A Role for Neutral Sphingomyelinase in Wound Healing Induced by Keratinocyte Proliferation upon 1α, 25-Dihydroxyvitamin D3 Treatment
Abstract
1. Introduction
2. Results
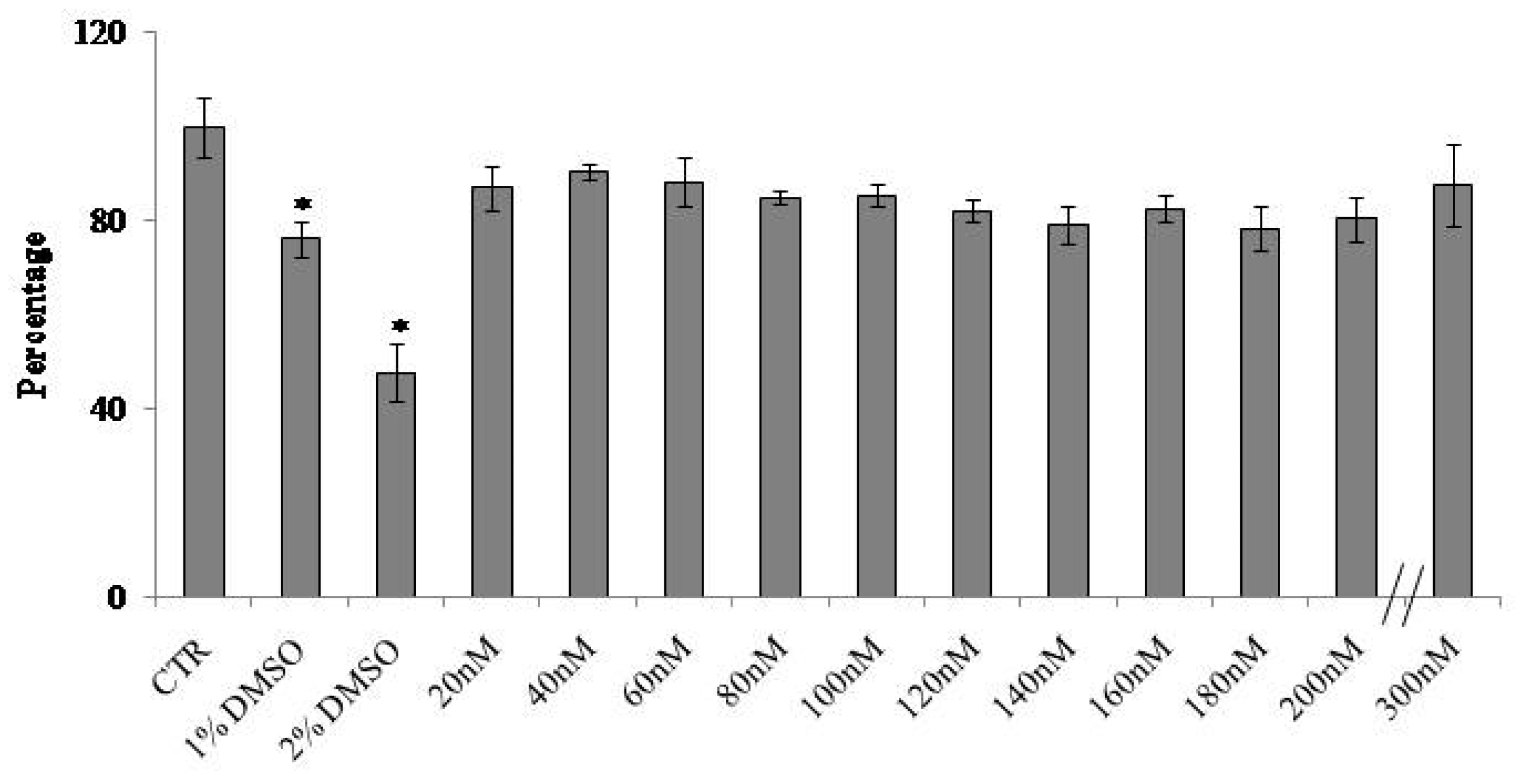
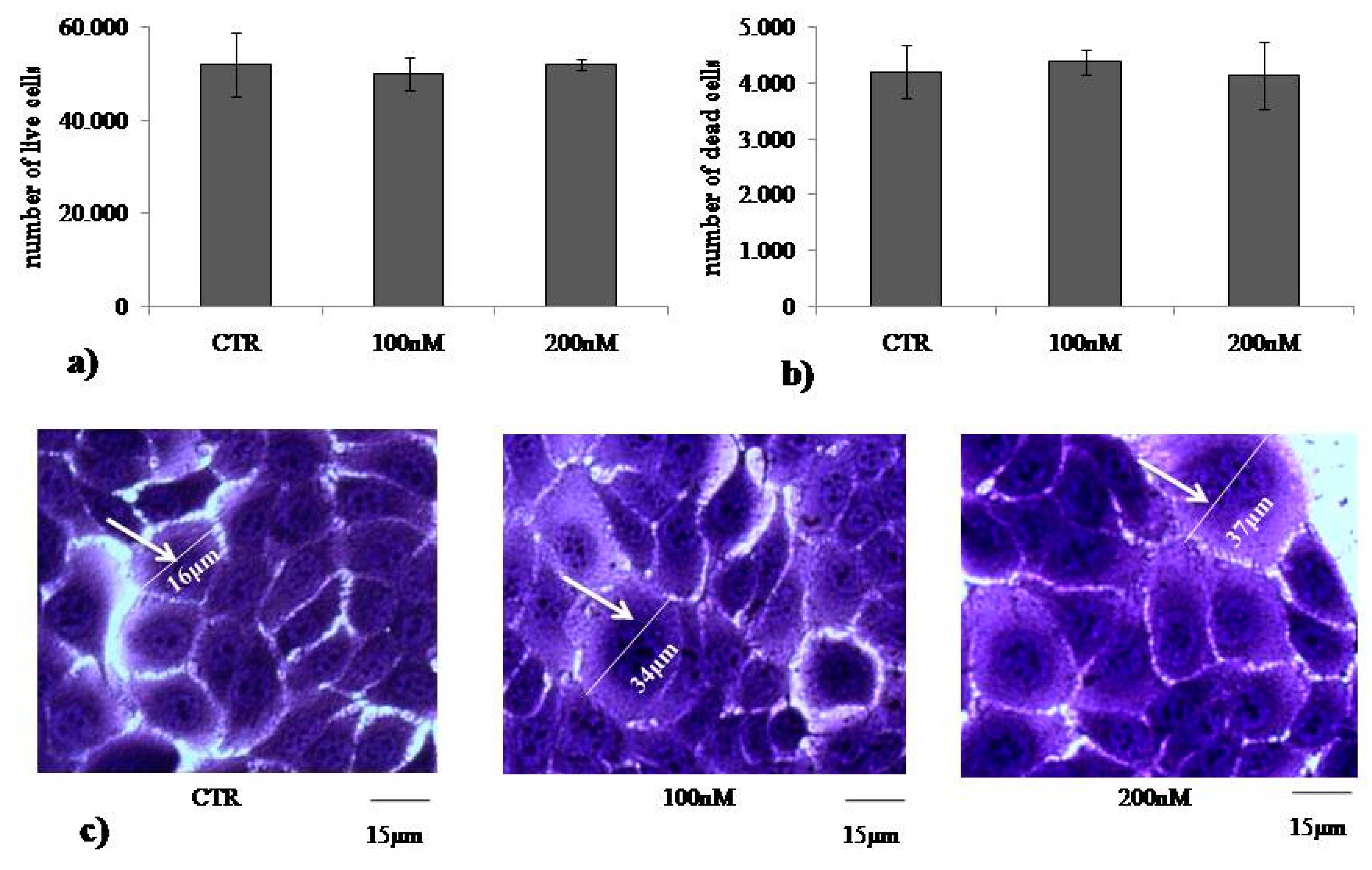
2.1. At Normal Cell Density, Keratinocytes Remain Vital and Increase in Cell Volume with Physiological and High Doses of VD3
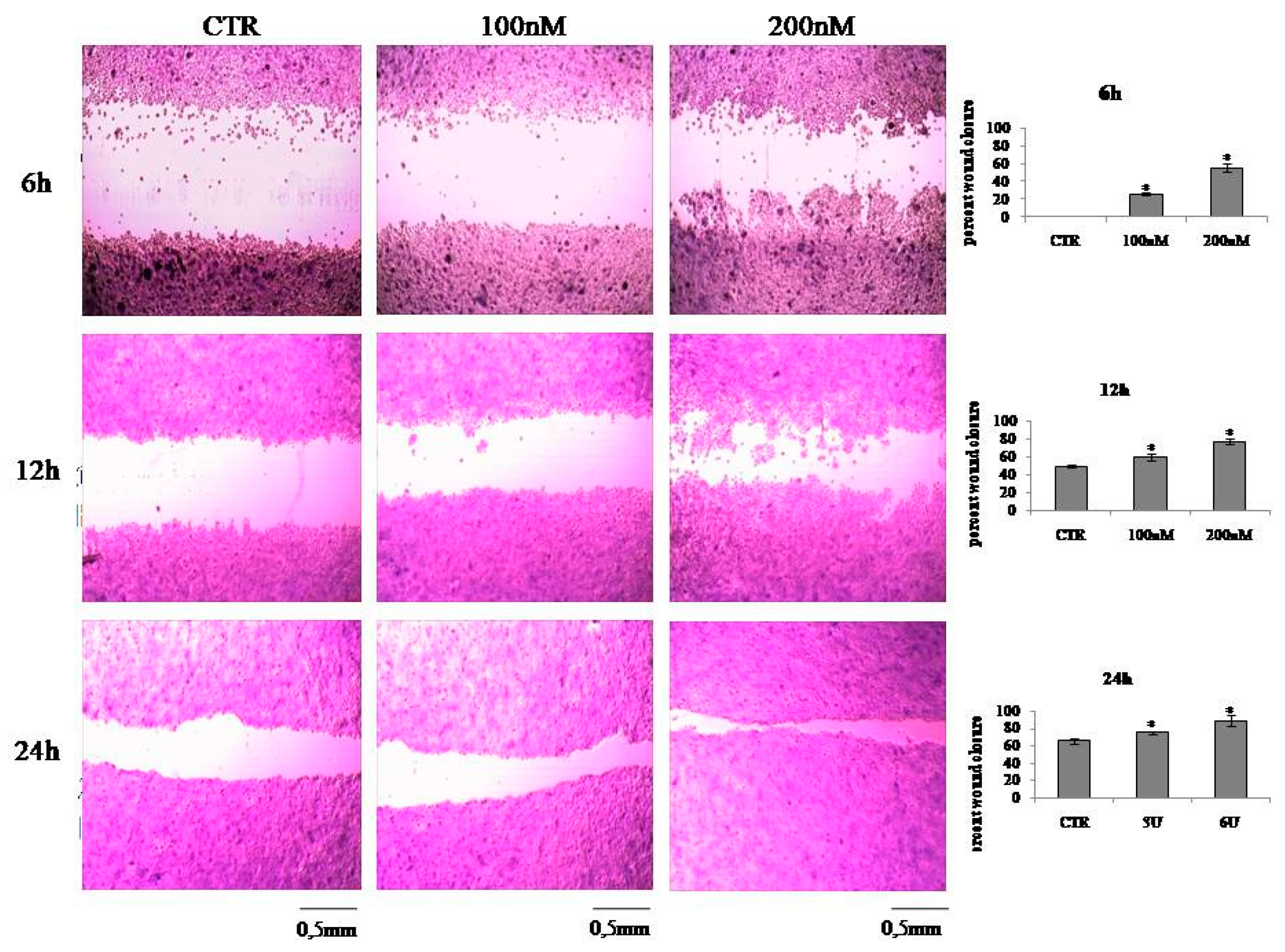
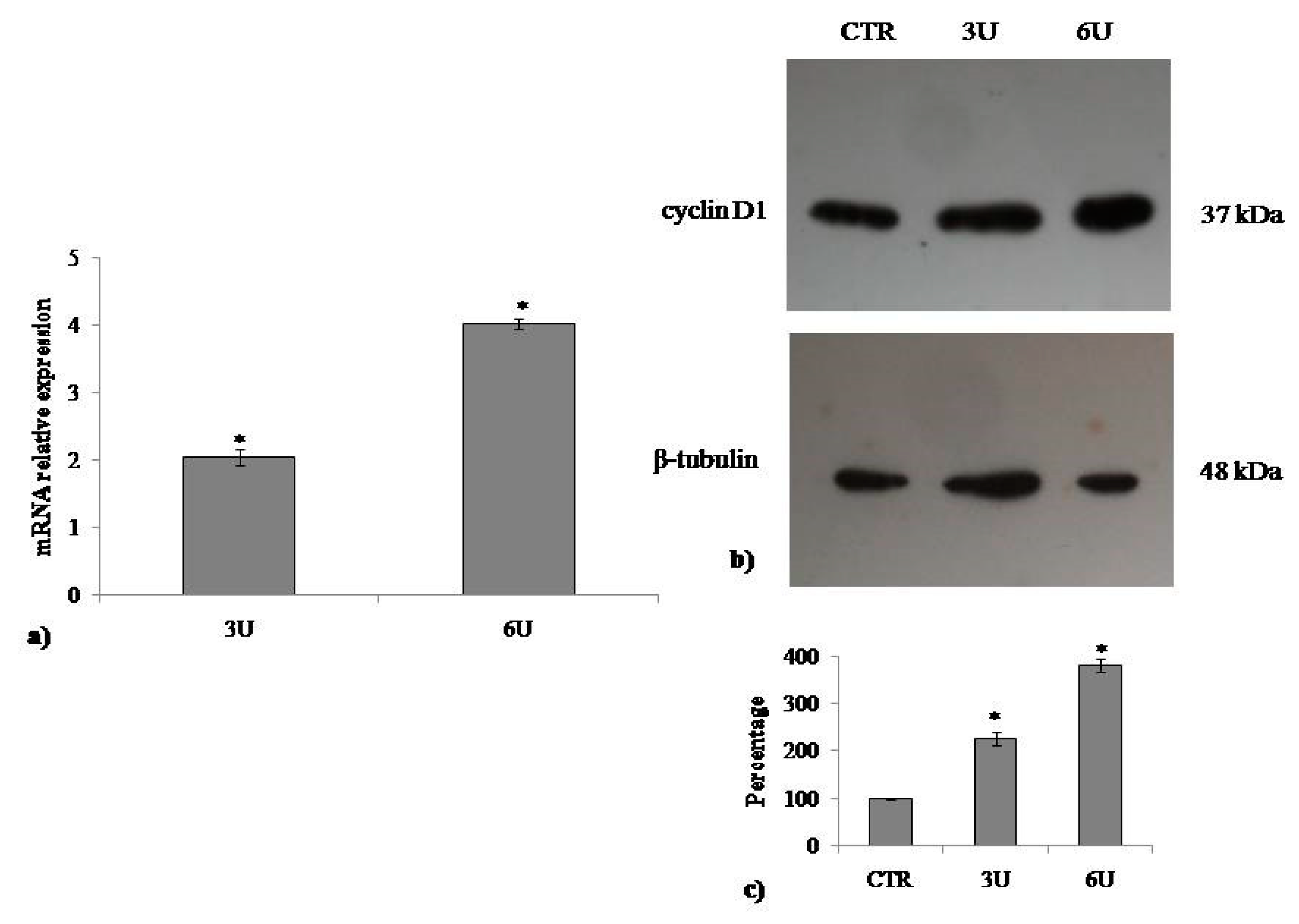
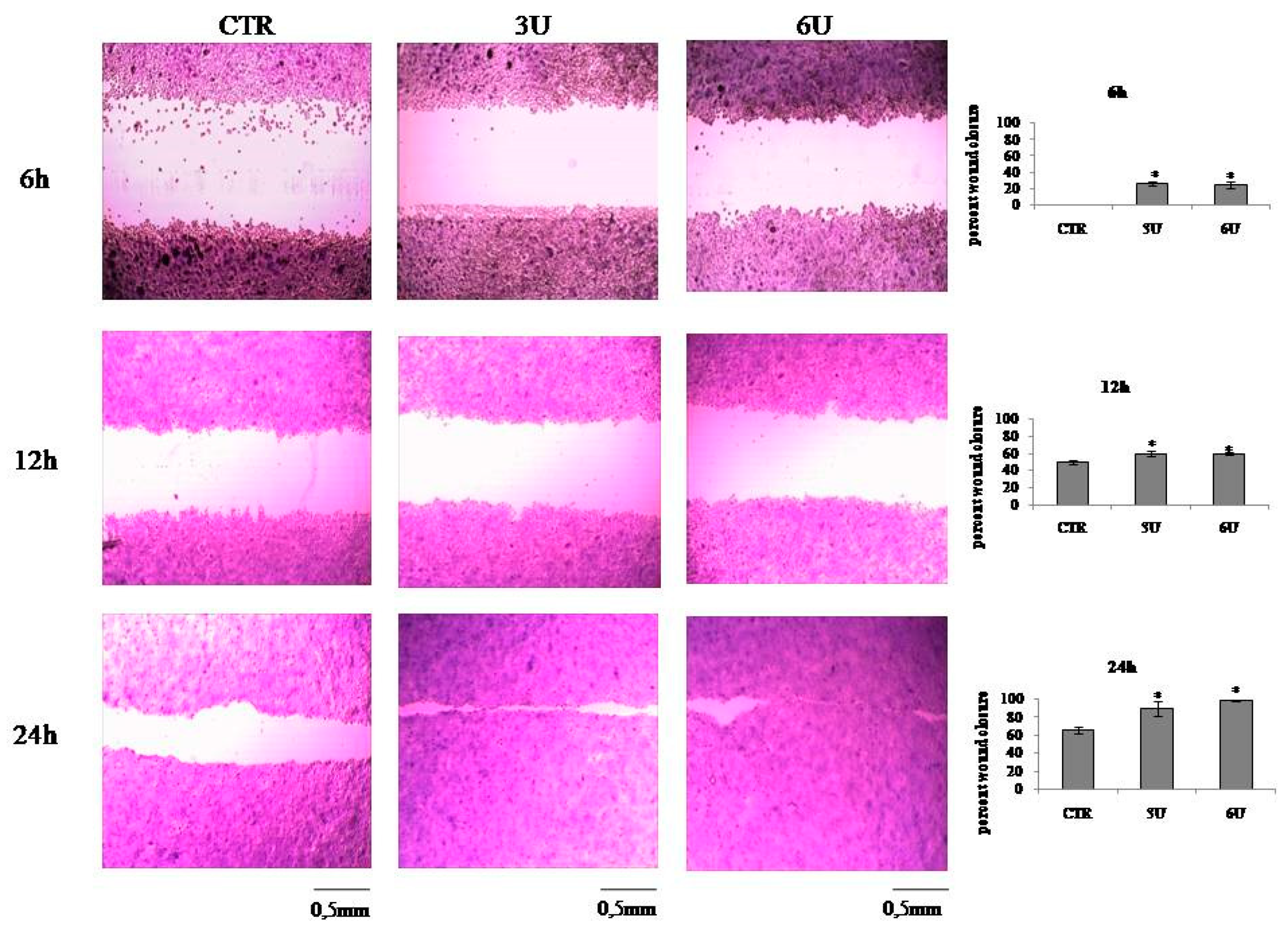
2.2. VD3 Promotes Keratinocite Proliferation in Wound Healing Simulation
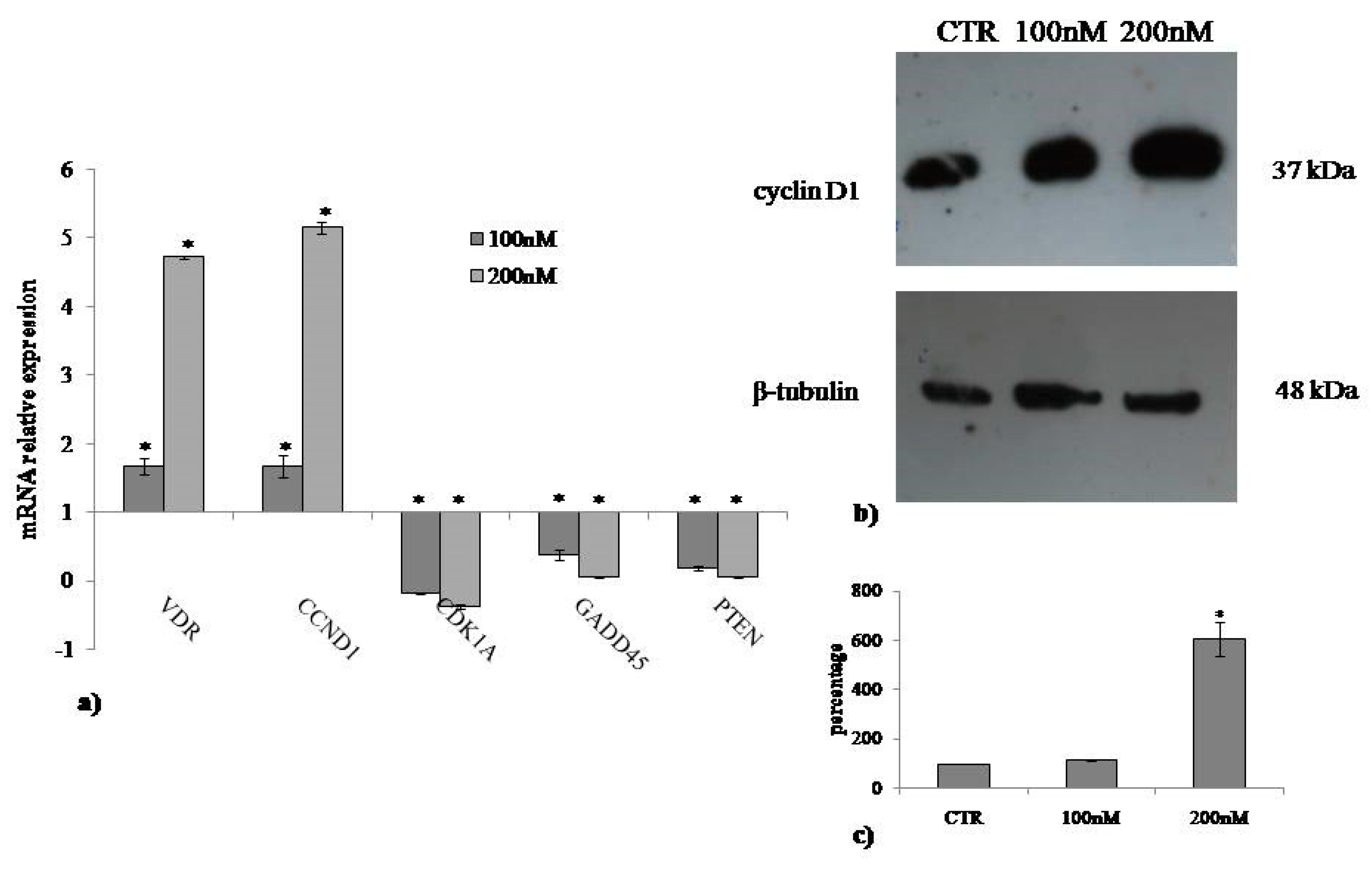
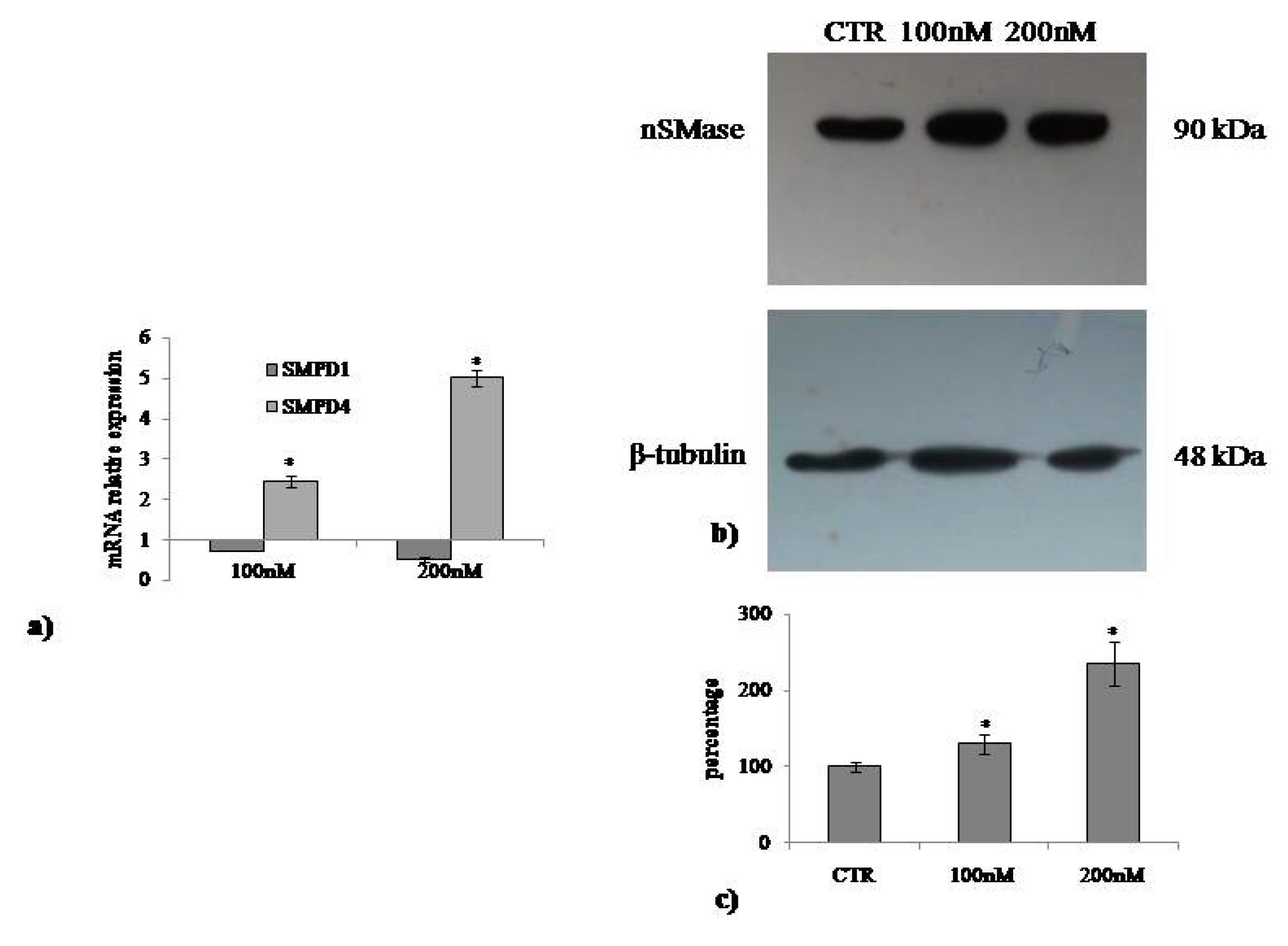
2.3. Involvement of Neutral Sphingomyelinase in VD3-induced Cell Proliferation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Treatments
4.3. Cell Viability
4.4. Trypan Blue Exclusion Assay
4.5. In Vitro Wound Healing Assay
4.6. Quantitative Real-Time RT-PCR
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yousef, H.; Alhajj, M.; Sharma, S. Anatomy, Skin (Integument), Epidermis. In StatPearls Treasure Island (FL); StatPearls Publishing: Petersburg, FL, USA, 2018. [Google Scholar]
- Fischer, M.; Glanz, D.; Urbatzka, M.; Brzoska, T.; Abels, C. Keratinocytes: A source of the transmitter L-glutamate in the epidermis. Exp. Dermatol. 2009, 18, 1064–1066. [Google Scholar] [CrossRef] [PubMed]
- Grando, S.A.; Kist, D.A.; Qi, M.; Dahl, M.V. Human Keratinocytes Synthesize, Secrete, and Degrade Acetylcholine. J. Investig. Dermatol. 1993, 101, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Barr, T.P.; Albrecht, P.J.; Hou, Q.; Mongin, A.A.; Strichartz, G.R.; Rice, F.L. Air-Stimulated ATP Release from Keratinocytes Occurs through Connexin Hemichannels. PLoS ONE 2013, 8, e56744. [Google Scholar] [CrossRef] [PubMed]
- Talagas, M.; Misery, L. Role of Keratinocytes in Sensitive Skin. Front. Med. 2019, 6, 108. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Shimada, M.; Matsumoto, Y.; Okino, A. DNA damage response after ionizing radiation exposure in skin keratinocytes derived from human induced pluripotent stem cells. Int. J. Radiat. Oncol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.; Tsai, M.; Lu, H.; Chang, C.; Cheng, K.; Jhan, M.; Hsieh, C. Enzymatic hydrolysates obtained from Trametes versicolor polysaccharopeptides protect human skin keratinocyte against AAPH-induced oxidative stress and inflammatory. J. Cosmet. Dermatol. 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bellón, T. Mechanisms of Severe Cutaneous Adverse Reactions: Recent Advances. Drug Saf. 2019, 42, 973–992. [Google Scholar] [CrossRef] [PubMed]
- Umar, M.; Sastry, K.S.; Al Ali, F.; Al-Khulaifi, M.; Wang, E.; Chouchane, A.I. Vitamin D and the Pathophysiology of Inflammatory Skin Diseases. Ski. Pharmacol. Physiol. 2018, 31, 74–86. [Google Scholar] [CrossRef]
- Barrea, L.; Savanelli, M.C.; Di Somma, C.; Napolitano, M.; Megna, M.; Colao, A.; Savastano, S. Vitamin D and its role in psoriasis: An overview of the dermatologist and nutritionist. Rev. Endocr. Metab. Disord. 2017, 18, 195–205. [Google Scholar] [CrossRef]
- Bikle, D.D. Vitamin D regulated keratinocyte differentiation. J. Cell. Biochem. 2004, 92, 436–444. [Google Scholar] [CrossRef]
- Chaiprasongsuk, A.; Janjetovic, Z.; Kim, T.K.; Holick, M.F.; Tuckey, R.C.; Panich, U.; Slominski, A.T. Slominsk, Protective effects of novel derivatives of vitamin D3 and lumisterol against UVB-induced damage in human keratinocytes involve activation of Nrf2 and p53 defense mechanisms. Redox. Biol. 2019, 24, 101206. [Google Scholar] [CrossRef] [PubMed]
- Cianferotti, L.; Cox, M.; Skorija, K.; DeMay, M.B. Vitamin D receptor is essential for normal keratinocyte stem cell function. Proc. Natl. Acad. Sci. USA 2007, 104, 9428–9433. [Google Scholar] [CrossRef]
- Garcia-Gil, M.; Pierucci, F.; Vestri, A.; Meacci, E.; Gil, M.D.L.M.G. Crosstalk between sphingolipids and vitamin D3: Potential role in the nervous system. Br. J. Pharmacol. 2017, 174, 605–627. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, A.; Macchiarulo, A.; Floridi, A.; Coletti, A.; Cataldi, S.; Codini, M.; Lazzarini, R.; Bartoccini, E.; Cascianelli, G.; Ambesi-Impiombato, F.S.; et al. Very-long-chain fatty acid sphingomyelin in nuclear lipid microdomains of hepatocytes and hepatoma cells: Can the exchange from C24:0 to C16:0 affect signal proteins and vitamin D receptor? Mol. Biol. Cell 2015, 26, 2418–2425. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, S.; Arcuri, C.; Hunot, S.; Légeron, F.-P.; Mecca, C.; Garcia-Gil, M.; Lazzarini, A.; Codini, M.; Beccari, T.; Tasegian, A.; et al. Neutral Sphingomyelinase Behaviour in Hippocampus Neuroinflammation of MPTP-Induced Mouse Model of Parkinson’s Disease and in Embryonic Hippocampal Cells. Mediat. Inflamm. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Koch, A.; Grammatikos, G.; Trautmann, S.; Schreiber, Y.; Thomas, D.; Bruns, F.; Pfeilschifter, J.; Badenhoop, K.; Penna-Martinez, M. Vitamin D Supplementation Enhances C18(dihydro)ceramide Levels in Type 2 Diabetes Patients. Int. J. Mol. Sci. 2017, 18, 1532. [Google Scholar] [CrossRef]
- Nejatian, N.; Trautmann, S.; Thomas, D.; Pfeilschifter, J.; Badenhoop, K.; Koch, A.; Penna-Martinez, M. Vitamin D effects on sphingosine 1-phosphate signaling and metabolism in monocytes from type 2 diabetes patients and controls. J. Steroid Biochem. Mol. Biol. 2019, 186, 130–135. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Vannini, S.; Cataldi, S.; Moretti, M.; Villarini, M.; Fioretti, B.; Albi, E.; Beccari, T.; Codini, M. In Vitro Protective Effects of Lycium barbarum Berries Cultivated in Umbria (Italy) on Human Hepatocellular Carcinoma Cells. Biolmed Res. Int. 2016, 2016, 7529521. [Google Scholar] [CrossRef]
- Bartoccini, E.; Marini, F.; Damaskopoulou, E.; Lazzarini, R.; Cataldi, S.; Cascianelli, G.; Gil Garcia, M.; Albi, E. Nuclear lipid microdomains regulate nuclear vitamin D3 uptake and influence embryonic hippocampal cell differentiation. Mol. Biol. Cell 2011, 22, 3022–3031. [Google Scholar] [CrossRef]
- Marini, F.; Bartoccini, E.; Cascianelli, G.; Voccoli, V.; Baviglia, M.G.; Magni, M.V.; Garcia-Gil, M.; Albi, E. Effect of 1alpha, 25-dihydroxyvitamin D3 in embryonic hippocampal cells. Hippocampus. 2010, 20, 696–705. [Google Scholar] [CrossRef]
- Yum, C.; Teegarden, D. Regulation of Vitamin D Receptor by 1, 25-dihydroxyvitamin D in Breast Cancer (P05-013-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef]
- Okazaki, T.; Bielawska, A.; Domae, N.; Bell, R.M.; Hannun, Y.A. Characteristics and partial purification of a novel cytosolic, magnesium-independent, neutralsphingomyelinase activated in the early signal transduction of 1 alpha, 25-dihydroxyvitamin D3-induced HL-60 cell differentiation. J. Biol. Chem. 1994, 269, 4070–4077. [Google Scholar]
- Conte, C.; Arcuri, C.; Cataldi, S.; Mecca, C.; Codini, M.; Ceccarini, M.R.; Patria, F.F.; Beccari, T.; Albi, E. Niemann-Pick Type A Disease: Behavior of Neutral Sphingomyelinase and Vitamin D Receptor. Int. J. Mol. Sci. 2019, 20, 2365. [Google Scholar] [CrossRef] [PubMed]
- Marino, R.; Misra, M. Extra-Skeletal Effects of Vitamin D. Nutrients 2019, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Suri, J.S.; Ezaz, G.; Lau, D.T.Y.; Kulai, T.B.; Malhi, H.; Tafesh, Z.H.; Fortune, B.; Russo, N.; Hirsova, P.; Schwartz, R.E.; et al. Hepatology Highlights. Hepatology 2019, 69, 2311–2314. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A.; Pithadia, D.J.; Lee, E.B.; Wu, J.J. Treatments for inverse psoriasis: A systematic review. J. Dermatol. Treat. 2019, 31, 1–8. [Google Scholar] [CrossRef]
- Hill, N.T.; Zhang, J.; Leonard, M.K.; Lee, M.; Shamma, H.N.; Kadakia, M. 1α, 25-Dihydroxyvitamin D₃ and the vitamin D receptor regulates ΔNp63α levels and keratinocyte proliferation. Cell Death Dis. 2015, 6, e1781. [Google Scholar] [CrossRef]
- Di Sante, G.; Di Rocco, A.; Pupo, C.; Casimiro, M.C.; Pestell, R.G. Hormone-induced DNA damage response and repair mediated by cyclin D1 in breast and prostate cancer. Oncotarget 2017, 8, 81803–81812. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Lazzarini, A.; Lazzarini, R.; Floridi, A.; Damaskopoulou, E.; Curcio, F.; Cataldi, S. Nuclear Lipid Microdomain as Place of Interaction between Sphingomyelin and DNA during Liver Regeneration. Int. J. Mol. Sci. 2013, 14, 6529–6541. [Google Scholar] [CrossRef] [PubMed]
- Codini, M.; Cataldi, S.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Floridi, A.; Lazzarini, R.; Curcio, F.; Beccari, T.; Albi, E. Gentamicin Arrests Cancer Cell Growth: The Intriguing Involvement of Nuclear Sphingomyelin Metabolism. Int. J. Mol. Sci. 2015, 16, 2307–2319. [Google Scholar] [CrossRef]
- Ceccarini, M.R.; Codini, M.; Cataldi, S.; Vannini, S.; Lazzarini, A.; Floridi, A.; Moretti, M.; Villarini, M.; Fioretti, B.; Beccari, T.; et al. Acid sphingomyelinase as target of Lycium Chinense: Promising new action for cell health. Lipids Health Dis. 2016, 15, 570. [Google Scholar] [CrossRef] [PubMed]
- Pagano, C.; Ceccarini, M.R.; Calarco, P.; Scuota, S.; Conte, C.; Primavilla, S.; Ricci, M.; Perioli, L. Bioadhesive polymeric films based on usnic acid for burn wound treatment: Antibacterial and cytotoxicity studies. Coll. Surf. B Biointerfaces 2019, 178, 488–499. [Google Scholar] [CrossRef] [PubMed]
- Albi, E.; Cataldi, S.; Ferri, I.; Sidoni, A.; Traina, G.; Fettucciari, K.; Ambesi-Impiombato, F.S.; Lazzarini, A.; Curcio, F.; Ceccarini, M.R.; et al. VDR independent induction of acid-sphingomyelinase by 1,23(OH)2 D3 in gastric cancer cells: Impact on apoptosis and cell morphology. Biochimie 2018, 146, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Codini, M.; Cataldi, S.; Lazzarini, A.; Tasegian, A.; Ceccarini, M.R.; Floridi, A.; Lazzarini, R.; Ambesi-Impiombato, F.S.; Curcio, F.; Beccari, T.; et al. Why high cholesterol levels help hematological malignancies: Role of nuclear lipid microdomains. Lipids Health Dis. 2016, 15, 7438. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patria, F.F.; Ceccarini, M.R.; Codini, M.; Conte, C.; Perioli, L.; Beccari, T.; Albi, E. A Role for Neutral Sphingomyelinase in Wound Healing Induced by Keratinocyte Proliferation upon 1α, 25-Dihydroxyvitamin D3 Treatment. Int. J. Mol. Sci. 2019, 20, 3634. https://doi.org/10.3390/ijms20153634
Patria FF, Ceccarini MR, Codini M, Conte C, Perioli L, Beccari T, Albi E. A Role for Neutral Sphingomyelinase in Wound Healing Induced by Keratinocyte Proliferation upon 1α, 25-Dihydroxyvitamin D3 Treatment. International Journal of Molecular Sciences. 2019; 20(15):3634. https://doi.org/10.3390/ijms20153634
Chicago/Turabian StylePatria, Federica Filomena, Maria Rachele Ceccarini, Michela Codini, Carmela Conte, Luana Perioli, Tommaso Beccari, and Elisabetta Albi. 2019. "A Role for Neutral Sphingomyelinase in Wound Healing Induced by Keratinocyte Proliferation upon 1α, 25-Dihydroxyvitamin D3 Treatment" International Journal of Molecular Sciences 20, no. 15: 3634. https://doi.org/10.3390/ijms20153634
APA StylePatria, F. F., Ceccarini, M. R., Codini, M., Conte, C., Perioli, L., Beccari, T., & Albi, E. (2019). A Role for Neutral Sphingomyelinase in Wound Healing Induced by Keratinocyte Proliferation upon 1α, 25-Dihydroxyvitamin D3 Treatment. International Journal of Molecular Sciences, 20(15), 3634. https://doi.org/10.3390/ijms20153634






