Behavioral Disturbances in Dementia and Beyond: Time for a New Conceptual Frame?
Abstract
1. Introduction
2. Behavioral Disturbances and Dementia Sub Types: Vascular Dementia (VaD), Alzheimer’s Disease (AD) and Mixed Dementia (MixD)
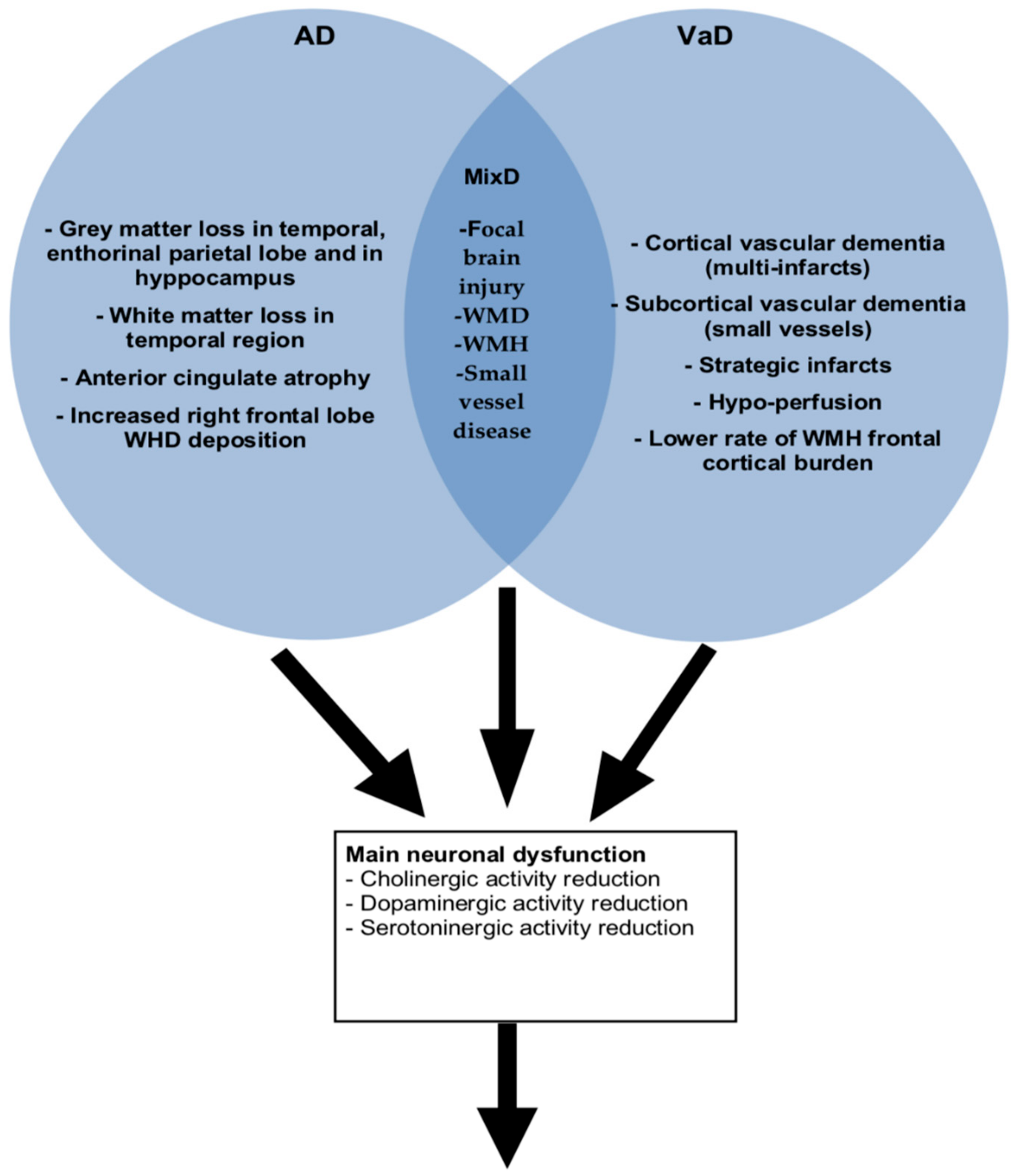
3. Neuroanatomic Findings Between Behavioral and Psychological Symptoms (BPSD) and Dementia Sub-Types (VaD, AD and MixD)
4. Antipsychotic Use across Dementia Sub-Types (VaD, AD Versus MixD)
5. Discussion and Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| VaD | vascular dementia |
| MixD | mixed type dementia |
| VCI | vascular cognitive impairment |
| ADL | activities of daily living |
| BPSD | behavioral and psychological symptoms of dementia |
| NPS | neuropsychiatric symptoms |
| AD | Alzheimer’s disease |
| MMSE | mini-mental state examination |
| WMD | white matter disease |
| WMH | white matter hyperintensities |
| BEHAVE-AD-K | Behavioral Pathology in Alzheimer’s Disease Rating Scale, Korean version |
References
- Rizzi, L.; Rosset, I.; Roriz-Cruz, M. Global epidemiology of dementia: Alzheimer’s and vascular types. BioMed Res. Int. 2014, 2014, 908915. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.D.; Yoshida, S.; Liu, Q.; Wu, C.L.; Wang, J.; Zhu, J.; Cai, D.F. Quantitative evaluation of severity of behavioral and psychological symptoms of dementia in patients with vascular dementia. Transl. Neurodegener. 2013, 2, 9. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Attems, J. Prevalence of dementia disorders in the oldest-old: An autopsy study. Acta Neuropathol. 2010, 119, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A. Basic mechanisms of neurodegeneration: A critical update. J. Cell. Mol. Med. 2010, 14, 457–487. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, P. Vascular cognitive disorder. Int. J. Geriatr. Psychiatry 1999, 14, 402–403. [Google Scholar] [CrossRef]
- O’Brien, J.T.; Erkinjuntti, T.; Reisberg, B.; Roman, G.; Sawada, T.; Pantoni, L.; Bowler, J.V.; Ballard, C.; DeCarli, C.; Gorelick, P.B.; et al. Vascular cognitive impairment. Lancet Neurol. 2003, 2, 89–98. [Google Scholar] [CrossRef]
- Wiesmann, M.; Kiliaan, A.J.; Claassen, J.A. Vascular aspects of cognitive impairment and dementia. J. Cereb. Blood Flow Metab. 2013, 33, 1696–1706. [Google Scholar] [CrossRef]
- Cummings, J.L.; Mahler, M.E. Cerebrovascular dementia. In Neurobehavioral Aspect of Cerebrovascular Disease; Bernstein, R.A., Brown, G.G., Eds.; Oxford University Press: New York, NY, USA, 1991. [Google Scholar]
- Toyama, K.; Spin, J.M.; Mogi, M.; Tsao, P.S. Therapeutic perspective on vascular cognitive impairment. Pharmacol. Res. 2019, 17, 104266. [Google Scholar] [CrossRef]
- Gupta, M.; Dasgupta, A.; Khwaja, G.A.; Chowdhury, D.; Patidar, Y.; Batra, A. Behavioural and phsychological symptoms in post stroke vascular cognitive impairment. Behav. Neurol. 2014, 2014, 430128. [Google Scholar] [CrossRef]
- Shin, I.S.; Carter, M.; Masterman, D.; Fairbanks, L.; Cummings, J.L. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am. J. Geriatr. Psychiatry 2005, 13, 469–474. [Google Scholar] [CrossRef]
- Drinka, T.J.K.; Smith, J.C.; Drinka, P.J. Correlates of depression and burden for informal caregivers of patients in a geriatrics referral clinic. J. Am. Geriatr. Soc. 1987, 35, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Steele, C.; Rovner, B.; Chase, G.A.; Folstein, M. Psychiatric symptoms and nursing home placement of patients with Alzheimer’s disease. Am. J. Psychiatry 1990, 147, 1049–1051. [Google Scholar] [PubMed]
- Aalten, P.; de Vugt, M.E.; Lousberg, R.; Korten, E.; Jaspers, N.; Senden, B.; Jolles, J.; Verhey, F.R. Behavioral problems in dementia: A factor analysis of the neuropsychiatric inventory. Dement. Geriatr. Cogn. Disord. 2003, 15, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J.L. The neuropsychiatric inventory: Assessing psychopathology in dementia patients. Neurology 1997, 48, S10–S16. [Google Scholar] [CrossRef] [PubMed]
- Sultzer, D.L.; Levin, H.S.; Mahler, M.E.; High, W.M.; Cummings, J.L. A Comparison of Psychiatric Symptoms in Vascular Dementia and Alzheimer’s Disease. Am. J. Psychiatry 1993, 150, 12. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Levin, H.S.; High, W.M.; Goethe, K.E.; Sisson, R.A.; Overall, J.E.; Rhoades, H.M.; Eisenberg, H.M.; Kalisky, Z.V.; Gary, H.E. The neurobehavioural rating scale: Assessment of the behavioural sequelae of head injury by the clinician. J. Neurol. Neurosurg. Psychiatry 1987, 50, 183–193. [Google Scholar] [CrossRef]
- Santos, M.A.; Bezerra, L.S.; Correia, C.D.; Bruscky, I.S. Neuropsychiatric symptoms in vascular dementia Epidemiologic and clinical aspects. Dement. Neuropsychol. 2018, 12, 40–44. [Google Scholar] [CrossRef]
- Hargrave, R.; Geck, L.C.; Reed, B.; Mungas, D. Affective behavioral disturbances in Alzheimer’s disease and ischaemic vascular disease. J. Neurol. Neurosurg. Psychiatry 2000, 68, 41–46. [Google Scholar] [CrossRef][Green Version]
- Krasuski, J.S.; Gaviria, M. Neuropsychiatric sequelae of ischemic cerebrovascular disease: Clinical and neuroanatomic correlates and implications for the concept of dementia. Neurol. Res. 1994, 16, 241–250. [Google Scholar] [CrossRef]
- Chui, H.C.; Victoroff, J.I.; Margolin, D.; Jagust, W.; Shankle, R.; Katzman, R. Criteria of the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s disease diagnostic and treatment centers. Neurology 1992, 42, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Saz, P.; López-Antón, R.; Dewey, M.E.; Ventura, T.; Martin, A.; Marcos, G.; De La Cámara, C.; Quintanilla, M.A.; Quetglas, B.; Bel, M.; et al. Prevalence and implications of psychopathological non-cognitive symptoms in dementia. Acta Psychiatr. Scand. 2009, 119, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Sadak, T.I.; Katon, J.; Beck, C.; Cochrane, B.B.; Borson, S. Key Neuropsychiatric Symptoms in Common Dementias: Prevalence and Implications for Caregivers, Clinicians, and Health Systems. Res. Gerontol. Nurs. 2014, 7, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Caputo, M.; Monastero, R.; Mariani, E.; Santucci, A.; Mangialasche, F.; Camarda, R.; Senin, U.; Me-cocci, P. Neuropsychiatric symptoms in 921 elderly subjects with dementia: A comparison between vascular and neurodegenerative types. Acta Psychiatr. Scand. 2008, 117, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Ballard, C.; Neill, D.; O’Brien, J.; McKeith, I.; Ince, P.; Perry, R. Anxiety, depression and psychosis in vascular dementia: Prevalence and associations. J. Affect. Disord. 2000, 59, 97–106. [Google Scholar] [CrossRef]
- Anor, C.J.; O’Connor, S.; Saund, A.; Tang-Wai, D.F.; Keren, R.; Tartaglia, M.C. Neuropsychiatric Symptoms in Alzheimer Disease, Vascular Dementia, and Mixed Dementia. Neurodegener. Dis. 2017, 17, 127–134. [Google Scholar] [CrossRef] [PubMed]
- D’Onofrio, G.; Sancarlo, D.; Panza, F.; Copetti, M.; Cascavilla, L.; Paris, F.; Seripa, D.; Matera, M.G.; Solfrizzi, V.; Pellegrini, F.; et al. Neuropsychiatric symptoms and functional status in Alzheimer’s disease and vascular dementia patients. Curr. Alzheimer Res. 2012, 9, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Echávarri, C.; Burgmans, S.; Uylings, H.; Cuesta, M.J.; Peralta, V.; Kamphorst, W.; Rozemuller, A.J.; Verhey, F.R. Neuropsychiatric symptoms in Alzheimer’s disease and vascular dementia. J. Alzheimers Dis. 2013, 33, 715–721. [Google Scholar] [CrossRef]
- Groves, W.C.; Brandt, J.; Steinberg, M.; Warren, A.; Rosenblatt, A.; Baker, A.; Lyketsos, C.G. Vascular Dementia and Alzheimer’s Disease: Is There a Difference? A Comparison of Symptoms by Disease Duration. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 305–315. [Google Scholar] [CrossRef]
- Federoff, J.P.; Starkstein, S.E.; Parikh, R.M.; Price, T.R.; Robinson, R.G. Are depressive symptoms nonspecific in patients with acute stroke? Am. J. Psychiatry 1991, 148, 1172–1176. [Google Scholar]
- Brown, F.W.; Lewine, R.J.; Hudgins, P.A.; Risch, S.C. White matter hyperintensity signals in psychiatric and nonpsychiatric subjects. Am. J. Psychiatry 1992, 149, 620–625. [Google Scholar] [PubMed]
- Wolfe, N.; Linn, R.; Babikian, V.L.; Knoefel, J.E.; Albert, M.L. Frontal systems impairment following multiple lacunar infarcts. Arch Neurol. 1990, 47, 1045–1059. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Simpkins, A.N.; Hitomi, E.; Dias, C.; Leigh, R. NIH Natural History of stroke investigators. White Matter Hyperintensity-Associated Blood-Brain Barrier Disruption and Vascular Risk Factors. J. Stroke Cerebrovasc. Dis. 2018, 27, 466–471. [Google Scholar] [CrossRef] [PubMed]
- Ting, S.K.; Hao, Y.; Chia, P.S.; Tan, E.K.; Hameed, S. Clinicopathological correlation of psychosis and brain vascular changes in Alzheimer’s disease. Sci. Rep. 2016, 6, 20858. [Google Scholar] [CrossRef] [PubMed]
- Peavy, G.M.; Salmon, D.P.; Edland, S.D.; Tam, S.; Hansen, L.A.; Masliah, E.; Galasko, D.; Hamilton, J.M. Neuropsychiatric features of frontal lobe dysfunction in autopsy-confirmed patients with lewy bodies and “pure” Alzheimer disease. Am. J. Geriatr. Psychiatry 2013, 21, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Lee, D.Y.; Choo, I.H.; Seo, E.H.; Kim, S.G.; Park, S.Y.; Woo, J.I. Microstructural alteration of the anterior cingulum is associated with apa- thy in Alzheimer disease. Am. J. Geriatr. Psychiatry 2011, 19, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Kee Hyung, P.; Lee, J.Y.; Na, D.L.; Kim, S.Y.; Cheong, H.K.; Moon, S.Y.; Shim, Y.S.; Park, K.W.; Ku, B.D.; Choi, S.H.; et al. Different associa- tions of periventricular and deep white matter lesions with cognition, neuropsychiatric symptoms, and daily activities in dementia. J. Geriatr. Psychiatry Neurol. 2011, 24, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ma, C.; Zhang, J.; Chen, Y.; Chen, K.; Zhang, Z. disrupted white matter structure underlies cognitive deficit in hypertensive patients. Eur. Radiol. 2016, 26, 2899–2907. [Google Scholar] [CrossRef]
- Mou, C.; Han, T.; Wang, M.; Jiang, M.; Liu, B.; Hu, J. Correlation of polymorphism of APOE and LRPgenes to cognitive impairment and behavioral and psychological symptoms of dementia in Alzheimer’s disease and vascular dementia. Int. J. Clin. Exp. Med. 2015, 8, 21679–21683. [Google Scholar]
- Ten, L.; Borson, S.; Kiyak, H.A.; Yamagishi, M. Behavioral disturbance, cognitive dysfunction, and functional skill. Prevalence and relationship in Alzheimer’s disease. J. Am. Geriatr. Soc. 1990, 37, 109–116. [Google Scholar] [CrossRef]
- Teri, L.; Logsdon, R.G.; Peskind, E.; Raskind, M.; Weiner, M.F.; Tractenberg, R.E.; Foster, N.L.; Schneider, L.S.; Sano, M.; Whitehouse, P.; et al. Treatment of agitation in AD: A randomized, placebo-controlled clinical trial. Neurology 2000, 55, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Small, G.W.; Rabins, P.V.; Barry, P.P.; Buckholtz, N.S.; DeKosky, S.T.; Ferris, S.H.; Finkel, S.I.; Gwyther, L.P.; Khachaturian, Z.S.; Lebowitz, B.D.; et al. Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. J. Am. Med. Assoc. 1997, 278, 1363–1371. [Google Scholar] [CrossRef]
- Folstein, M.F.; Bylsma, F.W. Noncognitive symptoms of Alzheimer Disease. In Alzheimer Disease, 2nd ed.; Terry, R.D., Katzmann, R., Bick, K.L., Sisodia, S.S., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 25–37. [Google Scholar]
- Reus, V.I.; Fochtmann, L.J.; Eyler, A.E.; Hilty, D.M.; Horvitz-Lennon, M.; Jibson, M.D.; Lopez, O.L.; Mahoney, J.; Pasic, J.; Tan, Z.S.; et al. The american psychiatric association practice guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Am. J. Psychiatry 2016, 173, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Na, R.; Yang, J.; Yeom, Y.; Kim, Y.J.; Byun, S.; Kim, K.; Kim, K.W. A Systematic Review and Meta-Analysis of Nonpharmacological Interventions for Moderate to Severe Dementia. Psychiatry Investig. 2019, 16, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Abraha, I.; Rimland, J.M.; Trotta, F.M.; Dell’Aquila, G.; Cruz-Jentoft, A.; Petrovic, M.; Gudmundsson, A.; Solza, R.; O’Mahony, D.; Guaita, A.; et al. Systematic review of systematic review of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open 2017, 7, e012759. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Albayrak, A.; van der Cammen, T.J. A systematic review of non-pharmacological interventions for BPSD in nursing home residents with dementia: From a perspective of ergonomics. International Psychogeriatrics. Int. Psychogeriatr. 2018, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Caspar, S.; David, E.D.; Douziech, A.; Scott, D.R. Nonpharmacological Management of Behavioral and Psychological Symptoms of Dementia: What Works, in What Circumstances, and Why? Innov. Aging 2018, 20, 2. [Google Scholar] [CrossRef] [PubMed]
- Lenzer, J. FDA warns about using antipsychotic drugs for dementia. BMJ 2005, 330, 922. [Google Scholar] [CrossRef] [PubMed]
- Katz, I.R.; Jeste, D.V.; Mintzer, J.E.; Clyde, C.; Napolitano, J.; Brecher, M. Comparison of risperidone and placebo for psychosis and behavioural disturbances associated with dementia: A randomized, double-blind trial. J. Clin. Psychiatry 1999, 60, 107–115. [Google Scholar] [CrossRef]
- McManus, D.Q.; Arvanitis, L.A.; Kowalcyk, B.B.; The Seroquel Trial 48 Study Group. Quetiapine, a novel antipsychotic: Experience in elderly patients with psychotic disorders. J. Clin. Psychiatry 1999, 60, 292–298. [Google Scholar] [CrossRef]
- Street, J.S.; Clark, W.S.; Gannon, K.S.; Cummings, J.L.; Bymaster, F.P.; Tamura, R.N.; Mitan, S.J.; Kadam, D.L.; Sanger, T.M.; Feldman, P.D.; et al. Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer Disease in nursing care facilities. Arch. Gen. Psychiatry 2000, 57, 968–976. [Google Scholar] [CrossRef] [PubMed]
- Wooltorton, E. Olanzapine (Zyprexa): Increased incidence of cerebrovascular events in dementia trials. CMAJ 2004, 170, 1395. [Google Scholar] [CrossRef] [PubMed]
- Mowat, D. CSM warning on atypical psychotics and stroke may be detrimental for dementia. BMJ 2004, 328, 1262. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moretti, R.; Torre, P.; Antonello, R.M.; Cattaruzza, T.; Cazzato, G.; Bava, A. Olanzapine as a possible treatment for anxiety due to vascular dementia: An open study. Am. J. Alzheimer’s Dis. Dement. 2004, 19, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Burón, J.A.; Diago, J.I.; Gallego, R. Risperidone in the treatment of behavioural and psychological symptoms of dementia in patients diagnosed with vascular or mixed-type dementia. Int. J. Psychiatry Clin. Pract. 2005, 9, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Suh, G.H.; Son, H.G.; Ju, Y.S.; Jcho, K.H.; Yeon, B.K.; Shin, Y.M.; Kee, B.S.; Choi, S.K. A randomized, double-blind, crossover comparison of risperidone and haloperidol in Korean dementia patients with behavioral disturbances. Am. J. Geriatr. Psychiatry 2004, 12, 509–516. [Google Scholar] [CrossRef]
- Davies, S.J.; Burhan, A.M.; Kim, D.; Gerretsen, P.; Graff-Guerrero, A.; Woo, V.L.; Kumar, S.; Colman, S.; Pollock, B.G.; Mulsant, B.H.; et al. Sequential drug treatment algorithm for agitation and aggression in Alzheimer’s and mixed dementia. J. Psychopharmacol. 2018, 32, 509–523. [Google Scholar] [CrossRef]
- Brodaty, H.; Ames, D.; Snowdon, J.; Woodward, M.; Kirwan, J.; Clarnette, R.; Lee, E.; Lyons, B.; Grossman, F. A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J. Clin. Psychiatry 2003, 64, 134–143. [Google Scholar] [CrossRef]
- Duran, J.C.; Greenspan, A.; Diago, J.I.; Gallego, R.; Martinez, G. Evaluation of risperidone in the treatment of behavioral and psychological symptoms and sleep disturbances associated with dementia. Int. Psychogeriatr. 2005, 17, 591–604. [Google Scholar] [CrossRef]
- Suh, G.H.; Greenspan, A.J.; Choi, S.K. Comparative efficacy of risperidone versus haloperidol on behavioural and psychological symptoms of dementia. Int. J. Geriatr. Psychiatry 2006, 21, 654–660. [Google Scholar] [CrossRef]
- Schneider, L.S.; Dagerman, K.; Insel, P.S. Efficacy and adverse effects of atypical antipsychotics for dementia: Meta-analysis of randomized, placebo-controlled trials. Am. J. Geriatr. Psychiatry 2006, 14, 191–221. [Google Scholar] [CrossRef] [PubMed]
- Cheung, G.; Stapelberg, J. Quetiapine for the treatment of behavioural and psychological symptoms of dementia (BPSD): A meta-analysis of randomised placebo-controlled trials. N. Z. Med. J. 2011, 124, 39–50. [Google Scholar] [PubMed]
- Mintzer, J.E.; Tune, L.E.; Breder, C.D.; Swanink, R.; Marcus, R.N.; McQuade, R.D.; Forbes, A. Aripiprazole for the treatment of psychoses in institutionalized patients with Alzheimer dementia: A multicenter, randomized, double-blind, placebo-controlled assessment of three fixed doses. Am. J. Geriatr. Psychiatry 2007, 15, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Liperoti, R.; Graziano Onder, D.; Landi, F.; Lapane, K.L.; Mor, V.; Bernabei, R.; Gambassi, G. All-cause mortality associated with atypical and conventional antipsychotics among nursing home residents with dementia: A retrospective cohort study. J. Clin. Psychiatry 2009, 70, 1340–1347. [Google Scholar] [CrossRef] [PubMed]
- Huybrechts, K.F.; Rothman, K.J.; Silliman, R.A.; Brookhart, M.A.; Schneeweiss, S. Risk of death and hospital admission for major medical events after initiation of psychotropic medications in older adults admitted to nursing homes. CMAJ 2011, 183, E411–E419. [Google Scholar] [CrossRef] [PubMed]
- Aizenstein, H.J.; Baskys, A.; Boldrini, M.; Butters, M.A.; Diniz, B.S.; Jaiswal, M.K.; Tene, O. Vascular depression consensus report—A critical update. BMC Med. 2016, 14, 161. [Google Scholar] [CrossRef]
- Lohner, V.; Brookes, R.L.; Hollocks, M.J.; Morris, R.G.; Markus, H.S. Apathy, but not depression, is associated with executive dysfunction in cerebral small vessel disease. PLoS ONE 2017, 12, e0176943. [Google Scholar] [CrossRef]
- Staekenborg, S.S.; Su, T.; van Straaten EC, W.; Lane, R.; Scheltens, P.; Barkhof, F.; van der Flier, W.M. Behavioural and psychological symptoms in vascular dementia; differences between small- and large-vessel disease. J. Neurol. Neurosurg. Psychiatry 2009, 81, 547–551. [Google Scholar] [CrossRef]
- Gupta, M.; Khwaja, G.; Patidar, Y.; Dasgupta, A.; Chowdhury, D.; Batra, A. The profile of behavioral and psychological symptoms in vascular cognitive impairment with and without dementia. Ann. Indian Acad. Neurol. 2013, 16, 599. [Google Scholar] [CrossRef]
- Tiel, C.; Sudo, F.K.; Alves, G.S.; Ericeira-Valente, L.; Moreira, D.M.; Laks, J.; Engelhardt, E. Neuropsychiatric symptoms in Vascular Cognitive Impairment: A systematic review. Dement. Neuropsychol. 2015, 9, 230–236. [Google Scholar] [CrossRef]
- Aigbogun, M.S.; Stellhorn, R.; Hartry, A.; Baker, R.A.; Fillit, H. treatment patterns and burden of behavioral disturbances in patients with dementia in the United states: A claims database analysis. BMC Neurol. 2019, 19, 33. [Google Scholar] [CrossRef]
- Lanctôt, K.L.; Amatniek, J.; Ancoli-Israel, S.; Arnold, S.E.; Ballard, C.; Cohen-Mansfield, J.; Boot, B. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2017, 3, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Fuh, J.-L. Neuropsychiatric profiles in patients with Alzheimer’s disease and vascular dementia. J. Neurol. Neurosurg. Psychiatry 2005, 76, 1337–1341. [Google Scholar] [CrossRef] [PubMed]
- Bettney, L.; Butt, S.; Morris, J.; Connolly, A.; McCollum, C.; Burns, A.; Purandare, N. Investigating the stability of neuropsychiatric sub-syndromes with progression of dementia. A 2-year prospective study. Int. J. Geriatr. Psychiatry 2012, 27, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Nagaraja, A.V.; Ratnavalli, E. Neuropsychiatric symptoms in dementia-frequency, relationship to dementia severity and comparison in Alzheimer’s disease, vascular dementia and frontotemporal dementia. J. Neurol. Sci. 2005, 236, 43–48. [Google Scholar] [CrossRef]
- Smith, L.H.; Mallucci, G.R. The unfolded protein response: Mechanisms and therapy for neurodegeneration. Brain 2016, 139, 2113–2121. [Google Scholar] [CrossRef] [PubMed]
- Freeman, O.J.; Mallucci, G.R. The UPR and synaptic dysfunction in neurodegeneration. Brain Res. 2016, 1648, 530–537. [Google Scholar] [CrossRef]
| Authors (Year) | Study Design | Study Population | N | Results | Reference |
|---|---|---|---|---|---|
| Sultzer et al. (1993) | Observational VaD versus AD | American older adults | 104 | Patients with VaD had more severe behavioural retardation, depression and anxiety. | [16] |
| Hargrave et al. (2000) | Observational on patient with VaD versus AD. | American older adults | 378 | Decreased affect/withdrawal and psychomotor retardation were the most prevalent symptoms in patients with VaD. | [20] |
| Saz et al. (2009) | Cross sectional VaD versus AD | Spanish (age >55 yrs) | 4.803 | AD patients had a higher rate of “negative-type” (anhedonia, psychomotor retardation). VaD patients reported increased “affective-type” symptoms. | [23] |
| Ballard et al. (2000) | Prospective VaD versus AD | English older adults | 184 | Anxiety and depression were more common in patients with VaD. | [26] |
| Echávarri et al. (2013) | Retrospective VaD versus AD | Spanish older adults | 80 | Agitation, depression and anxiety in both groups no significance differences. | [29] |
| Groves et al. (2000) | Retrospective VaD, versus AD | American (mean age 76 yrs) | 517 | VaD patients are more depressed and functionally impaired. | [30] |
| Santos et al. (2018) | Retrospective VaD versus MixD | Brazilian older adults (>60 yrs) | 53 | Patients with VaD had more apathy, irritability, anxiety and depression. Association between NPS and mild to moderate dementia. | [19] |
| Anor et al. (2017) | Observational VaD versus AD versus MixD | Canadian older adults | 180 |
| [27] |
| Caputo et al. (2008) | Observational VaD versus AD and Lewy Body dementia | Italian older adults | 921 | VaD had less disrupted behaviors. | [25] |
| D’Onofrio et al. (2012) | Observational | Italian older adults | 302 | AD patients show increased agitation/aggression and irritability/lability. | [28] |
| Sadak et al. (2014) | Retrospective VaD, Lewy body dementia, Frontotemporal dementia versus AD | Americans (>65 yrs) | 3768 | Prevalence of targeted behavioral and psychological symptoms (BPSD) varied according to the aetiology and severity of dementia. | [24] |
| Type of Antipsychotics | Type of Dementia | Mean Dose | Observation Frame | Targeted Symptoms | Side Effects | References |
|---|---|---|---|---|---|---|
| Olanzapine | Vascular cognitive impairment (VCI) | 2.5–5 mg/day | 6 months | Anxiety | somnolence, postural instability, and postural hypotension. | Moretti et al. [56] |
| Risperidone | VaD, AD, Mixed | 1–2 mg/day | 6 months | Aggression, agitation, apathy, depression dysphoria. | Hypotension sedation, paraesthesia. | Alfonso et al. [57] |
| Risperidone | VaD, AD, Mixed | 0.95 mg/day | 12 weeks | Aggression, psychotic symptoms | Somnolence, urinary tract infections. | Brodaty et al. [59] |
| Aripiprazole | AD, VaD, MixD | 2.5–10 mg/day | 10 weeks | Agitation, psychotic symptoms | Cerebrovascular adverse events. | Mintzer et al. [65] |
| Risperidone vs. Haloperidol | VaD, AD, Mixed | 1–1.5 mg/day | 18 weeks | Aggression, anxiety (Risperidone) | Extrapyramidal symptoms (Haloperidol). | Suh et al. [58] |
| Quetiapine | AD, VaD, Lewy body dementia | 300 mg/day | 12 weeks | Psychotic symptoms | NA | Cheung et al. [64] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ambrogio, F.; Martella, L.A.; Odetti, P.; Monacelli, F. Behavioral Disturbances in Dementia and Beyond: Time for a New Conceptual Frame? Int. J. Mol. Sci. 2019, 20, 3647. https://doi.org/10.3390/ijms20153647
Ambrogio F, Martella LA, Odetti P, Monacelli F. Behavioral Disturbances in Dementia and Beyond: Time for a New Conceptual Frame? International Journal of Molecular Sciences. 2019; 20(15):3647. https://doi.org/10.3390/ijms20153647
Chicago/Turabian StyleAmbrogio, Federico, Lucia Anna Martella, Patrizio Odetti, and Fiammetta Monacelli. 2019. "Behavioral Disturbances in Dementia and Beyond: Time for a New Conceptual Frame?" International Journal of Molecular Sciences 20, no. 15: 3647. https://doi.org/10.3390/ijms20153647
APA StyleAmbrogio, F., Martella, L. A., Odetti, P., & Monacelli, F. (2019). Behavioral Disturbances in Dementia and Beyond: Time for a New Conceptual Frame? International Journal of Molecular Sciences, 20(15), 3647. https://doi.org/10.3390/ijms20153647




