A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves
Abstract
1. Introduction
2. Results and Discussion
2.1. Condition Optimization
2.1.1. Optimization of UPLC Conditions
2.1.2. Optimization of HS-SPME Extraction Method
2.2. Identification of Active Compounds of E. Globulus Leaves
2.2.1. Aqueous Extracts from E. Globulus Leaves
2.2.2. Volatile Compounds of E. Globulus Leaves
2.3. Prediction of Active Components of E. Globulus Leaves
2.3.1. Docking Results
2.3.2. Docking Model
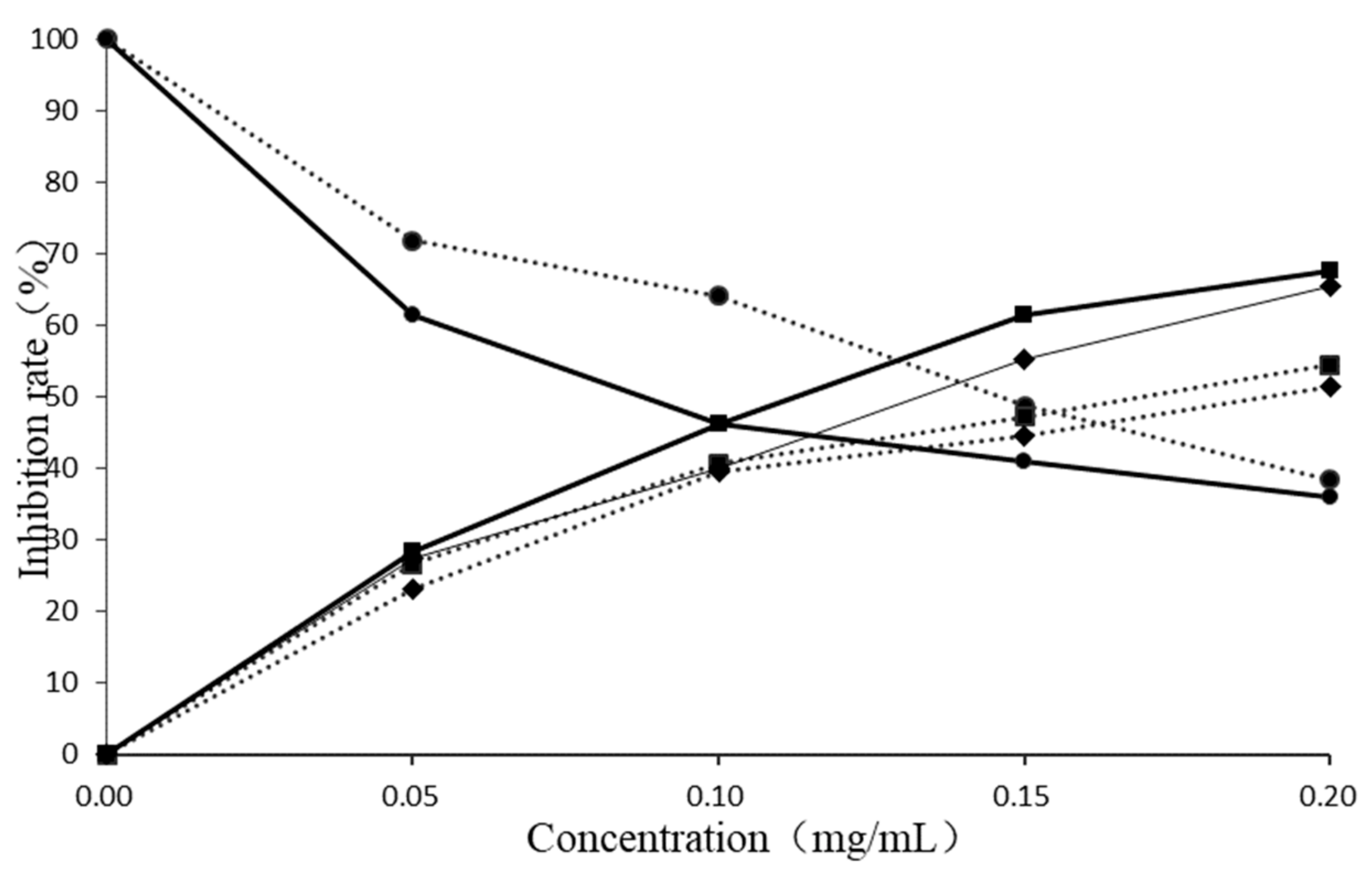
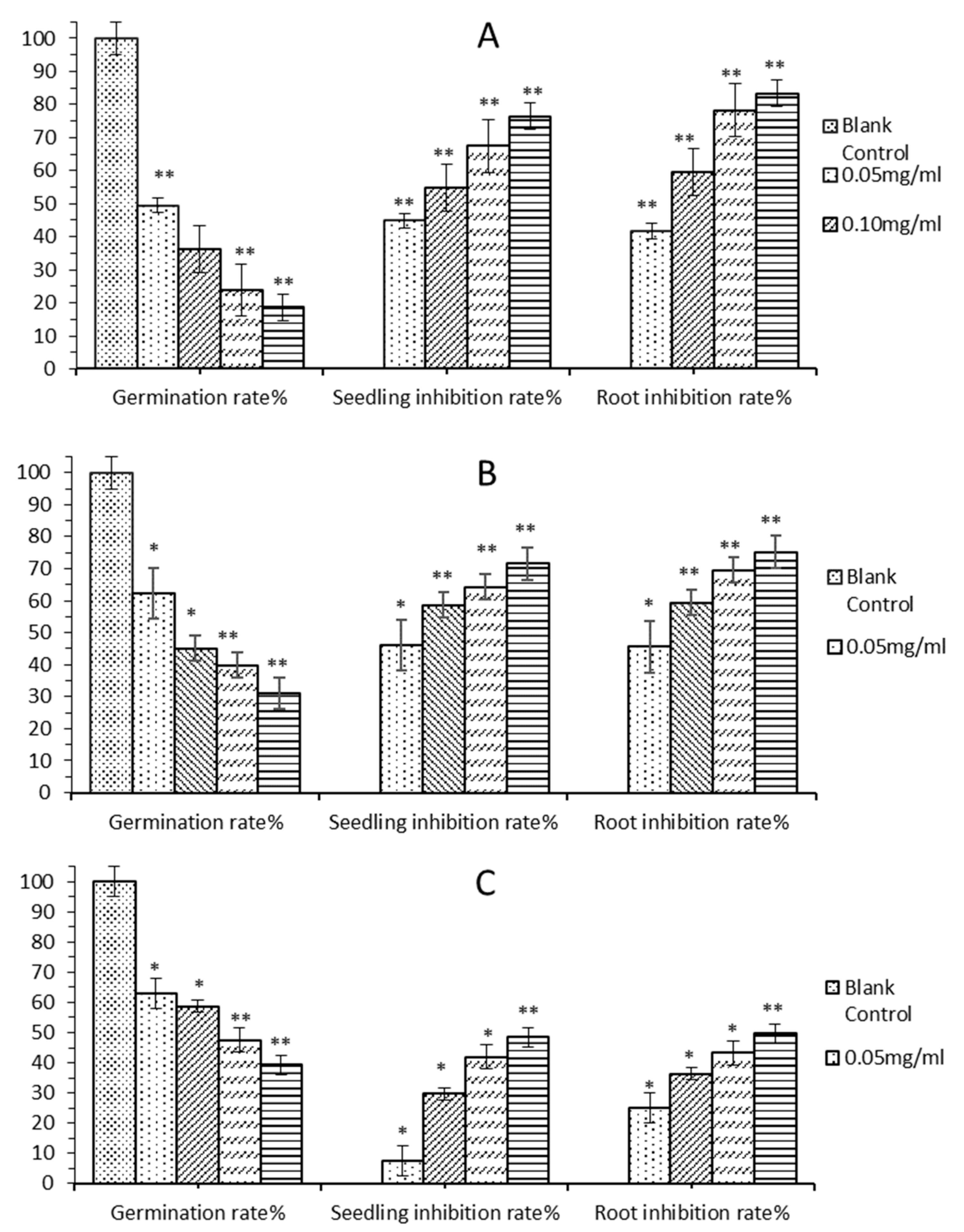
2.4. Activity Verification
2.4.1. Allelopathic Effect of E. Globulus Leaves Extract
2.4.2. Allelopathic Effects of Predicted Active Ingredients of E. Globulus Leaves
3. Experimental
3.1. Chemicals and Plant Materials
3.2. Extraction of Leaves
3.2.1. Extraction of Aqueous Extracts from E. Globulus Leaves
3.2.2. Extraction of Volatile Components from E. Globulus Leaves
3.3. GC-MS/MS, UPLC-MS/MS Analysis
3.3.1. UPLC-MS/MS Analysis of Aqueous Extracts from Leaves
3.3.2. GC-MS/MS Analysis of Volatile Components of Leaves
3.4. Molecular Docking
3.4.1. Preparation of ligands and receptors
3.4.2. Docking Method
3.5. Allelopathic Effects on Seeds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Luiz, B.; Claudinei, F.; Robson, T. Chemical variability and biological activities of Eucalyptus spp. essential oils. Molecules 2016, 21, 1671. [Google Scholar] [CrossRef]
- Dezsi, S.; Bădărău, A.; Bischin, C.; Vodnar, D.C.; Vlase, L. Antimicrobial and antioxidant activities and phenolic profile of Eucalyptus globulus Labill. and Corymbia ficifolia (F. Muell.) K.D. Hill & L.A.S. Johns. Leaves. Mol. 2015, 20, 4720–4734. [Google Scholar]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef]
- Tinnin, R.O.; Muller, C.H. The Allelopathic Influence of Avena fatua: The Allelopathic Mechanism. Bull. Torrey Bot. Club 1972, 99, 287. [Google Scholar] [CrossRef]
- Wang, H.; Gu, D.; Wang, M.; Guo, H.; Wu, H.; Tian, G. A strategy based on gas chromatography-mass spectrometry and virtual molecular docking for analysis and prediction of bioactive composition in natural product essential oil. J. Chromatogr. A 2017, 1501, 128–133. [Google Scholar] [CrossRef]
- Atanas, G.A.; Birgit, W.; Eva-Maria, P.W.; Thomas, L.; Christoph, W.; Pavel, U.; Veronika, T.; Limei, W.; Stefan, S.; Elke, H.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Yang, Y.; Abdulla, R.; Aisa, H.A. Characterization and identification of chemical compositions in the extract of Artemisia rupestris L. by liquid chromatography coupled to quadrupole time-of-flight tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2011, 26, 83–100. [Google Scholar] [CrossRef]
- Shen, J.; Xu, X.; Cheng, F.; Liu, H.; Luo, X.; Shen, J.; Chen, K.; Zhao, W.; Shen, X.; Jiang, H. Virtual screening on natural products for discovering active compounds and target information. Curr. Med. Chem. 2003, 10, 2327–2342. [Google Scholar] [CrossRef] [PubMed]
- Muegge, I.; Oloff, S. Advances in virtual screening. Drug Discov. Today Technol. 2007, 3, 405–411. [Google Scholar] [CrossRef]
- Borges, A.; Ferreira, C.; Saavedra, M.J. Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist. 2013, 19, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Chapuis-Lardy, L.; Contour-Ansel, D.; Bernhard-Reversat, F. High-performance liquid chromatography of water-soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci. 2002, 163, 217–222. [Google Scholar] [CrossRef]
- Sónia, A.O.; Santos Vilela, C.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Ultra-high performance liquid chromatography coupled to mass spectrometry applied to the identification of valuable phenolic compounds from Eucalyptuswood. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 938, 65–74. [Google Scholar]
- Lee, Y.G.; Cho, J.Y.; Kim, C.M.; Lee, S.H.; Kim, W.S.; Jeon, T.I. Coumaroyl quinic acid derivatives and flavonoids from immature pear (Pyrus pyrifolia nakai) fruit. Food Sci. Biotechnol. 2013, 22, 803–810. [Google Scholar] [CrossRef]
- Barry, K.M.; Davies, N.W.; Mohammed, C.L. Identification of hydrolysable tannins in the reaction zone of Eucalyptus nitens wood by high performance liquid chromatography-electrospray ionisation mass spectroscopy. Phytochem. Anal. 2010, 12, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Sónia, A.O.; Santos Juan, J.V.; Freire, C.S.R.; Rosário, M.; Domingues, M.; Neto, C.P.; Silvestre, A.J.D. Phenolic composition and antioxidant activity of Eucalyptus grandis, E. urograndis (E. grandis × E. urophylla) and E. maidenii bark extracts. Ind. Crop. Prod. 2012, 39, 120–127. [Google Scholar]
- Gao, W.N.; Luo, J.G.; Kong, L.Y. Quality evaluation of Hypericum japonicum by using high-performance liquid chromatography coupled with photodiode array detector and electrospray ionization tandem mass spectrometry. Biomed. Chromatogr. BMC 2010, 23, 1022–1030. [Google Scholar] [CrossRef]
- Tian, L.W.; Yang, C.R.; Zhang, Y.J. Phenolic Compounds from the Fresh Leaves of Eucalyptus maideni. Helv. Chim. Acta 2010, 93, 2194–2202. [Google Scholar] [CrossRef]
- Conde, E.; Cadahía, E.; García-Vallejo, M.C. Low molecular weight polyphenols in leaves of Eucalyptus camaldulensis, E. globulus and E. rudis. Phytochem. Anal. 2015, 8, 186–193. [Google Scholar] [CrossRef]
- Al-Sayed, E.; Singab, A.N.; Ayoub, N.; Martiskainen, O.; Sinkkonen, J.; Pihlaja, K. HPLC–PDA–ESI–MS/MS profiling and chemopreventive potential of Eucalyptus gomphocephala DC. Food Chem. 2012, 133, 1017–1024. [Google Scholar] [CrossRef]
- Jong-Pyung, K.; In-Kyoung, L.; Bong-Sik, Y.; Sung-Hyun, C.; Gyu-Seop, S.; Hiroyuki, K.; Ick-Dong, Y. Ellagic acid rhamnosides from the stem bark of Eucalyptus globulus. Phytochemistry 2001, 57, 587–591. [Google Scholar]
- Ting-Ting, C.; Bing-Sheng, H.; Xiao-Nong, Z.; Dan, Z.; Ming-Ya, L.I.; Xiao-Xiong, P. GC-MS analysis of volatile constituents of essential oil from Corymbia citriodora Hook. f. Leaves. J. Mod. Med. Health 2012, 1, 3–5. [Google Scholar]
- Paik, S.Y.; Koh, K.H.; Beak, S.M.; Paek, S.H.; Kim, J.A. The essential oils from Zanthoxylum schinifolium pericarp induce apoptosis of HepG2 human hepatoma cells through increased production of reactive oxygen species. Biol. Pharm. Bull. 2005, 28, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Bounihi, A.; Hajjaj, G.; Alnamer, R.; Cherrah, Y.; Zellou, A. In Vivo Potential Anti-Inflammatory Activity of Melissa officinalis L. Essential Oil. Adv. Pharmacol. Sci. 2013, 2013, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Abebe, W.; Sousa, S.M.; Duarte, V.G.; Machado, M.I.L.; Matos, F.J.A. Analgesic and anti-inflammatory effects of essential oils of Eucalyptus. J. Ethnopharmacol. 2003, 89, 277–283. [Google Scholar] [CrossRef]
- Ramezani, S.H.; Batish, H.P.; Kohli, D.R.; Kohli, R.K. Antifungal activity of the volatile oil of Eucalyptus citriodora. Fitoterapia 2002, 73, 261–262. [Google Scholar] [CrossRef]
- Zhu, F.; Shi, Z.; Qin, C.; Tao, L.; Liu, X.; Xu, F. Therapeutic target database update 2012: A resource for facilitating target-oriented drug discovery. Nucleic Acids Res. 2012, 40, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Sakata, I.; Iwamura, H. Synthesis and properties of menthyl glyosides. J. Agric. Chem. Soc. Jpn. 1979, 43, 6. [Google Scholar]
- Fu, Y.; Wang, Y.; Zhang, B. Systems pharmacology for traditional Chinese medicine with application to cardio-cerebrovascular diseases. J. Tradit. Chin. Med. Sci. 2014, 1, 84–91. [Google Scholar] [CrossRef][Green Version]
- Venkatachalam, C.M.; Jiang, X.; Oldfield, T.; Waldman, M. LigandFit: a novel method for the shape-directed rapid docking of ligands to protein active sites. J. Mol. Graph. Model. 2003, 21, 289–307. [Google Scholar] [CrossRef]
- André Krammer Kirchhoff, P.D.; Jiang, X.; Venkatachalam, C.M.; Waldman, M. LigScore: A novel scoring function for predicting binding affinities. J. Mol. Graph. Model. 2005, 23, 395–407. [Google Scholar]
- Gehlhaar, D.K.; Verkhivker, G.M.; Rejto, P.A.; Sherman, C.J.; Fogel, D.R.; Fogel, L.J. Molecular recognition of the inhibitor AG-1343 by HIV-1 protease: Conformationally flexible docking by evolutionary programming. Chem. Biol. 1995, 2, 317–324. [Google Scholar] [CrossRef]
- Jain, A.N. Scoring noncovalent protein-ligand interactions: A continuous differentiable function tuned to compute binding affinities. J. Comput. -Aided Mol. Des. 1996, 10, 427–440. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zou, X. Advances and challenges in protein-ligand docking. Int. J. Mol. Sci. 2010, 11, 3016. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.B.; Richardson, D. Bioassays for allelopathy: Measuring treatment responses with independent controls. J. Chem. Ecol. 1988, 14, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Terzı, I. Allelopathic effects of juglone and walnut leaf and fruit hull extracts on seed germination and seedling growth in muskmelon and cucumber. Asian J. Chem. 2009, 21, 1840–1846. [Google Scholar]


| NO. | Identification | TR/min | Formula | [M-H]− | MS2 | Relative Content (%) | Literature |
|---|---|---|---|---|---|---|---|
| 1 | Citric Acid | 2.09 | C6H8O7 | 191.0166 | 173.0061/111.0061/87.0063 | 0.19 | [12] |
| 2 | Quinic acid | 3.30 | C7H12O6 | 191.0529 | 147.0270/129.0165/85.0271 | 2.40 | [11] |
| 3 | Glucogallic acid | 6.13 | C13H16O10 | 331.0634 | 271.0425/169.0112 | 0.01 | [11] |
| 4 | Gallic acid | 6.31 | C7H6O5 | 169.0111 | 125.0216 | 2.81 | [11] |
| 5 | Ferulic acid | 6.85 | C10H10O4 | 193.0543 | 148.0589 | 0.01 | [11] |
| 6 | Protocatechuic acid glucoside | 12.96 | C13H16O9 | 315.0685 | 153.0164 | 0.01 | [11] |
| 7 | Protocatechuic acid | 13.38 | C7H6O4 | 153.0164 | 109.0269 | 0.15 | [11] |
| 8 | Gentiopic glucoside | 17.38 | C13H16O9 | 315.0676 | 153.0162 | 0.01 | [13] |
| 9 | 2-O-caffeoylquinic acid | 19.82 | C16H18O9 | 353.0838 | 191.0529/179.0319/135.0424 | 0.02 | [11] |
| 10 | 2,5-dihydroxybenzoic acid | 22.70 | C7H6O4 | 153.0164 | 109.0269 | 0.16 | [11] |
| 11 | Chlorogenic acid | 26.93 | C16H18O8 | 337.0894 | 191.0528/163.0369/119.0474 | 0.02 | [14] |
| 12 | 2-O-Coumaroylquinic Acid | 27.99 | C16H18O8 | 337.0894 | 163.0370/119.0475/119.0528 | 0.09 | [13] |
| 13 | 3,3′-Di-O-ellagic acid 4′-glucoside | 29.61 | C34H24O22 | 783.0594 | 765.0491/597.0453/300.9952 | 0.32 | [14] |
| 14 | 3-O-trans-caffeoylquinic acid | 30.17 | C16H18O9 | 353.0838 | 191.0531/179.0320 | 0.28 | [15] |
| 15 | Caffeic acid | 31.33 | C9H8O4 | 179.0318 | 135.0422 | 0.15 | [11] |
| 16 | cis-3-O-Coumaroylquinic Acid | 32.31 | C16H18O9 | 353.0838 | 191.0530/179.0320/173.0423/135.0421 | 0.01 | [15] |
| 17 | 2-O-trans-Coumaroylquinic Acid | 34.63 | C16H18O8 | 337.0894 | 173.0424/191.0527/163.0370 | 0.01 | [13] |
| 18 | Apigenin-7-O-β-D-glucopyranoside | 36.11 | C21H20O10 | 431.0968 | 385.1826/205.1199153.0891 | 0.10 | [15] |
| 19 | epigallocatechin | 36.55 | C15H14O7 | 305.0662 | 225.1100/96.9575 | 0.09 | [15] |
| 20 | Apigenin-7-O-galactoside | 37.40 | C21H20O10 | 431.0969 | 367.0140/179.0532/89.0219 | 0.08 | [16] |
| 21 | 3-O-Coumaroylquinic Acid | 38.50 | C16H18O8 | 337.0894 | 191.0529/173.0424/93.0320 | 0.02 | [15] |
| 22 | 5,7-hydroxyl-2-(1-methylpropyl)isopropyl-chroone-8-β-D-galactoside | 42.53 | C9H8O3 | 163.0370 | 119.0475 | 0.07 | [11] |
| 23 | 4-O-Coumaroylquinic Acid | 45.57 | C16H18O8 | 337.0894 | 191.0530/161.0370 | 0.03 | [13] |
| 24 | Ellagic acid-3-rhamnoside | 50.84 | C20H16O12 | 447.0547 | 300.9955 | 0.01 | [15] |
| 25 | Benzoic acid | 51.11 | C7H6O3 | 137.0216 | 93.0321 | 0.04 | [11] |
| 26 | Ellagic acid | 51.52 | C14H6O8 | 300.9953 | 300.9953 | 0.19 | [14] |
| 27 | 5,7-Hydroxyl-2-(1-methylpropyl)isopropyl-chroone-8-β-D-glucoside | 52.61 | C19H24O9 | 395.1311 | 275.0891/247.0947 | 0.03 | [16] |
| 28 | Quercetin-3-O-glucuronide | 52.70 | C21H18O13 | 477.0616 | 301.0318 | 0.17 | [17] |
| 29 | 5,7-Hydroxyl-2-(1-methylpropyl)isopropyl-chroone-8-β-D-galactoside | 54.02 | C19H24O9 | 395.1286 | 275.0891/247.0934 | 0.03 | [16] |
| 30 | Quercetin-3-O-arabinoside | 54.68 | C20H18O11 | 433.0710 | 301.0339 | 0.01 | [18] |
| 31 | kaempferol-7-O-glucuronide | 56.90 | C21H18O12 | 461.0666 | 286.0371 | 0.07 | [18] |
| 32 | Apigenin-7-O-glucuronide | 58.42 | C21H18O11 | 445.0767 | 269.0425/113.0217 | 0.09 | [19] |
| 33 | 3-O-methyl ellagic acid-O-glucopyranoside | 58.83 | C21H18O12 | 461.0675 | 315.0109 | 0.02 | [20] |
| 34 | 3,4-Dihydroxyhydrocinnamic acid | 61.82 | C9H10O4 | 181.0838 | 137.0943 | 0.01 | [20] |
| NO. | Identification | TR(min) | Formula | RI | Relative Content (%) |
|---|---|---|---|---|---|
| 1 | a-Pinene | 6.63 | C10H16 | 933 | 9.8 |
| 2 | Camphene | 7.27 | C10H16 | 950 | 2.43 |
| 3 | β-pinene | 7.39 | C10H16 | 981 | 0.44 |
| 4 | 3-Carene | 8.52 | C10H16 | 995 | 0.80 |
| 5 | α-Terpinolene | 11.20 | C10H16 | 1020 | 0.17 |
| 6 | D-Limonene | 11.51 | C10H16 | 1032 | 2.59 |
| 7 | Eucalyptol | 11.99 | C10H18O | 1039 | 21.49 |
| 8 | Ocimene | 12.42 | C10H16 | 1043 | 0.45 |
| 9 | Gamma terpinene | 12.51 | C10H16 | 1061 | 0.52 |
| 10 | Rosenoxide | 12.63 | C10H18O | 1112 | 0.01 |
| 11 | Fenchyl alcohol | 12.83 | C10H18O | 1119 | 1.83 |
| 12 | Camphor | 13.77 | C10H16O | 1151 | 0.15 |
| 13 | Isopulegol | 13.88 | C10H18O | 1155 | 0.41 |
| 14 | Borneol | 14.39 | C10H18O | 1173 | 2.23 |
| 15 | (-)-4-Terpineol | 14.67 | C10H18O | 1182 | 0.86 |
| 16 | (2R,5R)-2-Methyl-5-(prop-1-en-2-yl)cyclohexanone | 14.99 | C10H16O | 1193 | 5.15 |
| 17 | α-Terpineol | 15.12 | C10H18O | 1198 | 0.22 |
| 18 | Myrtenol | 15.26 | C10H16O | 1202 | 0.43 |
| 19 | Dihydrocarvone | 15.51 | C10H16O | 1210 | 0.13 |
| 20 | 2-Oxabicyclo[2.2.2]octan-6-ol, 1,3,3-trimethyl- | 15.99 | C10H18O2 | 1224 | 0.31 |
| 21 | Citronellol | 16.59 | C10H20O | 1242 | 0.16 |
| 22 | D-Carvone | 16.66 | C10H14O | 1244 | 0.38 |
| 23 | Menthone | 17.03 | C10H16O | 1256 | 0.12 |
| 24 | Citral | 17.42 | C10H16O | 1267 | 0.20 |
| 25 | (-)-β-elemene | 23.18 | C15H24 | 1395 | 0.16 |
| 26 | β-Caryophyllene | 25.08 | C15H24 | 1430 | 2.42 |
| 27 | Caryophyllene | 25.42 | C15H24 | 1436 | 0.14 |
| 28 | α-Caryophyllene | 26.81 | C15H24 | 1462 | 0.37 |
| 29 | (+)-aromadendrene | 27.21 | C15H24 | 1469 | 0.57 |
| 30 | B-cadinene | 27.75 | C15H24 | 1479 | 0.17 |
| 31 | β-selinene | 28.42 | C15H24 | 1491 | 0.13 |
| 32 | 1H-Cycloprop[e]azulene,1a,2,3,5,6,7,7a,7b-octahydro-1,1,4,7-tetramethyl-(1aR,7R,7aS,7bR)- | 28.83 | C15H24 | 1498 | 0.32 |
| 33 | γ-cadinene | 29.67 | C15H24 | 1517 | 0.13 |
| 34 | δ-cadinene | 30.11 | C15H24 | 1527 | 1.52 |
| 35 | 1H-Cycloprop[e]azulen-4-ol,decahydro-1,1,4,7-tetramethyl-,(1aR,4R,4aR,7R,7aS,7bS)- | 32.69 | C15H26O | 1586 | 0.27 |
| No. | Compound | Lig Score1 | Lig Score2 | PLP1 | PLP2 | Jain | PMF | Dock Score |
|---|---|---|---|---|---|---|---|---|
| 1 | Gallic acid | 1.74 | 2.14 | 22.78 | 21.7 | −0.43 | 57.46 | 89.142 |
| 2 | Glucogallic acid | 0.7 | 0.6 | 4.7 | 9.22 | −1.91 | 29.48 | 82.628 |
| 3 | Citric acid | 2.07 | 1.95 | 14.4 | 20.6 | −1.29 | 43.64 | 76.598 |
| 4 | 5,7-Hydroxyl-2-(1-methylpropyl)isopropyl-chroone-8-β-D-galactoside | 2.38 | 2.93 | 31.52 | 30.75 | 0.46 | 107.74 | 72.267 |
| 5 | 3,4-DihydroxyhYdrocinnamic acid | 1.93 | 2.29 | 23.41 | 38 | 0.41 | 65.15 | 66.73 |
| 6 | 3-O-trans-Coumaroylquinic acid | 2.26 | 2.68 | 26.18 | 37.87 | 1.4 | 87.69 | 66.699 |
| 7 | Caffeic acid | 1.9 | 2.4 | 25.45 | 38.58 | 0.71 | 71.17 | 64.25 |
| 8 | Quinic acid | 1.9 | 1.58 | 11.05 | 14.78 | 1.13 | 43.47 | 62.489 |
| 9 | 5,7-Hydroxyl-2-(1-methylpropyl)isopropyl-chroone-8-β-D-glucoside | 1.67 | 1.43 | 32.81 | 36.31 | 1.17 | 104.72 | 61.902 |
| 10 | 3-O-trans-Caffeoylquinic acid | 2.51 | 2.78 | 30.51 | 42.8 | 1.29 | 96 | 56.973 |
| 11 | cis-3-O-Coumaroylquinic acid | 1.93 | 3.05 | 29.34 | 41.58 | 1.66 | 92.73 | 55.67 |
| 12 | 2-O-trans-Coumaroylquinic acid | 3.23 | 2.94 | 26.54 | 38.98 | 0.64 | 89.46 | 53.726 |
| 13 | Chlorogenic acid | 2.17 | 2.8 | 25.04 | 41.94 | 1 | 101.15 | 52.818 |
| 14 | 2-O-Caffeoylquinic acid | 2.47 | 3.11 | 38.91 | 45.61 | 0.59 | 94.34 | 52.714 |
| 15 | Menthone | 1.5 | 2.04 | 12.32 | 12.45 | −1.09 | 46.31 | 52.166 |
| 16 | Protocatechuic acid glucoside | 2.21 | 3.3 | 29.87 | 46.23 | 0.76 | 67.21 | 49.487 |
| 17 | 2,5-Dihydroxybenzoic acid | 1.56 | 2.47 | 13.35 | 14.67 | −0.78 | 40.9 | 48.15 |
| 18 | Benzoic acid | 0.89 | 1.94 | 7.06 | 7.86 | −1.07 | 40.42 | 47.551 |
| 19 | Ferulic acid | 1.74 | 3.07 | 17.6 | 28.85 | 0.38 | 59.74 | 47.087 |
| 20 | Gentiopicroside | 2.53 | 1.03 | 3.63 | 17.49 | 2.44 | 69.56 | 46.111 |
| 21 | Protocatechuic acid | 1.83 | 2.73 | 17.32 | 31.89 | 1.8 | 49.28 | 42.911 |
| 22 | Epigallocatechin | 2.1 | 3.23 | 39.58 | 51.66 | 1.8 | 88.34 | 40.993 |
| 23 | Ellagic acid | 2.65 | 3.3 | 34.44 | 36.64 | -0.18 | 92.63 | 32.576 |
| 24 | Isopulegol | 2.3 | 3.01 | 30.07 | 34.74 | 1.51 | 59.87 | 25.029 |
| 25 | Rose oxide | 1.2 | 2.89 | 17.82 | 28.09 | 1.56 | 61.5 | 22.21 |
| 26 | Menthone | 1.5 | 2.93 | 15.7 | 16 | 0.61 | 46.76 | 21.555 |
| 27 | Citral | 1.51 | 2.97 | 22.2 | 30.08 | 0.84 | 58.13 | 21.089 |
| 28 | 2,5-dihydroxybenzoic acid | 1.3 | 2.92 | 24.93 | 25.85 | 0.95 | 55.84 | 20.39 |
| 29 | Fenchyl alcohol | 1.16 | 2.7 | 23.37 | 22.92 | 0.14 | 56.24 | 19.142 |
| 30 | Myrtenol | 1.41 | 2.83 | 19.58 | 18.61 | 0.72 | 50.72 | 19.131 |
| 31 | Dihydrocarvone | 0.45 | 2.8 | 24.48 | 25.09 | −0.13 | 60.37 | 19.017 |
| 32 | (2R,5R)-2-Methyl-5-(prop-1-en-2-yl)cyclohexanone | 0.43 | 2.78 | 25.81 | 25.34 | −0.1 | 61.49 | 18.259 |
| 33 | 3-Carene | 0.45 | 2.79 | 26.47 | 28.15 | 1.66 | 59.34 | 17.182 |
| 34 | d-Limonene | 0.38 | 2.66 | 24.21 | 25.95 | −0.02 | 60.25 | 17.138 |
| 35 | D-Carvone | 0.51 | 2.89 | 27.49 | 27.13 | −0.04 | 58.65 | 17.117 |
| 36 | γ-Terpinene | 0.42 | 2.73 | 25.09 | 26.37 | 0.71 | 61.9 | 17.116 |
| 37 | Citronellol | 1.27 | 2.85 | 25.08 | 28.96 | −0.99 | 57.52 | 16.85 |
| 38 | α-Terpineol | 2.02 | 3.26 | 25.42 | 30.86 | 2.09 | 57.13 | 16.766 |
| 39 | Camphene | 0.35 | 2.61 | 24.46 | 23.88 | 0.71 | 57.78 | 15.827 |
| 40 | Ocimene | 0.58 | 3.01 | 24.62 | 28.25 | 0.4 | 61.88 | 14.504 |
| 41 | Terpinyl acetate | 0.93 | 2.87 | 26.47 | 25.1 | −0.4 | 63.72 | 14.124 |
| 42 | β-Elemene | 0.53 | 2.91 | 26.79 | 25.52 | −0.82 | 68.55 | 13.708 |
| 43 | β-Eudesmol | 1 | 2.83 | 29.84 | 35.44 | 0.68 | 74.6 | 12.253 |
| 44 | Globulol | 0.37 | 2.68 | 16.5 | 18.05 | −1.69 | 18.5 | 11.926 |
| 45 | Borneol | 1.19 | 2.69 | 13.59 | 15.4 | −0.07 | 46.34 | 11.754 |
| 46 | α-Terpinolene | 0.16 | 2.28 | 17.95 | 28.24 | 1.39 | 62.53 | 11.285 |
| 47 | β-Pinene | 0.19 | 2.32 | 25.08 | 26.6 | 0.51 | 56.72 | 10.315 |
| 48 | Cineole | 0.18 | 1.6 | 24.83 | 27.37 | 1.77 | 58.45 | 9.034 |
| 49 | α-Pinene | 0.09 | 2.17 | 24.55 | 25.87 | 0.77 | 59.29 | 7.723 |
| 50 | Camphanone | 0.52 | 1.89 | 23.33 | 26.3 | 0.76 | 58.95 | 4.111 |
| 51 | γ-Cadinene | 0.31 | 2.5 | 24.9 | 28.05 | 2.53 | 75.53 | 4.041 |
| 52 | β-Cadinene | -0.24 | 1.62 | 23.67 | 37.92 | 0.96 | 60.27 | 2.74 |
| Compound | Absolute Energy | Binding Mode | H-Bind Number | Residues Involved in H-Bond Formation |
|---|---|---|---|---|
| Gallic acid | 15.277 |  | 3 | Lys 35 |
| Glucogallic acid | 13.502 | 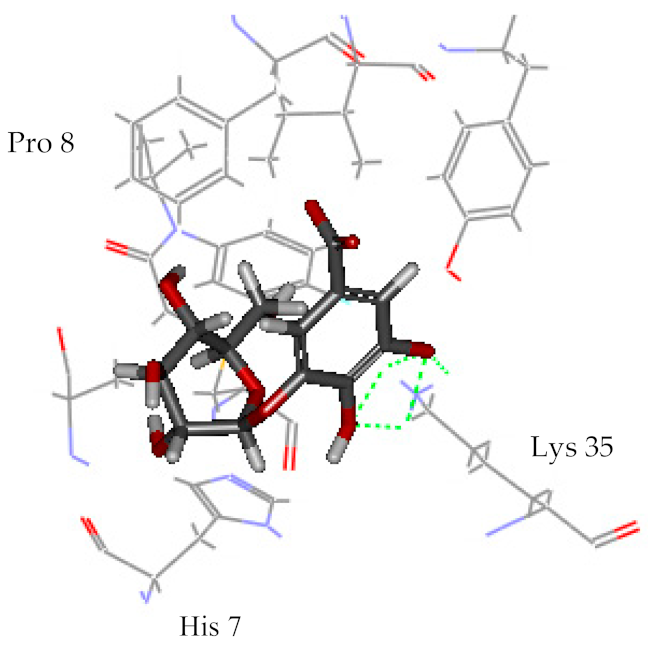 | 5 | Lys 35 |
| Citric acid | 12.893 | 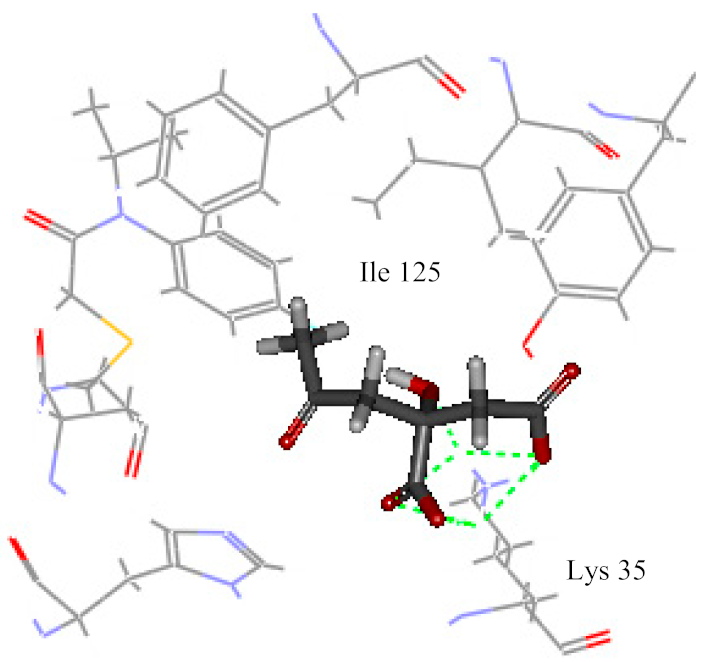 | 5 | Lys 35 |
| Isopulegol | 4.431 |  | 2 | Lys 35 Tyr 126 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, M.; Lei, Q.; Zang, N.; Zhang, H. A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves. Int. J. Mol. Sci. 2019, 20, 3875. https://doi.org/10.3390/ijms20163875
Pan M, Lei Q, Zang N, Zhang H. A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves. International Journal of Molecular Sciences. 2019; 20(16):3875. https://doi.org/10.3390/ijms20163875
Chicago/Turabian StylePan, Meng, Qicheng Lei, Ning Zang, and Hong Zhang. 2019. "A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves" International Journal of Molecular Sciences 20, no. 16: 3875. https://doi.org/10.3390/ijms20163875
APA StylePan, M., Lei, Q., Zang, N., & Zhang, H. (2019). A Strategy Based on GC-MS/MS, UPLC-MS/MS and Virtual Molecular Docking for Analysis and Prediction of Bioactive Compounds in Eucalyptus Globulus Leaves. International Journal of Molecular Sciences, 20(16), 3875. https://doi.org/10.3390/ijms20163875





