Loss of Glycine N-Methyltransferase Associates with Angiopoietin-Like Protein 8 Expression in High Fat-Diet-Fed Mice
Abstract
1. Introduction
2. Results
2.1. Increase of Plasma Insulin in High-Fat Diet Mice
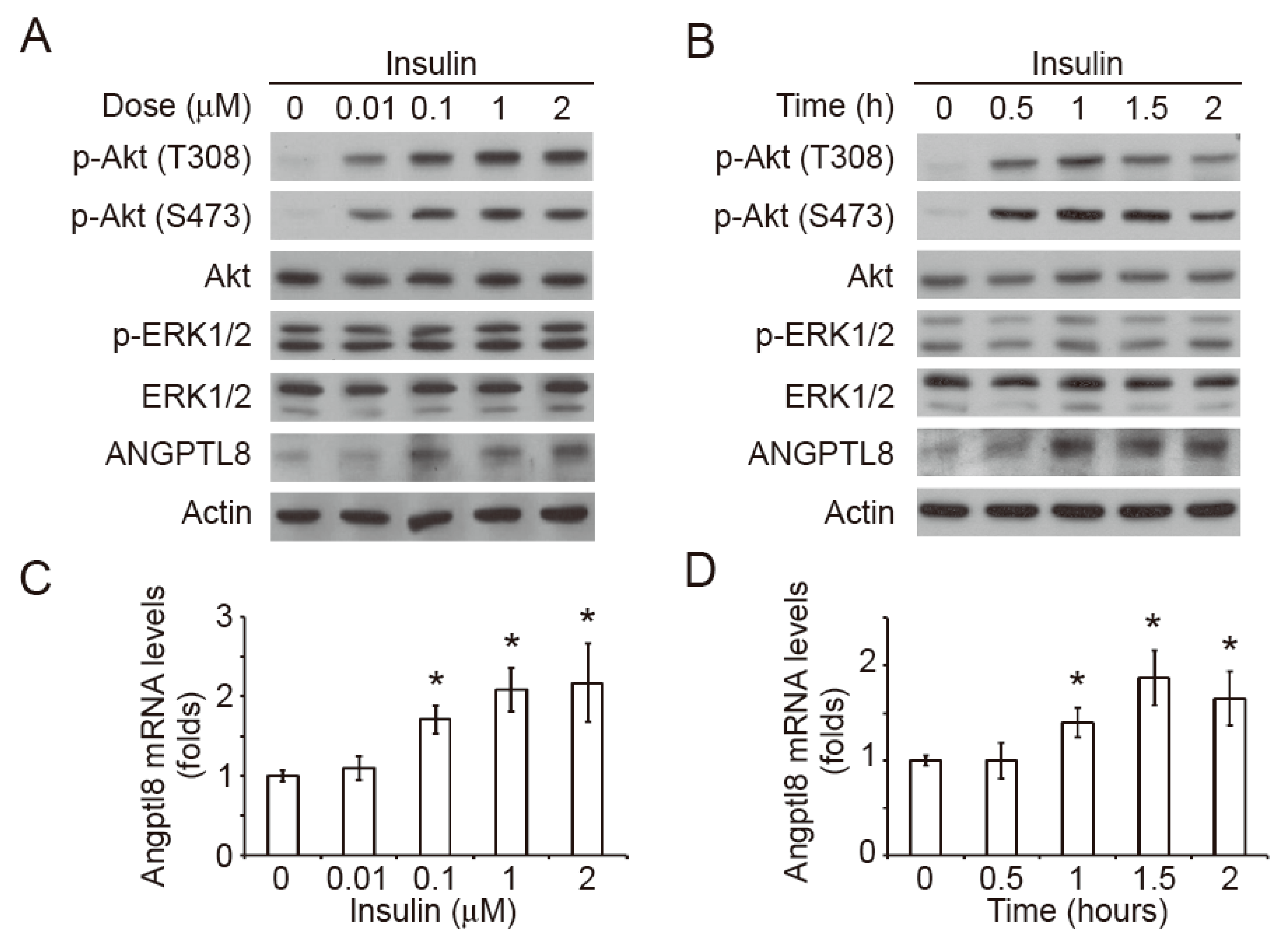
2.2. Insulin Induces Akt Phosphorylation and Angptl8 Expression
2.3. Insulin-Induced Angptl8 is Mediated by PI3K/Akt Signaling Pathway
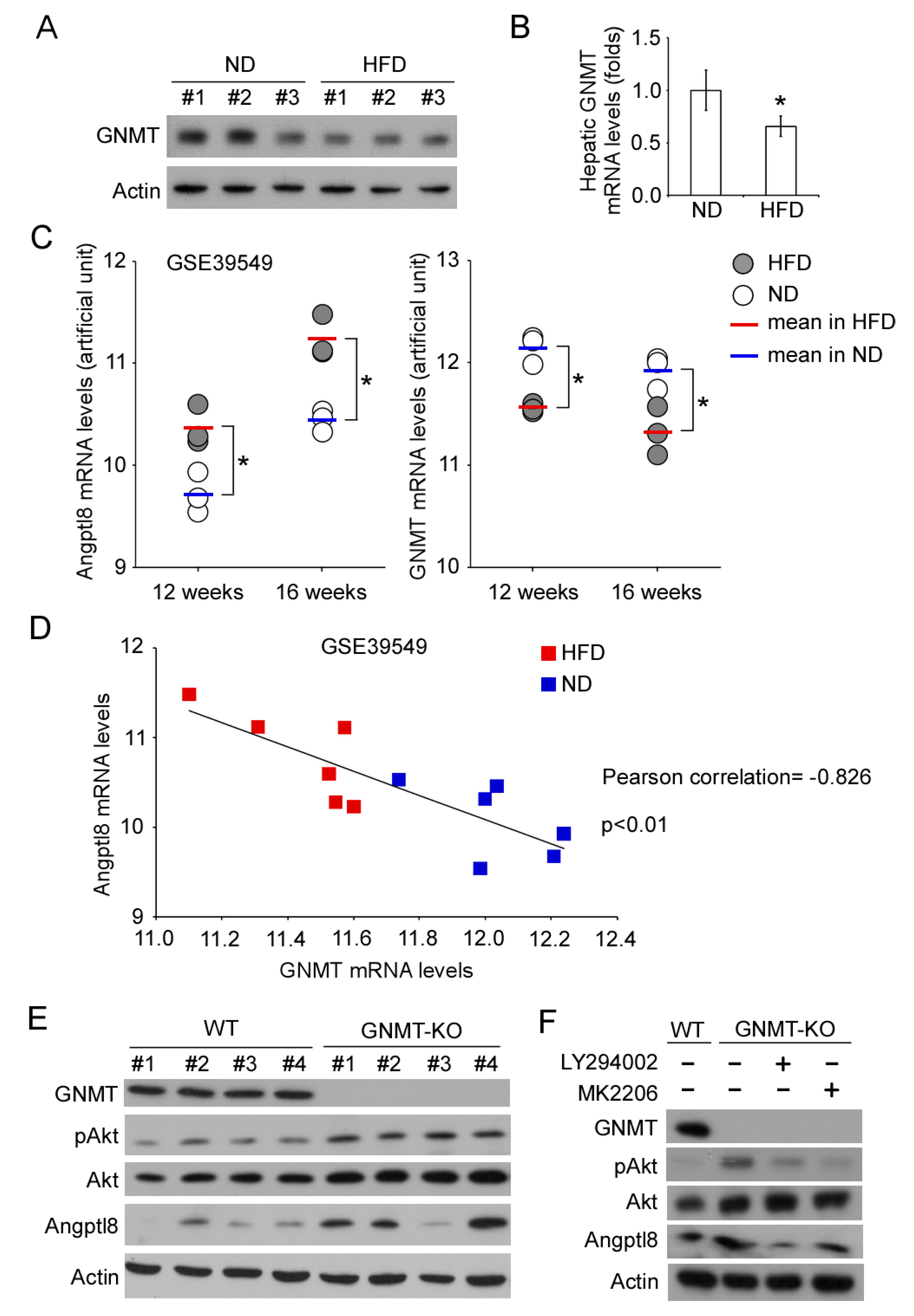
2.4. GNMT/PI3K/Akt Signaling Cascade Regulates Angptl8 Expression
3. Discussion
4. Materials and Methods
4.1. Animal Study
4.2. Biochemical Analysis and ELISA
4.3. Primary Hepatocyte Culture
4.4. Plasmids and Transfection
4.5. Western Blot
4.6. Quantitative Real-Time PCR
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BMI | Body mass index |
| FER | Food efficiency ratio |
| GNMT | Glycine N-methyltransferase |
| HFD | High fat diet |
| HSI | Hepatosomatic index |
| ND | Normal diet |
| SAM | s-adenosylmethionine |
| SAH | s-adenosylhomocysteine |
| TG | Triglyceride |
References
- Patel, T.P.; Rawal, K.; Bagchi, A.K.; Akolkar, G.; Bernardes, N.; Dias, D.D.S.; Gupta, S.; Singal, P.K. Insulin resistance: An additional risk factor in the pathogenesis of cardiovascular disease in type 2 diabetes. Heart Fail. Rev. 2016, 21, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Hojlund, K. Metabolism and insulin signaling in common metabolic disorders and inherited insulin resistance. Dan. Med. J. 2014, 61, B4890. [Google Scholar] [PubMed]
- Armani, A.; Berry, A.; Cirulli, F.; Caprio, M. Molecular mechanisms underlying metabolic syndrome: The expanding role of the adipocyte. FASEB J. 2017, 31, 4240–4255. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, H.M.; McDonald, M.R.; Sullivan, J.A.; Tsao, R.; Meckling, K.A. Proteomic Profiles of Adipose and Liver Tissues from an Animal Model of Metabolic Syndrome Fed Purple Vegetables. Nutrients 2018, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Vila, I.K.; Park, M.K.; Setijono, S.R.; Yao, Y.; Kim, H.; Badin, P.M.; Choi, S.; Narkar, V.; Choi, S.W.; Chung, J.; et al. A muscle-specific UBE2O/AMPKalpha2 axis promotes insulin resistance and metabolic syndrome in obesity. JCI Insight 2019, 4, 128269. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M. The Impact of Organokines on Insulin Resistance, Inflammation, and Atherosclerosis. Endocrinol Metab. (Seoul) 2016, 31, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Siddiqui, N.; Katsumura, S.; Rouya, C.; Larsson, O.; Nagashima, T.; Hekmatnejad, B.; Takahashi, A.; Kiyonari, H.; Zang, M.; et al. Hepatic posttranscriptional network comprised of CCR4-NOT deadenylase and FGF21 maintains systemic metabolic homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 7973–7981. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhu, Q.; Wang, W.; Qi, J.; He, Y.; Wang, Y.; Lu, Y.; Wu, H.; Ding, Y.; Sun, Y. Elevated chemerin induces insulin resistance in human granulosa-lutein cells from polycystic ovary syndrome patients. FASEB J. 2019. [Google Scholar] [CrossRef]
- Delitala, A.P.; Scuteri, A.; Fiorillo, E.; Lakatta, E.G.; Schlessinger, D.; Cucca, F. Role of Adipokines in the Association between Thyroid Hormone and Components of the Metabolic Syndrome. J. Clin. Med. 2019, 8, 764. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Mondal, S.A.; Kumar, M.; Dutta, D. Proinflammatory and antiinflammatory attributes of fetuin-a: A novel hepatokine modulating cardiovascular and glycemic outcomes in metabolic syndrome. Endocr. Pract. 2014, 20, 1345–1351. [Google Scholar] [CrossRef]
- Siddiqa, A.; Ahmad, J.; Ali, A.; Paracha, R.Z.; Bibi, Z.; Aslam, B. Structural characterization of ANGPTL8 (betatrophin) with its interacting partner lipoprotein lipase. Comput. Biol. Chem. 2016, 61, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R. Lipasin, a novel nutritionally-regulated liver-enriched factor that regulates serum triglyceride levels. Biochem. Biophys. Res. Commun. 2012, 424, 786–792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Abou-Samra, A.B. Emerging roles of Lipasin as a critical lipid regulator. Biochem. Biophys. Res. Commun. 2013, 432, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Quagliarini, F.; Gusarova, V.; Gromada, J.; Valenzuela, D.M.; Cohen, J.C.; Hobbs, H.H. Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis. Proc. Natl. Acad. Sci. USA 2013, 110, 16109–16114. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, V.; Banfi, S.; Alexa-Braun, C.A.; Shihanian, L.M.; Mintah, I.J.; Lee, J.S.; Xin, Y.; Su, Q.; Kamat, V.; Cohen, J.C.; et al. ANGPTL8 Blockade With a Monoclonal Antibody Promotes Triglyceride Clearance, Energy Expenditure, and Weight Loss in Mice. Endocrinology 2017, 158, 1252–1259. [Google Scholar] [CrossRef]
- Lee, Y.H.; Lee, S.G.; Lee, C.J.; Kim, S.H.; Song, Y.M.; Yoon, M.R.; Jeon, B.H.; Lee, J.H.; Lee, B.W.; Kang, E.S.; et al. Association between betatrophin/ANGPTL8 and non-alcoholic fatty liver disease: Animal and human studies. Sci. Rep. 2016, 6, 24013. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Panga, J.R.; Satyanarayana, M.V. Circulating Angiopoietin-like 8 protein (ANGPTL8/Betatrophin) in patients with polycystic ovary syndrome: A systematic review and multi effect size meta-analysis. Gynecol. Endocrinol. 2019, 35, 190–197. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Cherian, P.; Qaddoumi, M.G.; AlKhairi, I.; Sriraman, D.; Alanbaei, M.; Abubaker, J. Increased plasma and adipose tissue levels of ANGPTL8/Betatrophin and ANGPTL4 in people with hypertension. Lipids Health Dis. 2018, 17, 35. [Google Scholar] [CrossRef]
- Abu-Farha, M.; Al-Khairi, I.; Cherian, P.; Chandy, B.; Sriraman, D.; Alhubail, A.; Al-Refaei, F.; AlTerki, A.; Abubaker, J. Increased ANGPTL3, 4 and ANGPTL8/betatrophin expression levels in obesity and T2D. Lipids Health Dis. 2016, 15, 181. [Google Scholar] [CrossRef]
- Hu, H.; Yuan, G.; Wang, X.; Sun, J.; Gao, Z.; Zhou, T.; Yin, W.; Cai, R.; Ye, X.; Wang, Z. Effects of a diet with or without physical activity on angiopoietin-like protein 8 concentrations in overweight/obese patients with newly diagnosed type 2 diabetes: A randomized controlled trial. Endocr. J. 2019, 66, 89–105. [Google Scholar] [CrossRef]
- Maurer, L.; Brachs, S.; Decker, A.M.; Brachs, M.; Leupelt, V.; Jumpertz von Schwartzenberg, R.; Ernert, A.; Bobbert, T.; Krude, H.; Spranger, J.; et al. Weight Loss Partially Restores Glucose-Driven Betatrophin Response in Humans. J. Clin. Endocrinol. Metab. 2016, 101, 4014–4020. [Google Scholar] [CrossRef] [PubMed]
- DiStefano, J.K. Angiopoietin-like 8 (ANGPTL8) expression is regulated by miR-143-3p in human hepatocytes. Gene 2019, 681, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Mysore, R.; Ortega, F.J.; Latorre, J.; Ahonen, M.; Savolainen-Peltonen, H.; Fischer-Posovszky, P.; Wabitsch, M.; Olkkonen, V.M.; Fernandez-Real, J.M.; Haridas, P.A.N. MicroRNA-221-3p Regulates Angiopoietin-Like 8 (ANGPTL8) Expression in Adipocytes. J. Clin. Endocrinol. Metab. 2017, 102, 4001–4012. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Chen, X.; Zhang, Z.; Zhang, J.; Yang, Y.; Liu, Z.; Xie, J.; Shao, S.; Zhou, X.; Hu, S.; et al. Insulin upregulates betatrophin expression via PI3K/Akt pathway. Sci. Rep. 2017, 7, 5594. [Google Scholar] [CrossRef] [PubMed]
- Dang, F.; Wu, R.; Wang, P.; Wu, Y.; Azam, M.S.; Xu, Q.; Chen, Y.; Liu, Y. Fasting and Feeding Signals Control the Oscillatory Expression of Angptl8 to Modulate Lipid Metabolism. Sci. Rep. 2016, 6, 36926. [Google Scholar] [CrossRef] [PubMed]
- Pacana, T.; Cazanave, S.; Verdianelli, A.; Patel, V.; Min, H.K.; Mirshahi, F.; Quinlivan, E.; Sanyal, A.J. Dysregulated Hepatic Methionine Metabolism Drives Homocysteine Elevation in Diet-Induced Nonalcoholic Fatty Liver Disease. PLoS ONE 2015, 10, e0136822. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Chantar, M.L.; Vazquez-Chantada, M.; Ariz, U.; Martinez, N.; Varela, M.; Luka, Z.; Capdevila, A.; Rodriguez, J.; Aransay, A.M.; Matthiesen, R.; et al. Loss of the glycine N-methyltransferase gene leads to steatosis and hepatocellular carcinoma in mice. Hepatology 2008, 47, 1191–1199. [Google Scholar] [CrossRef]
- Liao, Y.J.; Liu, S.P.; Lee, C.M.; Yen, C.H.; Chuang, P.C.; Chen, C.Y.; Tsai, T.F.; Huang, S.F.; Lee, Y.H.; Chen, Y.M. Characterization of a glycine N-methyltransferase gene knockout mouse model for hepatocellular carcinoma: Implications of the gender disparity in liver cancer susceptibility. Int. J. Cancer 2009, 124, 816–826. [Google Scholar] [CrossRef]
- Lu, S.C.; Mato, J.M. S-adenosylmethionine in liver health, injury, and cancer. Physiol. Rev. 2012, 92, 1515–1542. [Google Scholar] [CrossRef]
- Li, C.H.; Yen, C.H.; Chen, Y.F.; Lee, K.J.; Fang, C.C.; Zhang, X.; Lai, C.C.; Huang, S.F.; Lin, H.K.; Arthur Chen, Y.M. Characterization of the GNMT-HectH9-PREX2 tripartite relationship in the pathogenesis of hepatocellular carcinoma. Int. J. Cancer 2017, 140, 2284–2297. [Google Scholar] [CrossRef]
- Fine, B.; Hodakoski, C.; Koujak, S.; Su, T.; Saal, L.H.; Maurer, M.; Hopkins, B.; Keniry, M.; Sulis, M.L.; Mense, S.; et al. Activation of the PI3K pathway in cancer through inhibition of PTEN by exchange factor P-REX2a. Science 2009, 325, 1261–1265. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.Y.; Shin, S.K.; Cho, Y.Y.; Jung, U.J.; Kim, E.; Park, T.; Park, J.H.; Yun, J.W.; McGregor, R.A.; Park, Y.B.; et al. Time-course microarrays reveal early activation of the immune transcriptome and adipokine dysregulation leads to fibrosis in visceral adipose depots during diet-induced obesity. BMC Genomics 2012, 13, 450. [Google Scholar] [CrossRef] [PubMed]
- Do, G.M.; Oh, H.Y.; Kwon, E.Y.; Cho, Y.Y.; Shin, S.K.; Park, H.J.; Jeon, S.M.; Kim, E.; Hur, C.G.; Park, T.S.; et al. Long-term adaptation of global transcription and metabolism in the liver of high-fat diet-fed C57BL/6J mice. Mol. Nutr. Food Res. 2011, 55, S173–S185. [Google Scholar] [CrossRef] [PubMed]
- Abu-Farha, M.; Abubaker, J.; Tuomilehto, J. ANGPTL8 (betatrophin) role in diabetes and metabolic diseases. Diabetes Metab. Res. Rev. 2017, 33, e2919. [Google Scholar] [CrossRef] [PubMed]
- Vatner, D.F.; Goedeke, L.; Camporez, J.G.; Lyu, K.; Nasiri, A.R.; Zhang, D.; Bhanot, S.; Murray, S.F.; Still, C.D.; Gerhard, G.S.; et al. Angptl8 antisense oligonucleotide improves adipose lipid metabolism and prevents diet-induced NAFLD and hepatic insulin resistance in rodents. Diabetologia 2018, 61, 1435–1446. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Feng, M.; Zhang, S.; Dong, Z.; Wang, Y.; Zhang, W.; Liu, C. Angptl8 mediates food-driven resetting of hepatic circadian clock in mice. Nat. Commun. 2019, 10, 3518. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Nakata, M.; Shinozaki, A.; Yang, Y.; Zhang, B.; Yada, T. Betatrophin expression is promoted in obese hyperinsulinemic type 2 but not type 1 diabetic mice. Endocr. J. 2016, 63, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Stefanyk, L.E.; Dyck, D.J. The interaction between adipokines, diet and exercise on muscle insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Wu, X.; Song, Y.; Luo, R.; Yin, H.; Zhan, S.; Li, S.; Wang, K.; Zhang, Y.; Yang, C. Angiopoietin-like protein 8 expression and association with extracellular matrix metabolism and inflammation during intervertebral disc degeneration. J. Cell. Mol. Med. 2019, 23, 5737–5750. [Google Scholar] [CrossRef]
- Dijk, W.; Kersten, S. Regulation of lipid metabolism by angiopoietin-like proteins. Curr. Opin. Lipidol. 2016, 27, 249–256. [Google Scholar] [CrossRef]
- Christopoulou, E.; Elisaf, M.; Filippatos, T. Effects of Angiopoietin-Like 3 on Triglyceride Regulation, Glucose Homeostasis, and Diabetes. Dis. Markers 2019, 2019, 8. [Google Scholar] [CrossRef] [PubMed]
- Aryal, B.; Singh, A.K.; Zhang, X.; Varela, L.; Rotllan, N.; Goedeke, L.; Chaube, B.; Camporez, J.P.; Vatner, D.F.; Horvath, T.L.; et al. Absence of ANGPTL4 in adipose tissue improves glucose tolerance and attenuates atherogenesis. JCI Insight 2018, 3, e97918. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Una, M.; Varela-Rey, M.; Cano, A.; Fernandez-Ares, L.; Beraza, N.; Aurrekoetxea, I.; Martinez-Arranz, I.; Garcia-Rodriguez, J.L.; Buque, X.; Mestre, D.; et al. Excess S-adenosylmethionine reroutes phosphatidylethanolamine towards phosphatidylcholine and triglyceride synthesis. Hepatology 2013, 58, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Maria Del Bas, J.; Rodriguez, B.; Puiggros, F.; Marine, S.; Rodriguez, M.A.; Morina, D.; Armengol, L.; Caimari, A.; Arola, L. Hepatic accumulation of S-adenosylmethionine in hamsters with non-alcoholic fatty liver disease associated with metabolic syndrome under selenium and vitamin E deficiency. Clin. Sci. (Lond) 2019, 133, 409–423. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Ramos, D.; Fernandez-Tussy, P.; Lopitz-Otsoa, F.; Gutierrez-de-Juan, V.; Navasa, N.; Barbier-Torres, L.; Zubiete-Franco, I.; Simon, J.; Fernandez, A.F.; Arbelaiz, A.; et al. MiR-873-5p acts as an epigenetic regulator in early stages of liver fibrosis and cirrhosis. Cell Death Dis. 2018, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Botezatu, A.; Bleotu, C.; Nastase, A.; Anton, G.; Bacalbasa, N.; Duda, D.; Dima, S.O.; Popescu, I. Epigenetic Silencing of GNMT Gene in Pancreatic Adenocarcinoma. Cancer Genomics Proteomics 2015, 12, 21–30. [Google Scholar] [PubMed]
- Liao, Y.J.; Chen, K.H.; Huang, S.F.; Chen, T.L.; Wang, C.K.; Chien, C.H.; Tsai, T.F.; Liu, S.P.; Chen, Y.M. Deficiency of glycine N-methyltransferase results in deterioration of cellular defense to stress in mouse liver. Proteomics Clin. Appl. 2010, 4, 394–406. [Google Scholar] [CrossRef]
- Yang, M.H.; Liao, C.C.; Hung, J.H.; Lai, X.T.; Yen, C.H.; Chen, Y.A. Utilizing proteomic approach to identify nuclear translocation related serine kinase phosphorylation site of GNMT as downstream effector for benzo[a]pyrene. J. Food Drug Anal. 2019, 27, 603–609. [Google Scholar] [CrossRef]
- Carrasco, M.; Rabaneda, L.G.; Murillo-Carretero, M.; Ortega-Martinez, S.; Martinez-Chantar, M.L.; Woodhoo, A.; Luka, Z.; Wagner, C.; Lu, S.C.; Mato, J.M.; et al. Glycine N-methyltransferase expression in the hippocampus and its role in neurogenesis and cognitive performance. Hippocampus 2014, 24, 840–852. [Google Scholar] [CrossRef]
- Liu, S.P.; Li, Y.S.; Chen, Y.J.; Chiang, E.P.; Li, A.F.; Lee, Y.H.; Tsai, T.F.; Hsiao, M.; Huang, S.F.; Chen, Y.M. Glycine N-methyltransferase-/- mice develop chronic hepatitis and glycogen storage disease in the liver. Hepatology 2007, 46, 1413–1425. [Google Scholar] [CrossRef]
- Wang, S.; Chen, L.; Wang, Q.; He, Z.; Chen, S.; Zhang, H.; Li, H.; Guo, P.; Li, Q.; Zhang, R.; et al. Strain differences between CD-1 and C57BL/6 mice in expression of metabolic enzymes and DNA methylation modifications of the primary hepatocytes. Toxicology 2019, 412, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Lin, Y.S.; Wang, C.Y.; Tsai, C.C.; Tseng, H.C.; Chen, C.L.; Lu, P.J.; Chen, P.S.; Qian, L.; Hong, J.S.; et al. Glycogen synthase kinase-3 negatively regulates anti-inflammatory interleukin-10 for lipopolysaccharide-induced iNOS/NO biosynthesis and RANTES production in microglial cells. Immunology 2009, 128, e275–e286. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.Y.; Lin, C.H.; Jan, Y.H.; Su, C.Y.; Yao, Y.C.; Cheng, H.C.; Hsu, T.I.; Wang, P.S.; Su, W.P.; Yang, C.J.; et al. Huntingtin-Interacting Protein-1 Is an Early-Stage Prognostic Biomarker of Lung Adenocarcinoma and Suppresses Metastasis via Akt-mediated Epithelial-Mesenchymal Transition. Am. J. Respir. Crit. Care Med. 2016, 193, 869–880. [Google Scholar] [CrossRef] [PubMed]
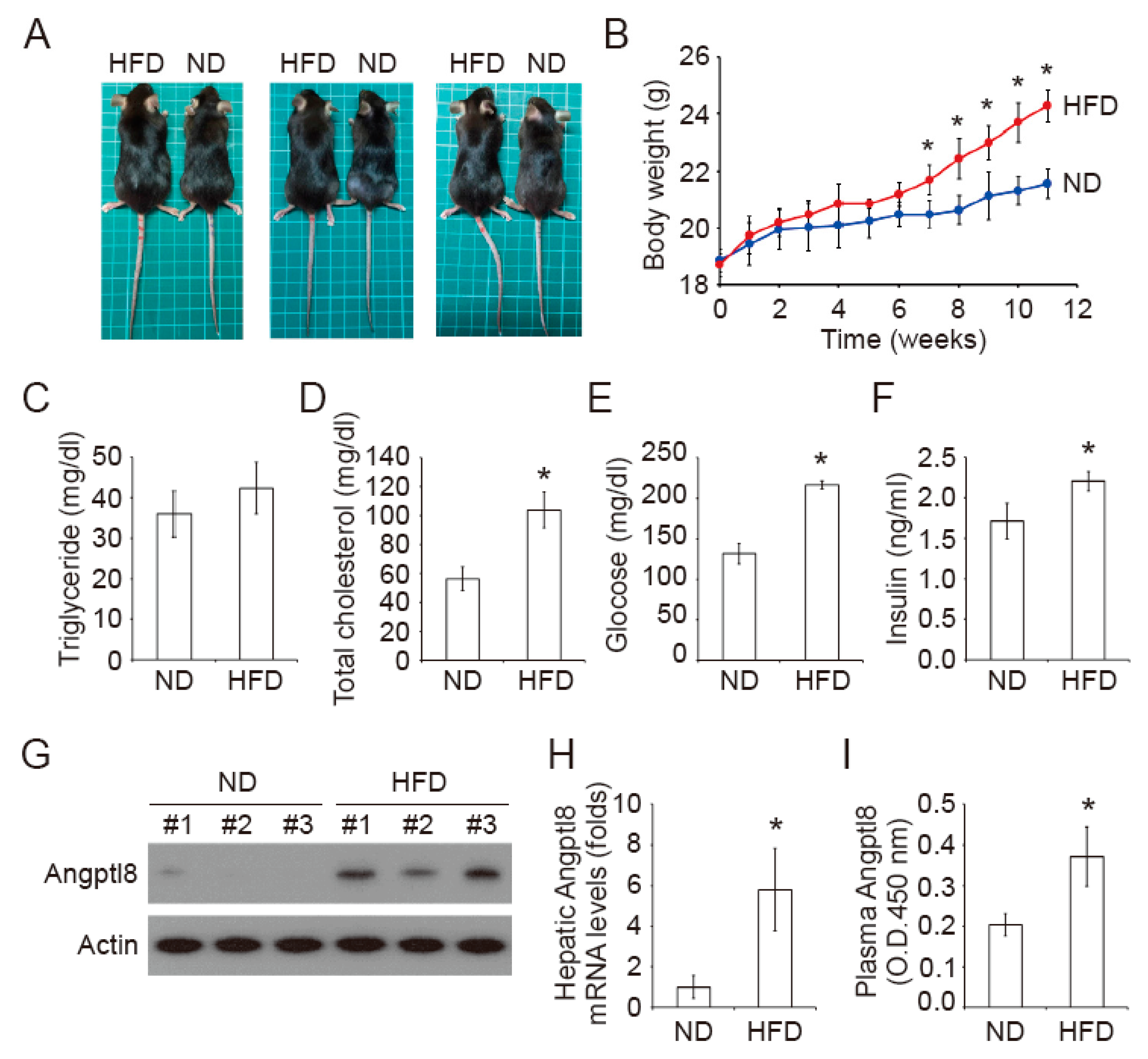

| Groups | ND | HFD |
|---|---|---|
| Food consumption (g/week) | 19.74 ± 2.17 | 17.17 ± 1.92 |
| Weight gain (g) | 2.71 ± 0.35 | 5.18 ± 0.79 * |
| FER (%) | 8.01 ± 0.87 | 17.60 ± 1.35 * |
| BMI (g.cm−2) | 0.30 ± 0.01 | 0.32 ± 0.02 * |
| Liver weight (g) | 1.02 ± 0.10 | 1.93 ± 0.29 * |
| HSI (%) | 4.74 ± 0.45 | 7.87 ± 0.64 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.-W.; Chen, C.-J.; Yen, C.-H.; Chen, Y.-M.A.; Liu, Y.-P. Loss of Glycine N-Methyltransferase Associates with Angiopoietin-Like Protein 8 Expression in High Fat-Diet-Fed Mice. Int. J. Mol. Sci. 2019, 20, 4223. https://doi.org/10.3390/ijms20174223
Huang J-W, Chen C-J, Yen C-H, Chen Y-MA, Liu Y-P. Loss of Glycine N-Methyltransferase Associates with Angiopoietin-Like Protein 8 Expression in High Fat-Diet-Fed Mice. International Journal of Molecular Sciences. 2019; 20(17):4223. https://doi.org/10.3390/ijms20174223
Chicago/Turabian StyleHuang, Jian-Wei, Chao-Ju Chen, Chia-Hung Yen, Yi-Ming Arthur Chen, and Yu-Peng Liu. 2019. "Loss of Glycine N-Methyltransferase Associates with Angiopoietin-Like Protein 8 Expression in High Fat-Diet-Fed Mice" International Journal of Molecular Sciences 20, no. 17: 4223. https://doi.org/10.3390/ijms20174223
APA StyleHuang, J.-W., Chen, C.-J., Yen, C.-H., Chen, Y.-M. A., & Liu, Y.-P. (2019). Loss of Glycine N-Methyltransferase Associates with Angiopoietin-Like Protein 8 Expression in High Fat-Diet-Fed Mice. International Journal of Molecular Sciences, 20(17), 4223. https://doi.org/10.3390/ijms20174223







