Roles of Glutamate Receptors in Parkinson’s Disease
Abstract
1. Introduction
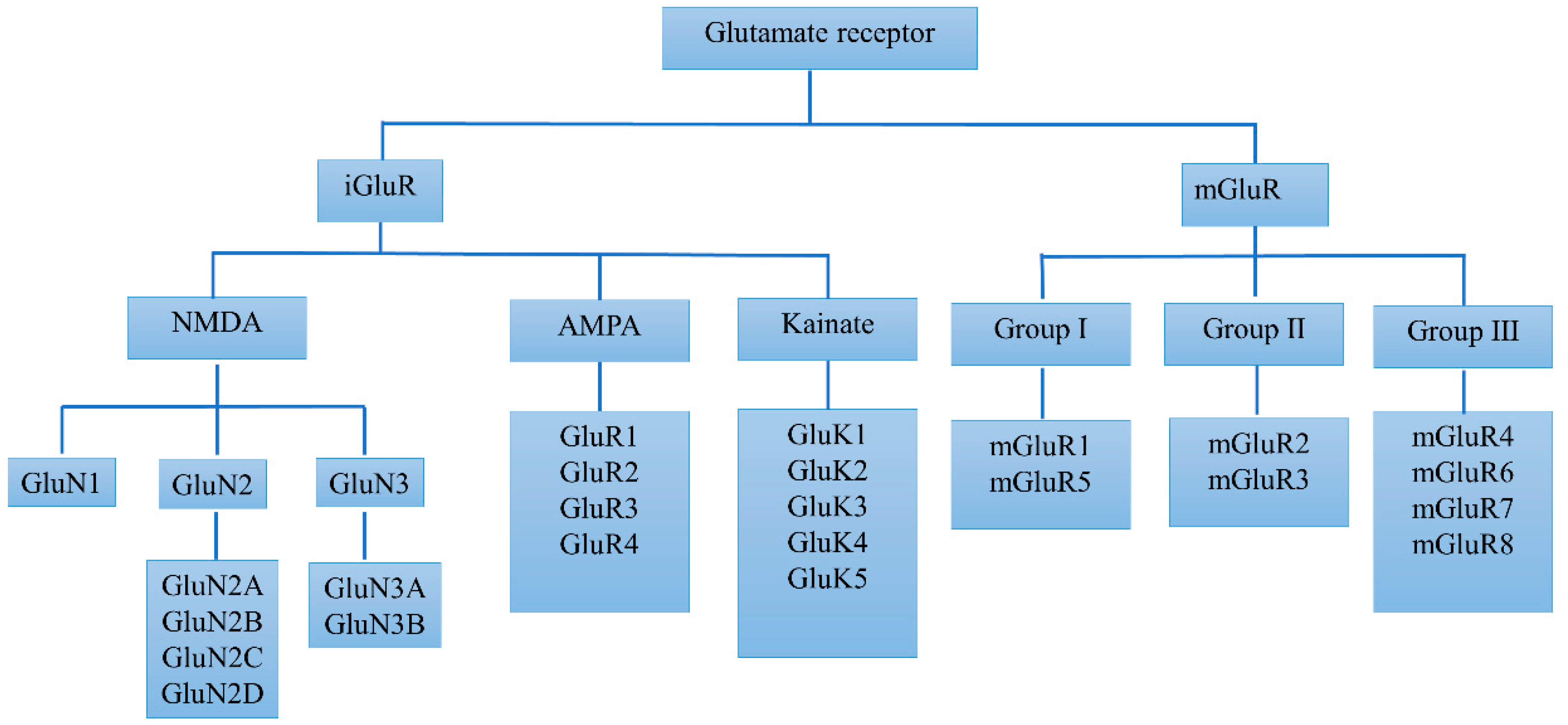
2. Basic Biology and Alterations of Glutamate Receptors in PD
2.1. Basic Biology and Alterations of iGluRs in PD
2.2. Basic Biology and Alterations of mGluRs in PD
3. Key Targets of Glutamate Receptors in PD Treatment
3.1. Key Targets of iGluRs in PD Treatment
3.2. Key Targets of mGluRs in PD Treatment
4. Clinical Trials Targeting Glutamate Receptors in PD
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| SNc | substantia nigra pars compacta |
| L-DOPA | l-3,4-dihydroxyphenylalanine |
| CNS | central nervous system |
| iGluRs | ionotropic glutamate receptors |
| mGluRs | metabotropic glutamate receptors |
| NMDA | N-methyl-d-aspartate |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| KA | kainate |
| KA receptor | KAR |
| NSCs | neural stem cells |
| 6-OHDA | 6-hydroxydopamine |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| DHPG | 3,5-dihydroxyphenylglycine |
| LID | levodopa-induced dyskinesia |
| NBQX | 2,3-dihydroxy-6-nitro-7-sulfamoyl-benzo[f]quinoxaline-2,3-dione |
| MPEP | 2-methyl-6-(phenylethynyl)-pyridine |
| MTEP | 3-[(2-methyl-1,3-thiazol-4-yl) ethynyl] pyridine |
| EMQMCM | (3-ethyl-2-methyl-quinoline-6-yl)-(4-methoxy-cyclohexyl)-methanone methanesulfonate |
| 2R,4R-APDC | (2R,4R)-4-aminopyrrolidine-2,4-dicarboxylate |
| PHCCC | Phenyl-7-(hydroxyamino) cyclopropopa[b] chrome-1a-carboxamide |
| DCPG | (S)-3,4-dicarboxyphenylglycine |
| NAMs | Negative allosteric modulators |
| PAMs | Positive allosteric modulators |
References
- De, P.E.; Lees, A.J.; Holton, J.L.; Warner, T.T. Prognosis and neuropathologic correlation of clinical subtypes of Parkinson disease. JAMA Neurol. 2019, 76, 470–479. [Google Scholar]
- Dickson, D.W. Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 2018, 46, S30–S33. [Google Scholar] [CrossRef]
- Chen, J.J.; Swope, D.M. Pharmacotherapy for Parkinson’s disease. Pharmacotherapy 2007, 27, 161S–173S. [Google Scholar] [CrossRef]
- Bastide, M.F.; Meissner, W.G.; Picconi, B.; Fasano, S.; Fernagut, P.O.; Feyder, M.; Francardo, V.; Alcacer, C.; Ding, Y.; Brambilla, R.; et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease. Prog. Neurobiol. 2015, 132, 96–168. [Google Scholar] [CrossRef]
- Blandini, F.; Porter, R.H.; Greenamyre, J.T. Glutamate and Parkinson’s disease. Mol. Neurobiol. 1996, 12, 73–94. [Google Scholar] [CrossRef]
- Mironova, Y.S.; Zhukova, I.A.; Zhukova, N.G.; Alifirova, V.M.; Izhboldina, O.P.; Latypova, A.V. Parkinson’s disease and glutamate excitotoxicity. Zhurnal nevrologii i psikhiatrii imeni SS Korsakova 2018, 118, 50–54. [Google Scholar] [CrossRef]
- Carrillo, M.P.; Silva, A.D.; Villaseñor, A.K. Glutamate in Parkinson’s disease: Role of antiglutamatergic drugs. Basal Ganglia 2013, 3, 147–157. [Google Scholar] [CrossRef]
- Pin, J.P.; Duvoisin, R. The metabotropic glutamate receptors: Structure and functions. Neuropharmacology 1995, 34, 1–26. [Google Scholar] [CrossRef]
- Jourdain, V.A.; Morin, N.; Grégoire, L.; Morissette, M.; Di, P.T. Changes in glutamate receptors in dyskinetic parkinsonian monkeys after unilateral subthalamotomy. J. Neurosurg. 2015, 123, 1383–1393. [Google Scholar] [CrossRef]
- Götz, T.; Kraushaar, U.; Geiger, J.; Lübke, J.; Berger, T.; Jonas, P. Functional properties of AMPA and NMDA receptors expressed in identified types of basal ganglia neurons. J. Neurosci. 1997, 17, 204–215. [Google Scholar] [CrossRef]
- Mothet, J.P.; Matildé, L.B.; Billard, J.M. Time and space profiling of NMDA receptor co-agonist functions. J. Neurochem. 2015, 135, 210–225. [Google Scholar] [CrossRef]
- Seeburg, P.H.; Burnashev, N.; Köhr, G.; Kuner, T.; Sprengel, R.; Monyer, H. The NMDA receptor channel: Molecular design of a coincidence detector. Recent Prog. Horm. Res. 1995, 50, 19–34. [Google Scholar]
- Mody, I.; MacDonald, J.F. NMDA receptor-dependent excitotoxicity: The role of intracellular Ca2+ release. Trends Pharm. Sci. 1995, 16, 356–359. [Google Scholar] [CrossRef]
- Plutino, S.; Sciaccaluga, M.; Fucile, S. Extracellular mild acidosis decreases the Ca2+ permeability of the human NMDA receptors. Cell Calcium 2019, 80, 63–70. [Google Scholar] [CrossRef]
- Mellone, M.; Stanic, J.; Hernandez, L.F.; Iglesias, E.; Zianni, E.; Longhi, A.; Prigent, A.; Picconi, B.; Calabresi, P.; Hirsch, E.C.; et al. NMDA receptor GluN2A/GluN2B subunit ratio as synaptic trait of levodopa-induced dyskinesias: From experimental models to patients. Front. Cell. Neurosci. 2015, 9, 245. [Google Scholar] [CrossRef]
- Gan, J.; Qi, C.; Mao, L.M.; Liu, Z. Changes in surface expression of N-methyl-D-aspartate receptors in the striatum in a rat model of Parkinson’s disease. Drug Des. Dev. Ther. 2014, 8, 165. [Google Scholar]
- Mellone, M.; Zianni, E.; Stanic, J.; Campanelli, F.; Marino, G.; Ghiglieri, V.; Longhi, A.; Thiolot, M.L.; Li, Q.; Calabresi, P.; et al. NMDA receptor GluN2D subunit participates to levodopa-induced dyskinesia pathophysiology. Neurobiol. Dis. 2019, 121, 338–349. [Google Scholar] [CrossRef]
- Guo, H.; Camargo, L.M.; Yeboah, F.; Digan, M.E.; Niu, H.; Pan, Y.; Reiling, S.; Soler-Llavina, G.; Weihofen, W.A.; Wang, H.R.; et al. A NMDA-receptor calcium influx assay sensitive to stimulation by glutamate and glycine/D-serine. Sci. Rep. 2017, 7, 11608. [Google Scholar] [CrossRef]
- Martin, L.J.; Blackstone, C.D.; Levey, A.I.; Huganir, R.L.; Price, D.L. AMPA glutamate receptor subunits are differentially distributed in rat brain. Neuroscience 1993, 53, 327–358. [Google Scholar] [CrossRef]
- Hollmann, M.; Hartley, M.; Heinemann, S. Ca2+ permeability of KA-AMPA--gated glutamate receptor channels depends on subunit composition. Science 1991, 252, 851–853. [Google Scholar] [CrossRef]
- Tamano, H.; Morioka, H.; Nishio, R.; Takeuchi, A.; Takeda, A. AMPA-induced extracellular Zn2+ influx into nigral dopaminergic neurons causes movement disorder in rats. Neurotoxicology 2018, 69, 23–28. [Google Scholar] [CrossRef]
- Chang, Y.; Du, C.; Han, L.; Lv, S.; Zhang, J.; Bian, G.; Tang, G.; Liu, Y.; Chen, T.; Liu, J. Enhanced AMPA receptor-mediated excitatory transmission in the rodent rostromedial tegmental nucleus following lesion of the nigrostriatal pathway. Neurochem. Int. 2019, 122, 85–93. [Google Scholar] [CrossRef]
- Ouattara, B.; Hoyer, D.; Grégoire, L.; Morissette, M.; Gasparini, F.; Gomez-Mancilla, B.; Paolo, T.D. Changes of AMPA receptors in MPTP monkeys with levodopa-induced dyskinesias. Neuroscience 2010, 167, 1160–1167. [Google Scholar] [CrossRef]
- Calon, F.; Rajput, A.H.; Hornykiewicz, O.; Bédard, P.J.; Di, P.T. Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiol. Dis. 2003, 14, 404–416. [Google Scholar] [CrossRef]
- Hadzic, M.; Jack, A.; Wahle, P. Ionotropic glutamate receptors: Which ones, when, and where in the mammalian neocortex. J. Comp. Neurol. 2017, 525, 976–1033. [Google Scholar] [CrossRef]
- Pinheiro, P.; Mulle, C. Kainate receptors. Cell Tissue Res. 2006, 326, 457–482. [Google Scholar] [CrossRef]
- Evans, A.J.; Gurung, S.; Henley, J.M.; Nakamura, Y.; Wilkinson, K.A. Exciting times: New advances towards understanding the regulation and roles of kainate receptors. Neurochem. Res. 2019, 44, 572–584. [Google Scholar] [CrossRef]
- Kieval, J.Z.; Hubert, G.W.; Charara, A.; Paré, J.F.; Smith, Y. Subcellular and subsynaptic localization of presynaptic and postsynaptic kainate receptor subunits in the monkey striatum. J. Neurosci. 2001, 21, 8746–8757. [Google Scholar] [CrossRef]
- Jin, X.T.; Smith, Y. Activation of presynaptic kainate receptors suppresses GABAergic synaptic transmission in the rat globus pallidus. Neuroscience 2007, 149, 338–349. [Google Scholar] [CrossRef][Green Version]
- Lauri, S.E.; Vesikansa, A.; Segerstråle, M.; Collingridge, G.L.; Isaac, J.T.; Taira, T. Functional maturation of CA1 synapses involves activity-dependent loss of tonic kainate receptor-mediated inhibition of glutamate release. Neuron 2006, 50, 415–429. [Google Scholar] [CrossRef]
- Daw, M.I.; Scott, H.L.; Isaac, J.T. Developmental synaptic plasticity at the thalamocortical input to barrel cortex: Mechanisms and roles. Mol. Cell. Neurosci. 2007, 34, 493–502. [Google Scholar] [CrossRef][Green Version]
- Maraschi, A.; Ciammola, A.; Folci, A.; Sassone, F.; Ronzitti, G.; Cappelletti, G.; Silani, V.; Sato, S.; Hattori, N.; Mazzanti, M.; et al. Parkin regulates kainate receptors by interacting with the GluK2 subunit. Nat. Commun. 2014, 5, 5182. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Smith, Y. Metabotropic glutamate receptors: Targets for neuroprotective therapies in Parkinson disease. Curr. Opin. Pharm. 2018, 38, 72–80. [Google Scholar] [CrossRef]
- Mishina, M.; Suzuki, M.; Ishii, K.; Sakata, M.; Wagatsuma, K.; Ishibashi, K.; Toyohara, J.; Zhang, M.R.; Kimura, K.; Ishiwata, K. Density of metabotropic glutamate receptors subtype 1 in Parkinson’s disease compared to healthy elderly—A ITMM PET study-. J. Neurol. Sci. 2017, 381, 806–807. [Google Scholar] [CrossRef]
- Yamasaki, T.; Fujinaga, M.; Kawamura, K.; Furutsuka, K.; Nengaki, N.; Shimoda, Y.; Shiomi, S.; Takei, M.; Hashimoto, H.; Yui, J.; et al. Dynamic changes in striatal mGluR1 but not mGluR5 during pathological progression of Parkinson’s disease in human alpha-synuclein A53T transgenic rats: A multi-PET imaging study. J. Neurosci. 2016, 36, 375–384. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nomura, S.; Ohishi, H.; Sugihara, H.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. Neurosci. Lett. 1993, 163, 53–57. [Google Scholar] [CrossRef]
- Romano, C.; Sesma, M.A.; McDonald, C.T.; O’malley, K.; VandenPol, A.N.; Olney, J.W. Distribution of metabotropic glutamate receptor mGluR5 immunoreactivity in rat brain. J. Comp. Neurol. 1995, 355, 455–469. [Google Scholar] [CrossRef]
- Kang, Y.; Henchcliffe, C.; Verma, A.; Vallabhajosula, S.; He, B.; Kothari, P.J.; Pryor, K.O.; Mozley, P.D. 18F-FPEB PET/CT Shows mGluR5 Upregulation in Parkinson’s Disease. J. Neuroimaging 2019, 29, 97–103. [Google Scholar] [CrossRef]
- García-Montes, J.R.; Solís, O.; Enríquez, T.J.; Ruiz, D.I.; Drucker, C.R.; Moratalla, R. Genetic Knockdown of mGluR5 in Striatal D1R-Containing Neurons Attenuates l-DOPA-Induced Dyskinesia in Aphakia Mice. Mol. Neurobiol. 2019, 56, 4037–4050. [Google Scholar] [CrossRef]
- Crabbé, M.; Vander, P.A.; Weerasekera, A.; Himmelreich, U.; Baekelandt, V.; Van, L.K.; Casteels, C. Altered mGluR5 binding potential and glutamine concentration in the 6-OHDA rat model of acute Parkinson’s disease and levodopa-induced dyskinesia. Neurobiol. Aging 2018, 61, 82–92. [Google Scholar] [CrossRef]
- Bradley, S.; Challiss, R. Defining protein kinase/phosphatase isoenzymic regulation of mGlu5 receptor-stimulated phospholipase C and Ca2+ responses in astrocytes. Br. J. Pharm. 2011, 164, 755–771. [Google Scholar] [CrossRef]
- Sarantis, K.; Tsiamaki, E.; Kouvaros, S.; Papatheodoropoulos, C.; Angelatou, F. Adenosine A2A receptors permit mGluR5-evoked tyrosine phosphorylation of NR 2B (Tyr1472) in rat hippocampus: A possible key mechanism in NMDA receptor modulation. J. Neurochem. 2015, 135, 714–726. [Google Scholar] [CrossRef]
- Ohishi, H.; Ogawa-Meguro, R.; Shigemoto, R.; Kaneko, T.; Nakanishi, S.; Mizuno, N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR2 and mGluR3, in rat cerebellar cortex. Neuron 1994, 13, 55–66. [Google Scholar] [CrossRef]
- Tamaru, Y.; Nomura, S.; Mizuno, N.; Shigemoto, R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: Differential location relative to pre-and postsynaptic sites. Neuroscience 2001, 106, 481–503. [Google Scholar] [CrossRef]
- Johnson, K.A.; Mateo, Y.; Lovinger, D.M. Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology 2017, 117, 114–123. [Google Scholar] [CrossRef]
- Jia, Y.J.; Deng, J.H.; Zhang, W.Z.; Sun, Z.L.; Yang, J.; Yu, Y.; Gong, X.L.; Jia, J.; Wang, X.M. The role of group II metabotropic glutamate receptors in the striatum in electroacupuncture treatment of Parkinsonian rats. CNS Neurosci. Ther. 2017, 23, 23–32. [Google Scholar] [CrossRef]
- Lin, C.H.; You, J.R.; Wei, K.C.; Gean, P.W. Stimulating ERK/PI3K/NFκB signaling pathways upon activation of mGluR2/3 restores OGD-induced impairment in glutamate clearance in astrocytes. Eur. J. Neurosci. 2014, 39, 83–96. [Google Scholar] [CrossRef]
- Shi, K.; Liu, X.; Hou, L.; Qiao, D.; Lin, X. Effects of exercise on mGluR-mediated glutamatergic transmission in the striatum of hemiparkinsonian rats. Neurosci. Lett. 2019, 705, 143–150. [Google Scholar] [CrossRef]
- Nakajima, Y.; Iwakabe, H.; Akazawa, C.; Nawa, H.; Shigemoto, R.; Mizuno, N.; Nakanishi, S. Molecular characterization of a novel retinal metabotropic glutamate receptor mGluR6 with a high agonist selectivity for L-2-amino-4-phosphonobutyrate. J. Biol. Chem. 1993, 268, 11868–11873. [Google Scholar]
- Kinoshita, A.; Shigemoto, R.; Ohishi, H.; Vander, P.H.; Mizuno, N. Immunohistochemical localization of metabotropic glutamate receptors, mGluR7a and mGluR7b, in the central nervous system of the adult rat and mouse: A light and electron microscopic study. J. Comp. Neurol. 1998, 393, 332–352. [Google Scholar] [CrossRef]
- Corti, C.; Restituito, S.; Rimland, J.M.; Brabet, I.; Corsi, M.; Pin, J.P.; Ferraguti, F. Cloning and characterization of alternative mRNA forms for the rat metabotropic glutamate receptors mGluR7 and mGluR8. Eur. J. Neurosci. 1998, 10, 3629–3641. [Google Scholar] [CrossRef]
- Ferraguti, F.; Shigemoto, R. Metabotropic glutamate receptors. Cell Tissue Res. 2006, 326, 483–504. [Google Scholar] [CrossRef]
- Gerlai, R.; Roder, J.C.; Hampson, D.R. Altered spatial learning and memory in mice lacking the mGluR4 subtype of metabotropic glutamate receptor. Behav. Neurosci. 1998, 112, 525. [Google Scholar] [CrossRef]
- Avdeeva, N.V.; Sidorova, S.A.; Gudyrev, O.S.; Ol’ga, A.O.; Golubev, I.V. Mechanism of neuroprotective effect of mGluR4 agonists. Res. Results Pharmacol. 2019, 5, 43. [Google Scholar] [CrossRef][Green Version]
- Betts, M.J.; O’neill, M.J.; Duty, S. Allosteric modulation of the group III mGlu4 receptor provides functional neuroprotection in the 6-hydroxydopamine rat model of Parkinson’s disease. Br. J. Pharm. 2012, 166, 2317–2330. [Google Scholar] [CrossRef]
- Charvin, D.; Di, P.T.; Bezard, E.; Halldin, C.; Duvey, G.; Gregoire, L.; Takano, A.; Pioli, E.; Medori, R.; Conquet, F. A Novel mglur4 compound alleviates motor symptoms in primate models of parkinson’s disease. J. Neurol. Sci. 2017, 381, 97. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, L.; Chen, Y.; Shi, H.; Huang, X.; Wang, Y.; Wei, Y.; Xue, W.; Han, J. Effect and mechanism of mGluR6 on the biological function of rat embryonic neural stem cells. Biosci. Biotechnol. Biochem. 2019, 83, 1027–1034. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Chen, Y.; Shi, H.; Huang, X.; Wang, Y.; Wang, Y.; Wei, Y.; Xue, W.; Han, J. Effect of mGluR7 on proliferation of human embryonic neural stem cells. Medicine 2019, 98, e14683. [Google Scholar] [CrossRef]
- Feng, S.; Xiao, J.; Han, F.; Chen, L.; Gao, W.; Mao, G.; Huang, H. Neurorestorative clinical application standards for the culture and quality control of neural progenitor/precursor cells (version 2017). J. Neurorestoratol. 2018, 6, 65–68. [Google Scholar] [CrossRef]
- Johnson, K.A.; Conn, P.J.; Niswender, C.M. Glutamate receptors as therapeutic targets for Parkinson’s disease. CNS Neurol. Disord. Drug Targets 2009, 8, 475–491. [Google Scholar] [CrossRef]
- Bubser, M.; Zadow, B.; Kronthaler, U.O.; Felsheim, U.; Rückert, N.G.; Schmidt, W.J. Behavioural pharmacology of the non-competitive NMDA antagonists dextrorphan and ADCI: Relations between locomotor stimulation, anticataleptic potential and forebrain dopamine metabolism. Naunyn-Schmiedebergs Arch Pharm. 1997, 355, 767–773. [Google Scholar] [CrossRef]
- Melo-Thomas, L.; Gil-Martínez, A.L.; Cuenca, L.; Estrada, C.; Gonzalez-Cuello, A.; Schwarting, R.K.; Herrero, M.T. Electrical stimulation or MK-801 in the inferior colliculus improve motor deficits in MPTP-treated mice. Neurotoxicology 2018, 65, 38–43. [Google Scholar] [CrossRef]
- Konieczny, J.; Ossowska, K.; Schulze, G.; Coper, H.; Wolfarth, S. L-701,324, a selective antagonist at the glycine site of the NMDA receptor, counteracts haloperidol-induced muscle rigidity in rats. Psychopharmacology 1999, 143, 235–243. [Google Scholar] [CrossRef]
- Bartlett, M.J.; Joseph, R.M.; LePoidevin, L.M.; Parent, K.L.; Laude, N.D.; Lazarus, L.B.; Heien, M.L.; Estevez, M.; Sherman, S.J.; Falk, T. Long-term effect of sub-anesthetic ketamine in reducing L-DOPA-induced dyskinesias in a preclinical model. Neurosci. Lett. 2016, 612, 121–125. [Google Scholar] [CrossRef]
- Nash, J.E.; Fox, S.H.; Henry, B.; Hill, M.P.; Peggs, D.; McGuire, S.; Maneuf, Y.; Hille, C.; Brotchie, J.M.; Crossman, A.R. Anti-Parkinsonian actions of ifenprodil in the MPTP-lesioned marmoset model of Parkinson’s disease. Exp. Neurol. 2000, 165, 136–142. [Google Scholar] [CrossRef]
- Bortolanza, M.; Bariotto-Dos-Santos, K.D.; Dos-Santos-Pereira, M.; da-Silva, C.A.; Del-Bel, E. Antidyskinetic Effect of 7-Nitroindazole and Sodium Nitroprusside Associated with Amantadine in a Rat Model of Parkinson’s Disease. Neurotox. Res. 2016, 30, 88–100. [Google Scholar] [CrossRef]
- Flores, A.J.; Bartlett, M.J.; Root, B.K.; Parent, K.L.; Heien, M.L.; Porreca, F.; Polt, R.; Sherman, S.J.; Falk, T. The Combination of the Opioid Glycopeptide MMP-2200 and a NMDA Receptor Antagonist Reduced L-Dopa-Induced Dyskinesia and MMP-2200 by Itself Reduced Dopamine Receptor 2-Like Agonist-Induced Dyskinesia. Neuropharmacology 2018, 141, 260–271. [Google Scholar] [CrossRef]
- Blanchet, P.J.; Konitsiotis, S.; Whittemore, E.R.; Zhou, Z.L.; Woodward, R.M.; Chase, T.N. Differing effects ofN-methyl-D-aspartate receptor subtype selective antagonists on dyskinesias in levodopa-treated 1-methyl-4-phenyl-tetrahydropyridine monkeys. J. Pharm. Exp. Ther. 1999, 290, 1034–1040. [Google Scholar]
- Starr, M. Anti-Parkinsonian actions of glutamate antagonists-alone and with L-DOPA: A review of evidence and suggestions for possible mechanisms. J. Neural. Transm. Parkinson’s Dis. Dement. Sect. 1995, 10, 141–185. [Google Scholar] [CrossRef]
- Jin, D.H.; Jung, Y.W.; Ko, B.H.; Moon, I.S. Immunoblot Analyses on the Differential Djstri~ ution of NR2A and NR2B Subunits in the Adult Rat Brain. Mol. Cells 1997, 7, 749–754. [Google Scholar]
- Schito, A.M.; Pizzuti, A.; Di, M.E.; Schenone, A.; Ratti, A.; Defferrari, R.; Bellone, E.; Mancardi, G.; Ajmar, F.; Mandich, P. mRNA distribution in adult human brain of GRIN2B, a N-methyl-D-aspartate (NMDA) receptor subunit. Neurosci. Lett. 1997, 239, 49–53. [Google Scholar] [CrossRef]
- Steece-Collier, K.; Chambers, L.K.; Jaw-Tsai, S.S.; Menniti, F.S.; Greenamyre, J.T. Anti-Parkinsonian actions of CP-101,606, an antagonist of NR2B subunit-containing N-methyl-d-aspartate receptors. Exp. Neurol. 2000, 163, 239–243. [Google Scholar] [CrossRef]
- Igarashi, M.; Habata, T.; Akita, H.; Noda, K.; Ogata, M.; Saji, M. The NR2B Antagonist, Ifenprodil, Corrects the L-Dopa-Induced Deficit of Bilateral Movement and Reduces C-Fos Expression in the Subthalamic Nucleus of Hemiparkinsonian Rats. Neurosci. Res. 2015, 96, 45–53. [Google Scholar] [CrossRef]
- Michel, A.; Downey, P.; Van, D.X.; De, W.C.; Schwarting, R.; Scheller, D. Behavioural Assessment of the A2a/NR2B Combination in the Unilateral 6-OHDA-Lesioned Rat Model: A New Method to Examine the Therapeutic Potential of Non-Dopaminergic Drugs. PLoS ONE 2015, 10, e0135949. [Google Scholar] [CrossRef]
- Michel, A.; Nicolas, J.M.; Rose, S.; Jackson, M.; Colman, P.; Briône, W.; Sciberras, D.; Muglia, P.; Scheller, D.K.; Citron, M.; et al. Anti-Parkinsonian Effects of the “Radiprodil and Tozadenant” Combination in MPTP-Treated Marmosets. PLoS ONE 2017, 12, e0182887. [Google Scholar] [CrossRef]
- Hartrampf, F.W.; Barber, D.M.; Gottschling, K.; Leippe, P.; Hollmann, M.; Trauner, D. Development of a photoswitchable antagonist of NMDA receptors. Tetrahedron 2017, 73, 4905–4912. [Google Scholar] [CrossRef]
- Klockgether, T.; Turski, L.; Honor, T.; Zhang, Z.; Gash, D.M.; Kurlan, R.; Greenamyre, T. The AMPA receptor antagonist NBQX has anti-Parkinsonian effects in monoamine-depleted rats and MPTP-treated monkeys. Ann. Neurol. 1991, 30, 717–723. [Google Scholar] [CrossRef]
- Löschmann, P.; Kunow, M.; Wachtel, H. Synergism of NBQX with dopamine agonists in the 6-OHDA rat model of Parkinson’s disease. J. Neural Transm. 1992, 38, 55–64. [Google Scholar]
- Wachtel, H.; Kunow, M.; Löschmann, P.A. NBQX (6-nitro-sulfamoyl-benzo-quinoxaline-dione) and CPP (3-carboxy-piperazin-propyl phosphonic acid) potentiate dopamine agonist induced rotations in substantia nigra lesioned rats. Neurosci. Lett. 1992, 142, 179–182. [Google Scholar] [CrossRef]
- Brotchie, J.M. Nondopaminergic mechanisms in levodopa-induced dyskinesia. Mov. Disord. 2005, 20, 919–931. [Google Scholar] [CrossRef]
- Paul, D.; Allakonda, L.; Sahu, A.; Surendran, S.; Satheeshkumar, N. Pharmacokinetics and brain uptake study of novel AMPA receptor antagonist perampanel in SD rats using a validated UHPLC-QTOF-MS method. J. Pharm. Biomed. Anal. 2018, 149, 234–241. [Google Scholar] [CrossRef]
- Lattanzi, S.; Grillo, E.; Brigo, F.; Silvestrini, M. Efficacy and safety of perampanel in Parkinson’s disease. A systematic review with meta-analysis. J. Neurol. 2018, 265, 733–740. [Google Scholar] [CrossRef]
- Löscher, W.; Lehmann, H.; Behl, B.; Seemann, D.; Teschendorf, H.J.; Hofmann, H.P.; Lubisch, W.; Höger, T.; Lemaire, H.G.; Groß, G. A new pyrrolyl-quinoxalinedione series of non-NMDA glutamate receptor antagonists: Pharmacological characterization and comparison with NBQX and valproate in the kindling model of epilepsy. Eur. J. Neurosci. 1999, 11, 250–262. [Google Scholar] [CrossRef]
- Bortolotto, Z.A.; Clarke, V.R.; Delany, C.M.; Parry, M.C.; Smolders, I.; Vignes, M.; Ho, K.H.; Miu, P.; Brinton, B.T.; Fantaske, R.; et al. Kainate receptors are involved in synaptic plasticity. Nature 1999, 402, 297. [Google Scholar] [CrossRef]
- O’Neill, M.J.; Bond, A.; Ornstein, P.L.; Ward, M.A.; Hicks, C.A.; Hoo, K.; Bleakman, D.; Lodge, D. Decahydroisoquinolines: Novel competitive AMPA/kainate antagonists with neuroprotective effects in global cerebral ischaemia. Neuropharmacology 1998, 37, 1211–1222. [Google Scholar] [CrossRef]
- Pourmirbabaei, S.; Dolatshahi, M.; Rahmani, F. Pathophysiological clues to therapeutic applications of glutamate mGlu5 receptor antagonists in levodopa-induced dyskinesia. Eur. J. Pharm. 2019, 855, 149–159. [Google Scholar] [CrossRef]
- Battaglia, G.; Busceti, C.L.; Molinaro, G.; Biagioni, F.; Storto, M.; Fornai, F.; Nicoletti, F.; Bruno, V. Endogenous activation of mGlu5 metabotropic glutamate receptors contributes to the development of nigro-striatal damage induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine in mice. J. Neurosci. 2004, 24, 828–835. [Google Scholar] [CrossRef]
- Huang, Y.; Shu, H.; Li, L.; Zhen, T.; Zhao, J.; Zhou, X.; Luo, W. L-DOPA-Induced Motor Impairment and Overexpression of Corticostriatal Synaptic Components Are Improved by the mGluR5 Antagonist MPEP in 6-OHDA-Lesioned Rats. ASN Neuro 2018, 10, 11. [Google Scholar] [CrossRef]
- Johnston, T.H.; Fox, S.H.; McIldowie, M.J.; Piggott, M.J.; Brotchie, J.M. Reduction of L-DOPA-induced dyskinesia by the selective metabotropic glutamate receptor 5 antagonist 3-[(2-methyl-1, 3-thiazol-4-yl) ethynyl] pyridine in the 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine-lesioned macaque model of Parkinson’s disease. J. Pharm. Exp. Ther. 2010, 333, 865–873. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Bogenpohl, J.W.; Alagille, D.; Delevich, K.; Tamagnan, G.; Votaw, J.R.; Wichmann, T.; Smith, Y. Metabotropic glutamate receptor 5 antagonist protects dopaminergic and noradrenergic neurons from degeneration in MPTP-treated monkeys. Brain 2011, 134, 2057–2073. [Google Scholar] [CrossRef]
- Turle-Lorenzo, N.; Breysse, N.; Baunez, C.; Amalric, M. Functional interaction between mGlu 5 and NMDA receptors in a rat model of Parkinson’s disease. Psychopharmacology 2005, 179, 117–127. [Google Scholar] [CrossRef]
- Grégoire, L.; Morin, N.; Ouattara, B.; Gasparini, F.; Bilbe, G.; Johns, D.; Vranesic, I.; Sahasranaman, S.; Mancilla, B.G.; Paolo, T.D. The acute anti-Parkinsonian and antidyskinetic effect of AFQ056, a novel metabotropic glutamate receptor type 5 antagonist, in l-Dopa-treated parkinsonian monkeys. Parkinsonism Relat. Disord. 2011, 17, 270–276. [Google Scholar] [CrossRef]
- Bezard, E.; Pioli, E.Y.; Li, Q.; Girard, F.; Mutel, V.; Keywood, C.; Tison, F.; Rascol, O.; Poli, S.M. The mGluR5 negative allosteric modulator dipraglurant reduces dyskinesia in the MPTP macaque model. Mov. Disord. 2014, 29, 1074–1079. [Google Scholar] [CrossRef]
- Tison, F.; Keywood, C.; Wakefield, M.; Durif, F.; Corvol, J.C.; Eggert, K.; Lew, M.; Isaacson, S.; Bezard, E.; Poli, S.M.; et al. A phase 2a trial of the novel mglur5-negative allosteric modulator dipraglurant for levodopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2016, 31, 1373–1380. [Google Scholar] [CrossRef]
- Dekundy, A.; Pietraszek, M.; Schaefer, D.; Cenci, M.A.; Danysz, W. Effects of group I metabotropic glutamate receptors blockade in experimental models of Parkinson’s disease. Brain Res. Bull. 2006, 69, 318–326. [Google Scholar] [CrossRef]
- Masilamoni, G.J.; Smith, Y. Neuroprotective Properties of Glutamate Metabotropic Glutamate Receptors in Parkinson’s Disease and Other Brain Disorders. In mGLU Receptors; Humana Press: Cham, Switzerland, 2017; pp. 103–127. [Google Scholar]
- Woolley, M.L.; Pemberton, D.J.; Bate, S.; Corti, C.; Jones, D.N.C. The mGlu2 but not the mGlu3 receptor mediates the actions of the mGluR2/3 agonist, LY379268, in mouse models predictive of antipsychotic activity. Psychopharmacology 2008, 196, 431–440. [Google Scholar] [CrossRef]
- Wang, L.; Kitai, S.T.; Xiang, Z. Modulation of excitatory synaptic transmission by endogenous glutamate acting on presynaptic group II mGluRs in rat substantia nigra compacta. J. Neurosci. Res. 2005, 82, 778–787. [Google Scholar] [CrossRef]
- Vernon, A.C.; Palmer, S.; Datla, K.P.; Zbarsky, V.; Croucher, M.J.; Dexter, D.T. Neuroprotective effects of metabotropic glutamate receptor ligands in a 6-hydroxydopamine rodent model of Parkinson’s disease. Eur. J. Neurosci. 2005, 22, 1799–1806. [Google Scholar] [CrossRef]
- Picconi, B.; Pisani, A.; Centonze, D.; Battaglia, G.; Storto, M.; Nicoletti, F.; Bernardi, G.; Calabresi, P. Striatal metabotropic glutamate receptor function following experimental parkinsonism and chronic levodopa treatment. Brain 2002, 125, 2635–2645. [Google Scholar] [CrossRef]
- Battaglia, G.; Busceti, C.L.; Molinaro, G.; Biagioni, F.; Traficante, A.; Nicoletti, F.; Bruno, V. Pharmacological activation of mGlu4 metabotropic glutamate receptors reduces nigrostriatal degeneration in mice treated with 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine. J. Neurosci. 2006, 26, 7222–7229. [Google Scholar] [CrossRef]
- Iderberg, H.; Maslava, N.; Thompson, A.D.; Bubser, M.; Niswender, C.M.; Hopkins, C.R.; Lindsley, C.W.; Conn, P.J.; Jones, C.K.; Cenci, M.A. Pharmacological stimulation of metabotropic glutamate receptor type 4 in a rat model of Parkinson’s disease and L-DOPA-induced dyskinesia: Comparison between a positive allosteric modulator and an orthosteric agonist. Neuropharmacology 2015, 95, 121–129. [Google Scholar] [CrossRef]
- Panarese, J.D.; Engers, D.W.; Wu, Y.J.; Bronson, J.J.; Macor, J.E.; Chun, A. Discovery of VU2957 (valiglurax): An mGlu4 positive allosteric modulator evaluated as a preclinical candidate for the treatment of Parkinson’s disease. ACS Med. Chem. Lett. 2018, 10, 255–260. [Google Scholar] [CrossRef]
- Lopez, S.; Turle-Lorenzo, N.; Acher, F.; De, L.E.; Mele, A.; Amalric, M. Targeting group III metabotropic glutamate receptors produces complex behavioral effects in rodent models of Parkinson’s disease. J. Neurosci. 2007, 27, 6701–6711. [Google Scholar] [CrossRef]
- Stoof, J.; Booij, J.; Drukarch, B. Amantadine as N-methyl-D-aspartic acid receptor antagonist: New possibilities for therapeutic applications? Clin. Neurol. Neurosurg. 1992, 94, 4–6. [Google Scholar] [CrossRef]
- Uitti, R.J.; Rajput, A.H.; Ahlskog, J.E.; Offord, K.P.; Schroeder, D.R.; Ho, M.M.; Prasad, M.; Rajput, A.; Basran, P. Amantadine treatment is an independent predictor of improved survival in Parkinson’s disease. Neurology 1996, 46, 1551–1556. [Google Scholar] [CrossRef]
- Fox, S.H.; Metman, L.V.; Nutt, J.G.; Brodsky, M.; Factor, S.A.; Lang, A.E.; Pope, L.E.; Knowles, N.; Siffert, J. Trial of dextromethorphan/quinidine to treat levodopa-induced dyskinesia in Parkinson’s disease. Mov. Disord. 2017, 32, 893–903. [Google Scholar] [CrossRef]
- Majláth, Z.; Vécsei, L. NMDA antagonists as Parkinson’s disease therapy: Disseminating the evidence. Neurodegen. Dis. Manag. 2014, 4, 23–30. [Google Scholar] [CrossRef]
- Herring, W.J.; Assaid, C.; Budd, K.; Vargo, R.; Mazenko, R.S.; Lines, C.; Ellenbogen, A.D.; Verhagen, M.L. A phase Ib randomized controlled study to evaluate the effectiveness of a single-dose of the NR2B selective N-methyl-D-aspartate antagonist MK-0657 on levodopa-induced dyskinesias and motor symptoms in patients with Parkinson disease. Clin. Neuropharmacol. 2017, 40, 255–260. [Google Scholar] [CrossRef]
- Eggert, K.; Squillacote, D.; Barone, P.; Dodel, R.; Katzenschlager, R.; Emre, M.; Lees, A.J.; Rascol, O.; Poewe, W.; Tolosa, E.; et al. Safety and efficacy of perampanel in advanced Parkinson’s disease: A randomized, placebo-controlled study. Mov. Disord. 2010, 25, 896–905. [Google Scholar] [CrossRef]
- Lees, A.; Fahn, S.; Eggert, K.M.; Jankovic, J.; Lang, A.; Micheli, F.; Mouradian, M.M.; Oertel, W.H.; Olanow, C.W.; Poewe, W.; et al. Perampanel, an AMPA antagonist, found to have no benefit in reducing “off” time in Parkinson’s disease. Mov. Disord. 2012, 27, 284–288. [Google Scholar] [CrossRef]
- Rascol, O.; Barone, P.; Behari, M.; Emre, M.; Giladi, N.; Olanow, C.W.; Ruzicka, E.; Bibbiani, F.; Squillacote, D.; Patten, A.; et al. Perampanel in Parkinson disease fluctuations: A double-blind randomized trial with placebo and entacapone. Clin. Neuropharmacol. 2012, 35, 15–20. [Google Scholar] [CrossRef]
- Stocchi, F.; Rascol, O.; Destee, A.; Hattori, N.; Hauser, R.A.; Lang, A.E.; Poewe, W.; Stacy, M.; Tolosa, E.; Gao, H.; et al. AFQ056 in Parkinson patients with levodopa-induced dyskinesia: 13-week, randomized, dose-finding study. Mov. Disord. 2013, 28, 1838–1846. [Google Scholar] [CrossRef]
- Trenkwalder, C.; Stocchi, F.; Poewe, W.; Dronamraju, N.; Kenney, C.; Shah, A.; Raison, F.; Graf, A. Mavoglurant in Parkinson’s patients with l-Dopa-induced dyskinesias: Two randomized phase 2 studies. Mov. Disord. 2016, 31, 1054–1058. [Google Scholar] [CrossRef]
- Olanow, C.W. Can we achieve neuroprotection with currently available anti-parkinsonian interventions? Neurology 2009, 72, S59–S64. [Google Scholar] [CrossRef]
- Schapira, A.H.; Obeso, J. Timing of treatment initiation in Parkinson’s disease: A need for reappraisal? Ann. Neurol. 2006, 59, 559–562. [Google Scholar] [CrossRef]
- Kieburtz, K.; Olanow, C.W. Translational experimental therapeutics: The translation of laboratory-based discovery into disease-related therapy. Mt. Sinai J. Med. 2007, 74, 7–14. [Google Scholar] [CrossRef]
- Nuzzo, T.; Punzo, D.; Devoto, P.; Rosini, E.; Paciotti, S.; Sacchi, S.; Li, Q.; Thiolat, M.L.; Véga, C.; Carella, M.; et al. The levels of the NMDA receptor co-agonist D-serine are reduced in the substantia nigra of MPTP-lesioned macaques and in the cerebrospinal fluid of Parkinson’s disease patients. Sci. Rep. 2019, 9, 8898. [Google Scholar] [CrossRef]
- Vanle, B.; Olcott, W.; Jimenez, J.; Bashmi, L.; Danovitch, I.; IsHak, W.W. NMDA antagonists for treating the non-motor symptoms in Parkinson’s disease. Transl. Psychiatr. 2018, 8, 117. [Google Scholar] [CrossRef]
- Bennouar, K.E.; Uberti, M.A.; Melon, C.; Bacolod, M.D.; Jimenez, H.N.; Cajina, M.; Goff, L.K.; Doller, D.; Gubellini, P. Synergy between L-DOPA and a novel positive allosteric modulator of metabotropic glutamate receptor 4: Implications for Parkinson’s disease treatment and dyskinesia. Neuropharmacology 2013, 66, 158–169. [Google Scholar] [CrossRef]
- Le Poul, E.; Bolea, C.; Girard, F.; Poli, S.; Charvin, D.; Campo, B.; Bortoli, J.; Bessif, A.; Luo, B.; Koser, A.J.; et al. A potent and selective metabotropic glutamate receptor 4 positive allosteric modulator improves movement in rodent models of Parkinson’s disease. J. Pharm. Exp. Ther. 2012, 343, 167–177. [Google Scholar] [CrossRef]
| Types | Changes during PD Process | Antiparkinsonian Treatment and Compounds | References |
|---|---|---|---|
| NMDA | GluN1, GluN2A, B, D: increase | NAMs: MK-801, dextrorphan, L-701324, SDZ220-581, MDL100,453, et al. GluN2B-selective NAMs: Ifenprodil, traxoprodil, Radiprodil Effects: improve PD motor symptoms, synergistically increase the anti-Parkinsonian efficiency of dopaminergic agents, reduce LIDs | [15,16,17,60,61,62,63,64,72,73,74,75] |
| AMPA | Increase | NAMs: NBQX, talampanel Effects: improve motor deficits, reduce LIDs | [23,77,78,79] |
| KA | GluK2: increase | NAMs: NBQX and CNQX (share with AMPA receptor), LU97175, LY382884, LY377770 Effects: no direct anti-Parkinsonian study targeting KAR | [32,83,84,85] |
| mGluR1/5 | mGluR1: dynamically changed mGluR5: increase | NAMs of mGluR5: MPEP, MTEP, AFQ056, ADX48621 Effects: limite the extent of nigrostriatal damage, alleviate LIDs | [35,38,88,89,90,92,93,94] |
| mGluR2/3 | mGluR2/3: decrease | PAMs: LY379268, (2R, 4R)-APDC, DCG-IV Effects: reduce the extent toxicity and corticostriatal transmission of 6-OHDA | [46,99,100] |
| mGluR4,6,7,8 | lacking mGluR4 impairs learning ability of complex motor tasks | PAMs of mGluR4: PHCCC, ADX88178, VU0364770, Lu AF21934 Effects: reduce the extent nigrostriatal toxicity and dosage of L-DOPA | [101,102,120,121] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Zhang, S.; Fu, P.; Zhang, Z.; Lin, K.; Ko, J.K.-S.; Yung, K.K.-L. Roles of Glutamate Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 4391. https://doi.org/10.3390/ijms20184391
Zhang Z, Zhang S, Fu P, Zhang Z, Lin K, Ko JK-S, Yung KK-L. Roles of Glutamate Receptors in Parkinson’s Disease. International Journal of Molecular Sciences. 2019; 20(18):4391. https://doi.org/10.3390/ijms20184391
Chicago/Turabian StyleZhang, Zhu, Shiqing Zhang, Pengfei Fu, Zhang Zhang, Kaili Lin, Joshua Ka-Shun Ko, and Ken Kin-Lam Yung. 2019. "Roles of Glutamate Receptors in Parkinson’s Disease" International Journal of Molecular Sciences 20, no. 18: 4391. https://doi.org/10.3390/ijms20184391
APA StyleZhang, Z., Zhang, S., Fu, P., Zhang, Z., Lin, K., Ko, J. K.-S., & Yung, K. K.-L. (2019). Roles of Glutamate Receptors in Parkinson’s Disease. International Journal of Molecular Sciences, 20(18), 4391. https://doi.org/10.3390/ijms20184391






