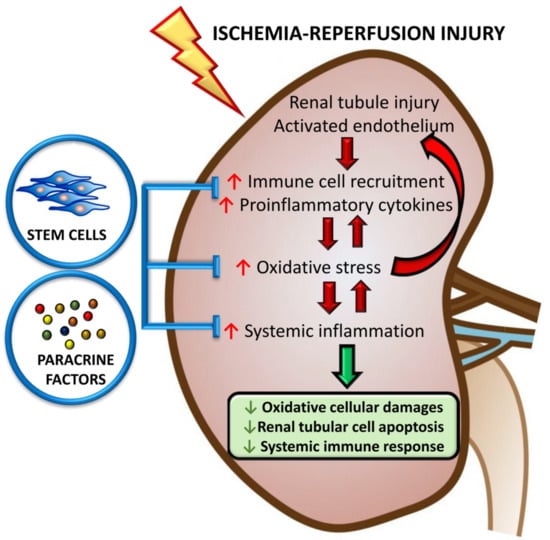
The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury
Abstract
1. Introduction
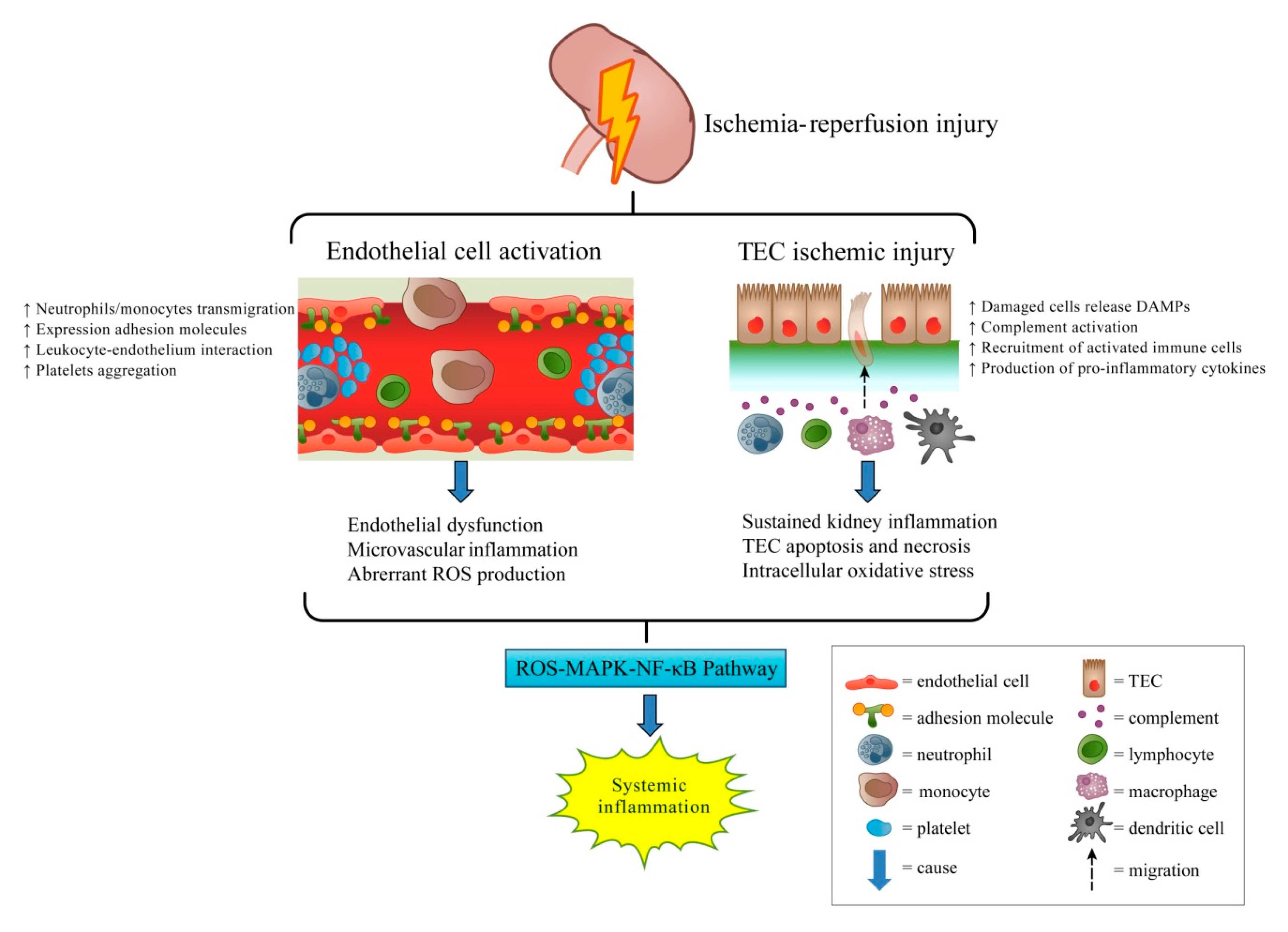
2. Immune Responses and Inflammation in IR-AKI
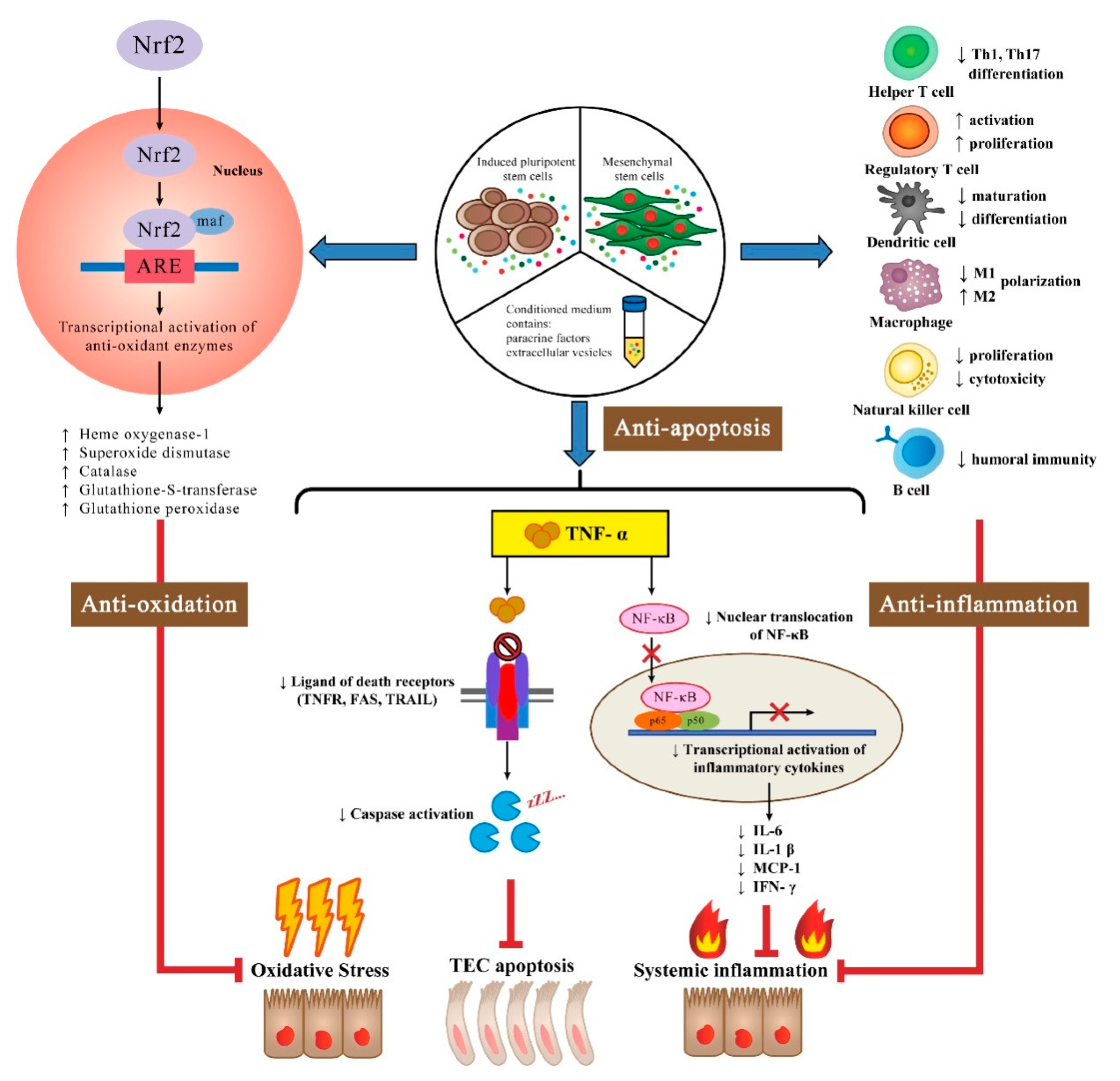
Immunomodulatory Effects of Stem Cells
3. Oxidative Stress in IR-AKI
Antioxidant Effects of Stem Cells
4. Apoptosis of TEC in IR-AKI
Anti-Apoptotic Effects of Stem Cells
5. Stem Cells in the Context of Clinical Use
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Rabb, H.; Griffin, M.D.; McKay, D.B.; Swaminathan, S.; Pickkers, P.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Acute Dialysis Quality Initiative Consensus, X.W.G. Inflammation in AKI: Current Understanding, Key Questions, and Knowledge Gaps. J. Am. Soc. Nephrol. 2016, 27, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Malek, M.; Nematbakhsh, M. Renal ischemia/reperfusion injury; from pathophysiology to treatment. J. Ren. Inj. Prev. 2015, 4, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Holderied, A.; Kumar, S.V.; Anders, H.J. Targeting Inflammation in So-Called Acute Kidney Injury. Semin. Nephrol. 2016, 36, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Huen, S.C.; Cantley, L.G. Macrophages in Renal Injury and Repair. Annu. Rev. Physiol. 2017, 79, 449–469. [Google Scholar] [CrossRef] [PubMed]
- Bonavia, A.; Singbartl, K. A review of the role of immune cells in acute kidney injury. Pediatr. Nephrol. 2018, 33, 1629–1639. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Pino, C.J.; Humes, H.D. Stem cell technology for the treatment of acute and chronic renal failure. Transl. Res. 2010, 156, 161–168. [Google Scholar] [CrossRef][Green Version]
- Pavyde, E.; Usas, A.; Maciulaitis, R. Regenerative pharmacology for the treatment of acute kidney injury: Skeletal muscle stem/progenitor cells for renal regeneration? Pharmacol. Res. 2016, 113, 802–807. [Google Scholar] [CrossRef]
- Zhao, H.; Alam, A.; Soo, A.P.; George, A.J.T.; Ma, D. Ischemia-Reperfusion Injury Reduces Long Term Renal Graft Survival: Mechanism and Beyond. EBioMedicine 2018, 28, 31–42. [Google Scholar] [CrossRef]
- Aghajani Nargesi, A.; Lerman, L.O.; Eirin, A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: Current status and looming challenges. Stem Cell Res. Ther. 2017, 8, 273. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Zou, C. Mesenchymal Stem Cells in Renal Ischemia-Reperfusion Injury: Biological and Therapeutic Perspectives. Curr. Stem Cell Res. Ther. 2017, 12, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; Dong, Z. Renoprotective approaches and strategies in acute kidney injury. Pharmacol. Ther. 2016, 163, 58–73. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Dong, Z.; Harris, R.; Murray, P.; Parikh, S.M.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Acute Dialysis Quality Initiative XIII Working Group. Cellular and Molecular Mechanisms of AKI. J. Am. Soc. Nephrol. 2016, 27, 1288–1299. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhou, H.; Ni, M.; Wang, X.; Busuttil, R.; Kupiec-Weglinski, J.; Zhai, Y. Innate Immune Regulations and Liver Ischemia-Reperfusion Injury. Transplantation 2016, 100, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Kumar, S.V.; Lech, M.; Desai, J.; Anders, H.J. How Kidney Cell Death Induces Renal Necroinflammation. Semin. Nephrol. 2016, 36, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Bolisetty, S.; Zarjou, A.; Agarwal, A. Heme Oxygenase 1 as a Therapeutic Target in Acute Kidney Injury. Am. J. Kidney Dis. 2017, 69, 531–545. [Google Scholar] [CrossRef]
- Chung, S.; Overstreet, J.M.; Li, Y.; Wang, Y.; Niu, A.; Wang, S.; Fan, X.; Sasaki, K.; Jin, G.N.; Khodo, S.N.; et al. TGF-beta promotes fibrosis after severe acute kidney injury by enhancing renal macrophage infiltration. JCI Insight 2018, 3. [Google Scholar] [CrossRef]
- Yi, T.; Song, S.U. Immunomodulatory properties of mesenchymal stem cells and their therapeutic applications. Arch. Pharm. Res. 2012, 35, 213–221. [Google Scholar] [CrossRef]
- Casiraghi, F.; Perico, N.; Cortinovis, M.; Remuzzi, G. Mesenchymal stromal cells in renal transplantation: Opportunities and challenges. Nat. Rev. Nephrol. 2016, 12, 241–253. [Google Scholar] [CrossRef]
- Gao, F.; Chiu, S.M.; Motan, D.A.; Zhang, Z.; Chen, L.; Ji, H.L.; Tse, H.F.; Fu, Q.L.; Lian, Q. Mesenchymal stem cells and immunomodulation: Current status and future prospects. Cell Death Dis. 2016, 7, e2062. [Google Scholar] [CrossRef] [PubMed]
- Ankrum, J.A.; Ong, J.F.; Karp, J.M. Mesenchymal stem cells: Immune evasive, not immune privileged. Nat. Biotechnol. 2014, 32, 252–260. [Google Scholar] [CrossRef] [PubMed]
- McTaggart, S.J.; Atkinson, K. Mesenchymal stem cells: Immunobiology and therapeutic potential in kidney disease. Nephrology 2007, 12, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Benvenuto, F.; Ferrari, S.; Gerdoni, E.; Gualandi, F.; Frassoni, F.; Pistoia, V.; Mancardi, G.; Uccelli, A. Human mesenchymal stem cells promote survival of T cells in a quiescent state. Stem Cells 2007, 25, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Chen, X.; Huang, Y.; Li, W.; Li, J.; Cao, K.; Cao, G.; Zhang, L.; Li, F.; Roberts, A.I.; et al. Phylogenetic distinction of iNOS and IDO function in mesenchymal stem cell-mediated immunosuppression in mammalian species. Cell Death Differ. 2014, 21, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Castro-Manrreza, M.E.; Montesinos, J.J. Immunoregulation by mesenchymal stem cells: Biological aspects and clinical applications. J. Immunol. Res. 2015, 2015, 394917. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.M.; Ritter, T.; Ceredig, R.; Griffin, M.D. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res. Ther. 2011, 2, 34. [Google Scholar] [CrossRef] [PubMed]
- Fitzsimmons, R.E.B.; Mazurek, M.S.; Soos, A.; Simmons, C.A. Mesenchymal Stromal/Stem Cells in Regenerative Medicine and Tissue Engineering. Stem Cells Int. 2018, 2018, 8031718. [Google Scholar] [CrossRef] [PubMed]
- Cahill, E.F.; Tobin, L.M.; Carty, F.; Mahon, B.P.; English, K. Jagged-1 is required for the expansion of CD4+ CD25+ FoxP3+ regulatory T cells and tolerogenic dendritic cells by murine mesenchymal stromal cells. Stem Cell Res. Ther. 2015, 6, 19. [Google Scholar] [CrossRef]
- Ethokic, J.M.; Tomic, S.Z.; Colic, M.J. Cross-Talk Between Mesenchymal Stem/Stromal Cells and Dendritic Cells. Curr. Stem Cell Res. Ther. 2016, 11, 51–65. [Google Scholar]
- Reinders, M.E.; Hoogduijn, M.J. NK Cells and MSCs: Possible Implications for MSC Therapy in Renal Transplantation. J. Stem Cell Res. Ther. 2014, 4, 1000166. [Google Scholar] [CrossRef] [PubMed]
- Rosado, M.M.; Bernardo, M.E.; Scarsella, M.; Conforti, A.; Giorda, E.; Biagini, S.; Cascioli, S.; Rossi, F.; Guzzo, I.; Vivarelli, M.; et al. Inhibition of B-cell proliferation and antibody production by mesenchymal stromal cells is mediated by T cells. Stem Cells Dev. 2015, 24, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.B.; Zhang, X.L.; Tang, Y.L.; Ma, G.S.; Shen, C.X.; Wei, Q.; Zhu, Q.; Yao, Y.Y.; Liu, N.F. Effects of heme oxygenase-1 gene modulated mesenchymal stem cells on vasculogenesis in ischemic swine hearts. Chin. Med. J. 2011, 124, 401–407. [Google Scholar] [PubMed]
- Wang, X.; Wang, S.; Zhou, Y.; Obulkasim, H.; Zhang, Z.H.; Dai, B.; Zhu, W.; Shi, X.L. BMMSCs protect against liver ischemia/reperfusion injury via HO1 mediated autophagy. Mol. Med. Rep. 2018, 18, 2253–2262. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, Y.; Wang, W.; Liu, Z.; Meng, J.; Han, Z. Mesenchymal Stem Cells Modified with Heme Oxygenase-1 Have Enhanced Paracrine Function and Attenuate Lipopolysaccharide-Induced Inflammatory and Oxidative Damage in Pulmonary Microvascular Endothelial Cells. Cell. Physiol. Biochem. 2018, 49, 101–122. [Google Scholar] [CrossRef]
- Zarjou, A.; Kim, J.; Traylor, A.M.; Sanders, P.W.; Balla, J.; Agarwal, A.; Curtis, L.M. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am. J. Physiol. Ren. Physiol. 2011, 300, F254–F262. [Google Scholar] [CrossRef]
- Liu, D.; Xu, J.; Liu, O.; Fan, Z.; Liu, Y.; Wang, F.; Ding, G.; Wei, F.; Zhang, C.; Wang, S. Mesenchymal stem cells derived from inflamed periodontal ligaments exhibit impaired immunomodulation. J. Clin. Periodontol. 2012, 39, 1174–1182. [Google Scholar] [CrossRef]
- Najar, M.; Krayem, M.; Meuleman, N.; Bron, D.; Lagneaux, L. Mesenchymal Stromal Cells and Toll-Like Receptor Priming: A Critical Review. Immune Netw. 2017, 17, 89–102. [Google Scholar] [CrossRef]
- Waterman, R.S.; Tomchuck, S.L.; Henkle, S.L.; Betancourt, A.M. A new mesenchymal stem cell (MSC) paradigm: Polarization into a pro-inflammatory MSC1 or an Immunosuppressive MSC2 phenotype. PLoS ONE 2010, 5, e10088. [Google Scholar] [CrossRef]
- Levin, S.; Pevsner-Fischer, M.; Kagan, S.; Lifshitz, H.; Weinstock, A.; Gataulin, D.; Friedlander, G.; Zipori, D. Divergent levels of LBP and TGFbeta1 in murine MSCs lead to heterogenic response to TLR and proinflammatory cytokine activation. Stem Cell Rev. 2014, 10, 376–388. [Google Scholar] [CrossRef]
- Schnabel, L.V.; Abratte, C.M.; Schimenti, J.C.; Felippe, M.J.; Cassano, J.M.; Southard, T.L.; Cross, J.A.; Fortier, L.A. Induced pluripotent stem cells have similar immunogenic and more potent immunomodulatory properties compared with bone marrow-derived stromal cells in vitro. Regen. Med. 2014, 9, 621–635. [Google Scholar] [CrossRef]
- Lee, P.Y.; Chien, Y.; Chiou, G.Y.; Lin, C.H.; Chiou, C.H.; Tarng, D.C. Induced pluripotent stem cells without c-Myc attenuate acute kidney injury via downregulating the signaling of oxidative stress and inflammation in ischemia-reperfusion rats. Cell Transplant. 2012, 21, 2569–2585. [Google Scholar] [CrossRef]
- Yen, B.L.; Chang, C.J.; Liu, K.J.; Chen, Y.C.; Hu, H.I.; Bai, C.H.; Yen, M.L. Brief report—Human embryonic stem cell-derived mesenchymal progenitors possess strong immunosuppressive effects toward natural killer cells as well as T lymphocytes. Stem Cells 2009, 27, 451–456. [Google Scholar] [CrossRef]
- Gao, W.X.; Sun, Y.Q.; Shi, J.; Li, C.L.; Fang, S.B.; Wang, D.; Deng, X.Q.; Wen, W.; Fu, Q.L. Effects of mesenchymal stem cells from human induced pluripotent stem cells on differentiation, maturation, and function of dendritic cells. Stem Cell Res. Ther. 2017, 8, 48. [Google Scholar] [CrossRef]
- Tan, Z.; Su, Z.Y.; Wu, R.R.; Gu, B.; Liu, Y.K.; Zhao, X.L.; Zhang, M. Immunomodulative effects of mesenchymal stem cells derived from human embryonic stem cells in vivo and in vitro. J. Zhejiang Univ. Sci. B 2011, 12, 18–27. [Google Scholar] [CrossRef]
- Korthuis, R.J. Mechanisms of I/R-Induced Endothelium-Dependent Vasodilator Dysfunction. Adv. Pharmacol. 2018, 81, 331–364. [Google Scholar] [CrossRef]
- Gonzalez-Vicente, A.; Garvin, J.L. Effects of Reactive Oxygen Species on Tubular Transport along the Nephron. Antioxidants 2017, 6, 23. [Google Scholar] [CrossRef]
- Marko, L.; Vigolo, E.; Hinze, C.; Park, J.K.; Roel, G.; Balogh, A.; Choi, M.; Wubken, A.; Cording, J.; Blasig, I.E.; et al. Tubular Epithelial NF-kappaB Activity Regulates Ischemic AKI. J. Am. Soc. Nephrol. 2016, 27, 2658–2669. [Google Scholar] [CrossRef]
- Shih, Y.C.; Lee, P.Y.; Cheng, H.; Tsai, C.H.; Ma, H.; Tarng, D.C. Adipose-derived stem cells exhibit antioxidative and antiapoptotic properties to rescue ischemic acute kidney injury in rats. Plast. Reconstr. Surg. 2013, 132, 940e–951e. [Google Scholar] [CrossRef]
- Zhang, J.B.; Wang, X.Q.; Lu, G.L.; Huang, H.S.; Xu, S.Y. Adipose-derived mesenchymal stem cells therapy for acute kidney injury induced by ischemia-reperfusion in a rat model. Clin. Exp. Pharmacol. Physiol. 2017, 44, 1232–1240. [Google Scholar] [CrossRef]
- Havakhah, S.; Sankian, M.; Kazemzadeh, G.H.; Sadri, K.; Bidkhori, H.R.; Naderi-Meshkin, H.; Ebrahimzadeh Bideskan, A.; Niazmand, S.; Bahrami, A.R.; Khajavi Rad, A. In vivo effects of allogeneic mesenchymal stem cells in a rat model of acute ischemic kidney injury. Iran. J. Basic Med. Sci. 2018, 21, 824–831. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, T.M.; Kim, K.S.; Lee, S.; Cho, J.; Park, J.B.; Kwon, G.Y.; Kim, S.J. Renal Ischemia-Reperfusion Injury in a Diabetic Monkey Model and Therapeutic Testing of Human Bone Marrow-Derived Mesenchymal Stem Cells. J. Diabetes Res. 2018, 2018, 5182606. [Google Scholar] [CrossRef]
- Orbay, H.; Tobita, M.; Mizuno, H. Mesenchymal stem cells isolated from adipose and other tissues: Basic biological properties and clinical applications. Stem Cells Int. 2012, 2012, 461718. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Zhu, W.; Ren, H.Z.; Zhao, X.; Wang, S.; Ma, H.C.; Shi, X.L. Mesenchymal stem cells increase expression of heme oxygenase-1 leading to anti-inflammatory activity in treatment of acute liver failure. Stem Cell Res. Ther. 2017, 8, 70. [Google Scholar] [CrossRef]
- Sung, P.H.; Chang, C.L.; Tsai, T.H.; Chang, L.T.; Leu, S.; Chen, Y.L.; Yang, C.C.; Chua, S.; Yeh, K.H.; Chai, H.T.; et al. Apoptotic adipose-derived mesenchymal stem cell therapy protects against lung and kidney injury in sepsis syndrome caused by cecal ligation puncture in rats. Stem Cell Res. Ther. 2013, 4, 155. [Google Scholar] [CrossRef]
- Du, T.; Cheng, J.; Zhong, L.; Zhao, X.F.; Zhu, J.; Zhu, Y.J.; Liu, G.H. The alleviation of acute and chronic kidney injury by human Wharton’s jelly-derived mesenchymal stromal cells triggered by ischemia-reperfusion injury via an endocrine mechanism. Cytotherapy 2012, 14, 1215–1227. [Google Scholar] [CrossRef]
- Zhang, D.; Fu, L.; Wang, L.; Lin, L.; Yu, L.; Zhang, L.; Shang, T. Therapeutic benefit of mesenchymal stem cells in pregnant rats with angiotensin receptor agonistic autoantibody-induced hypertension: Implications for immunomodulation and cytoprotection. Hypertens. Pregnancy 2017, 36, 247–258. [Google Scholar] [CrossRef]
- Camara, N.O.; Soares, M.P. Heme oxygenase-1 (HO-1), a protective gene that prevents chronic graft dysfunction. Free Radic. Biol. Med. 2005, 38, 426–435. [Google Scholar] [CrossRef]
- Liu, N.; Wang, H.; Han, G.; Tian, J.; Hu, W.; Zhang, J. Alleviation of apoptosis of bone marrow-derived mesenchymal stem cells in the acute injured kidney by heme oxygenase-1 gene modification. Int. J. Biochem. Cell Biol. 2015, 69, 85–94. [Google Scholar] [CrossRef]
- Turkseven, S.; Kruger, A.; Mingone, C.J.; Kaminski, P.; Inaba, M.; Rodella, L.F.; Ikehara, S.; Wolin, M.S.; Abraham, N.G. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H701–H707. [Google Scholar] [CrossRef]
- Tsai, M.T.; Tarng, D.C. Beyond a Measure of Liver Function-Bilirubin Acts as a Potential Cardiovascular Protector in Chronic Kidney Disease Patients. Int. J. Mol. Sci. 2018, 20, 117. [Google Scholar] [CrossRef]
- Liu, H.; McTaggart, S.J.; Johnson, D.W.; Gobe, G.C. Anti-oxidant pathways are stimulated by mesenchymal stromal cells in renal repair after ischemic injury. Cytotherapy 2012, 14, 162–172. [Google Scholar] [CrossRef]
- Agarwal, A.; Bolisetty, S. Adaptive responses to tissue injury: Role of heme oxygenase-1. Trans. Am. Clin. Climatol. Assoc. 2013, 124, 111–122. [Google Scholar]
- Liu, N.; Wang, H.; Han, G.; Cheng, J.; Hu, W.; Zhang, J. Enhanced proliferation and differentiation of HO-1 gene-modified bone marrow-derived mesenchymal stem cells in the acute injured kidney. Int. J. Mol. Med. 2018, 42, 946–956. [Google Scholar] [CrossRef]
- Mates, J.M. Effects of antioxidant enzymes in the molecular control of reactive oxygen species toxicology. Toxicology 2000, 153, 83–104. [Google Scholar] [CrossRef]
- Zhuo, W.; Liao, L.; Xu, T.; Wu, W.; Yang, S.; Tan, J. Mesenchymal stem cells ameliorate ischemia-reperfusion-induced renal dysfunction by improving the antioxidant/oxidant balance in the ischemic kidney. Urol. Int. 2011, 86, 191–196. [Google Scholar] [CrossRef]
- Fahmy, S.R.; Soliman, A.M.; El Ansary, M.; Elhamid, S.A.; Mohsen, H. Therapeutic efficacy of human umbilical cord mesenchymal stem cells transplantation against renal ischemia/reperfusion injury in rats. Tissue Cell 2017, 49, 369–375. [Google Scholar] [CrossRef]
- Inan, M.; Bakar, E.; Cerkezkayabekir, A.; Sanal, F.; Ulucam, E.; Subasi, C.; Karaoz, E. Mesenchymal stem cells increase antioxidant capacity in intestinal ischemia/reperfusion damage. J. Pediatr. Surg. 2017, 52, 1196–1206. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Huang, Y.; Wang, F.; Miao, S.; Liu, G.; Chen, M.; Zhu, Y. Mesenchymal Stromal Cell-Derived Extracellular Vesicles Protect Against Acute Kidney Injury Through Anti-Oxidation by Enhancing Nrf2/ARE Activation in Rats. Kidney Blood Press. Res. 2016, 41, 119–128. [Google Scholar] [CrossRef]
- Zhang, G.; Zou, X.; Miao, S.; Chen, J.; Du, T.; Zhong, L.; Ju, G.; Liu, G.; Zhu, Y. The anti-oxidative role of micro-vesicles derived from human Wharton-Jelly mesenchymal stromal cells through NOX2/gp91(phox) suppression in alleviating renal ischemia-reperfusion injury in rats. PLoS ONE 2014, 9, e92129. [Google Scholar] [CrossRef]
- Tarng, D.C.; Tseng, W.C.; Lee, P.Y.; Chiou, S.H.; Hsieh, S.L. Induced Pluripotent Stem Cell-Derived Conditioned Medium Attenuates Acute Kidney Injury by Downregulating the Oxidative Stress-Related Pathway in Ischemia-Reperfusion Rats. Cell Transplant. 2016, 25, 517–530. [Google Scholar] [CrossRef]
- Borkan, S.C. The Role of BCL-2 Family Members in Acute Kidney Injury. Semin. Nephrol. 2016, 36, 237–250. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Kazachenko, A.V.; Vyssokikh, M.Y.; Vasileva, A.K.; Tcvirkun, D.V.; Isaev, N.K.; Kirpatovsky, V.I.; Zorov, D.B. The role of mitochondria in oxidative and nitrosative stress during ischemia/reperfusion in the rat kidney. Kidney Int. 2007, 72, 1493–1502. [Google Scholar] [CrossRef]
- Jacobs, K.M.; Bhave, S.R.; Ferraro, D.J.; Jaboin, J.J.; Hallahan, D.E.; Thotala, D. GSK-3beta: A Bifunctional Role in Cell Death Pathways. Int. J. Cell Biol. 2012, 2012, 930710. [Google Scholar] [CrossRef]
- Devarapu, S.K.; Grill, J.F.; Xie, J.; Weidenbusch, M.; Honarpisheh, M.; Vielhauer, V.; Anders, H.J.; Mulay, S.R. Tumor necrosis factor superfamily ligand mRNA expression profiles differ between humans and mice during homeostasis and between various murine kidney injuries. J. Biomed. Sci. 2017, 24, 77. [Google Scholar] [CrossRef]
- Wu, C.C.; Bratton, S.B. Regulation of the intrinsic apoptosis pathway by reactive oxygen species. Antioxid. Redox Signal. 2013, 19, 546–558. [Google Scholar] [CrossRef]
- Dorstyn, L.; Akey, C.W.; Kumar, S. New insights into apoptosome structure and function. Cell Death Differ. 2018, 25, 1194–1208. [Google Scholar] [CrossRef]
- Humphreys, B.D.; Cantaluppi, V.; Portilla, D.; Singbartl, K.; Yang, L.; Rosner, M.H.; Kellum, J.A.; Ronco, C.; Acute Dialysis Quality Initiative XIII Working Group. Targeting Endogenous Repair Pathways after AKI. J. Am. Soc. Nephrol. 2016, 27, 990–998. [Google Scholar] [CrossRef]
- Rowart, P.; Erpicum, P.; Detry, O.; Weekers, L.; Gregoire, C.; Lechanteur, C.; Briquet, A.; Beguin, Y.; Krzesinski, J.M.; Jouret, F. Mesenchymal Stromal Cell Therapy in Ischemia/Reperfusion Injury. J. Immunol. Res. 2015, 2015, 602597. [Google Scholar] [CrossRef]
- Fleig, S.V.; Humphreys, B.D. Rationale of mesenchymal stem cell therapy in kidney injury. Nephron Clin. Pract. 2014, 127, 75–80. [Google Scholar] [CrossRef]
- Bianchi, F.; Sala, E.; Donadei, C.; Capelli, I.; La Manna, G. Potential advantages of acute kidney injury management by mesenchymal stem cells. World J. Stem Cells 2014, 6, 644–650. [Google Scholar] [CrossRef]
- Naji, A.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Eitoku, M.; Suganuma, N. Mesenchymal stem/stromal cell function in modulating cell death. Stem Cell Res. Ther. 2019, 10, 56. [Google Scholar] [CrossRef]
- Gnecchi, M.; Danieli, P.; Malpasso, G.; Ciuffreda, M.C. Paracrine Mechanisms of Mesenchymal Stem Cells in Tissue Repair. Methods Mol. Biol. 2016, 1416, 123–146. [Google Scholar] [CrossRef]
- Borger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Li, N.; Long, B.; Han, W.; Yuan, S.; Wang, K. microRNAs: Important regulators of stem cells. Stem Cell Res. Ther. 2017, 8, 110. [Google Scholar] [CrossRef]
- Nargesi, A.A.; Lerman, L.O.; Eirin, A. Mesenchymal Stem Cell-derived Extracellular Vesicles for Renal Repair. Curr. Gene Ther. 2017, 17, 29–42. [Google Scholar] [CrossRef]
- Osafune, K. Cell therapy for kidney injury: Different options and mechanisms--kidney progenitor cells. Nephron Exp. Nephrol. 2014, 126, 64. [Google Scholar] [CrossRef]
- Toyohara, T.; Mae, S.; Sueta, S.; Inoue, T.; Yamagishi, Y.; Kawamoto, T.; Kasahara, T.; Hoshina, A.; Toyoda, T.; Tanaka, H.; et al. Cell Therapy Using Human Induced Pluripotent Stem Cell-Derived Renal Progenitors Ameliorates Acute Kidney Injury in Mice. Stem Cells Transl. Med. 2015, 4, 980–992. [Google Scholar] [CrossRef]
- Li, Q.; Tian, S.F.; Guo, Y.; Niu, X.; Hu, B.; Guo, S.C.; Wang, N.S.; Wang, Y. Transplantation of induced pluripotent stem cell-derived renal stem cells improved acute kidney injury. Cell Biosci. 2015, 5, 45. [Google Scholar] [CrossRef]
- Ko, S.F.; Chen, Y.T.; Wallace, C.G.; Chen, K.H.; Sung, P.H.; Cheng, B.C.; Huang, T.H.; Chen, Y.L.; Li, Y.C.; Chang, H.W.; et al. Inducible pluripotent stem cell-derived mesenchymal stem cell therapy effectively protected kidney from acute ischemia-reperfusion injury. Am. J. Transl. Res. 2018, 10, 3053–3067. [Google Scholar]
- Shen, W.C.; Chou, Y.H.; Huang, H.P.; Sheen, J.F.; Hung, S.C.; Chen, H.F. Induced pluripotent stem cell-derived endothelial progenitor cells attenuate ischemic acute kidney injury and cardiac dysfunction. Stem Cell Res. Ther. 2018, 9, 344. [Google Scholar] [CrossRef]
- Wu, D.P.; He, D.L.; Li, X.; Liu, Z.H. Differentiations of transplanted mouse spermatogonial stem cells in the adult mouse renal parenchyma in vivo. Acta Pharmacol. Sin. 2008, 29, 1029–1034. [Google Scholar] [CrossRef]
- De Chiara, L.; Fagoonee, S.; Ranghino, A.; Bruno, S.; Camussi, G.; Tolosano, E.; Silengo, L.; Altruda, F. Renal cells from spermatogonial germline stem cells protect against kidney injury. J. Am. Soc. Nephrol. 2014, 25, 316–328. [Google Scholar] [CrossRef]
- Togel, F.E.; Westenfelder, C. Kidney protection and regeneration following acute injury: Progress through stem cell therapy. Am. J. Kidney Dis. 2012, 60, 1012–1022. [Google Scholar] [CrossRef]
- Swaminathan, M.; Stafford-Smith, M.; Chertow, G.M.; Warnock, D.G.; Paragamian, V.; Brenner, R.M.; Lellouche, F.; Fox-Robichaud, A.; Atta, M.G.; Melby, S.; et al. Allogeneic Mesenchymal Stem Cells for Treatment of AKI after Cardiac Surgery. J. Am. Soc. Nephrol. 2018, 29, 260–267. [Google Scholar] [CrossRef]
- Tan, J.; Wu, W.; Xu, X.; Liao, L.; Zheng, F.; Messinger, S.; Sun, X.; Chen, J.; Yang, S.; Cai, J.; et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA 2012, 307, 1169–1177. [Google Scholar] [CrossRef]
- Pan, G.H.; Chen, Z.; Xu, L.; Zhu, J.H.; Xiang, P.; Ma, J.J.; Peng, Y.W.; Li, G.H.; Chen, X.Y.; Fang, J.L.; et al. Low-dose tacrolimus combined with donor-derived mesenchymal stem cells after renal transplantation: A prospective, non-randomized study. Oncotarget 2016, 7, 12089–12101. [Google Scholar] [CrossRef]
- Miller, B.L.K.; Garg, P.; Bronstein, B.; LaPointe, E.; Lin, H.; Charytan, D.M.; Tilles, A.W.; Parekkadan, B. Extracorporeal Stromal Cell Therapy for Subjects With Dialysis-Dependent Acute Kidney Injury. Kidney Int. Rep. 2018, 3, 1119–1127. [Google Scholar] [CrossRef]
- Rota, C.; Morigi, M.; Imberti, B. Stem Cell Therapies in Kidney Diseases: Progress and Challenges. Int. J. Mol. Sci. 2019, 20, 2790. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, K.-H.; Tseng, W.-C.; Yang, C.-Y.; Tarng, D.-C. The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury. Int. J. Mol. Sci. 2019, 20, 3529. https://doi.org/10.3390/ijms20143529
Lee K-H, Tseng W-C, Yang C-Y, Tarng D-C. The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury. International Journal of Molecular Sciences. 2019; 20(14):3529. https://doi.org/10.3390/ijms20143529
Chicago/Turabian StyleLee, Kuo-Hua, Wei-Cheng Tseng, Chih-Yu Yang, and Der-Cherng Tarng. 2019. "The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury" International Journal of Molecular Sciences 20, no. 14: 3529. https://doi.org/10.3390/ijms20143529
APA StyleLee, K.-H., Tseng, W.-C., Yang, C.-Y., & Tarng, D.-C. (2019). The Anti-Inflammatory, Anti-Oxidative, and Anti-Apoptotic Benefits of Stem Cells in Acute Ischemic Kidney Injury. International Journal of Molecular Sciences, 20(14), 3529. https://doi.org/10.3390/ijms20143529