Cyclin–CDK Complexes are Key Controllers of Capacitation-Dependent Actin Dynamics in Mammalian Spermatozoa
Abstract
1. Introduction
2. Results
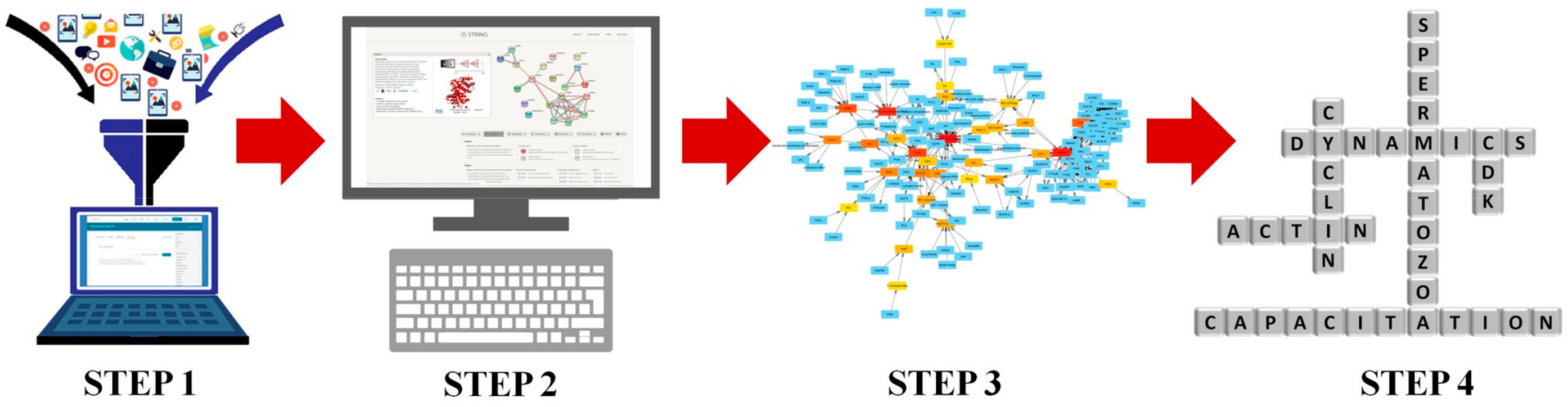
2.1. Networks Creation, Analysis, and Visualization
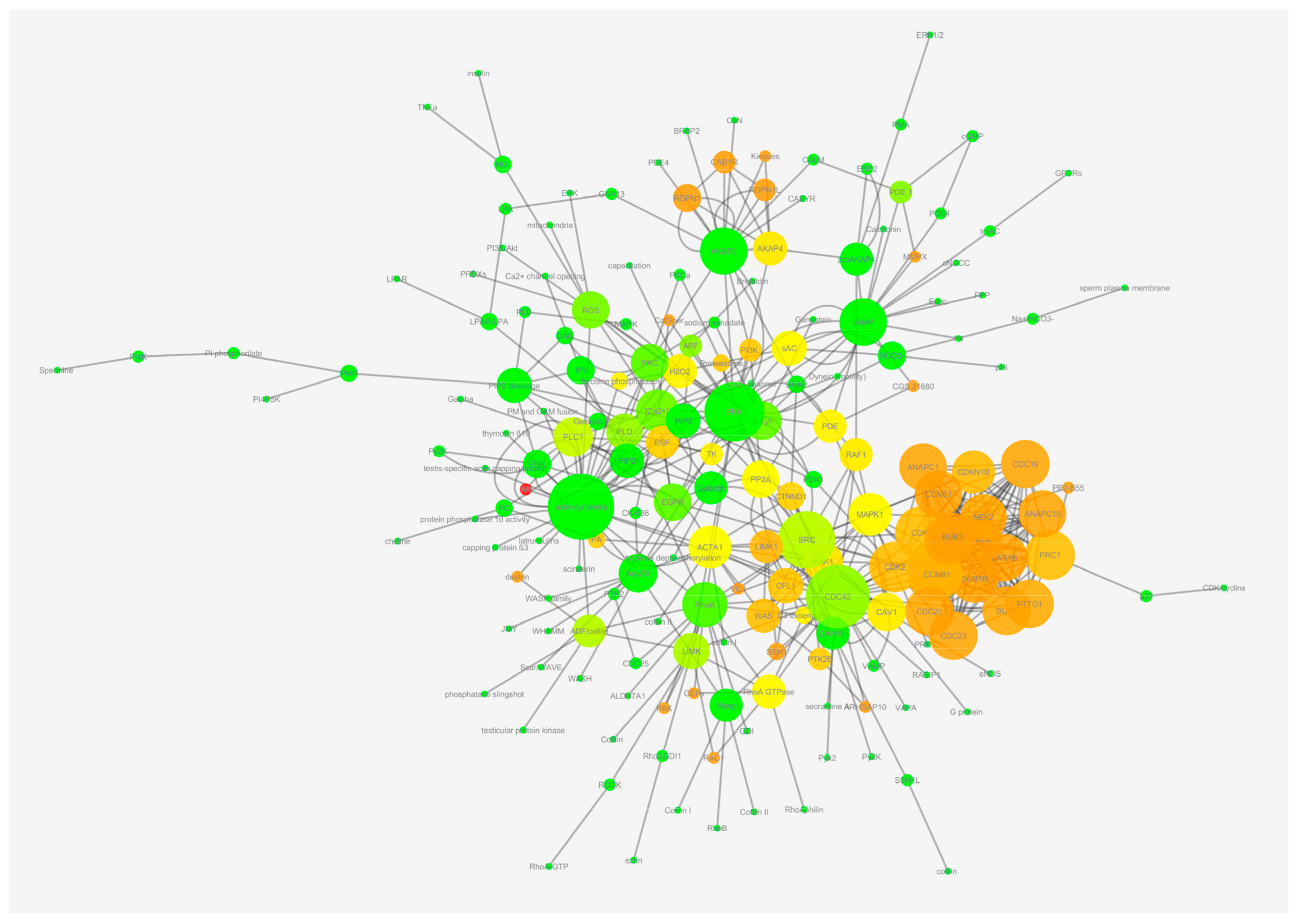
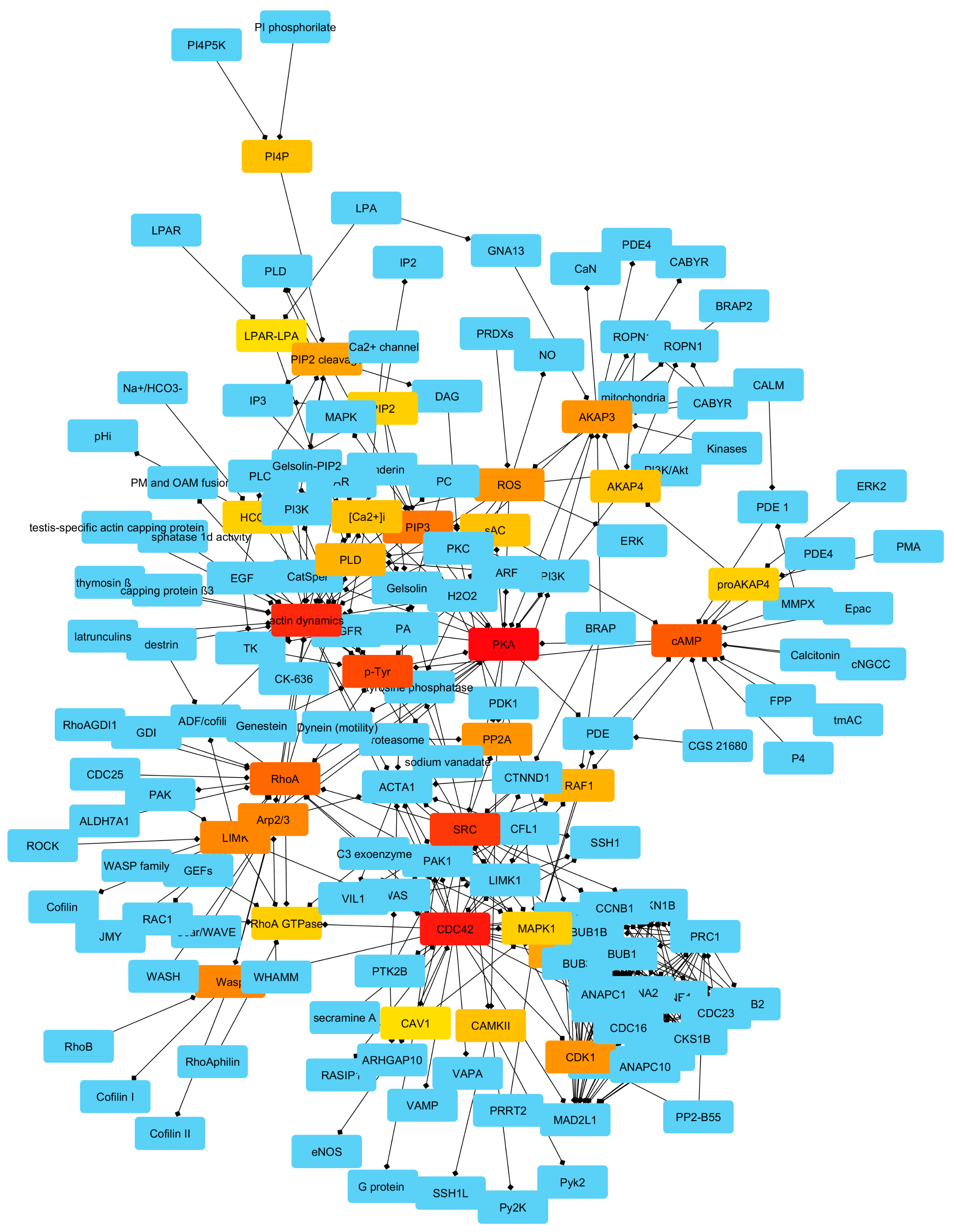
2.2. Identification of Controllers within the Network
2.3. Targeted vs. Random Attack to MN
3. Discussion
4. Materials and Methods
4.1. Data Collection, Network Creation, and General Analysis
- Molecule involved in biochemical reaction (source): molecules participating in the interaction as source;
- Interaction: kind of interaction between the molecules or the structures;
- Molecule or anatomical structure involved in biochemical reaction (target): molecules participating to the interaction as target;
- Alias: eventual aliases;
- Role: physiological and/or pathological role of the molecule/reaction related to fertility;
- Reference: article reporting the above mentioned data;
- Notes: any further information that could be useful in the study.
4.2. Identification of Predicted Interactions with STRING
4.3. Network Exhaustive Analysis and Visualization
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barratt, C.L.R.; Björndahl, L.; De Jonge, C.J.; Lamb, D.J.; Osorio Martini, F.; McLachlan, R.; Oates, R.D.; van der Poel, S.; St John, B.; Sigman, M.; et al. The diagnosis of male infertility: An analysis of the evidence to support the development of global WHO guidance—Challenges and future research opportunities. Hum. Reprod. Update 2017, 23, 660–680. [Google Scholar] [CrossRef] [PubMed]
- Winters, B.R.; Walsh, T.J. The epidemiology of male infertility. Urol. Clin. North Am. 2014, 41, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Ring, J.D.; Lwin, A.A.; Köhler, T.S. Current medical management of endocrine-related male infertility. Asian J. Androl. 2016, 18, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Inhorn, M.C.; Patrizio, P. Infertility around the globe: New thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 2015, 21, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Suarez, S.S. Mammalian sperm interactions with the female reproductive tract. Cell Tissue Res. 2016, 363, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Sostaric, E.; Dieleman, S.J.; van de Lest, C.H.A.; Colenbrander, B.; Vos, P.L.A.M.; Garcia-Gil, N.; Gadella, B.M. Sperm binding properties and secretory activity of the bovine oviduct immediately before and after ovulation. Mol. Reprod. Dev. 2008, 75, 60–74. [Google Scholar] [CrossRef] [PubMed]
- Leahy, T.; Gadella, B.M. New insights into the regulation of cholesterol efflux from the sperm membrane. Asian J. Androl. 2000, 17, 561–567. [Google Scholar]
- Ickowicz, D.; Finkelstein, M.; Breitbart, H. Mechanism of sperm capacitation and the acrosome reaction: Role of protein kinases. Asian J. Androl. 2012, 14, 816–821. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Xia, J.; Aoki, F.; Sakai, S.; Kohmoto, K.; Arnoult, C.; Cardullo, R.; Lemos, J.; Florman, H.; Arnoult, C.; et al. Calcium signaling through CatSper channels in mammalian fertilization. Physiol. (Bethesda) 2010, 25, 165–175. [Google Scholar] [CrossRef] [PubMed]
- Navarrete, F.A.1.; García-Vázquez, F.A.; Alvau, A.; Escoffier, J.; Krapf, D.; Sánchez-Cárdenas, C.; Salicioni, A.M.; Darszon, A.V.P. Biphasic role of calcium in mouse sperm capacitation signaling pathways. J. Cell. Physiol. 2015, 230, 1758–1769. [Google Scholar] [CrossRef] [PubMed]
- Hunter, R.H.F.; Petersen, H.H.; Greve, T. Ovarian follicular fluid, progesterone and Ca2+ ion influences on sperm release from the Fallopian tube reservoir. Mol. Reprod. Dev. 1999, 54, 283–291. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A.; Nixon, B. Are sperm capacitation and apoptosis the opposite ends of a continuum driven by oxidative stress? Asian J. Androl. 2015, 17, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Romarowski, A.; Battistone, M.A.; La Spina, F.A.; Puga Molina, L.; Del, C.; Luque, G.M.; Vitale, A.M.; Cuasnicu, P.S.; Visconti, P.E.; Krapf, D.; et al. PKA-dependent phosphorylation of LIMK1 and Cofilin is essential for mouse sperm acrosomal exocytosis. Dev. Biol. 2015, 405, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, J.; Diaz, E.S.; Morales, P. Kinases, phosphatases and proteases during sperm capacitation. Cell Tissue Res. 2012, 349, 765–782. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Rubinstein, S.; Gur, Y.; Breitbart, H. Crosstalk between protein kinase A and C regulates phospholipase D and F-actin formation during sperm capacitation. Dev. Biol. 2004, 267, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Correa, L.M.; Thomas, A.; Meyers, S.A. The macaque sperm actin cytoskeleton reorganizes in response to osmotic stress and contributes to morphological defects and decreased motility. Biol. Reprod. 2007, 77, 942–953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Brener, E.; Rubinstein, S.; Cohen, G.; Shternall, K.; Rivlin, J.; Breitbart, H. Remodeling of the Actin Cytoskeleton During Mammalian Sperm Capacitation and Acrosome Reaction. Biol. Reprod. 2002, 68, 837–845. [Google Scholar] [CrossRef]
- Delgado-Buenrostro, N.L.; Mújica, A.; Chiquete-Felix, N.; Déciga-Alcaraz, A.; Medina-Reyes, E.I.; Uribe-Carvajal, S.; Chirino, Y.I. Role of Wasp and the small GTPases RhoA, RhoB, and Cdc42 during capacitation and acrosome reaction in spermatozoa of English guinea pigs. Mol. Reprod. Dev. 2016, 83, 927–937. [Google Scholar] [CrossRef]
- Bernabò, N.; Valbonetti, L.; Greco, L.; Capacchietti, G.; Ramal Sanchez, M.; Palestini, P.; Botto, L.; Mattioli, M.; Barboni, B. Aminopurvalanol A, a Potent, Selective, and Cell Permeable Inhibitor of Cyclins/Cdk Complexes, Causes the Reduction of in Vitro Fertilizing Ability of Boar Spermatozoa, by Negatively Affecting the Capacitation-Dependent Actin Polymerization. Front. Physiol. 2017, 8, 1097. [Google Scholar] [CrossRef]
- Ordinelli, A.; Bernabò, N.; Orsini, M.; Mattioli, M.; Barboni, B. Putative human sperm Interactome: A networks study. BMC Syst. Biol. 2018, 12, 52. [Google Scholar] [CrossRef]
- Breitbart, H.; Rotman, T.; Rubinstein, S.; Etkovitz, N. Role and regulation of PI3K in sperm capacitation and the acrosome reaction. Mol. Cell. Endocrinol. 2010, 314, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Breitbart, H.; Finkelstein, M. Biochemical and Biophysical Research Communications Actin cytoskeleton and sperm function. Biochem. Biophys. Res. Commun. 2018, 506, 372–377. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Tyagi, M.; Teng, S.; Shoemaker, B.A.; Hashimoto, K.; Alexov, E.; Wuchty, S.; Panchenko, A.R. Cancer Missense Mutations Alter Binding Properties of Proteins and Their Interaction Networks. PLoS ONE 2013, 8, e66273. [Google Scholar] [CrossRef] [PubMed]
- Sharan, R.; Ideker, T. Modeling cellular machinery through biological network comparison. Nat. Biotechnol. 2006, 24, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, I.; Bernabò, N.; Todisco, G.; Lucidi, P.; Berardinelli, P. Role of Actin in Spermatozoa Function Through Biological Network Theory; Springer: Berlin/Heidelberg, Germany, 2012; Volume 9783642232, ISBN 978364223. [Google Scholar]
- Bernabò, N.; Greco, L.; Ordinelli, A.; Mattioli, M.; Barboni, B. Capacitation-Related Lipid Remodeling of Mammalian Spermatozoa Membrane Determines the Final Fate of Male Gametes: A Computational Biology Study. OMICS 2015, 19, 712–721. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, N.; Agostino, R.D.; Ordinelli, A.; Mattioli, M. Systems Biology in Reproductive Medicine The maturation of murine spermatozoa membranes within the epididymis, a computational biology perspective. Syst. Biol. Reprod. Med. 2016, 62, 299–308. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bernabò, N.; Mattioli, M.; Barboni, B. Signal transduction in the activation of spermatozoa compared to other signalling pathways: A biological networks study. Int. J. Data Min. Bioinform. 2015, 12, 59. [Google Scholar] [CrossRef] [PubMed]
- Bernabò, N.; Berardinelli, P.; Mauro, A.; Russo, V.; Lucidi, P.; Mattioli, M.; Barboni, B. The role of actin in capacitation-related signaling: An in silico and in vitro study. BMC Syst. Biol. 2011, 5, 47. [Google Scholar] [CrossRef]
- Latora, V.; Marchiori, M. Efficient Behavior of Small-World Networks. Phys. Rev. Lett. 2001, 87, 198701. [Google Scholar] [CrossRef]
- Amaral, A.; Castillo, J.; Ramalho-Santos, J.; Oliva, R. The combined human sperm proteome: Cellular pathways and implications for basic and clinical science. Hum. Reprod. Update 2014, 20, 40–62. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Brinkworth, M.; Iles, D. Paternal DNA packaging in spermatozoa: More than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 2010, 139, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.; Ramalho-Santos, J. The Male Gamete Is Not a Somatic Cell—The Possible Meaning of Varying Sperm RNA Levels. Antioxid. Redox Signal. 2013, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Gur, Y.; Breitbart, H. Protein synthesis in sperm: Dialog between mitochondria and cytoplasm. Mol. Cell. Endocrinol. 2008, 282, 45–55. [Google Scholar] [CrossRef]
- Gur, Y.; Breitbart, H. Mammalian sperm translate nuclear-encoded proteins by mitochondrial-type ribosomes. Genes Dev. 2006, 20, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Baker, M.A. The ’omics revolution and our understanding of sperm cell biology. Asian J. Androl. 2011, 13, 6–10. [Google Scholar] [CrossRef]
- Gadella, B.M.; Harrison, R.A.P. The capacitating agent bicarbonate induces protein kinase A-dependent changes in phospholipid transbilayer behavior in the sperm plasma membrane. Development 2000, 2420, 2407–2420. [Google Scholar]
- Botto, L.; Bernabò, N.; Palestini, P.; Barboni, B. Bicarbonate induces membrane reorganization and CBR1 and TRPV1 endocannabinoid receptor migration in lipid microdomains in capacitating boar spermatozoa. J. Membr. Biol. 2010, 238, 33–41. [Google Scholar] [CrossRef]
- Barboni, B.; Bernabò, N.; Palestini, P.; Botto, L.; Pistilli, M.G.; Charini, M.; Tettamanti, E.; Battista, N.; Maccarrone, M.; Mattioli, M. Type-1 Cannabinoid receptors reduce membrane fluidity of capacitated boar sperm by impairing their activation by bicarbonate. PLoS ONE 2011, 6, e23038. [Google Scholar] [CrossRef]
- Brouwers, J.F.; Boerke, A.; Silva, P.F.N.; Garcia-Gil, N.; van Gestel, R.A.; Helms, J.B.; van de Lest, C.H.A.; Gadella, B.M. Mass spectrometric detection of cholesterol oxidation in bovine sperm. Biol. Reprod. 2011, 85, 128–136. [Google Scholar] [CrossRef]
- Gadella, B.M.; Luna, C. Cell biology and functional dynamics of the mammalian sperm surface. Theriogenology 2014, 81, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Kaldis, P. Cdks, cyclins and CKIs: Roles beyond cell cycle regulation. Development 2013, 140, 3079–3093. [Google Scholar] [CrossRef] [PubMed]
- Hydbring, P.; Malumbres, M.; Sicinski, P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016, 17, 280–292. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A. The Cytoskeleton and Cell Signaling: Component Localization and Mechanical Coupling. Physiol. Rev. 1998, 78, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Shafrir, Y.; ben-Avraham, D.; Forgacs, G. Trafficking and signaling through the cytoskeleton: A specific mechanism. J. Cell Sci. 2000, 113, 2747–2757. [Google Scholar] [PubMed]
- Bernabò, N.; Ordinelli, A.; Ramal Sanchez, M.; Mattioli, M.; Barboni, B. Networks Models of Actin Dynamics during Spermatozoa Postejaculatory Life: A Comparison among Human-Made and Text Mining-Based Models. Biomed Res. Int. 2016, 2016. [Google Scholar] [CrossRef]
- Szklarczyk, D.; Franceschini, A.; Wyder, S.; Forslund, K.; Heller, D.; Huerta-Cepas, J.; Simonovic, M.; Roth, A.; Santos, A.; Tsafou, K.P.; et al. STRING v10: Protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015, 43, D447–D452. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8, S11. [Google Scholar] [CrossRef]
- Pržulj, N.; Wigle, D.A.; Jurisica, I. Functional topology in a network of protein interactions. 2004, 20, 340–348. Bioinformatics 2004, 20, 340–348. [Google Scholar]
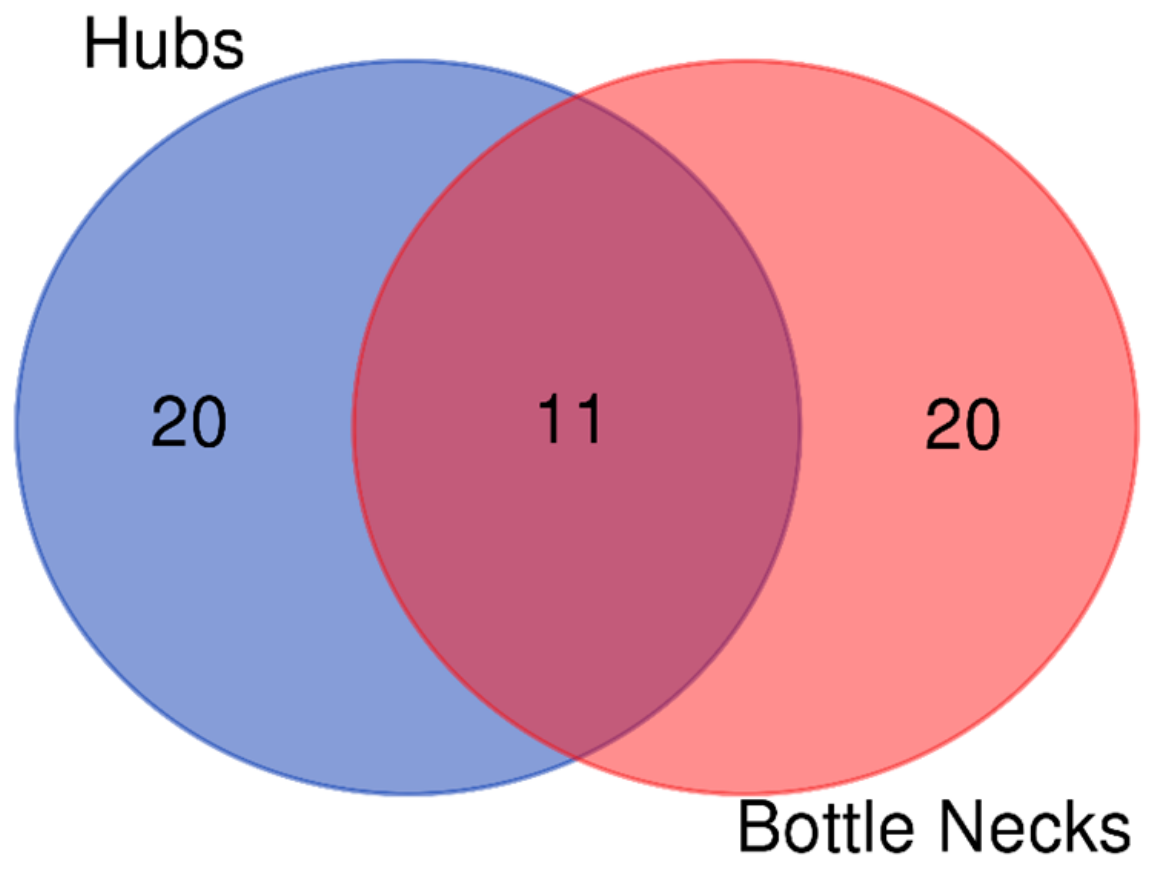
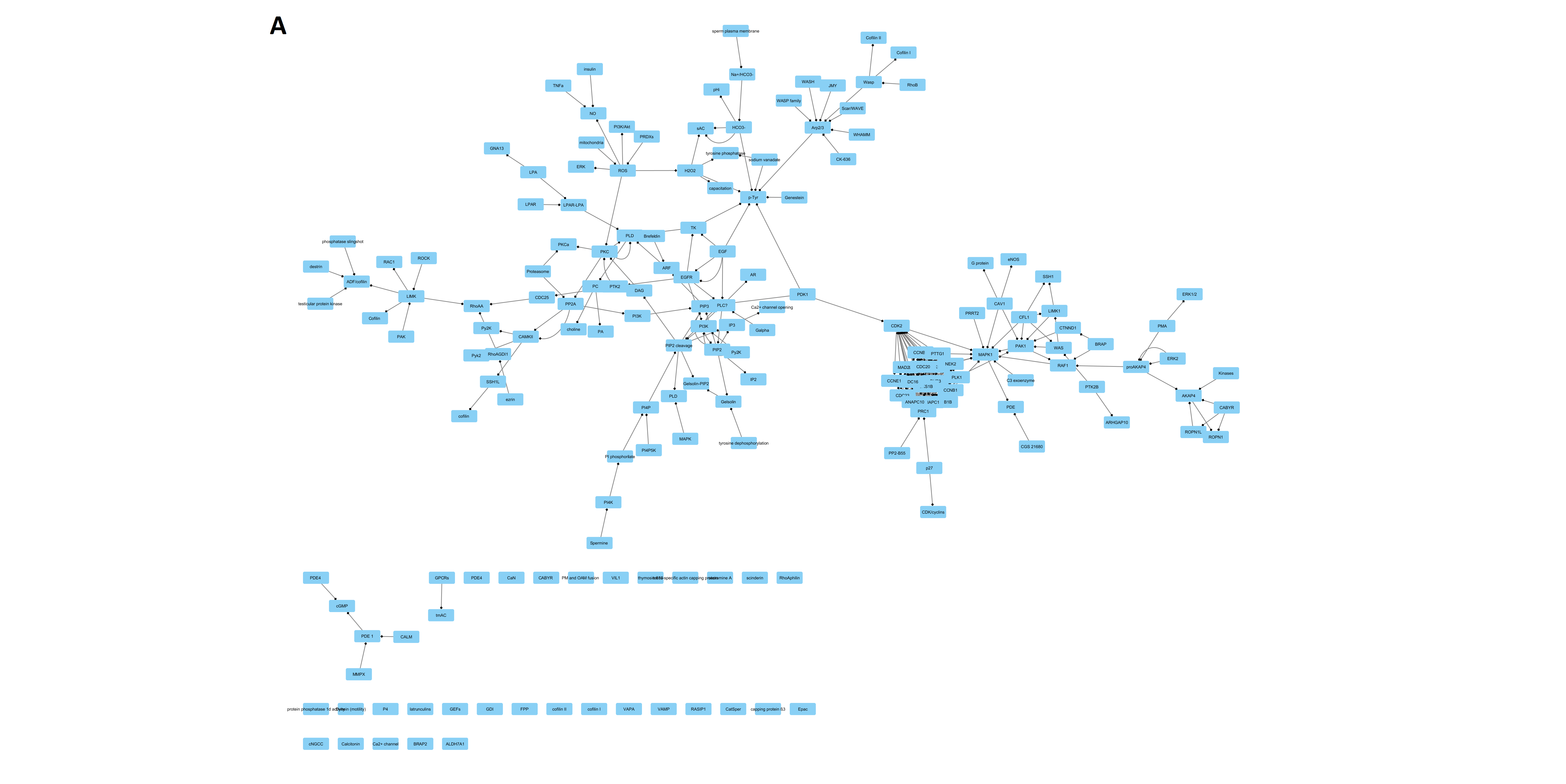
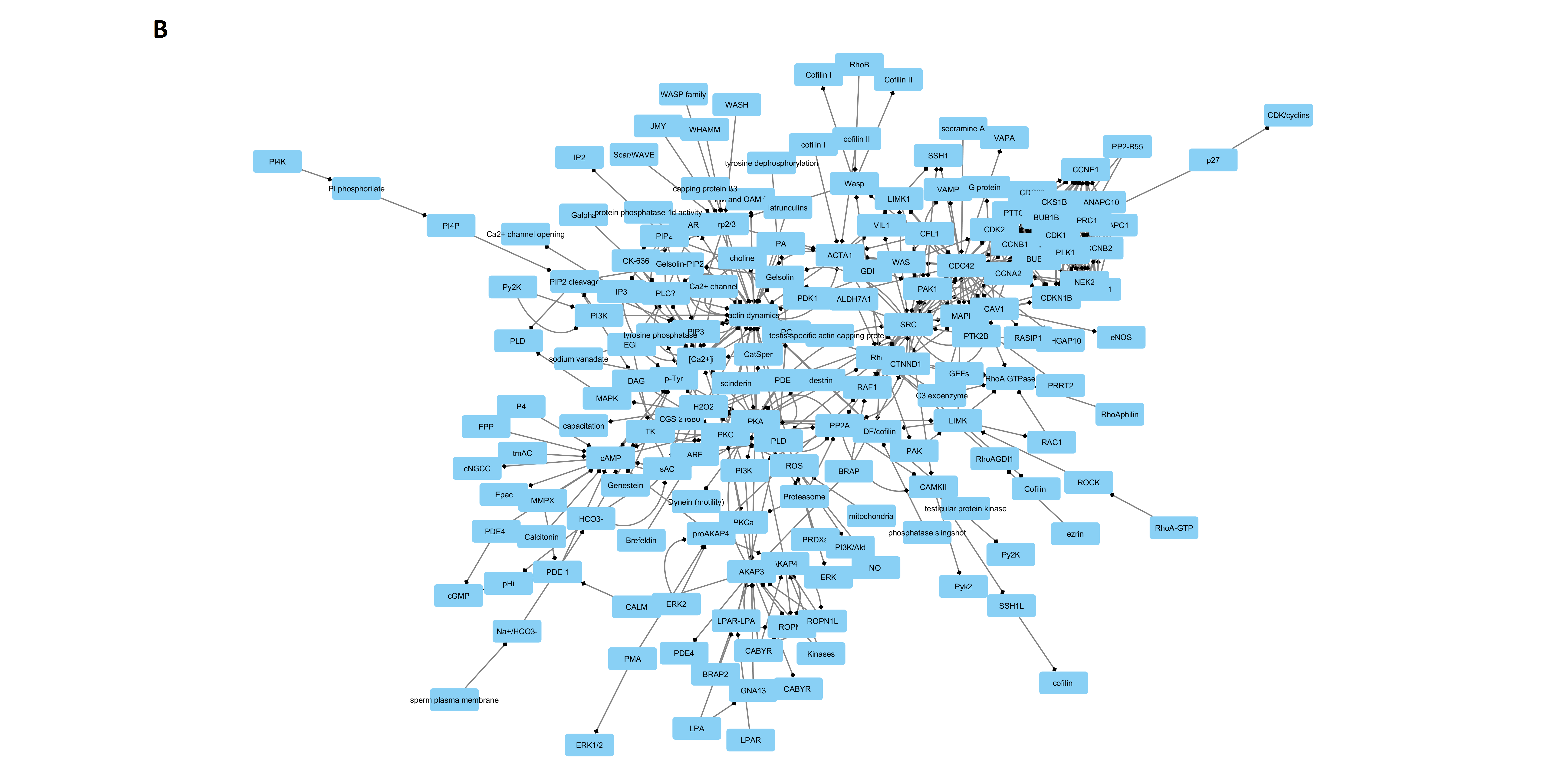
| Parameter | DB Network | STRING Network | Merged Network |
|---|---|---|---|
| N of nodes | 167 | 49 | 188 |
| N of edges | 274 | 287 | 558 |
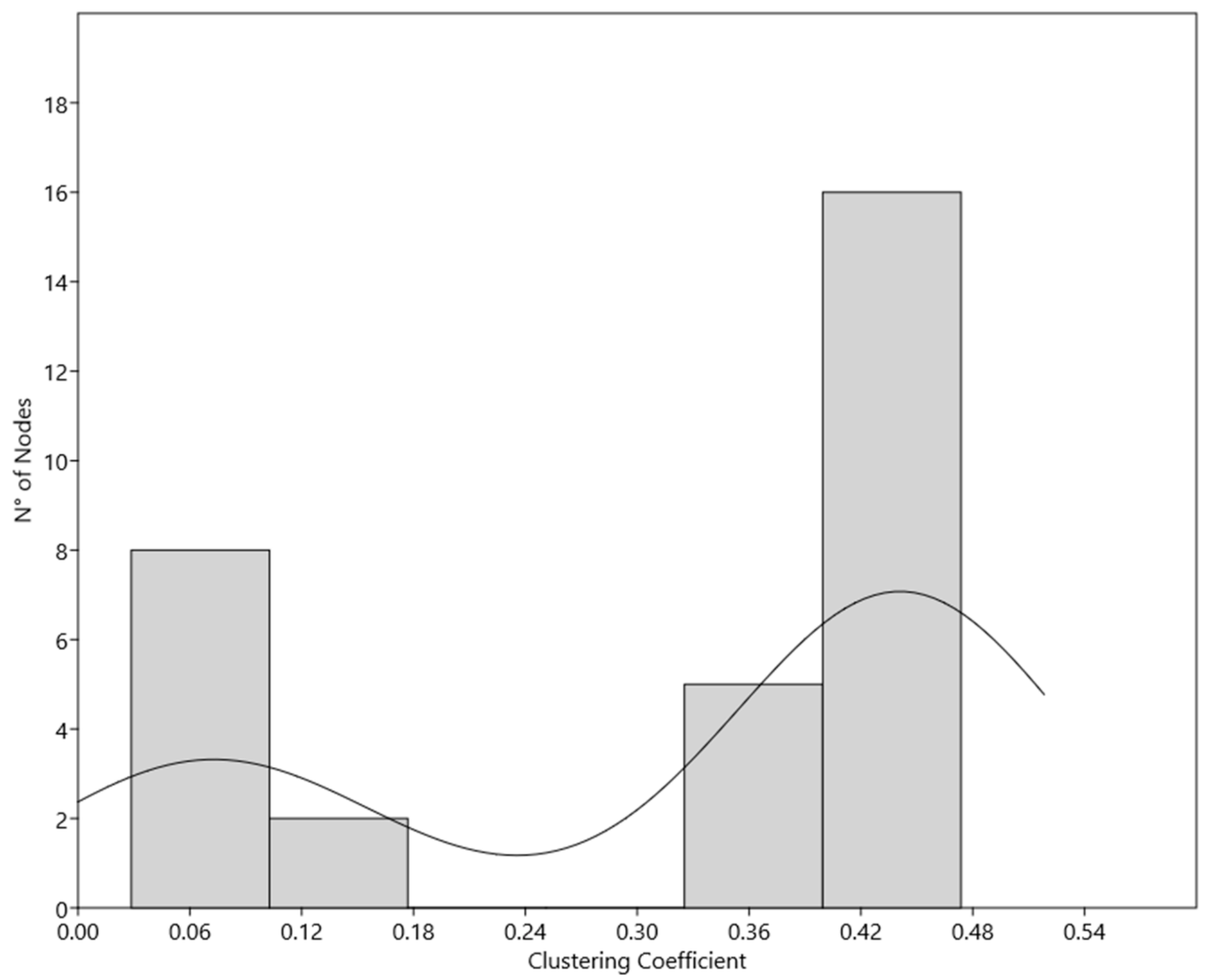
| Clustering Coefficient | 0.037 | 0.341 | 0.127 |
| Diameter | 14 | 5 | 12 |
| Shortest Path Length | 8837 (31%) | 436 (19%) | 14832 (41%) |
| Characteristic Path Length | 5.760 | 1.505 | 5.246 |
| Averaged Number of Neighbors | 2.922 | 11.633 | 5.471 |
| In Degree | |||
| Exponent (γ) | −1.314 | −0.608 | −1.183 |
| Coefficient of Correlation (r) | 0.996 | 0.734 | 0.988 |
| Coefficient of Determination (R2) | 0.910 | 0.527 | 0.871 |
| Out Degree | |||
| Exponent (γ) | −1.708 | −0.675 | −1.314 |
| Coefficient of Correlation (r) | 0.989 | 0.935 | 0.991 |
| Coefficient of Determination (R2) | 0.885 | 0.735 | 0.879 |
| Node Degree vs. Cluster. Coeff. | |||
| Coefficient of Determination (R2) | 0.333 | 0.085 | 0.003 |
| Node Name | Node Degree |
|---|---|
| AD | 36 |
| CDC42 | 34 |
| PKA | 30 |
| SRC | 28 |
| CDK1 | 24 |
| CCNB1 | 23 |
| CCNA2 | 22 |
| CDK2 | 22 |
| PLK1 | 22 |
| PRC1 | 21 |
| BUB1 | 20 |
| BUB1B | 20 |
| CCNB2 | 20 |
| MAD2L1 | 20 |
| CDC16 | 20 |
| CDC20 | 20 |
| CDC23 | 20 |
| PTTG1 | 20 |
| AKAP3 | 19 |
| ANAPC1 | 19 |
| CCNE1 | 19 |
| CKS1B | 19 |
| ANAPC10 | 19 |
| BUB3 | 19 |
| cAMP | 19 |
| NEK2 | 18 |
| [Ca2+]i | 15 |
| ACTA1 | 15 |
| CDKN1B | 15 |
| MAPK1 | 15 |
| RhoA | 13 |
| Node Name | Clustering Coefficient | |
|---|---|---|
| Subpopulation 1 | CCNE1 | 0,4737 |
| ANAPC1 | 0,4708 | |
| CKS1B | 0,4708 | |
| ANAPC10 | 0,4708 | |
| CCNB2 | 0,4684 | |
| CDC16 | 0,4684 | |
| CDC23 | 0,4684 | |
| BUB3 | 0,4591 | |
| BUB1 | 0,4579 | |
| BUB1B | 0,4579 | |
| MAD2L1 | 0,4579 | |
| CDC20 | 0,4579 | |
| PTTG1 | 0,4474 | |
| NEK2 | 0,4444 | |
| CCNA2 | 0,4048 | |
| PLK1 | 0,4048 | |
| CDKN1B | 0,3952 | |
| CCNB1 | 0,3893 | |
| PRC1 | 0,3789 | |
| CDK2 | 0,3788 | |
| CDK1 | 0,3775 | |
| Subpopulation 2 | MAPK1 | 0,1429 |
| ACTA1 | 0,1346 | |
| SRC | 0,1000 | |
| CDC42 | 0,0860 | |
| [Ca2+]i | 0,0758 | |
| RhoA | 0,0641 | |
| PKA | 0,0498 | |
| AKAP3 | 0,0381 | |
| AD | 0,0331 | |
| cAMP | 0,0286 |
| Name | Bottleneck Score |
|---|---|
| PKA | 81 |
| CDC42 | 59 |
| AD | 57 |
| SRC | 40 |
| p-Tyr | 22 |
| cAMP | 16 |
| RhoA | 14 |
| PIP3 | 12 |
| LIMK | 11 |
| Wasp | 11 |
| Arp2/3 | 11 |
| ROS | 8 |
| AKAP3 | 8 |
| PP2A | 8 |
| CDK1 | 8 |
| PIP2 cleavage | 7 |
| PLK1 | 7 |
| PLD | 6 |
| RAF1 | 6 |
| CAMKII | 5 |
| [Ca2+]i | 5 |
| PI4P | 5 |
| sAC | 5 |
| AKAP4 | 5 |
| PIP2 | 4 |
| proAKAP4 | 4 |
| MAPK1 | 4 |
| RhoA GTPase | 4 |
| HCO3- | 4 |
| CAV1 | 3 |
| LPAR-LPA | 3 |
| Node Name | Node Degree | Bottleneck Score | Function (with Focus on Mammalian Spermatozoa Physiology) |
|---|---|---|---|
| AD | 36 | 82 | |
| PKA | 30 | 88 | Key effector of the bicarbonate-dependent cAMP/protein kinase A (PKA) pathway that leads to the control of p-Tyr of sperm proteins during capacitation. Its activation is correlated to a myriad of biochemical events. |
| CDC42 | 34 | 34 | Controller of cell cycle, controller of sperm AD. |
| SRC | 28 | 23 | A non-receptor tyrosine kinase protein that in humans is encoded by the SRC gene. This protein phosphorylates specific tyrosine residues in other tyrosine kinases. An elevated level of activity of c-Src tyrosine kinase is suggested to be linked to cancer progression by promoting other signals. Mutations in this gene could be involved in the malignant progression of colon cancer. |
| CCNA2 | 22 | 14 | Controller of cell cycle, controller of sperm AD. |
| cAMP | 19 | 13 | Second messenger of the bicarbonate-dependent cAMP/protein kinase A (PKA) pathway. |
| AKAP3 | 19 | 13 | It is expressed in spermatozoa and localized to the acrosomal region of the sperm head, as well as the length of the principal piece. It may function as a regulator of motility, capacitation, and the acrosome reaction (AR) |
| CDK1 | 24 | 6 | Controller of cell cycle, controller of sperm AD. |
| ACTA1 | 15 | 15 | Polymerizes and depolymerizes during capacitation. |
| BUB1 | 20 | 7 | It plays a key role in the establishment of the mitotic spindle checkpoint and chromosome congression. |
| [Ca2+]i | 15 | 12 | Second messenger involved in virtually all the biochemical event related to the capacitation. |
| RhoA | 13 | 12 | It interacts with proteins involved in capacitation and the AR, and RhoA signaling in sperm may be targeted by AKAPs. |
| Parameter | Definition |
|---|---|
| Connected Components | Number of networks in which any two vertices are connected to each other by links, and which is connected to no additional vertices in the network. |
| Number of nodes | Total number of molecules involved. |
| Number of edges | Total number of interactions found. |
| Clustering coefficient | Calculated as CI = 2nI/kI(kI–1), where nI is the number of links connecting the kI neighbors of node I to each other. It is a measure of how the nodes tend to form clusters. |
| Network diameter | The longest of all the calculated shortest paths in a network. |
| Shortest paths | The length of the shortest path between two nodes n and m is L (n, m). The shortest path length distribution gives the number of node pairs (n, m) with L(n,m) = k for k = 1,2,… |
| Characteristic path length | Expected distance between two connected nodes. |
| Averaged number of neighbors | Mean number of connections of each node. |
| Node degree | It is the number of interaction of each node. |
| Node degree distribution | It represents the probability that a selected node has k links. |
| γ | Exponent of node degree equation. |
| R2 | Coefficient of determination of node degree vs. number of nodes, on logarithmized data. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernabò, N.; Ramal-Sanchez, M.; Valbonetti, L.; Machado-Simoes, J.; Ordinelli, A.; Capacchietti, G.; Taraschi, A.; Barboni, B. Cyclin–CDK Complexes are Key Controllers of Capacitation-Dependent Actin Dynamics in Mammalian Spermatozoa. Int. J. Mol. Sci. 2019, 20, 4236. https://doi.org/10.3390/ijms20174236
Bernabò N, Ramal-Sanchez M, Valbonetti L, Machado-Simoes J, Ordinelli A, Capacchietti G, Taraschi A, Barboni B. Cyclin–CDK Complexes are Key Controllers of Capacitation-Dependent Actin Dynamics in Mammalian Spermatozoa. International Journal of Molecular Sciences. 2019; 20(17):4236. https://doi.org/10.3390/ijms20174236
Chicago/Turabian StyleBernabò, Nicola, Marina Ramal-Sanchez, Luca Valbonetti, Juliana Machado-Simoes, Alessandra Ordinelli, Giulia Capacchietti, Angela Taraschi, and Barbara Barboni. 2019. "Cyclin–CDK Complexes are Key Controllers of Capacitation-Dependent Actin Dynamics in Mammalian Spermatozoa" International Journal of Molecular Sciences 20, no. 17: 4236. https://doi.org/10.3390/ijms20174236
APA StyleBernabò, N., Ramal-Sanchez, M., Valbonetti, L., Machado-Simoes, J., Ordinelli, A., Capacchietti, G., Taraschi, A., & Barboni, B. (2019). Cyclin–CDK Complexes are Key Controllers of Capacitation-Dependent Actin Dynamics in Mammalian Spermatozoa. International Journal of Molecular Sciences, 20(17), 4236. https://doi.org/10.3390/ijms20174236







