Incomplete Spinal Cord Injury Reverses the Level-Dependence of Spinal Cord Injury Immune Deficiency Syndrome
Abstract
1. Introduction
2. Results
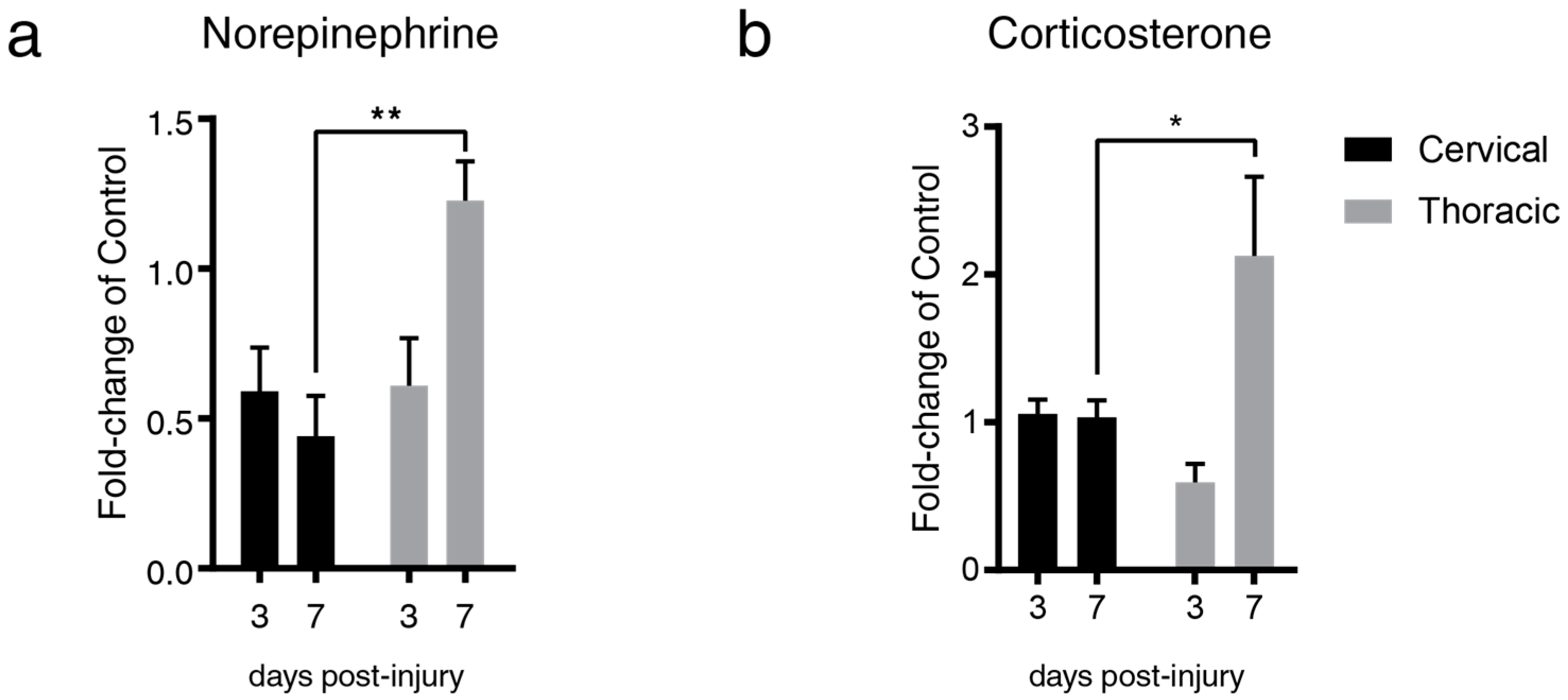
2.1. Lesion Level-Dependent SCI Effects on Splenic Norepinephrine and Cortisol Levels
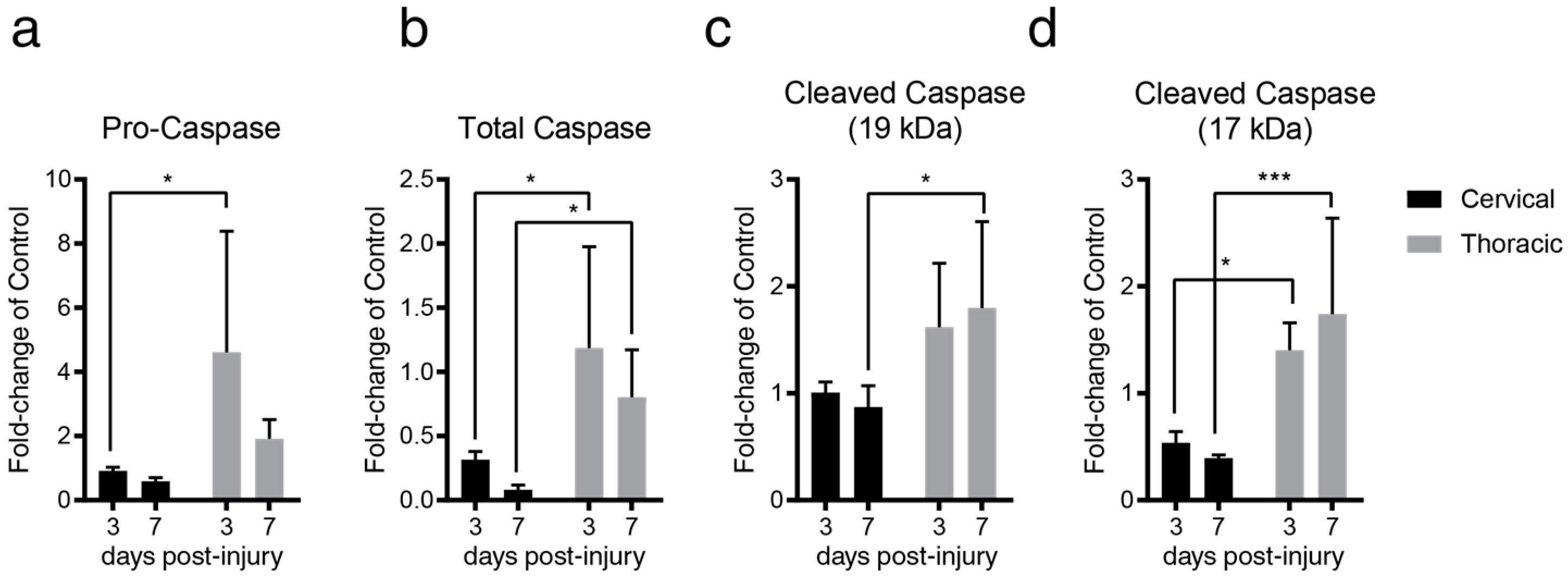
2.2. Lesion Level-Dependent SCI Effects on Splenocyte Apoptosis
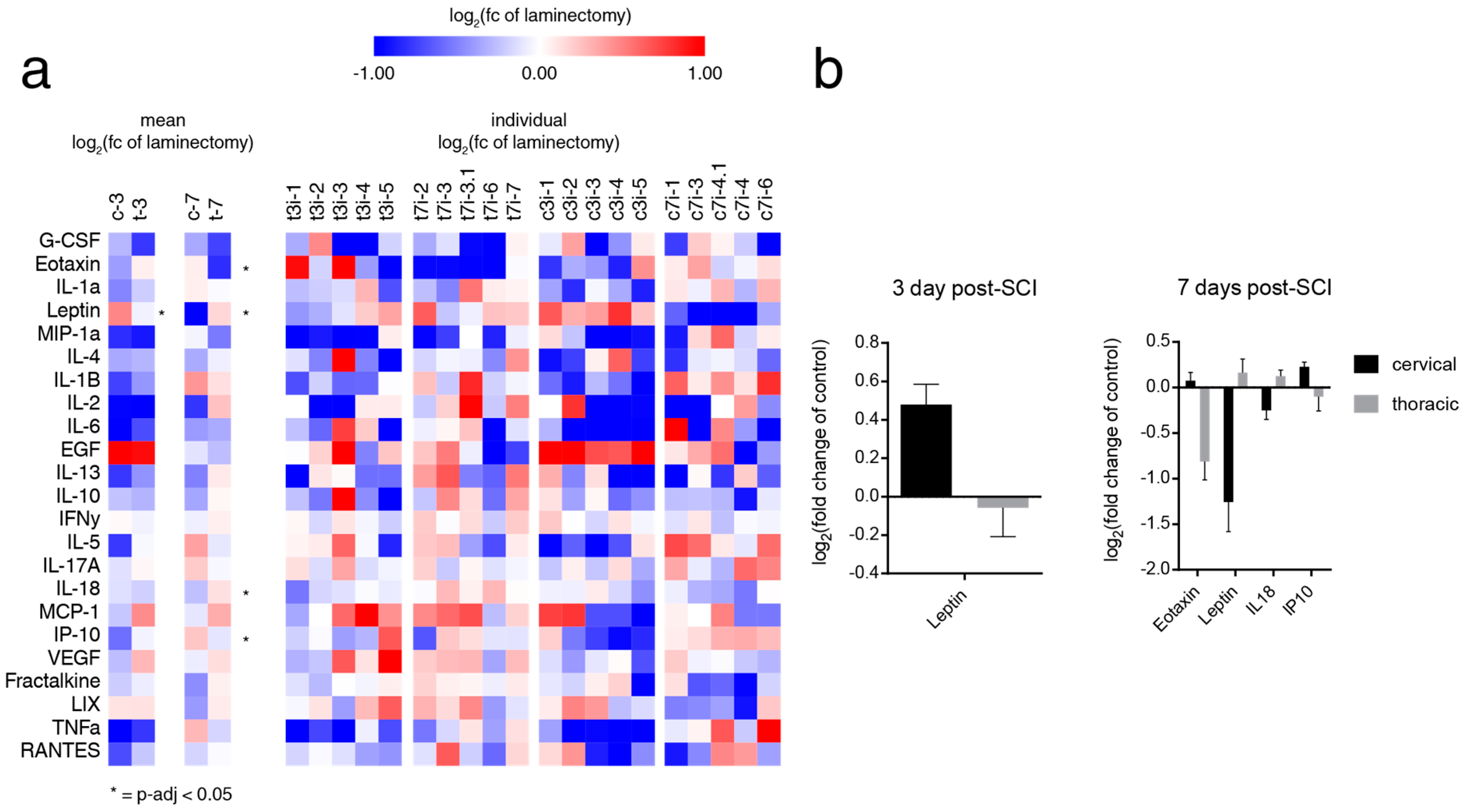
2.3. SCI induces Level-Dependent Splenic Cytokine/Chemokine Changes
3. Discussion
3.1. Incomplete SCI Alters Level Dependence of Several Hallmarks of SCI-IDS through Localized Denervation and Partial Preservation of Sympathetic Tone
3.2. Elevations in Splenic Norepinephrine and Corticosterone Result in Splenocyte Apoptosis and a Reduction of Key Leukocyte and Lymphocyte Generation and Trafficking Molecules
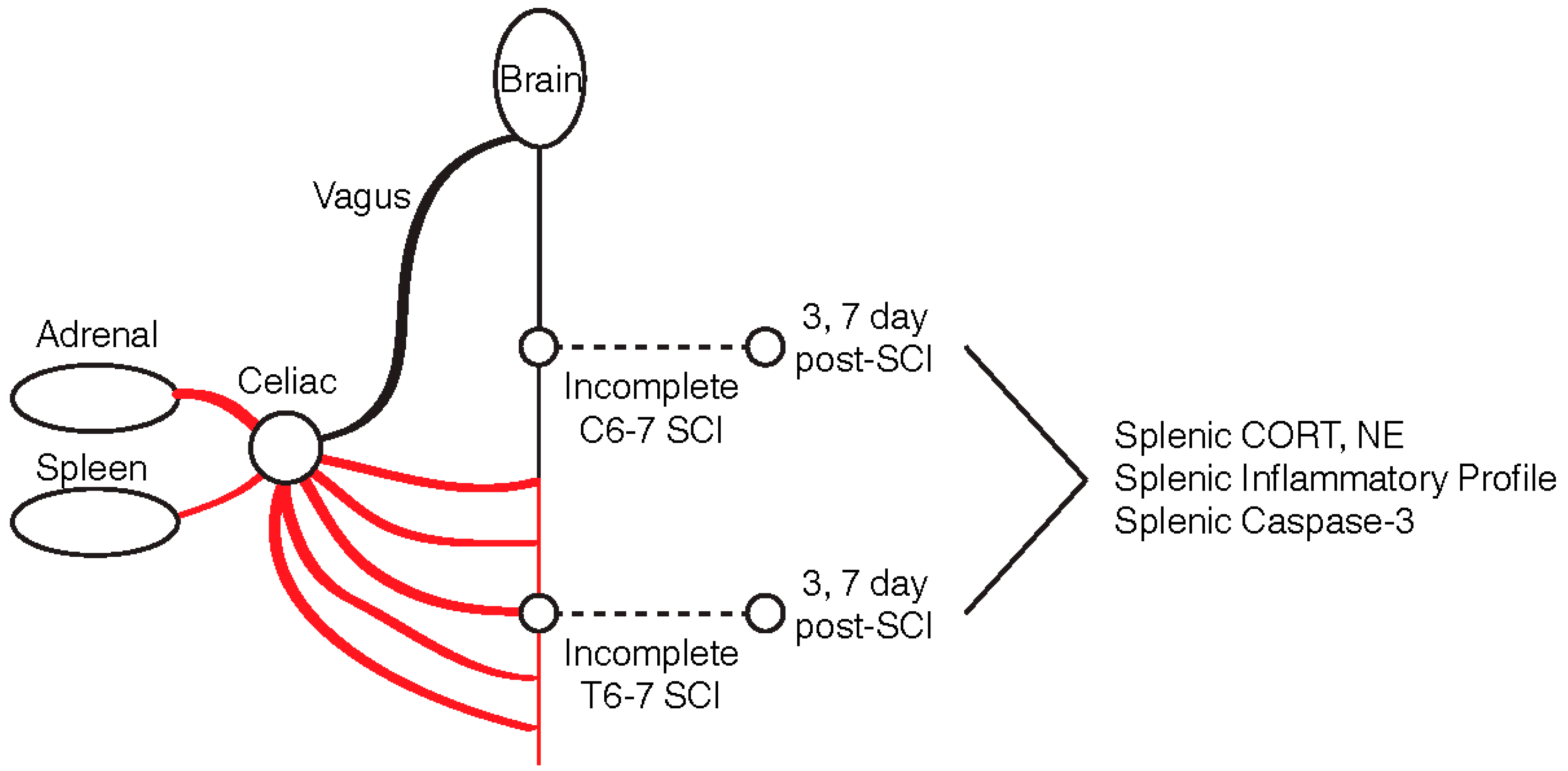
4. Materials and Methods
4.1. Clip-Compression SCI and Spleen Extraction
4.2. Protein Extraction from the Spleen
4.3. Quantitative ELISA for Norepinephrine and Corticosterone
4.4. Western Blot
4.5. High-Throughput Luminex Array for Cytokines and Chemokines
4.6. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| SCI | Spinal cord injury |
| SCI-IDS | Spinal cord injury-induced immunodeficiency syndrome |
| NE | Norepinephrine |
| CORT | Corticosterone |
| tSCI | Thoracic spinal cord injury |
| cSCI | Cervical spinal cord injury |
| SPN | Spinal preganglionic neurons |
| TBST | Tris-buffered saline with 0.1% Tween-20 |
| GMCSF | Granulocyte-macrophage colony-stimulating factor |
| IL1α | Interleukin 1 alpha |
| IL1β | Interleukin 1 beta |
| IL2 | Interleukin-2 |
| IL17A | Interleukin-17A |
| IL18 | Interleukin-18 |
| MCP1 | Monocyte chemoattractant protein-1 |
| IP10 | Interferon gamma-induced protein 10 |
| LIX | Lipopolysaccharide-induced CXC chemokine |
| MIP2 | macrophage inflammatory protein 2 |
| RANTES | regulated on activation, normal T cell expressed and secreted |
| GCSF | Granulocyte - colony stimulating factor |
| IL4 | Interleukin-4 |
| IL10 | Interleukin-10 |
| IL12 | Interleukin-12 |
| IL5 | Interleukin-5 |
References
- Riegger, T.; Conrad, S.; Schluesener, H.J.; Kaps, H.P.; Badke, A.; Baron, C.; Gerstein, J.; Dietz, K.; Abdizahdeh, M.; Schwab, J.M. Immune depression syndrome following human spinal cord injury (SCI): A pilot study. Neuroscience 2009, 158, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Brommer, B.; Engel, O.; Kopp, M.A.; Watzlawick, R.; Muller, S.; Pruss, H.; Chen, Y.; DeVivo, M.J.; Finkenstaedt, F.W.; Dirnagl, U.; et al. Spinal cord injury-induced immune deficiency syndrome enhances infection susceptibility dependent on lesion level. Brain 2016, 139, 692–707. [Google Scholar] [CrossRef] [PubMed]
- Kopp, M.A.; Druschel, C.; Meisel, C.; Liebscher, T.; Prilipp, E.; Watzlawick, R.; Cinelli, P.; Niedeggen, A.; Schaser, K.D.; Wanner, G.A.; et al. The SCIentinel study--prospective multicenter study to define the spinal cord injury-induced immune depression syndrome (SCI-IDS)--study protocol and interim feasibility data. BMC Neurol. 2013, 13, 168. [Google Scholar] [CrossRef] [PubMed]
- Noble, B.T.; Brennan, F.H.; Popovich, P.G. The spleen as a neuroimmune interface after spinal cord injury. J. Neuroimmunol 2018, 321, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pruss, H.; Tedeschi, A.; Thiriot, A.; Lynch, L.; Loughhead, S.M.; Stutte, S.; Mazo, I.B.; Kopp, M.A.; Brommer, B.; Blex, C.; et al. Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat. Neurosci. 2017, 20, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Riegger, T.; Conrad, S.; Liu, K.; Schluesener, H.J.; Adibzahdeh, M.; Schwab, J.M. Spinal cord injury-induced immune depression syndrome (SCI-IDS). Eur J. Neurosci. 2007, 25, 1743–1747. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Guan, Z.; Reader, B.; Shawler, T.; Mandrekar-Colucci, S.; Huang, K.; Weil, Z.; Bratasz, A.; Wells, J.; Powell, N.D.; et al. Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J. Neurosci. 2013, 33, 12970–12981. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Sanders, V.M.; Jones, T.B.; Malarkey, W.B.; Popovich, P.G. Impaired antibody synthesis after spinal cord injury is level dependent and is due to sympathetic nervous system dysregulation. Exp. Neurol. 2007, 207, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Lucin, K.M.; Sanders, V.M.; Popovich, P.G. Stress hormones collaborate to induce lymphocyte apoptosis after high level spinal cord injury. J. Neurochem. 2009, 110, 1409–1421. [Google Scholar] [CrossRef]
- Ueno, M.; Ueno-Nakamura, Y.; Niehaus, J.; Popovich, P.G.; Yoshida, Y. Silencing spinal interneurons inhibits immune suppressive autonomic reflexes caused by spinal cord injury. Nat. Neurosci. 2016, 19, 784–787. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol 2014, 6, 309–331. [Google Scholar] [PubMed]
- Wilcox, J.T.; Satkunendrarajah, K.; Nasirzadeh, Y.; Laliberte, A.M.; Lip, A.; Cadotte, D.W.; Foltz, W.D.; Fehlings, M.G. Generating level-dependent models of cervical and thoracic spinal cord injury: Exploring the interplay of neuroanatomy, physiology, and function. Neurobiol. Dis. 2017, 105, 194–212. [Google Scholar] [CrossRef]
- Hong, J.; Chang, A.; Zavvarian, M.M.; Wang, J.; Liu, Y.; Fehlings, M.G. Level-Specific Differences in Systemic Expression of Pro- and Anti-Inflammatory Cytokines and Chemokines after Spinal Cord Injury. Int. J. Mol. Sci. 2018, 19, 2167. [Google Scholar] [CrossRef]
- Zha, J.; Smith, A.; Andreansky, S.; Bracchi-Ricard, V.; Bethea, J.R. Chronic thoracic spinal cord injury impairs CD8+ T-cell function by up-regulating programmed cell death-1 expression. J. Neuroinflammation 2014, 11, 65. [Google Scholar] [CrossRef]
- Norden, D.M.; Bethea, J.R.; Jiang, J. Impaired CD8 T cell antiviral immunity following acute spinal cord injury. J. Neuroinflammation 2018, 15, 149. [Google Scholar] [CrossRef] [PubMed]
- Jahnsen, F.L.; Haye, R.; Gran, E.; Brandtzaeg, P.; Johansen, F.E. Glucocorticosteroids inhibit mRNA expression for eotaxin, eotaxin-2, and monocyte-chemotactic protein-4 in human airway inflammation with eosinophilia. J. Immunol. 1999, 163, 1545–1551. [Google Scholar]
- Dufour, J.H.; Dziejman, M.; Liu, M.T.; Leung, J.H.; Lane, T.E.; Luster, A.D. IFN-gamma-inducible protein 10 (IP-10; CXCL10)-deficient mice reveal a role for IP-10 in effector T cell generation and trafficking. J. Immunol. 2002, 168, 3195–3204. [Google Scholar] [CrossRef]
- Gezici, A.R.; Ergun, R.; Karakas, A.; Gunduz, B. Serum Leptin Levels Following Acute Experimental Spinal Cord Injury. J. Spinal Cord Med. 2009, 32, 416–421. [Google Scholar] [CrossRef]
- Latifi, S.; Koushki, D.; Norouzi Javidan, A.; Matin, M.; Sabour, H. Changes of leptin concentration in plasma in patients with spinal cord injury: A meta-analysis. Spinal Cord 2013, 51, 728–731. [Google Scholar] [CrossRef]
- Ramirez, O.; Garza, K.M. Leptin deficiency in vivo enhances the ability of splenic dendritic cells to activate T cells. Int Immunol 2014, 26, 627–636. [Google Scholar] [CrossRef]
- Kristo, C.; Godang, K.; Ueland, T.; Lien, E.; Aukrust, P.; Froland, S.S.; Bollerslev, J. Raised serum levels of interleukin-8 and interleukin-18 in relation to bone metabolism in endogenous Cushing’s syndrome. Eur. J. Endocrinol 2002, 146, 389–395. [Google Scholar] [CrossRef][Green Version]
- Takahashi, H.K.; Iwagaki, H.; Mori, S.; Yoshino, T.; Tanaka, N.; Nishibori, M. Beta 2-adrenergic receptor agonist induces IL-18 production without IL-12 production. J. Neuroimmunol 2004, 151, 137–147. [Google Scholar] [CrossRef]
- Seagard, J.L.; Hopp, F.A.; Bosnjak, Z.J.; Osborn, J.L.; Kampine, J.P. Sympathetic efferent nerve activity in conscious and isoflurane-anesthetized dogs. Anesthesiology 1984, 61, 266–270. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.; Chang, A.; Liu, Y.; Wang, J.; Fehlings, M.G. Incomplete Spinal Cord Injury Reverses the Level-Dependence of Spinal Cord Injury Immune Deficiency Syndrome. Int. J. Mol. Sci. 2019, 20, 3762. https://doi.org/10.3390/ijms20153762
Hong J, Chang A, Liu Y, Wang J, Fehlings MG. Incomplete Spinal Cord Injury Reverses the Level-Dependence of Spinal Cord Injury Immune Deficiency Syndrome. International Journal of Molecular Sciences. 2019; 20(15):3762. https://doi.org/10.3390/ijms20153762
Chicago/Turabian StyleHong, James, Alex Chang, Yang Liu, Jian Wang, and Michael G. Fehlings. 2019. "Incomplete Spinal Cord Injury Reverses the Level-Dependence of Spinal Cord Injury Immune Deficiency Syndrome" International Journal of Molecular Sciences 20, no. 15: 3762. https://doi.org/10.3390/ijms20153762
APA StyleHong, J., Chang, A., Liu, Y., Wang, J., & Fehlings, M. G. (2019). Incomplete Spinal Cord Injury Reverses the Level-Dependence of Spinal Cord Injury Immune Deficiency Syndrome. International Journal of Molecular Sciences, 20(15), 3762. https://doi.org/10.3390/ijms20153762





