.; Patti, C.; Ferrero, S.; Barbero, D.; Evangelista, A.; Spina, M.; Molinari, A.; Rigacci, L.; et al. Rituximab, bendamustine, and low-dose cytarabine as induction therapy in elderly patients with mantle cell lymphoma: A multicentre, phase 2 trial from Fondazione Italiana Linfomi. Lancet Haematol. 2017, 4, e15–e23. [Google Scholar] [CrossRef]
- Obr, A.; Prochazka, V.; Papajik, T.; Klener, P., Jr.; Janikova, A.; Salek, D.; Belada, D.; Pytlik, R.; Sykorova, A.; Mocikova, H.; et al. Maintenance rituximab in newly diagnosed mantle cell lymphoma patients: A real world analysis from the Czech lymphoma study group registry(dagger). Leuk. Lymphoma 2019, 60, 748–755. [Google Scholar] [CrossRef]
- Chen, R.W.; Palmer, J.M.; Tomassetti, S.; Popplewell, L.L.; Alluin, J.; Chomchan, P.; Nademanee, A.P.; Siddiqi, T.; Tsai, N.C.; Chen, L.; et al. Multi-center phase II trial of bortezomib and rituximab maintenance combination therapy in patients with mantle cell lymphoma after consolidative autologous stem cell transplantation. J. Hematol. Oncol. 2018, 11, 87. [Google Scholar] [CrossRef]
- Till, B.G. Maintenance Therapy in Diffuse Large B Cell Lymphoma and Mantle Cell Lymphoma. Curr. Treat. Options Oncol. 2018, 19, 45. [Google Scholar] [CrossRef]
- Klener, P.; Fronkova, E.; Kalinova, M.; Belada, D.; Forsterova, K.; Pytlik, R.; Blahovcova, P.; Simkovic, M.; Salek, D.; Mocikova, H.; et al. Potential loss of prognostic significance of minimal residual disease assessment after R-CHOP-based induction in elderly patients with mantle cell lymphoma in the era of rituximab maintenance. Hematol. Oncol. 2018, 36, 773–778. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Tisi, M.C.; Evangelista, A.; Di Rocco, A.; Zoellner, A.K.; Zilioli, V.R.; Hohaus, S.; Sciarra, R.; Re, A.; Tecchio, C.; et al. Time to progression of mantle cell lymphoma after high-dose cytarabine-based regimens defines patients risk for death. Br. J. Haematol. 2019, 185, 940–944. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Hoster, E.; Delfau-Larue, M.H.; Beldjord, K.; Bottcher, S.; Asnafi, V.; Plonquet, A.; Siebert, R.; Callet-Bauchu, E.; Andersen, N.; et al. Molecular remission is an independent predictor of clinical outcome in patients with mantle cell lymphoma after combined immunochemotherapy: A European MCL intergroup study. Blood 2010, 115, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- Andersen, N.S.; Pedersen, L.B.; Laurell, A.; Elonen, E.; Kolstad, A.; Boesen, A.M.; Pedersen, L.M.; Lauritzsen, G.F.; Ekanger, R.; Nilsson-Ehle, H.; et al. Pre-emptive treatment with rituximab of molecular relapse after autologous stem cell transplantation in mantle cell lymphoma. J. Clin. Oncol. 2009, 27, 4365–4370. [Google Scholar] [CrossRef] [PubMed]
- Pott, C.; Bruggemann, M.; Ritgen, M.; van der Velden, V.H.J.; van Dongen, J.J.M.; Kneba, M. MRD Detection in B-Cell Non-Hodgkin Lymphomas Using Ig Gene Rearrangements and Chromosomal Translocations as Targets for Real-Time Quantitative PCR. Methods Mol. Biol. (CliftonN.J.) 2019, 1956, 199–228. [Google Scholar] [CrossRef]
- Smith, A.; Roman, E.; Appleton, S.; Howell, D.; Johnson, R.; Burton, C.; Patmore, R. Impact of novel therapies for mantle cell lymphoma in the real world setting: A report from the UK’s Haematological Malignancy Research Network (HMRN). Br. J. Haematol. 2018, 181, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Hamadani, M.; Fenske, T.S.; Costa, L.J. Incidence and survival trends in mantle cell lymphoma. Br. J. Haematol. 2018, 181, 703–706. [Google Scholar] [CrossRef]
- Klanova, M.; Lorkova, L.; Vit, O.; Maswabi, B.; Molinsky, J.; Pospisilova, J.; Vockova, P.; Mavis, C.; Lateckova, L.; Kulvait, V.; et al. Downregulation of deoxycytidine kinase in cytarabine-resistant mantle cell lymphoma cells confers cross-resistance to nucleoside analogs gemcitabine, fludarabine and cladribine, but not to other classes of anti-lymphoma agents. Mol. Cancer 2014, 13, 159. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.L.; Champlin, R.E.; Wang, M.L. Targeting Bruton’s tyrosine kinase with ibrutinib in B-cell malignancies. Clin. Pharmacol. Ther. 2015, 97, 455–468. [Google Scholar] [CrossRef]
- Wang, M.; Rule, S.; Zinzani, P.L.; Goy, A.; Casasnovas, O.; Smith, S.D.; Damaj, G.; Doorduijn, J.; Lamy, T.; Morschhauser, F.; et al. Acalabrutinib in relapsed or refractory mantle cell lymphoma (ACE-LY-004): A single-arm, multicentre, phase 2 trial. Lancet 2018, 391, 659–667. [Google Scholar] [CrossRef]
- Wang, M.L.; Rule, S.; Martin, P.; Goy, A.; Auer, R.; Kahl, B.S.; Jurczak, W.; Advani, R.H.; Romaguera, J.E.; Williams, M.E.; et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N. Engl. J. Med. 2013, 369, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Dreyling, M.; Jurczak, W.; Jerkeman, M.; Silva, R.S.; Rusconi, C.; Trneny, M.; Offner, F.; Caballero, D.; Joao, C.; Witzens-Harig, M.; et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet 2016, 387, 770–778. [Google Scholar] [CrossRef]
- Martin, P.; Maddocks, K.; Leonard, J.P.; Ruan, J.; Goy, A.; Wagner-Johnston, N.; Rule, S.; Advani, R.; Iberri, D.; Phillips, T.; et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016, 127, 1559–1563. [Google Scholar] [CrossRef] [PubMed]
- Cheah, C.Y.; Chihara, D.; Romaguera, J.E.; Fowler, N.H.; Seymour, J.F.; Hagemeister, F.B.; Champlin, R.E.; Wang, M.L. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann. Oncol. 2015, 26, 1175–1179. [Google Scholar] [CrossRef] [PubMed]
- Epperla, N.; Hamadani, M.; Cashen, A.F.; Ahn, K.W.; Oak, E.; Kanate, A.S.; Calzada, O.; Cohen, J.B.; Farmer, L.; Ghosh, N.; et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol. Oncol. 2017, 35, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Dreger, P.; Michallet, M.; Bosman, P.; Dietrich, S.; Sobh, M.; Boumendil, A.; Nagler, A.; Scheid, C.; Cornelissen, J.; Niederwieser, D.; et al. Ibrutinib for bridging to allogeneic hematopoietic cell transplantation in patients with chronic lymphocytic leukemia or mantle cell lymphoma: A study by the EBMT Chronic Malignancies and Lymphoma Working Parties. Bone Marrow Transplant. 2019, 54, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Rule, S.; Cook, G.; Russell, N.H.; Hunter, A.; Robinson, S.; Morley, N.; Sureda, A.; Patrick, P.; Clifton-Hadley, L.; Adedayo, T.; et al. Allogeneic stem cell transplantation as part of front line therapy for Mantle cell lymphoma. Br. J. Haematol. 2019, 184, 999–1005. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Lu, P.; Guo, A.; Cheng, S.; Zong, H.; Martin, P.; Coleman, M.; Wang, Y.L. Characterization of ibrutinib-sensitive and -resistant mantle lymphoma cells. Br. J. Haematol. 2014, 166, 849–861. [Google Scholar] [CrossRef] [PubMed]
- Hershkovitz-Rokah, O.; Pulver, D.; Lenz, G.; Shpilberg, O. Ibrutinib resistance in mantle cell lymphoma: Clinical, molecular and treatment aspects. Br. J. Haematol. 2018, 181, 306–319. [Google Scholar] [CrossRef]
- Chiron, D.; Di Liberto, M.; Martin, P.; Huang, X.; Sharman, J.; Blecua, P.; Mathew, S.; Vijay, P.; Eng, K.; Ali, S.; et al. Cell-cycle reprogramming for PI3K inhibition overrides a relapse-specific C481S BTK mutation revealed by longitudinal functional genomics in mantle cell lymphoma. Cancer Discov. 2014, 4, 1022–1035. [Google Scholar] [CrossRef]
- Zhao, X.; Lwin, T.; Silva, A.; Shah, B.; Tao, J.; Fang, B.; Zhang, L.; Fu, K.; Bi, C.; Li, J.; et al. Unification of de novo and acquired ibrutinib resistance in mantle cell lymphoma. Nat. Commun. 2017, 8, 14920. [Google Scholar] [CrossRef] [PubMed]
- Compagno, M.; Wang, Q.; Pighi, C.; Cheong, T.C.; Meng, F.L.; Poggio, T.; Yeap, L.S.; Karaca, E.; Blasco, R.B.; Langellotto, F.; et al. Phosphatidylinositol 3-kinase delta blockade increases genomic instability in B cells. Nature 2017, 542, 489–493. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.; Bartlett, N.L.; Blum, K.A.; Park, S.; Maddocks, K.; Ruan, J.; Ridling, L.; Dittus, C.; Chen, Z.; Huang, X.; et al. A phase 1 trial of ibrutinib plus palbociclib in previously treated mantle cell lymphoma. Blood 2019, 133, 1201–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.L.; Lee, H.; Chuang, H.; Wagner-Bartak, N.; Hagemeister, F.; Westin, J.; Fayad, L.; Samaniego, F.; Turturro, F.; Oki, Y.; et al. Ibrutinib in combination with rituximab in relapsed or refractory mantle cell lymphoma: A single-centre, open-label, phase 2 trial. Lancet. Oncol. 2016, 17, 48–56. [Google Scholar] [CrossRef]
- Tam, C.S.; Anderson, M.A.; Pott, C.; Agarwal, R.; Handunnetti, S.; Hicks, R.J.; Burbury, K.; Turner, G.; Di Iulio, J.; Bressel, M.; et al. Ibrutinib plus Venetoclax for the Treatment of Mantle-Cell Lymphoma. N. Engl. J. Med. 2018, 378, 1211–1223. [Google Scholar] [CrossRef]
- Robak, P.; Robak, T. Bortezomib for the Treatment of Hematologic Malignancies: 15 Years Later. Drugs RD 2019, 19, 73–92. [Google Scholar] [CrossRef]
- Fisher, R.I.; Bernstein, S.H.; Kahl, B.S.; Djulbegovic, B.; Robertson, M.J.; de Vos, S.; Epner, E.; Krishnan, A.; Leonard, J.P.; Lonial, S.; et al. Multicenter phase II study of bortezomib in patients with relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2006, 24, 4867–4874. [Google Scholar] [CrossRef] [PubMed]
- Lamm, W.; Kaufmann, H.; Raderer, M.; Hoffmann, M.; Chott, A.; Zielinski, C.; Drach, J. Bortezomib combined with rituximab and dexamethasone is an active regimen for patients with relapsed and chemotherapy-refractory mantle cell lymphoma. Haematologica 2011, 96, 1008–1014. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Vose, J.M.; Kelly, J.L.; Young, F.; Bernstein, S.H.; Peterson, D.; Rich, L.; Blumel, S.; Proia, N.K.; Liesveld, J.; et al. The combination of bendamustine, bortezomib, and rituximab for patients with relapsed/refractory indolent and mantle cell non-Hodgkin lymphoma. Blood 2011, 117, 2807–2812. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Vose, J.M.; Czuczman, M.S.; Reeder, C.B.; Haioun, C.; Polikoff, J.; Tilly, H.; Zhang, L.; Prandi, K.; Li, J.; et al. Long-term follow-up of lenalidomide in relapsed/refractory mantle cell lymphoma: Subset analysis of the NHL-003 study. Ann. Oncol. 2013, 24, 2892–2897. [Google Scholar] [CrossRef] [PubMed]
- Goy, A.; Sinha, R.; Williams, M.E.; Kalayoglu Besisik, S.; Drach, J.; Ramchandren, R.; Zhang, L.; Cicero, S.; Fu, T.; Witzig, T.E. Single-agent lenalidomide in patients with mantle-cell lymphoma who relapsed or progressed after or were refractory to bortezomib: Phase II MCL-001 (EMERGE) study. J. Clin. Oncol. 2013, 31, 3688–3695. [Google Scholar] [CrossRef] [PubMed]
- Trneny, M.; Lamy, T.; Walewski, J.; Belada, D.; Mayer, J.; Radford, J.; Jurczak, W.; Morschhauser, F.; Alexeeva, J.; Rule, S.; et al. Lenalidomide versus investigator’s choice in relapsed or refractory mantle cell lymphoma (MCL-002; SPRINT): A phase 2, randomised, multicentre trial. Lancet Oncol. 2016, 17, 319–331. [Google Scholar] [CrossRef]
- Hagner, P.R.; Chiu, H.; Ortiz, M.; Apollonio, B.; Wang, M.; Couto, S.; Waldman, M.F.; Flynt, E.; Ramsay, A.G.; Trotter, M.; et al. Activity of lenalidomide in mantle cell lymphoma can be explained by NK cell-mediated cytotoxicity. Br. J. Haematol. 2017, 179, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hess, G.; Herbrecht, R.; Romaguera, J.; Verhoef, G.; Crump, M.; Gisselbrecht, C.; Laurell, A.; Offner, F.; Strahs, A.; Berkenblit, A.; et al. Phase III study to evaluate temsirolimus compared with investigator’s choice therapy for the treatment of relapsed or refractory mantle cell lymphoma. J. Clin. Oncol. 2009, 27, 3822–3829. [Google Scholar] [CrossRef] [PubMed]
- Davids, M.S.; Roberts, A.W.; Seymour, J.F.; Pagel, J.M.; Kahl, B.S.; Wierda, W.G.; Puvvada, S.; Kipps, T.J.; Anderson, M.A.; Salem, A.H.; et al. Phase I First-in-Human Study of Venetoclax in Patients with Relapsed or Refractory Non-Hodgkin Lymphoma. J. Clin. Oncol. 2017, 35, 826–833. [Google Scholar] [CrossRef]
- Eyre, T.A.; Walter, H.S.; Iyengar, S.; Follows, G.; Cross, M.; Fox, C.P.; Hodson, A.; Coats, J.; Narat, S.; Morley, N.; et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica 2019, 104, e68–e71. [Google Scholar] [CrossRef] [PubMed]
- Andorsky, D.J.; Kolibaba, K.S.; Assouline, S.; Forero-Torres, A.; Jones, V.; Klein, L.M.; Patel-Donnelly, D.; Smith, M.; Ye, W.; Shi, W.; et al. An open-label phase 2 trial of entospletinib in indolent non-Hodgkin lymphoma and mantle cell lymphoma. Br. J. Haematol. 2019, 184, 215–222. [Google Scholar] [CrossRef]
- Morschhauser, F.; Seymour, J.F.; Kluin-Nelemans, H.C.; Grigg, A.; Wolf, M.; Pfreundschuh, M.; Tilly, H.; Raemaekers, J.; van’t Veer, M.B.; Milpied, N.; et al. A phase II study of enzastaurin, a protein kinase C beta inhibitor, in patients with relapsed or refractory mantle cell lymphoma. Ann. Oncol. 2008, 19, 247–253. [Google Scholar] [CrossRef]
- Xu-Monette, Z.Y.; Zhou, J.; Young, K.H. PD-1 expression and clinical PD-1 blockade in B-cell lymphomas. Blood 2018, 131, 68–83. [Google Scholar] [CrossRef]
- Levin, A.; Shah, N.N. Chimeric antigen receptor modified T cell therapy in B cell non-Hodgkin lymphomas. Am. J. Hematol. 2019, 94, S18–S23. [Google Scholar] [CrossRef]
- Montico, B.; Lapenta, C.; Ravo, M.; Martorelli, D.; Muraro, E.; Zeng, B.; Comaro, E.; Spada, M.; Donati, S.; Santini, S.M.; et al. Exploiting a new strategy to induce immunogenic cell death to improve dendritic cell-based vaccines for lymphoma immunotherapy. Oncoimmunology 2017, 6, e1356964. [Google Scholar] [CrossRef] [PubMed]
- Cox, M.C.; Castiello, L.; Mattei, M.; Santodonato, L.; D’Agostino, G.; Muraro, E.; Martorelli, D.; Lapenta, C.; Di Napoli, A.; Di Landro, F.; et al. Clinical and Antitumor Immune Responses in Relapsed/Refractory Follicular Lymphoma Patients after Intranodal Injections of IFNalpha-Dendritic Cells and Rituximab. Clin. Cancer Res. 2019, 0709. [Google Scholar] [CrossRef]
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klener, P. Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 4417. https://doi.org/10.3390/ijms20184417
Klener P. Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma. International Journal of Molecular Sciences. 2019; 20(18):4417. https://doi.org/10.3390/ijms20184417
Chicago/Turabian StyleKlener, Pavel. 2019. "Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma" International Journal of Molecular Sciences 20, no. 18: 4417. https://doi.org/10.3390/ijms20184417
APA StyleKlener, P. (2019). Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma. International Journal of Molecular Sciences, 20(18), 4417. https://doi.org/10.3390/ijms20184417




