Dissecting the Proton Transport Pathway in Oral Squamous Cell Carcinoma: State of the Art and Theranostics Implications
Abstract
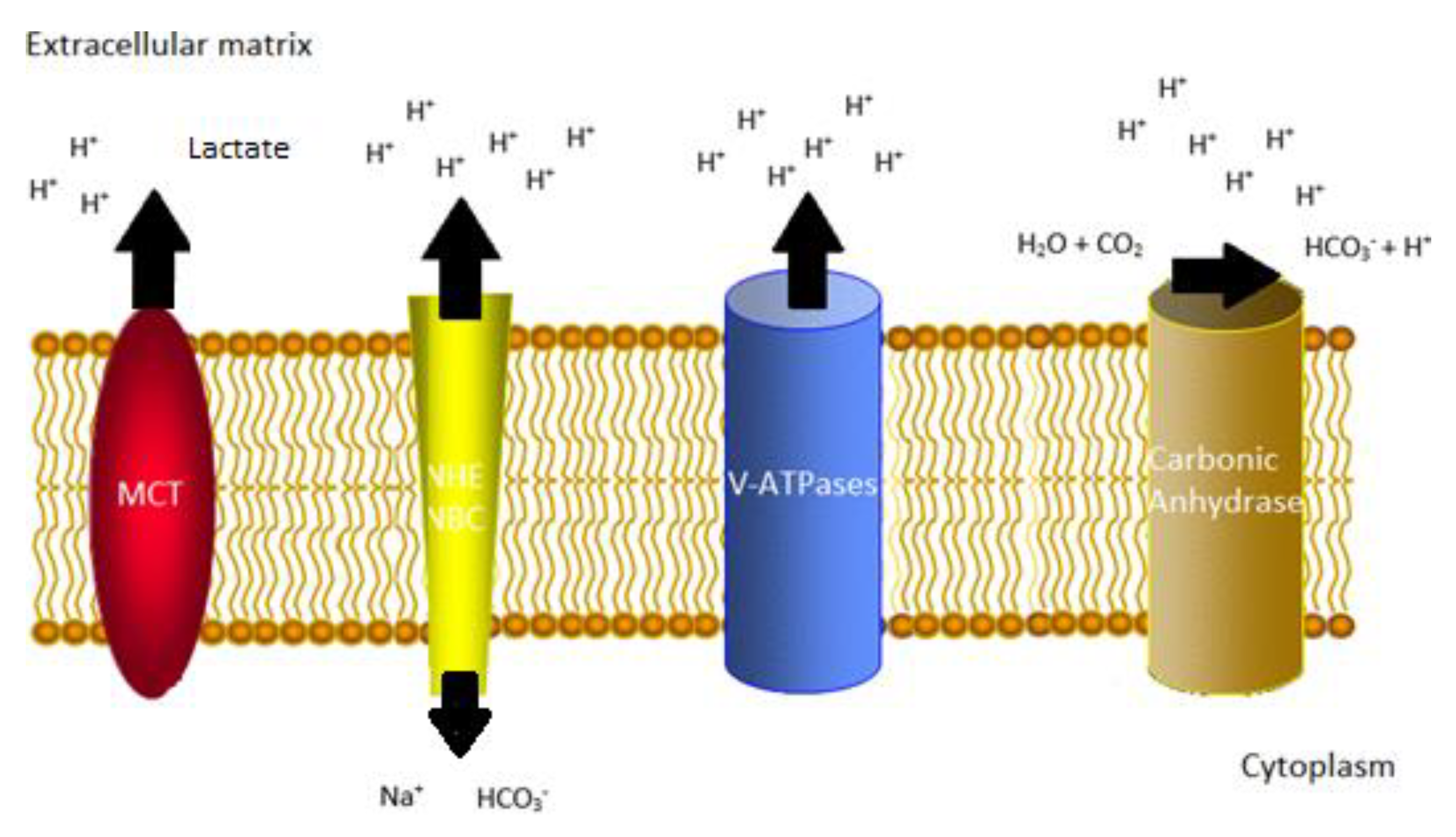
1. Introduction
2. Material and Methods
3. Results
3.1. Carbonic Anhydrases
3.1.1. Diagnostic and Prognostic Implications of Carbonic Anhydrases in Oral Squamous Cell Carcinoma and Oral Potential Malignant Disorders
3.1.2. Carbonic Anhydrases as a Therapeutic Target in Oral Squamous Cell Carcinoma
3.2. Monocarboxylate Transporters
3.2.1. Diagnostic and Prognostic Implications of Monocarboxylate Transporters in Oral Squamous Cell Carcinoma
3.2.2. Monocarboxylate Transporters as a Therapeutic Target in Oral Squamous Cell Carcinoma
3.3. Na+/H+ Exchangers and Sodium Bicarbonate Cotransporters
3.4. Vacuolar ATPases
3.4.1. Diagnostic and Prognostic Implications of Vacuolar ATPases in Oral Squamous Cell Carcinoma
3.4.2. Vacuolar ATPases as a Therapeutic Target in Oral Squamous Cell Carcinoma
4. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CA | carbonic anhydrase |
| CI | confidence interval |
| DFS | disease-free survival |
| ECM | extracellular matrix |
| FDA | Food and Drug Administration |
| HIF | hypoxia-inducible factor |
| HNC | head and neck cancer |
| HR | hazard ratio |
| IHC | immunohistochemistry |
| MCT | monocarboxylate transporter |
| MDR | multidrug resistance |
| MT | malignant transformation |
| MTOR | mammalian target of rapamycin |
| NBC | sodium bicarbonate cotransporters |
| NHE | Na+/H+ exchanger |
| OPMD | oral potentially malignant disorders |
| OSCC | oral squamous cell carcinoma |
| PPI | proton pump inhibitors |
| PTM | post-translation modification |
| Pgp | P-glycoprotein |
| TMA | tissue microarray |
| V-ATPase | vacuolar ATPase |
References
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef]
- Huber, V.; de Milito, A.; Harguindey, S.; Reshkin, S.J.; Wahl, M.L.; Rauch, C.; Chiesi, A.; Pouysségur, J.; Gatenby, R.A.; Rivoltini, L.; et al. Proton dynamics in cancer. J. Transl. Med. 2010, 15, 57. [Google Scholar] [CrossRef]
- Estrella, V.; Chen, T.; Lloyd, M.; Wojtkowiak, J.; Cornnell, H.H.; Ibrahim-Hashim, A.; Bailey, K.; Balagurunathan, Y.; Rothberg, J.M.; Sloane, B.F.; et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 2013, 73, 1524–1535. [Google Scholar] [CrossRef]
- Spugnini, E.P.; Sonveaux, P.; Stock, C.; Perez-Sayans, M.; De Milito, A.; Avnet, S.; Garcìa, A.G.; Harguindey, S.; Fais, S. Proton channels and exchangers in cancer. Biochim. Biophys. Acta. 2015, 1848, 2715–2726. [Google Scholar] [CrossRef] [PubMed]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug. Discov. 2011, 16, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Izumi, H.; Torigoe, T.; Ishiguchi, H.; Uramoto, H.; Yoshida, Y.; Tanabe, M.; Ise, T.; Murakami, T.; Yoshida, T.; Nomoto, M.; et al. Cellular pH regulators: Potentially promising molecular targets for cancer chemotherapy. Cancer Treat. Rev. 2003, 29, 541–549. [Google Scholar] [CrossRef]
- Maeda, H.; Khatami, M. Analyses of repeated failures in cancer therapy for solid tumors: Poor tumor-selective drug delivery, low therapeutic efficacy and unsustainable costs. Clin Transl Med. 2018, 7, 11. [Google Scholar] [CrossRef]
- Zheng, H.C. The molecular mechanisms of chemoresistance in cancers. Oncotarget 2017, 8, 59950–59964. [Google Scholar] [CrossRef]
- Evans, W.E.; Relling, M.V. Pharmacogenomics: Translating functional genomics into rational therapeutics. Science 1999, 286, 487–491. [Google Scholar] [CrossRef]
- Furuta, T.; Shirai, N.; Sugimoto, M.; Ohashi, K.; Ishizaki, T. Pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2004, 5, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Chi, A.C.; Day, T.A.; Neville, B.W. Oral cavity and oropharyngeal squamous cell carcinoma-an update. CA Cancer J. Clin. 2015, 65, 401–421. [Google Scholar] [CrossRef] [PubMed]
- Scully, C.; Bagan, J. Oral squamous cell carcinoma: Overview of current understanding of aetiopathogenesis and clinical implications. Oral Dis. 2009, 15, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Tilakaratne, W.M.; Jayasooriya, P.R.; Jayasuriya, N.S.; De Silva, R.K. Oral epithelial dysplasia: Causes, quantification, prognosis, and management challenges. Periodontology 2000 2019, 80, 126–147. [Google Scholar] [CrossRef] [PubMed]
- Gleber-Netto, F.O.; Braakhuis, B.J.; Triantafyllou, A.; Takes, R.P.; Kelner, N.; Rodrigo, J.P.; Strojan, P.; Vander Poorten, V.; Rapidis, A.D.; Rinaldo, A.; et al. Molecular events in relapsed oral squamous cell carcinoma: Recurrence vs. secondary primary tumor. Oral Oncol. 2015, 51, 738–744. [Google Scholar] [CrossRef]
- Perez-Sayans, M.; Garcia-Garcia, A.; Scozzafava, A.; Supuran, C.T. Inhibition of V-ATPase and carbonic anhydrases as interference strategy with tumor acidification processes. Curr. Pharm. Des. 2012, 18, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Simões-Sousa, S.; Granja, S.; Pinheiro, C.; Fernandes, D.; Longatto-Filho, A.; Laus, A.C.; Alves, C.D.; Suárez-Peñaranda, J.M.; Pérez-Sayáns, M.; Lopes Carvalho, A.; et al. Prognostic significance of monocarboxylate transporter expression in oral cavity tumors. Cell Cycle 2016, 15, 1865–1873. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug. Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Pilar, G.D.; Barros-Angueira, F.; Gándara-Rey, J.M.; García-García, A. Hypoxia-inducible factors in OSCC. Cancer Lett. 2011, 26, 1–8. [Google Scholar] [CrossRef]
- Pérez-Sayáns, M.; Supuran, C.T.; Pastorekova, S.; Suárez-Peñaranda, J.M.; Pilar, G.D.; Barros-Angueira, F.; Gándara-Rey, J.M.; García-García, A. The role of carbonic anhydrase IX in hypoxia control in OSCC. J. Oral Pathol. Med. 2013, 42, 1–8. [Google Scholar] [CrossRef]
- Pastorek, J.; Pastorek, J.; Pastoreková, S.; Callebaut, I.; Mornon, J.P.; Zelník, V.; Opavský, R.; Zat’ovicová, M.; Liao, S.; Portetelle, D.; et al. Cloning and characterization of MN, a human tumor-associated protein with a domain homologous to carbonic anhydrase and putative helix–loop–helix DNA binding segment. Oncogene 1994, 9, 2877–2888. [Google Scholar] [PubMed]
- Peridis, S.; Pilgrim, G.; Athanasopoulos, I.; Parpounas, K. Carbonic anhydrase-9 expression in head and neck cancer: A meta-analysis. Eur. Arch. Otorhinolaryngol. 2011, 268, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Shin, H.J.; Jung, K.Y.; Baek, S.K.; Shin, B.K.; Choi, J.; Kim, B.S.; Shin, S.W.; Kim, Y.H.; Kim, J.S.; et al. Prognostic value of carbonic anhydrase IX and Ki-67 expression in squamous cell carcinoma of the tongue. Jpn. J. Clin. Oncol. 2007, 37, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J.Y.; Park, J.Y.; Cha, I.H.; Kim, J.; Lee, S. Expression of carbonic anhydrase IX is associated with postoperative recurrence and poor prognosis in surgically treated oral squamous cell carcinoma. Hum. Pathol. 2008, 39, 1317–1322. [Google Scholar] [CrossRef] [PubMed]
- Roh, J.L.; Cho, K.J.; Kwon, G.Y.; Ryu, C.H.; Chang, H.W.; Choi, S.H.; Nam, S.Y.; Kim, S.Y. The prognostic value of hypoxia markers in T2-staged oral tongue cancer. Oral Oncol. 2009, 45, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Eckert, A.W.; Lautner, M.H.; Schütze, A.; Bolte, K.; Bache, M.; Kappler, M.; Schubert, J.; Taubert, H.; Bilkenroth, U. Co-expression of Hif1alpha and CAIX is associated with poor prognosis in oral squamous cell carcinoma patients. J. Oral. Pathol. Med. 2010, 39, 313–317. [Google Scholar] [PubMed]
- Kondo, Y.; Yoshikawa, K.; Omura, Y.; Shinohara, A.; Kazaoka, Y.; Sano, J.; Mizuno, Y.; Yokoi, T.; Yamada, S. Clinicopathological significance of carbonic anhydrase 9, glucose transporter-1, Ki-67 and p53 expression in oral squamous cell carcinoma. Oncol Rep. 2011, 25, 1227–1233. [Google Scholar] [PubMed]
- Brockton, N.T.; Klimowicz, A.C.; Bose, P.; Petrillo, S.K.; Konno, M.; Rudmik, L.; Dean, M.; Nakoneshny, S.C.; Matthews, T.W.; Chandarana, S.; et al. High stromal carbonic anhydrase IX expression is associated with nodal metastasis and decreased survival in patients with surgically-treated oral cavity squamous cell carcinoma. Oral Oncol. 2012, 48, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Pilar, G.D.; Supuran, C.T.; Pastorekova, S.; Barros-Angueira, F.; Gándara-Rey, J.M.; García-García, A. Expression of CA-IX is associated with advanced stage tumors and poor survival in oral squamous cell carcinoma patients. J. Oral. Pathol. Med. 2012, 41, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Han, S.; Han, H.Y.; Ryu, M.H.; Kim, K.Y.; Choi, E.J.; Cha, I.H.; Kim, J. Risk prediction for malignant conversion of oral epithelial dysplasia by hypoxia related protein expression. Pathology 2013, 45, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Hwa, J.S.; Kwon, O.J.; Park, J.J.; Woo, S.H.; Kim, J.P.; Ko, G.H.; Seo, J.H.; Kim, R.B. The prognostic value of immunohistochemical markers for oral tongue squamous cell carcinoma. Eur. Arch. Otorhinolaryngol. 2015, 272, 2953–2959. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.S.; Lin, C.W.; Chuang, C.Y.; Su, S.C.; Lin, S.H.; Yang, S.F. Carbonic anhydrase IX overexpression regulates the migration and progression in oral squamous cell carcinoma. Tumour Biol. 2015, 36, 9517–9524. [Google Scholar] [CrossRef] [PubMed]
- Peterle, G.T.; Maia, L.L.; Trivilin, L.O.; de Oliveira, M.M.; Dos Santos, J.G.; Mendes, S.O.; Stur, E.; Agostini, L.P.; Rocha, L.A.; Moysés, R.A.; et al. PAI-1, CAIX, and VEGFA expressions as prognosis markers in oral squamous cell carcinoma. J. Oral Pathol. Med. 2018, 47, 566–574. [Google Scholar] [CrossRef] [PubMed]
- Klimowicz, A.C.; Bose, P.; Petrillo, S.K.; Magliocco, A.M.; Dort, J.C.; Brockton, N.T. The prognostic impact of a combined carbonic anhydrase IX and Ki67 signature in oral squamous cell carcinoma. Br. J. Cancer. 2013, 109, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Brockton, N.T.; Lohavanichbutr, P.; Enwere, E.K.; Upton, M.P.; Kornaga, E.N.; Nakoneshny, S.C.; Bose, P.; Chen, C.; Dort, J.C. Impact of tumoral carbonic anhydrase IX and Ki-67 expression on survival in oral squamous cell carcinoma patients. Oncol. Lett. 2017, 14, 5434–5442. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Torres-López, M.; Supuran, C.T.; Gándara-Vila, P.; Gayoso-Diz, P.; Barros-Angueira, F.; Gallas-Torreira, M.; García-García, A. The use of CA-IX as a diagnostic method for oral leukoplakia. Biotech. Histochem. 2015, 90, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Kim, K.Y.; Zheng, Z.; Bazarsad, S.; Kim, J. Nomogram for risk prediction of malignant transformation in oral leukoplakia patients using combined biomarkers. Oral Oncol. 2017, 72, 132–139. [Google Scholar] [CrossRef]
- Yang, J.S.; Chen, M.K.; Yang, S.F.; Chang, Y.C.; Su, S.C.; Chiou, H.L.; Chien, M.H.; Lin, C.W. Increased expression of carbonic anhydrase IX in oral submucous fibrosis and oral squamous cell carcinoma. Clin Chem. Lab Med. 2014, 52, 1367–1377. [Google Scholar] [CrossRef]
- Eckert, A.W.; Horter, S.; Bethmann, D.; Kotrba, J.; Kaune, T.; Rot, S.; Bache, M.; Bilkenroth, U.; Reich, W.; Greither, T.; et al. Investigation of the Prognostic Role of Carbonic Anhydrase 9 (CAIX) of the Cellular mRNA/Protein Level or Soluble CAIX Protein in Patients with Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2019, 16, 20. [Google Scholar] [CrossRef]
- Torres López, M.; Pérez Sayáns, M.; Chamorro Petronacci, C.; Barros Angueira, F.; Gándara Vila, P.; Lorenzo Pouso, A.; García García, A. Determination and diagnostic value of CA9 mRNA in peripheral blood of patients with oral leukoplakia. J. Enzyme Inhib. Med. Chem. 2018, 33, 951–955. [Google Scholar] [CrossRef]
- Chien, M.H.; Yang, J.S.; Chu, Y.H.; Lin, C.H.; Wei, L.H.; Yang, S.F.; Lin, C.W. Impacts of CA9 gene polymorphisms and environmental factors on oral-cancer susceptibility and clinicopathologic characteristics in Taiwan. PLoS ONE 2012, 7, 51051. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Lin, Y.M.; Yeh, K.T.; Chen, M.K.; Chang, J.H.; Chen, C.J.; Chou, M.Y.; Yang, S.F.; Chien, M.H. Expression of carbonic anhydrases I/II and the correlation to clinical aspects of oral squamous cell carcinoma analyzed using tissue microarray. J. Oral Pathol. Med. 2012, 41, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Inci Gul, H.; Yamali, C.; Tugce Yasa, A.; Unluer, E.; Sakagami, H.; Tanc, M.; Supuran, C.T. Carbonic anhydrase inhibition and cytotoxicity studies of Mannich base derivatives of thymol. J. Enzyme Inhib. Med. Chem. 2016, 31, 1375–1380. [Google Scholar] [CrossRef] [PubMed]
- Yamali, C.; Gul, H.I.; Sakagami, H.; Supuran, C.T. Synthesis and bioactivities of halogen bearing phenolic chalcones and their corresponding bis Mannich bases. J. Enzyme Inhib. Med. Chem. 2016, 31, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Ozmen Ozgun, D.; Gul, H.I.; Yamali, C.; Sakagami, H.; Gulcin, I.; Sukuroglu, M.; Supuran, C.T. Synthesis and bioactivities of pyrazoline benzensulfonamides as carbonic anhydrase and acetylcholinesterase inhibitors with low cytotoxicity. Bioorg. Chem. 2019, 84, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, A.; Tóth, K.; Durrani, F.A.; Cao, S.; Slocum, H.K.; Chintala, S.; Rustum, Y.M. Hypoxia-specific drug tirapazamine does not abrogate hypoxic tumor cells in combination therapy with irinotecan and methylselenocysteine in well-differentiated human head and neck squamous cell carcinoma a253 xenografts. Neoplasia 2008, 10, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Chintala, S.; Tόth, K.; Cao, S.; Durrani, F.A.; Vaughan, M.M.; Jensen, R.L.; Rustum, Y.M. Se-methylselenocysteine sensitizes hypoxic tumor cells to irinotecan by targeting hypoxia-inducible factor 1alpha. Cancer Chemother. Pharmacol. 2010, 66, 899–911. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Zhou, M.; Ou, X.; Peng, B.; Yu, Y.; Kong, F.; Ouyang, Y.; He, Z. Identification of carbonic anhydrase 9 as a contributor to pingyangmycin-induced drug resistance in human tongue cancer cells. FEBS J. 2010, 277, 4506–4518. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Peng, C.; Jia, X.; Gu, Y.; Zhang, Z.; Deng, Y.; Wang, C.; Li, N.; Yin, J.; Liu, X.; et al. ZEB1 transcriptionally regulated carbonic anhydrase 9 mediates the chemoresistance of tongue cancer via maintaining intracellular pH. Mol. Cancer. 2015, 14, 84. [Google Scholar] [CrossRef]
- Dhup, S.; Dadhich, R.K.; Porporato, P.E.; Sonveaux, P. Multiple biological activities of lactic acid in cancer: Influences on tumor growth, angiogenesis and metastasis. Cur. Pharm. Des. 2012, 18, 1319–1330. [Google Scholar] [CrossRef]
- Le Floch, R.; Chiche, J.; Marchiq, I.; Naiken, T.; Ilc, K.; Murray, C.M.; Critchlow, S.E.; Roux, D.; Simon, M.P.; Pouysségur, J. CD147 subunit of lactate/H+ symporters MCT1 and hypoxia-inducible MCT4 is critical for energetics and growth of glycolytic tumors. Proc. Natl. Acad. Sci. USA 2011, 108, 16663–16668. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Tuluc, M.; Whitaker-Menezes, D.; Ames, J.A.; Anantharaman, A.; Butera, A.; Leiby, B.; Cognetti, D.M.; Sotgia, F.; Lisanti, M.P.; et al. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle 2013, 12, 1371–1384. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, Y.N.; Zhang, W.; Zhang, X.M.; Ding, X.; Li, H.Q.; Geng, M.; Xie, Z.Q.; Wu, H.M. Monocarboxylate transporter 4 facilitates cell proliferation and migration and is associated with poor prognosis in oral squamous cell carcinoma patients. PLoS ONE 2014, 30, e87904. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.H.; Therkildsen, M.H.; Dabelsteen, E. A reverse Warburg metabolism in oral squamous cell carcinoma is not dependent upon myofibroblasts. J. Oral Pathol. Med. 2015, 44, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Busk, M.; Walenta, S.; Mueller-Klieser, W.; Steiniche, T.; Jakobsen, S.; Horsman, M.R.; Overgaard, J. Inhibition of tumor lactate oxidation: Consequences for the tumor microenvironment. Radiother Oncol. 2011, 99, 404–411. [Google Scholar] [CrossRef] [PubMed]
- Tassone, P.; Domingo-Vidal, M.; Whitaker-Menezes, D.; Lin, Z.; Roche, M.; Tuluc, M.; Martinez-Outschoorn, U.; Curry, J. Metformin Effects on Metabolic Coupling and Tumor Growth in Oral Cavity Squamous Cell Carcinoma Coinjection Xenografts. Otolaryngol Head Neck Surg. 2018, 158, 867–877. [Google Scholar] [CrossRef] [PubMed]
- Mehibel, M.; Ortiz-Martinez, F.; Voelxen, N.; Boyers, A.; Chadwick, A.; Telfer, B.A.; Mueller-Klieser, W.; West, C.M.; Critchlow, S.E.; Williams, K.J.; et al. Statin-induced metabolic reprogramming in head and neck cancer: A biomarker for targeting monocarboxylate transporters. Sci. Rep. 2018, 14, 8, 16804. [Google Scholar] [CrossRef] [PubMed]
- Lebo, N.L.; Griffiths, R.; Hall, S.; Dimitroulakos, J.; Johnson-Obaseki, S. Effect of statin use on oncologic outcomes in head and neck squamous cell carcinoma. Head Neck 2018, 40, 1697–1706. [Google Scholar] [CrossRef]
- Schwab, A.; Fabian, A.; Hanley, P.J.; Stock, C. Role of ion channels and transporters in cell migration. Physiol. Rev. 2012, 92, 1865–1913. [Google Scholar] [CrossRef]
- Lee, S.P.; Chao, S.C.; Huang, S.F.; Chen, Y.L.; Tsai, Y.T.; Loh, S.H. Expressional and Functional Characterization of Intracellular pH Regulators and Effects of Ethanol in Human Oral Epidermoid Carcinoma Cells. Cell Physiol. Biochem. 2018, 47, 2056–2068. [Google Scholar] [CrossRef]
- Lv, C.; Yang, X.; Yu, B.; Ma, Q.; Liu, B.; Liu, Y. Blocking the Na+/H+ exchanger 1 with cariporide (HOE642) reduces the hypoxia-induced invasion of human tongue squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2012, 41, 1206–1210. [Google Scholar] [CrossRef] [PubMed]
- Kaminota, T.; Yano, H.; Shiota, K.; Nomura, N.; Yaguchi, H.; Kirino, Y.; Ohara, K.; Tetsumura, I.; Sanada, T.; Ugumori, T.; et al. Elevated Na+/H+ exchanger-1 expression enhances the metastatic collective migration of head and neck squamous cell carcinoma cells. Biochem. Biophys. Res. Commun. 2017, 22, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Nishi, T.; Forgac, M. The vacuolar (H+)-ATPases--nature’s most versatile proton pumps. Nat. Rev. Mol. Cell. Biol. 2002, 3, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Maxson, M.E.; Grinstein, S. The vacuolar-type H⁺-ATPase at a glance - more than a proton pump. J. Cell. Sci. 2014, 127, 4987–4993. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Somoza-Martín, J.M.; Barros-Angueira, F.; Diz, P.G.; Rey, J.M.; García-García, A. Multidrug resistance in oral squamous cell carcinoma: The role of vacuolar ATPases. Cancer Lett. 2010, 28, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Otero-Rey, E.M.; Somoza-Martín, M.; Barros-Angueira, F.; García-García, A. Intracellular pH regulation in oral squamous cell carcinoma is mediated by increased V-ATPase activity via over-expression of the ATP6V1C1 gene. Oral Oncol. 2008, 44, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Sayáns, M.; Reboiras-López, M.D.; Somoza-Martín, J.M.; Barros-Angueira, F.; Diz, P.G.; Rey, J.M.; García-García, A. Measurement of ATP6V1C1 expression in brush cytology samples as a diagnostic and prognostic marker in oral squamous cell carcinoma. Cancer. Biol. Ther. 2010, 9, 1057–1064. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Pérez-Sayáns García, M.; Rodríguez, M.J.; Antúnez-López, J.; Barros-Angueira, F.; Somoza-Martín, M.; Gándara-Rey, J.M.; Aguirre-Urízar, J.M. Immunohistochemical localization of C1 subunit of V-ATPase (ATPase C1) in oral squamous cell cancer and normal oral mucosa. Biotech. Histochem. 2012, 87, 133–139. [Google Scholar] [CrossRef]
- Pérez-Sayáns, M.; Suárez-Peñaranda, J.M.; Aguirre-Urízar, J.M.; Rodríguez-Tojo, M.J.; Barros-Angueira, F.; Gallas-Torreira, M.; García-García, A. The use of tissue microarrays for semiquantitative evaluation of ATPaseC1 expression is ineffective. Biotech. Histochem. 2015, 90, 439–444. [Google Scholar] [CrossRef]
- Pérez-Sayáns, M.; Somoza-Martín, J.M.; Barros-Angueira, F.; Rey, J.M.; García-García, A. V-ATPase inhibitors and implication in cancer treatment. Cancer Treat. Rev. 2009, 35, 707–713. [Google Scholar] [CrossRef]
- Calcinotto, A.; Filipazzi, P.; Grioni, M.; Iero, M.; De Milito, A.; Ricupito, A.; Cova, A.; Canese, R.; Jachetti, E.; Rossetti, M.; et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 2012, 72, 2746–2756. [Google Scholar] [CrossRef] [PubMed]
- Kiyoshima, T.; Yoshida, H.; Wada, H.; Nagata, K.; Fujiwara, H.; Kihara, M.; Hasegawa, K.; Someya, H.; Sakai, H. Chemoresistance to concanamycin A1 in human oral squamous cell carcinoma is attenuated by an HDAC inhibitor partly via suppression of Bcl-2 expression. PLoS ONE 2013, 8, e80998. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, C.; Roberg, K.; Grafström, R.C.; Ollinger, K. Intrinsic differences in cisplatin sensitivity of head and neck cancer cell lines: Correlation to lysosomal pH. Head Neck 2010, 32, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Matossian, M.; Vangelderen, C.; Papagerakis, P.; Zheng, L.; Wolf, G.T.; Papagerakis, S. In silico modeling of the molecular interactions of antacid medication with the endothelium: Novel therapeutic implications in head and neck carcinomas. Int. J. Immunopathol. Pharmacol. 2014, 27, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Papagerakis, S.; Bellile, E.; Peterson, L.A.; Pliakas, M.; Balaskas, K.; Selman, S.; Hanauer, D.; Taylor, J.M.; Duffy, S.; Wolf, G. Proton pump inhibitors and histamine 2 blockers are associated with improved overall survival in patients with head and neck squamous carcinoma. Cancer Prev. Res. (Phila) 2014, 7, 1258–1269. [Google Scholar] [CrossRef] [PubMed]
| Study | OSCC Cases | Positivity n (%) | Negativity n (%) | Quantification |
|---|---|---|---|---|
| Kim et al. (2007) [23] | 60 | 38 (63.3) | 22 (36.7) | CAIX (−) <10%; CAIX (+) >10% |
| Choi et al. (2008) [24] | 117 | 54 (46.2) a; 14 (11.9) b | 49 (41.9) | CAIX (−) <5%; CAIX (1+) 5–20%; CAIX (2+) >20% |
| Roh et al. (2009) [25] | 43 | 7 (16.3) a; 2 (4.6) b; 10 (23.6); 2 (4.6) c | 17 (39.5) | CAIX (−) 0%; CAIX (1+) 1–10%; CAIX (2+) 11–50%; CAIX (3+) 51–80%; CAIX (4+) 81–100% |
| Eckert et al. (2010) [26] | 80 | 21 (25) a; 11 (13.8) b; 2 (2.5) c | 46 (57.5) | CAIX (−) 1–10%; CAIX (1+) 11–50%; CAIX (2+) 51–80%; CAIX (3+) >80% |
| Kondo et al. (2011) [27] | 107 | 105 (98.1) | 2 (1.9) | CAIX (−) <10%; CAIX (+) >10% |
| Brockton et al. (2012) [28] | 61 | 16 (26.2) | 45 (73.8) | CAIX (−) AQUA score within the lower three quartiles; CAIX (+) AQUA score within the upper quartile* |
| Pérez-Sayáns et al. (2012) [29] | 50 | 18 (36.0) a; 23 (46.0) b | 9 (18.0) | CAIX (−) <10%; CAIX (1+) 10–50%; CAIX (2+) >50% |
| Zhang et al. (2013) [30] | 85 | 58 (58.2) | 27 (41.8) | CAIX (−) <5%; CAIX (+) >5% |
| Hwa et al. (2015) [31] | 25 | 5 (20.0) | 20 (80.0) | CAIX (−) <30%; CAIX (+) >30% |
| Yang et al. (2015) [32] | 271 | 113 (41.7) | 158 (58.3) | NR |
| Simões-Sousa et al. (2016) [17] | 124 | 78 (62.9) | 46 (37.1) | CAIX (−) <50%; CAIX (+) >50% |
| Peterle et al. (2018) [33] | 52 | 19 (36.5) a; 7 (13.5) b | 25 (48.1) | CAIX (−) 0%; CAIX (1+) 1–25%; CAIX (2+) >25% |
| Study | OSCC Cases | Positivity n (%) | Negativity n (%) | Quantification |
|---|---|---|---|---|
| Zhu et al. (2014) [53] | 99 | 41 (41.5) | 58 (58.5) | MCT4 (−), <5%; MCT4 (1+) 5–10% a; MCT4 (2+) 10–50%; MCT4 (3+), 50–75%; MCT4 (4+) >75% b |
| Jensen et al. (2015) [54] | 30 | MCT1: 10 (33.3); 20 (66.6). MCT4: 14 (46.7); 2 (6.6%). | MCT1: 0 (0). MCT4: 15 (50.0). | c |
| Simões-Sousa et al. (2016) [17] | 124 | MCT1: 120 (93.8). MCT2: 47(52.8). MCT4: 89 (71.2) | MCT1: 8 (6.3). MCT2: 42 (47.2). MCT4: 36 (28.8) | MCT1 (−) <50%; MCT1 (+) >50%. MCT2 (−) <50%; MCT2 (+) >50%. MCT4 (−) <50%; MCT4 (+) >50% |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenzo-Pouso, A.I.; Pérez-Sayáns, M.; Rodríguez-Zorrilla, S.; Chamorro-Petronacci, C.; García-García, A. Dissecting the Proton Transport Pathway in Oral Squamous Cell Carcinoma: State of the Art and Theranostics Implications. Int. J. Mol. Sci. 2019, 20, 4222. https://doi.org/10.3390/ijms20174222
Lorenzo-Pouso AI, Pérez-Sayáns M, Rodríguez-Zorrilla S, Chamorro-Petronacci C, García-García A. Dissecting the Proton Transport Pathway in Oral Squamous Cell Carcinoma: State of the Art and Theranostics Implications. International Journal of Molecular Sciences. 2019; 20(17):4222. https://doi.org/10.3390/ijms20174222
Chicago/Turabian StyleLorenzo-Pouso, Alejandro I., Mario Pérez-Sayáns, Samuel Rodríguez-Zorrilla, Cintia Chamorro-Petronacci, and Abel García-García. 2019. "Dissecting the Proton Transport Pathway in Oral Squamous Cell Carcinoma: State of the Art and Theranostics Implications" International Journal of Molecular Sciences 20, no. 17: 4222. https://doi.org/10.3390/ijms20174222
APA StyleLorenzo-Pouso, A. I., Pérez-Sayáns, M., Rodríguez-Zorrilla, S., Chamorro-Petronacci, C., & García-García, A. (2019). Dissecting the Proton Transport Pathway in Oral Squamous Cell Carcinoma: State of the Art and Theranostics Implications. International Journal of Molecular Sciences, 20(17), 4222. https://doi.org/10.3390/ijms20174222






