Bakuchiol Suppresses Inflammatory Responses Via the Downregulation of the p38 MAPK/ERK Signaling Pathway
Abstract
:1. Introduction
2. Results
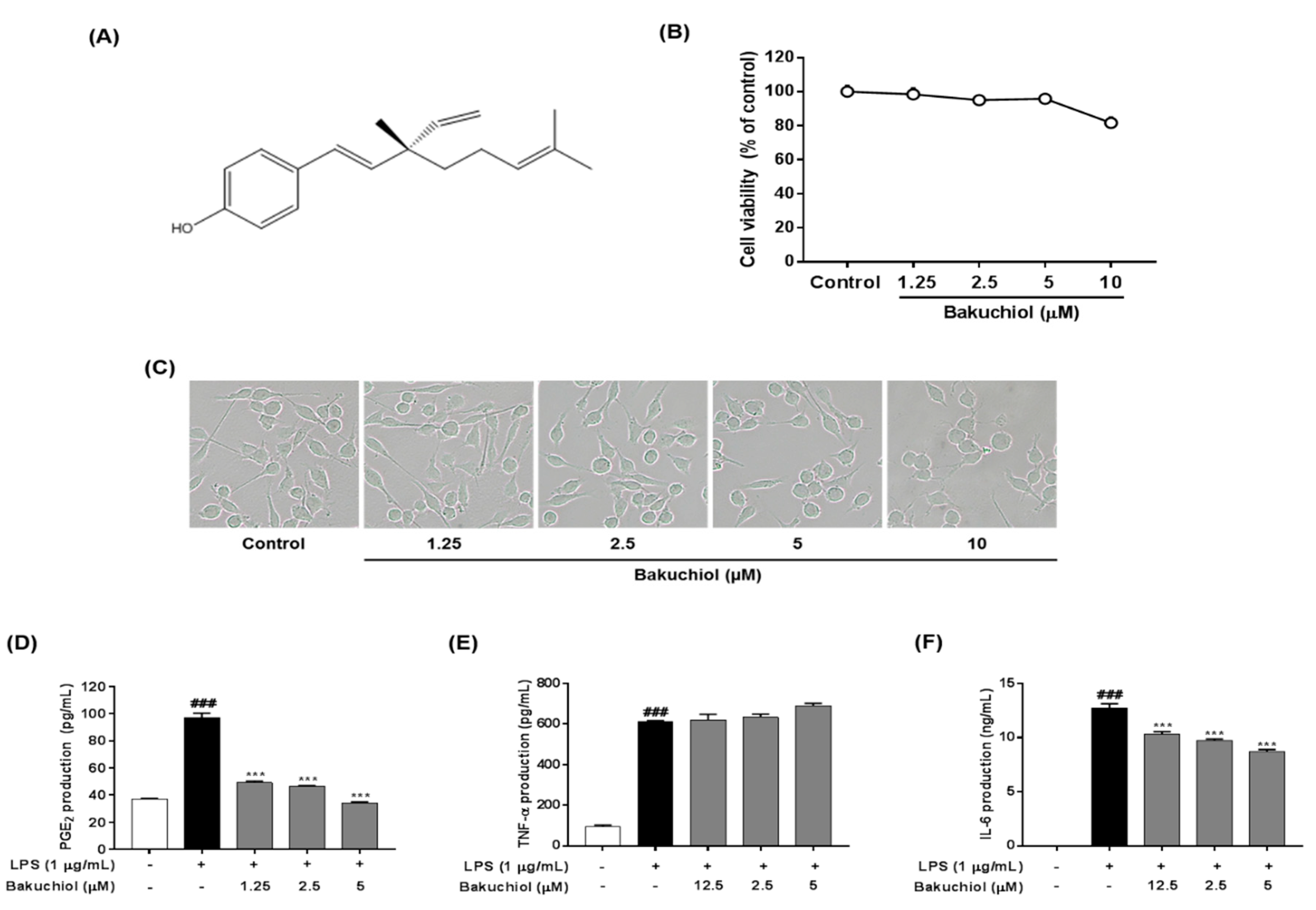
2.1. Effect of Bakuchiol on the Viability of BV-2 Microglia
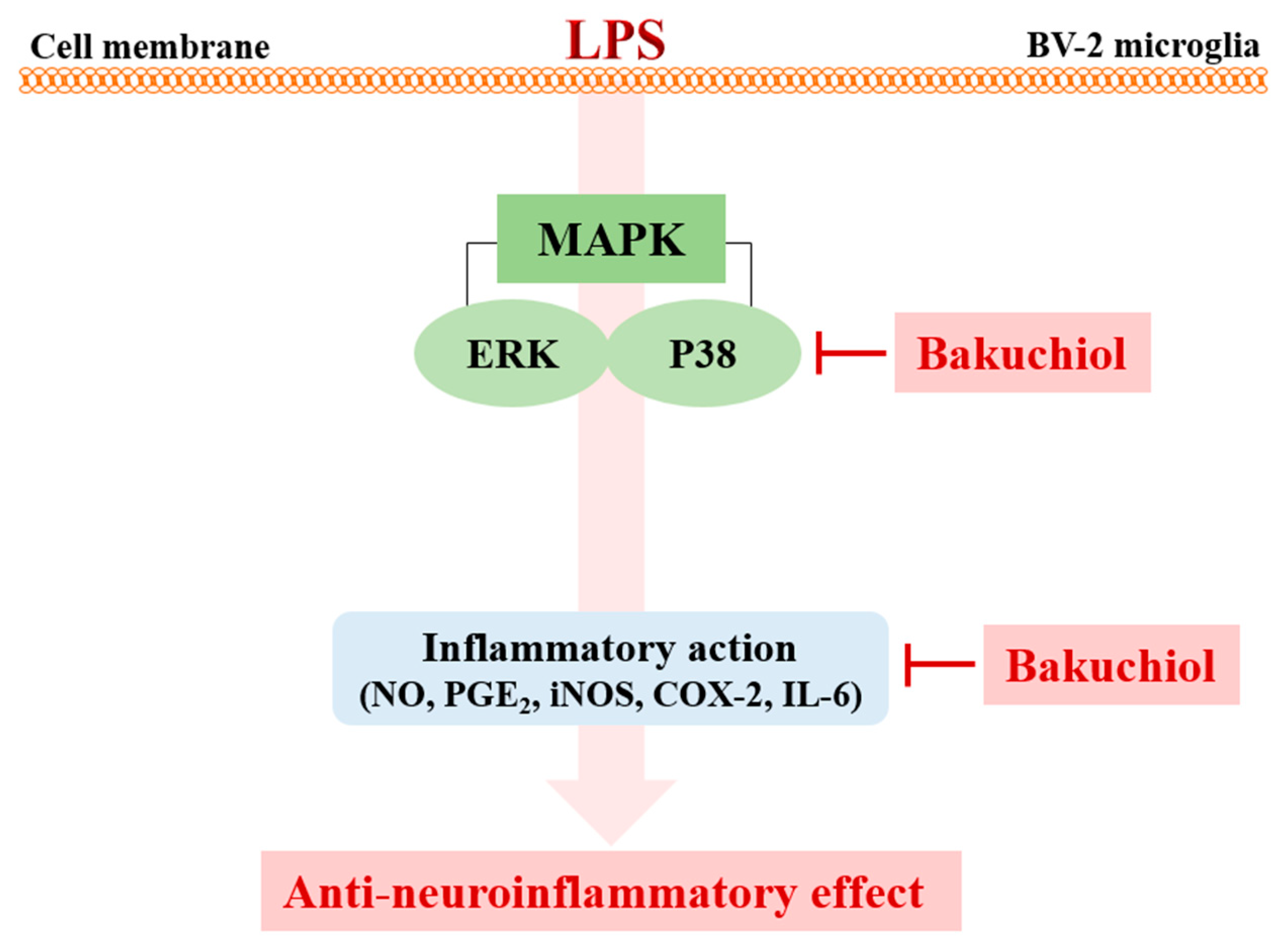
2.2. Bakuchiol Inhibits the Production of PGE2 and IL-6, But not TNF-α, in LPS-induced BV-2 Microglia
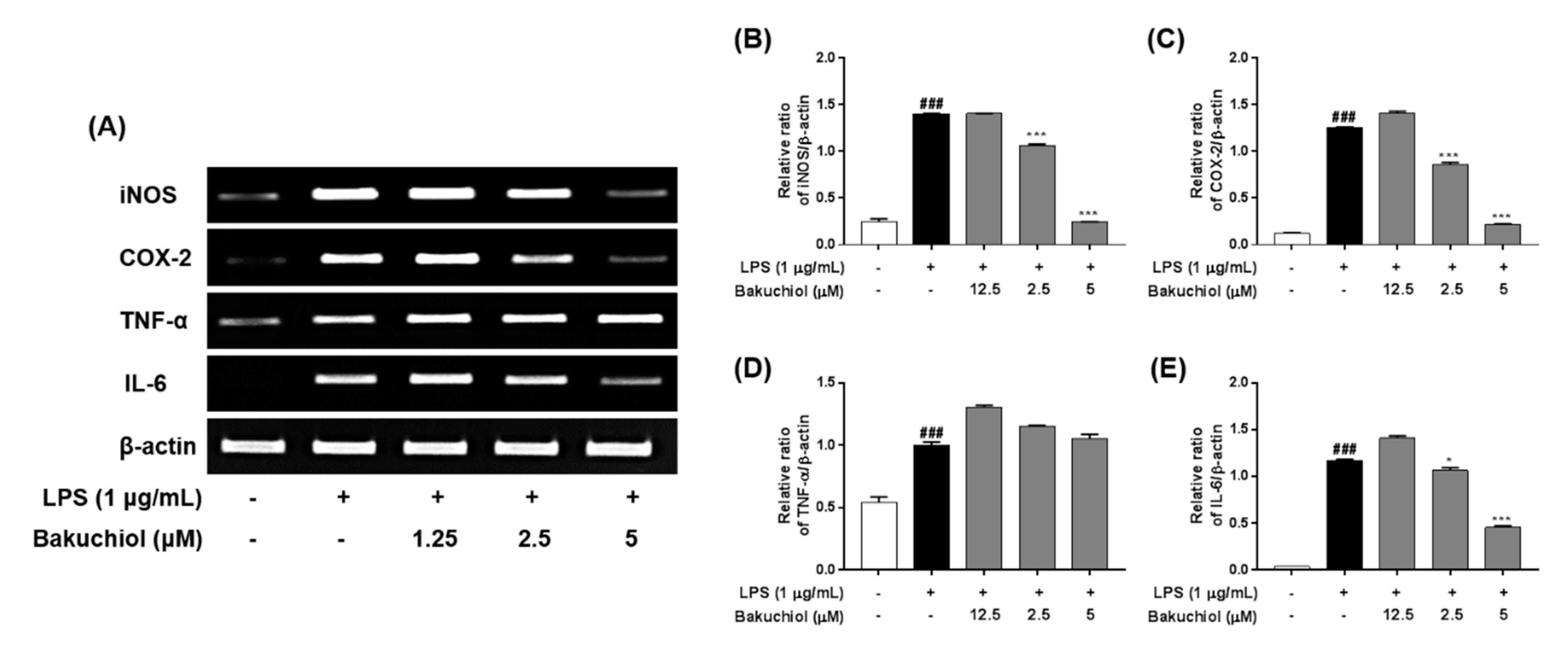
2.3. Bakuchiol Inhibits mRNA Expression of iNOS, COX2, and IL-6, But not TNF-α, in LPS-induced BV-2 Microglia
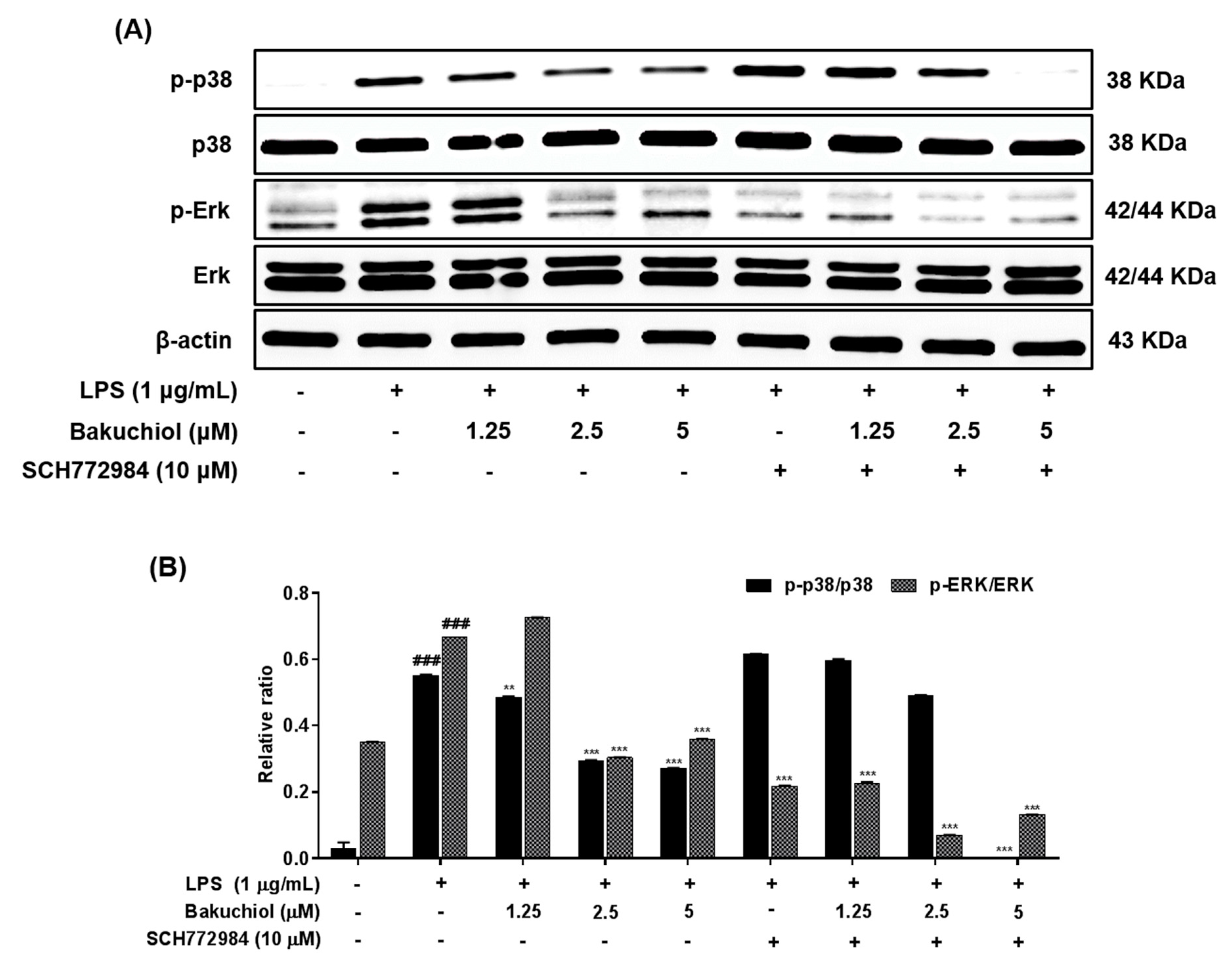
2.4. Bakuchiol Inhibits the Phosphorylation of p38 MAPK and ERK, but not of JNK, in LPS-induced BV-2 Microglia
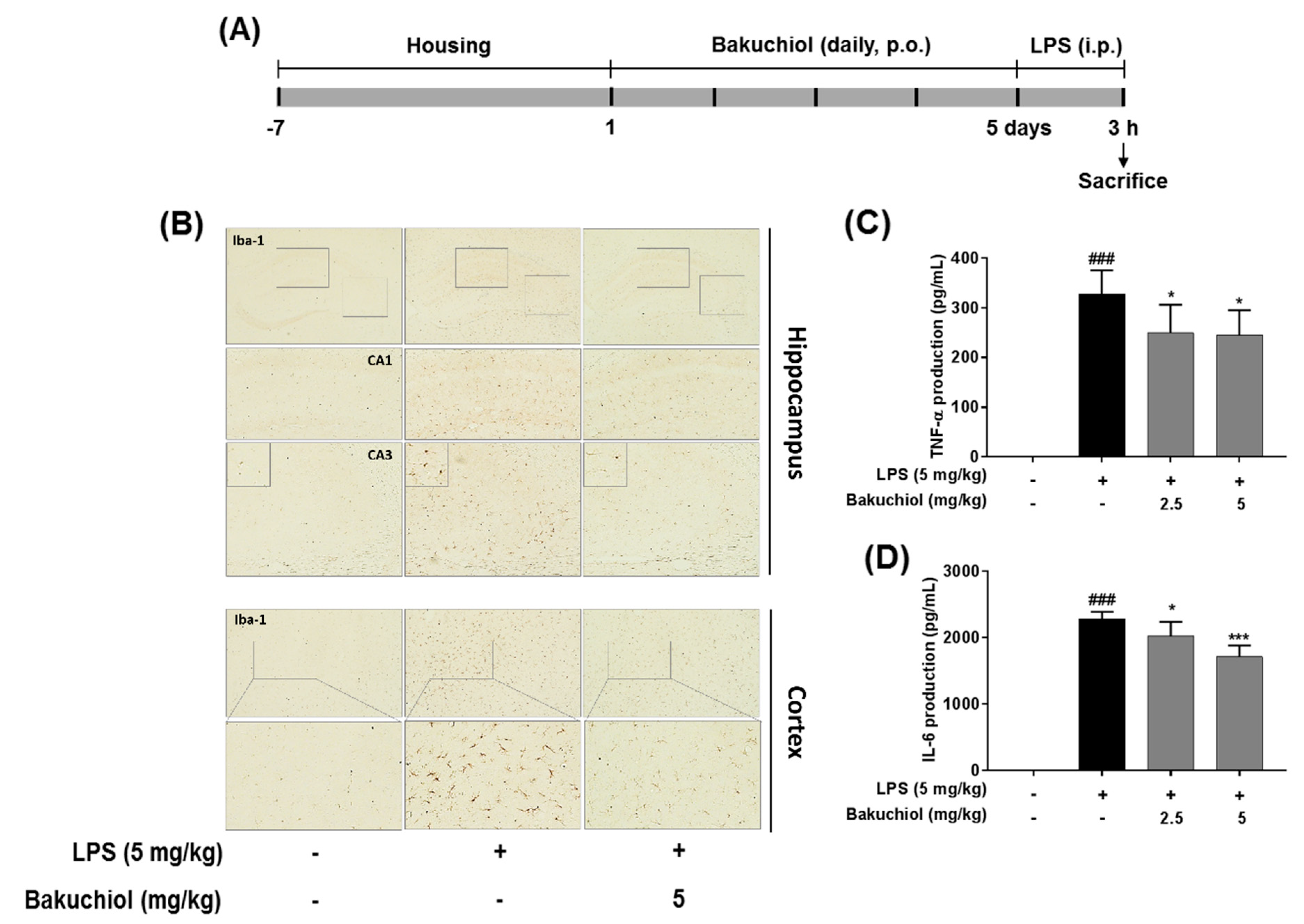
2.5. Effect on Bakuchiol on Microglial Activation and the Production of TNF-α and IL-6 in LPS-induced Mice
3. Discussion
4. Materials and Methods
4.1. Cell Culture, Animals, and Drug Administration
4.2. Cytotoxicity Assay
4.3. Enzyme-linked Immunosorbent Assay for PGE2, TNF-α, and IL-6
4.4. RNA Extraction and Reverse Transcription–Polymerase Chain Reaction Analysis
4.5. Western Blot Analysis
4.6. Immunohistochemistry
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| COX-2 | cycloxygenase-2 |
| ERK | extracellular signal-regulated kinase |
| IL-6 | interleukin-6 |
| IL-1β | interleukin-1β |
| iNOS | inducible nitric oxide synthase |
| JNK | c-Jun N-terminal kinase |
| LPS | lipopolysaccharide |
| MAPK | mitogen-activated protein kinase |
| PGE2 | prostaglandin E2 |
| TNF-α | tumor necrosis factor-α |
References
- Liu, B.; Hong, J.S. Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. J. Pharm. Exp. Ther. 2003, 304, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Joh, T.H. Microglia, major player in the brain inflammation: Their roles in the pathogenesis of Parkinson’s disease. Exp. Mol. Med. 2006, 38, 333–347. [Google Scholar] [CrossRef] [PubMed]
- Bonaiuto, C.; McDonald, P.P.; Rossi, F.; Cassatella, M.A. Activation of nuclear factor-κB by β-amyloid peptides and interferon-γ in murine microglia. J. Neuroimmunol. 1997, 77, 51–56. [Google Scholar] [CrossRef]
- Qin, L.; Li, G.; Qian, X.; Liu, Y.; Wu, X.; Liu, B.; Hong, J.S.; Block, M.L. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia 2005, 52, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Dheen, S.T.; Kaur, C.; Ling, E.A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Amor, S.; Puentes, F.; Baker, D.; van der Valk, P. Inflammation in neurodegenerative diseases. Immunology 2010, 129, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Jin, K.; Gao, L.; Lou, G.; Jin, Y.; Yu, Y.; Lou, Y. Anti-tumor effects of bakuchiol, an analogue of resveratrol, on human lung adenocarcinoma A549 cell line. Eur. J. Pharmacol. 2010, 643, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Ha, T.Y.; Kim, S.R.; Ahn, J.; Park, H.J.; Kim, S. Ethanol extract of Psoralea corylifolia L., and its main constituent, bakuchiol, reduce bone loss in ovariectomised Sprague-Dawley rats. Br. J. Nutr. 2009, 101, 1031–1039. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.A.; Shim, S.H.; Ahn, H.R.; Jung, S.H. Protective effects of the compounds isolated from the seed of Psoralea corylifolia on oxidative stress-induced retinal damage. Toxicol. Appl. Pharmacol. 2013, 269, 109–120. [Google Scholar] [CrossRef]
- Katsura, H.; Tsukiyama, R.I.; Suzuki, A.; Kobayashi, M. In vitro antimicrobial activities of bakuchiol against oral microorganisms. Antimicrob. Agents Chemother. 2001, 45, 3009–3013. [Google Scholar] [CrossRef]
- Seo, E.; Oh, Y.S.; Kim, D.; Lee, M.Y.; Chae, S.; Jun, H.S. Protective role of Psoralea corylifolia L. Seed extract against hepatic mitochondrial dysfunction induced by oxidative stress or aging. Evid. Based Complement. Alternat. Med. 2013, 2013, 678028. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Lee, S.; Choi, W.H.; Lee, Y.; Jo, Y.O.; Ha, T.Y. Isolation and anti-inflammatory activity of Bakuchiol from Ulmus davidiana var. japonica. J. Med. Food. 2010, 13, 1019–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Lim, H.S.; Lee, J.; Jeong, S.J. Quantitative analysis of Psoralea corylifolia Linne and its neuroprotective and anti-neuroinflammatory effects in HT22 hippocampal cells and BV-2 microglia. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms underlying inflammation in neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.A.; Das, A.; Ray, S.K.; Banik, N.L. Role of pro-inflammatory cytokines released from microglia in neurodegenerative diseases. Brain Res. Bull. 2012, 87, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Hyun, T.K.; Kim, M.O.; Lee, H.; Kim, Y.; Kim, E.; Kim, J.S. Evaluation of anti-oxidant and anticancer properties of Dendropanax morbifera Léveille. Food Chem. 2013, 141, 1947–1955. [Google Scholar] [CrossRef]
- Liu, N.; Zheng, J.X.; Zhuang, Y.S.; Zhou, Z.K.; Zhao, J.H.; Yang, L. Anti-Inflammatory Effects of Schisandrin B on LPS-Stimulated BV2 Microglia via Activating PPAR-γ. Inflammation 2017, 40, 1006–1011. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Park, J.S.; Kim, D.H.; Kang, J.L.; Kim, H.S. Anti-inflammatory mechanism of lonchocarpine in LPS- or poly(I:C)-induced neuroinflammation. Pharmacol. Res. 2017, 119, 431–442. [Google Scholar] [CrossRef]
- Bae, K.H. The Medicinal Plants of Korea; Kyo-Hak Publishing Co Ltd.: Seoul, Korea, 2000; p. 364. [Google Scholar]
- Meda, L.; Cassatella, M.A.; Szendrei, G.I.; Otvos, L., Jr.; Baron, P.; Villalba, M.; Ferrari, D.; Rossi, F. Activation of microglial cells by β-amyloid protein and interferon-γ. Nature 1995, 374, 647–650. [Google Scholar] [CrossRef]
- Kang, B.K.; Kim, M.K.; Kim, S.Y.; Lee, S.J.; Choi, Y.W.; Choi, B.T.; Shin, H.K. Anti-Neuroinflammatory Effects of Uncaria sinensis in LPS-Stimulated BV2 Microglia Cells and Focal Cerebral Ischemic Mice. Am. J. Chin. Med. 2015, 43, 1099–1115. [Google Scholar] [CrossRef]
- Inoue, K. The function of microglia through purinergic receptors: Neuropathic pain and cytokine release. Pharmacol. Ther. 2006, 109, 210–226. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Wu, G.Z.; Yang, N.; Shen, Y.H.; Tang, J.; Zhang, W.D. Delavatine A, an unusual isoquinoline alkaloid exerts anti-inflammation on LPS-induced proinflammatory cytokines production by suppressing NF-κB activation in BV-2 microglia. Biochem Biophys Res Commun. 2018, 502, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Raivich, G.; Bohatschek, M.; Kloss, C.U.; Werner, A.; Jones, L.L.; Kreutzberg, G.W. Neuroglial activation repertoire in the injured brain: Graded response, molecular mechanisms and cues to physiological function. Brain Res. Brain Res. Rev. 1999, 30, 77–105. [Google Scholar] [CrossRef]
- Han, L.; Yin, K.; Zhang, S.; Wu, Z.; Zhang, Q.; Pan, J.; Chen, B.; Li, J.; Tan, R.; Xu, Y. Dalesconols B inhibits lipopolysaccharide-induced inflammation and suppresses NF-kappaB and p38/JNK activation in microglial cells. Neurochem. Int. 2013, 62, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.L.; Puig, K.L.; Combs, C.K.; Rosenberger, T.A. Acetate reduces microglia inflammatory signaling in vitro. J. Neurochem. 2012, 123, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Ji, R.R.; Gereau, R.W.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Imai, Y.; Ibata, I.; Ito, D.; Ohsawa, K.; Kohsaka, S. A novel gene iba1 in the major histocompatibility complex class III region encoding an EF hand protein expressed in a monocytic lineage. Biochem. Biophys. Res. Commun. 1996, 224, 855–886. [Google Scholar] [CrossRef]
- Lim, H.S.; Kim, Y.J.; Kim, B.Y.; Park, G.; Jeong, S.J. The Anti-neuroinflammatory Activity of Tectorigenin Pretreatment via Downregulated NF-κB and ERK/JNK Pathways in BV-2 Microglial and Microglia Inactivation in Mice with Lipopolysaccharide. Front. Pharmacol. 2018, 9, 462. [Google Scholar] [CrossRef]

| Gene | Primer Sequences | |
|---|---|---|
| iNOS | Forward | 3′-CCTCCTCCACCCTAGCAAGT-5′ |
| Reverse | 3′-CACCCAAAGTGCTTCAGTCA-5′ | |
| COX-2 | Forward | 3′-AAGACTTGCCAGGCTGAACT-5′ |
| Reverse | 3′-CTTCTGCAGTCCAGGTTCAA-5′ | |
| TNF-α | Forward | 3′-TGGGTAGAGAATGGATGAAC-5′ |
| Reverse | 3′-GCCGATTTGGTATCTCATAC-5′ | |
| IL-6 | Forward | 3′-AAGAGACTTCCATCCAGTTG-5′ |
| Reverse | 3′-TCCAGGTAGCTATGGTACTC-5′ | |
| β-actin | Forward | 3′-TGTGATGGTGGGAATGGGTCAG-5′ |
| Reverse | 3′-TTTGATGTCACGCACGATTTCC-5′ | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-S.; Kim, Y.J.; Kim, B.-Y.; Jeong, S.-J. Bakuchiol Suppresses Inflammatory Responses Via the Downregulation of the p38 MAPK/ERK Signaling Pathway. Int. J. Mol. Sci. 2019, 20, 3574. https://doi.org/10.3390/ijms20143574
Lim H-S, Kim YJ, Kim B-Y, Jeong S-J. Bakuchiol Suppresses Inflammatory Responses Via the Downregulation of the p38 MAPK/ERK Signaling Pathway. International Journal of Molecular Sciences. 2019; 20(14):3574. https://doi.org/10.3390/ijms20143574
Chicago/Turabian StyleLim, Hye-Sun, Yu Jin Kim, Bu-Yeo Kim, and Soo-Jin Jeong. 2019. "Bakuchiol Suppresses Inflammatory Responses Via the Downregulation of the p38 MAPK/ERK Signaling Pathway" International Journal of Molecular Sciences 20, no. 14: 3574. https://doi.org/10.3390/ijms20143574
APA StyleLim, H.-S., Kim, Y. J., Kim, B.-Y., & Jeong, S.-J. (2019). Bakuchiol Suppresses Inflammatory Responses Via the Downregulation of the p38 MAPK/ERK Signaling Pathway. International Journal of Molecular Sciences, 20(14), 3574. https://doi.org/10.3390/ijms20143574





