Atractylodis Rhizoma Alba Attenuates Neuroinflammation in BV2 Microglia upon LPS Stimulation by Inducing HO-1 Activity and Inhibiting NF-κB and MAPK
Abstract
1. Introduction
2. Results
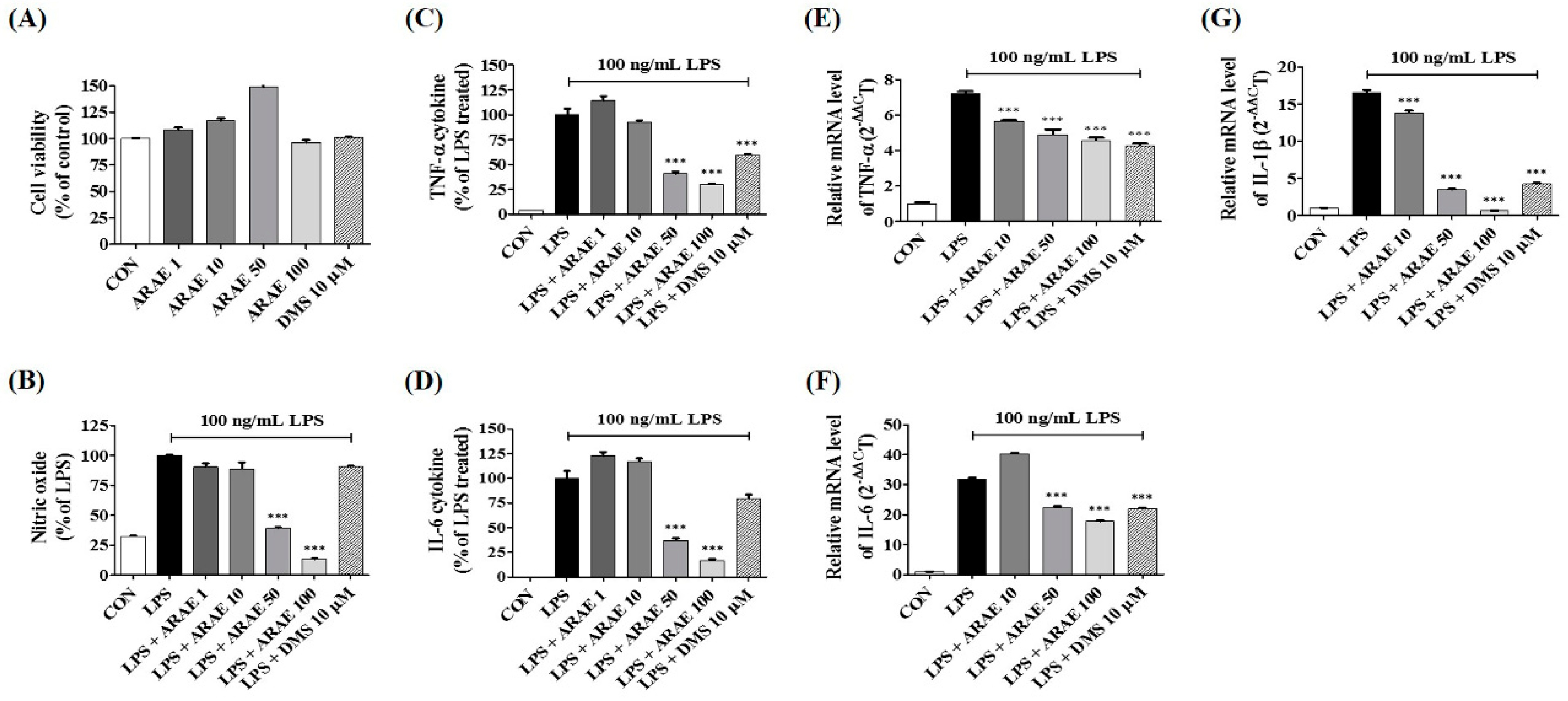
2.1. Effects of ARAE on the Viability of BV2 Microglia
2.2. Effects of ARAE on Secretion of NO and Production of Inflammatory Cytokines
2.3. Effects of ARAE on the Expression of iNOS and COX-2 and Induction of HO-1
2.4. Effects of ARAE on Transcriptional Activity of NF-κB in LPS-Stimulated BV2 Microglia
2.5. Effects of ARAE on the Activation of MAPK Induced by LPS in BV2 Microglia
2.6. Identification of the Components of ARAE Using HPLC-Diode Array UV/VIS Detector (DAD) Analysis
2.7. Validation of the Analytical HPLC Method
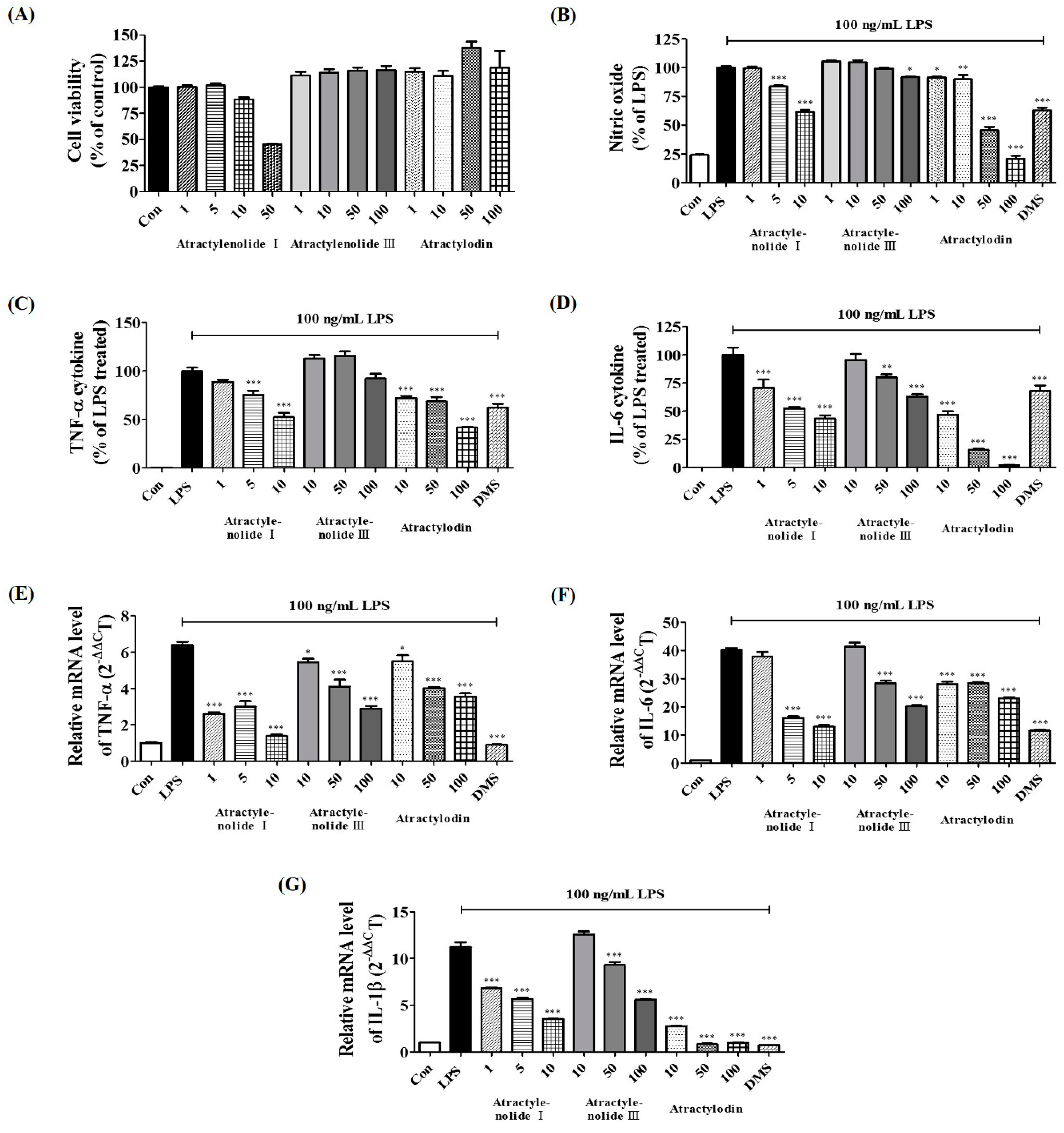
2.8. Verification of Neuroinflammatory Inhibitory Efficacy of Atractylenolide I, Atractylenolide III, and Atractylodin in LPS-Stimulated BV2 Microglia
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Cell Culture and Drug Treatment
4.3. Cell Viability Test
4.4. Determination of NO Production
4.5. Cytokine Determination
4.6. Preparation of Whole Cell, Cytosolic, and Nuclear Extracts
4.7. Western Blot Analyses
4.8. Total RNA Extraction and RT-PCR
4.9. Sample Preparation for HPLC Analysis
4.10. Optimization of Chromatographic Conditions
4.11. Validation of the Method
4.12. Preparation of the Main Components of ARAE
4.13. Statistical Analyses
4.14. Statistical Analysis Software
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gehrmann, J.; Matsumoto, Y.; Kreutzberg, G.W. Microglia: Intrinsic immuneffector cell of the brain. Brain Res. Brain Res. Rev. 1995, 20, 269–287. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.F.; Beach, T.G. Gene expression profiling of amyloid beta peptide-stimulated human postmortem brain microglia. Neurobiol. Aging 2001, 22, 957–966. [Google Scholar] [CrossRef]
- Glezer, I.; Simard, A.R.; Rivest, S. Neuroprotective role of the innate immune system by microglia. Neuroscience 2007, 147, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Kosovrasti, V.Y.; Nechev, L.V.; Amiji, M.M. Peritoneal macrophage-specific TNFalpha gene silencing in LPS-induced acute inflammation model using CD44 targeting hyaluronic acid nanoparticles. Mol. Pharm. 2016, 13, 3404–3416. [Google Scholar] [CrossRef] [PubMed]
- Tianzhu, Z.; Shumin, W. Esculin inhibits the inflammation of LPS-induced acute lung injury in mice via regulation of TLR/NF-kappaB pathways. Inflammation 2015, 38, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Kim, D.C.; Yoon, C.S.; Ko, W.M.; Lee, S.J.; Sohn, J.H.; Jang, J.H.; Ahn, J.S.; Kim, Y.C.; Oh, H. Anti-neuroinflammatory effects of citreohybridonol involving TLR4-MyD88-mediated inhibition of NF-small ka, CyrillicB and MAPK signaling pathways in lipopolysaccharide-stimulated BV2 cells. Neurochem. Int. 2016, 95, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Sohn, K.H.; Jo, M.J.; Cho, W.J.; Lee, J.R.; Cho, I.J.; Kim, S.C.; Kim, Y.W.; Jee, S.Y. Bojesodok-eum, a herbal prescription, ameliorates acute inflammation in association with the inhibition of NF-kappaB-mediated nitric oxide and proinflammatory cytokine production. Evid. Based Complement. Alternat. Med. 2012, 2012, 457370. [Google Scholar] [CrossRef]
- Cortez, M.; Carmo, L.S.; Rogero, M.M.; Borelli, P.; Fock, R.A. A high-fat diet increases IL-1, IL-6 and TNF-alpha production by increasing NF-kappaB and attenuating PPAR-gamma expression in bone marrow mesenchymal stem cells. Inflammation 2013, 36, 379–386. [Google Scholar] [CrossRef]
- Otterbein, L.E.; Bach, F.H.; Alam, J.; Soares, M.; Lu, H.T.; Wysk, M.; Davis, R.J.; Flavell, R.A.; Choi, A.M. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat. Med. 2000, 6, 422–428. [Google Scholar] [CrossRef]
- Suh, G.Y.; Jin, Y.; Yi, A.K.; Wang, X.M.; Choi, A.M. CCAAT/enhancer-binding protein mediates carbon monoxide-induced suppression of cyclooxygenase-2. Am. J. Respir. Cell Mol. Biol. 2006, 35, 220–226. [Google Scholar] [CrossRef]
- Oh, G.S.; Pae, H.O.; Lee, B.S.; Kim, B.N.; Kim, J.M.; Kim, H.R.; Jeon, S.B.; Jeon, W.K.; Chae, H.J.; Chung, H.T. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-kappaB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic. Biol. Med. 2006, 41, 106–119. [Google Scholar] [CrossRef] [PubMed]
- Bahar, E.; Kim, J.Y.; Yoon, H. Quercetin Attenuates Manganese-Induced Neuroinflammation by Alleviating Oxidative Stress through Regulation of Apoptosis, iNOS/NF-κB and HO-1/Nrf2 Pathways. Int. J. Mol. Sci. 2017, 18, 1989. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.S.; Choi, M.J.; Lee, Y.Y.; Moon, B.I.; Park, J.S.; Kim, H.S. Suppression of Lipopolysaccharide-Induced Neuroinflammation by Morin via MAPK, PI3K/Akt, and PKA/HO-1 Signaling Pathway Modulation. J. Agric. Food Chem. 2017, 65, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.G.; Jan, Y.S.; Tsai, P.W.; Norimoto, H.; Michihara, S.; Murayama, C.; Wang, C.C. Anti-inflammatory and antinociceptive constituents of Atractylodes japonica Koidzumi. J. Agric. Food Chem. 2016, 64, 2254–2262. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, J.; Han, W.; Mai, W.; Wang, L.; Li, Q.; Lin, M.; Bai, M.; Zhang, L.; Chen, D. Antioxidant ability and mechanism of rhizoma Atractylodes macrocephala. Molecules 2012, 17, 13457–13472. [Google Scholar] [CrossRef] [PubMed]
- Rosi, S.; Vazdarjanova, A.; Ramirez-Amaya, V.; Worley, P.F.; Barnes, C.A.; Wenk, G.L. Memantine protects against LPS-induced neuroinflammation, restores behaviorallyinduced gene expression and spatial learning in the rat. Neuroscience 2006, 142, 1303–1315. [Google Scholar] [CrossRef] [PubMed]
- Raber, J.; Sorg, O.; Horn, T.F.; Yu, N.; Koob, G.F.; Campbell, I.L.; Bloom, F.E. Inflammatory cytokines: Putative regulators of neuronal and neuro-endocrine function. Brain Res. Brain Res. Rev. 1998, 26, 320–326. [Google Scholar] [CrossRef]
- Rajapakse, N.; Kim, M.M.; Mendis, E.; Kim, S.K. Inhibition of inducible nitric oxide synthase and cyclooxygenase-2 in lipopolysaccharide-stimulated RAW264.7 cells by carboxybutyrylated glucosamine takes place via down-regulation of mitogen-activated protein kinase-mediated nuclear factor-kappaB signaling. Immunology 2008, 123, 348–357. [Google Scholar] [PubMed]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell. Signal. 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Rojo, L.E.; Fernandez, J.A.; Maccioni, A.A.; Jimenez, J.M.; Maccioni, R.B. Neuroinflammation: Implications for the pathogenesis and molecular diagnosis of Alzheimer’s disease. Arch. Med. Res. 2008, 39, 1–16. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Song, M.Y.; Lim, S.K.; Wang, J.H.; Kim, H. The Root of Atractylodes macrocephala Koidzumi Prevents Obesity and Glucose Intolerance and Increases Energy Metabolism in Mice. Int. J. Mol. Sci. 2018, 19, 278. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Jung, H.W.; Park, Y.K. The roots of Atractylodes japonica Koidzumi promote adipogenic differentiation via activation of the insulin signaling pathway in 3T3-L1 cells. BMC Complement. Altern. Med. 2012, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Tripathi, P.; Kashyap, L.; Singh, V. The role of nitric oxide in inflammatory reactions. FEMS Immunol. Med. Microbiol. 2007, 51, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Korbut, R.; Adamek-Guzik, T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003, 54, 469–487. [Google Scholar] [PubMed]
- Southan, G.J.; Szabo, C. Selective pharmacological inhibition of distinct nitric oxide synthase isoforms. Biochem. Pharmacol. 1996, 51, 383–394. [Google Scholar] [CrossRef]
- Ashino, T.; Yamanaka, R.; Yamamoto, M.; Shimokawa, H.; Sekikawa, K.; Iwakura, Y.; Shioda, S.; Numazawa, S.; Yoshida, T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol. Immunol. 2008, 45, 2106–2115. [Google Scholar] [CrossRef]
- Chapman, N.R.; Perkins, N.D. Inhibition of the RelA(p65) NF-kappaB subunit by Egr-1. J. Biol. Chem. 2000, 275, 4719–4725. [Google Scholar] [CrossRef]
- Brown, J.; Wang, H.; Hajishengallis, G.N.; Martin, M. TLR-signaling networks: An integration of adaptor molecules, kinases, and cross-talk. J. Dent. Res. 2011, 90, 417–427. [Google Scholar] [CrossRef]
- Kim, B.W.; Koppula, S.; Hong, S.S.; Jeon, S.B.; Kwon, J.H.; Hwang, B.Y.; Park, E.J.; Choi, D.K. Regulation of microglia activity by glaucocalyxin-A: Attenuation of lipopolysaccharide-stimulated neuroinflammation through NF-κB and p38 MAPK signaling pathways. PLoS One 2013, 8, e55792. [Google Scholar] [CrossRef]
- Kim, A.R.; Lee, M.S.; Choi, J.W.; Utsuki, T.; Kim, J.I.; Jang, B.C.; Kim, H.R. Phlorofucofuroeckol A suppresses expression of inducible nitric oxide synthase, cyclooxygenase-2, and proinflammatory cytokines via inhibition of nuclear factor-κB, c-Jun NH2-terminal kinases, and Akt in microglial cells. Inflammation 2013, 36, 259–271. [Google Scholar] [CrossRef] [PubMed]
- More, S.; Choi, D.K. Neuroprotective Role of Atractylenolide-I in an In Vitro and In Vivo Model of Parkinson’s Disease. Nutrients 2017, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Ji, G.Q.; Chen, R.Q.; Wang, L. Anti-inflammatory activity of atractylenolide III through inhibition of nuclear factor-κB and mitogen-activated protein kinase pathways in mouse macrophages. Immunopharmacol. Immunotoxicol. 2016, 38, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.; Fan, K.; Wang, K.; Bian, C. Atractylodin attenuates lipopolysaccharide-induced acute lung injury by inhibiting NLRP3 inflammasome and TLR4 pathways. J. Pharmacol. Sci. 2018, 136, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.H.; Oh, Y.C.; Cho, W.K.; Yim, N.H.; Ma, J.Y. Anti-Inflammatory Effect of Rhapontici Radix Ethanol Extract via Inhibition of NF-κB and MAPK and Induction of HO-1 in Macrophages. Mediators Inflamm. 2016, 2016, 7216912. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.S.; Xiang, Y.; Cui, Y.L.; Lin, K.M.; Zhang, X.F. Dietary blue pigments derived from genipin, attenuate inflammation by inhibiting LPS-induced iNOS and COX-2 expression via the NF-κB inactivation. PLoS One 2012, 7, e34122. [Google Scholar] [CrossRef] [PubMed]




| Analytes | Regression Equation | R2 | Content (mg/g) |
|---|---|---|---|
| Atractylenolide I (1) | y = 0.0572x − 0.122 | 0.9998 | 16.04287 ± 0.0175 |
| Atractylenolide III (2) | y = 0.7879x − 1.5886 | 0.9998 | 10.39714 ± 0.0712 |
| Atractylodin (3) | y = 0.4807x − 1.1052 | 0.9998 | 17.06388 ± 0.0702 |
| Target Gene | Primer Sequence |
|---|---|
| TNF-α | F: 5′-TTCTGTCTACTGAACTTCGGGGTGATCGGTCC-3′ |
| R: 5′-GTATGAGATAGCAAATCGGCTGACGGTGTGGG-3′ | |
| IL-6 | F: 5′-TCCAGTTGCCTTCTTGGGAC-3′ |
| R: 5′-GTGTAATTAAGCCTCCGACTTG-3′ | |
| IL-1β | F: 5′-ATGGCAACTGTTCCTGAACTCAACT-3′ |
| R: 5′-CAGGACAGGTATAGATTCTTTCCTTT-3′ | |
| iNOS | F: 5′-GGCAGCCTGTGAGACCTTTG-3′ |
| R: 5′-GCATTGGAAGTGAAGCGTTTC-3′ | |
| COX-2 | F: 5′-TGAGTACCGCAAACGCTTCTC-3′ |
| R: 5′-TGGACGAGGTTTTTCCACCAG-3′ | |
| HO-1 | F: 5′-TGAAGGAGGCCACCAAGGAGG-3′ |
| R: 5′-AGAGGTCACCCAGGTAGCGGG-3′ | |
| β-actin | F: 5′-AGAGGGAAATCGTGCGTGAC-3′ |
| R: 5′-CAATAGTGATGACCTGGCCGT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, Y.H.; Li, W.; Go, Y.; Oh, Y.-C. Atractylodis Rhizoma Alba Attenuates Neuroinflammation in BV2 Microglia upon LPS Stimulation by Inducing HO-1 Activity and Inhibiting NF-κB and MAPK. Int. J. Mol. Sci. 2019, 20, 4015. https://doi.org/10.3390/ijms20164015
Jeong YH, Li W, Go Y, Oh Y-C. Atractylodis Rhizoma Alba Attenuates Neuroinflammation in BV2 Microglia upon LPS Stimulation by Inducing HO-1 Activity and Inhibiting NF-κB and MAPK. International Journal of Molecular Sciences. 2019; 20(16):4015. https://doi.org/10.3390/ijms20164015
Chicago/Turabian StyleJeong, Yun Hee, Wei Li, Younghoon Go, and You-Chang Oh. 2019. "Atractylodis Rhizoma Alba Attenuates Neuroinflammation in BV2 Microglia upon LPS Stimulation by Inducing HO-1 Activity and Inhibiting NF-κB and MAPK" International Journal of Molecular Sciences 20, no. 16: 4015. https://doi.org/10.3390/ijms20164015
APA StyleJeong, Y. H., Li, W., Go, Y., & Oh, Y.-C. (2019). Atractylodis Rhizoma Alba Attenuates Neuroinflammation in BV2 Microglia upon LPS Stimulation by Inducing HO-1 Activity and Inhibiting NF-κB and MAPK. International Journal of Molecular Sciences, 20(16), 4015. https://doi.org/10.3390/ijms20164015






