Proteomic Techniques to Examine Neuronal Translational Dynamics
Abstract
1. Introduction
2. Metabolic Labeling of Nascent Peptides
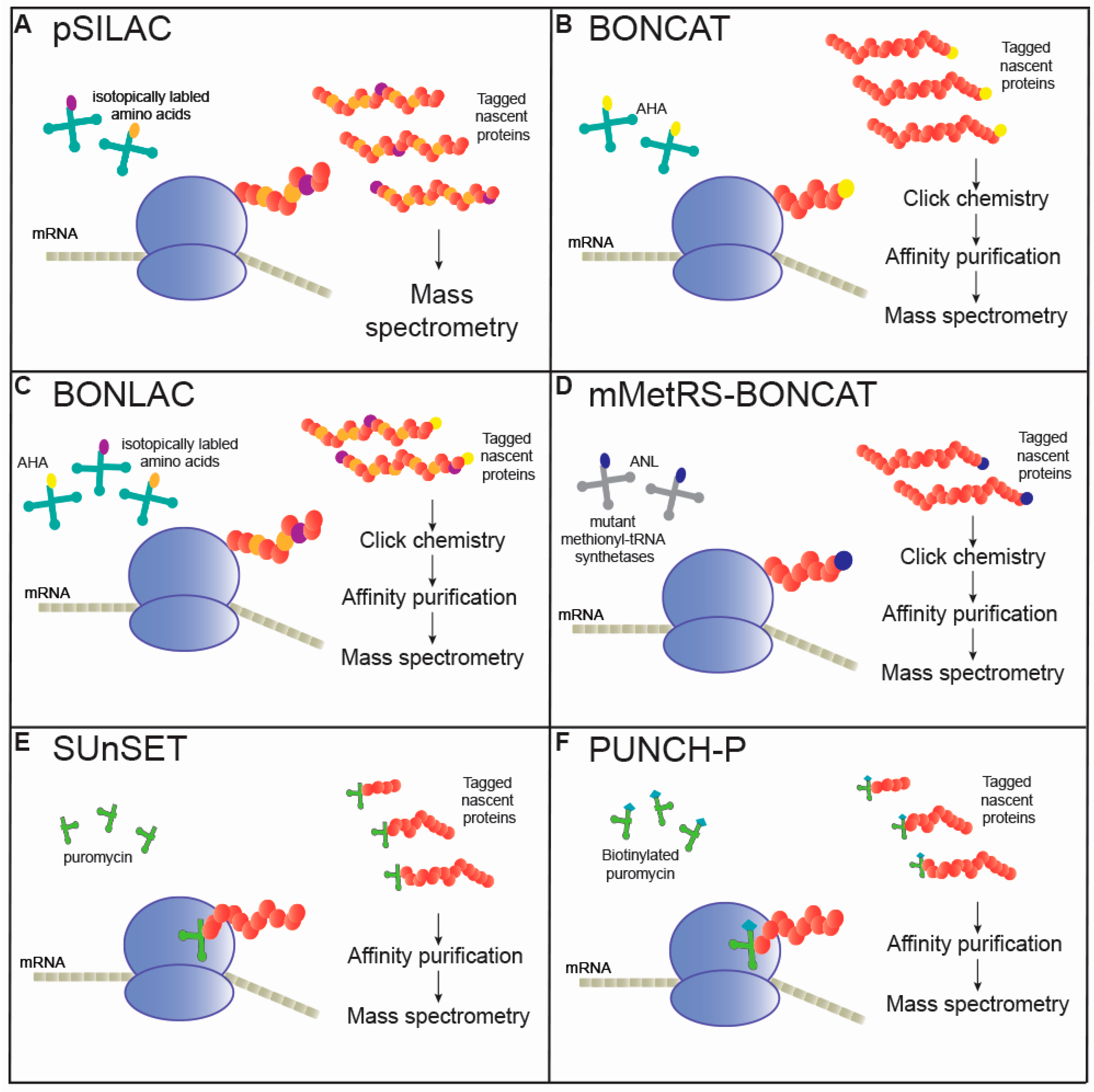
2.1. Pulsed Stable Isotope Labeling with Amino Acids
2.2. Non-Canonical Amino Acid Tagging (NCAT)
2.3. Combined BONCAT and SILAC (BONLAC)
2.4. Mutated MetRS-BONCAT
3. Puromycin Incorporation into Polypeptides
4. Technical Considerations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Flexner, J.B.; Flexner, L.B.; Stellar, E. Memory in mice as affected by intracerebral puromycin. Science 1963, 141, 57–59. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, P.J.; Abel, T. The role of protein synthesis in memory consolidation: Progress amid decades of debate. Neurobiol. Learn. Mem. 2008, 89, 293–311. [Google Scholar] [CrossRef]
- Jarome, T.J.; Helmstetter, F.J. Protein degradation and protein synthesis in long-term memory formation. Front. Mol. Neurosci. 2014, 7, 61. [Google Scholar] [CrossRef] [PubMed]
- Abraham, W.C.; Williams, J.M. LTP maintenance and its protein synthesis-dependence. Neurobiol. Learn. Mem. 2008, 89, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Buffington, S.A.; Huang, W.; Costa-Mattioli, M. Translational control in synaptic plasticity and cognitive dysfunction. Annu. Rev. Neurosci. 2014, 37, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Kapur, M.; Monaghan, C.E.; Ackerman, S.L. Regulation of mRNA Translation in Neurons-A Matter of Life and Death. Neuron 2017, 96, 616–637. [Google Scholar] [CrossRef] [PubMed]
- Hafner, A.S.; Donlin-Asp, P.G.; Leitch, B.; Herzog, E.; Schuman, E.M. Local protein synthesis is a ubiquitous feature of neuronal pre- and postsynaptic compartments. Science 2019, 364, eaau3644. [Google Scholar] [CrossRef] [PubMed]
- Khalil, B.; Morderer, D.; Price, P.L.; Liu, F.; Rossoll, W. mRNP assembly, axonal transport, and local translation in neurodegenerative diseases. Brain Res. 2018, 1693, 75–91. [Google Scholar] [CrossRef]
- Kim, E.; Jung, H. Local protein synthesis in neuronal axons: Why and how we study. BMB Rep. 2015, 48, 139–146. [Google Scholar] [CrossRef]
- Taylor, A.M.; Wu, J.; Tai, H.C.; Schuman, E.M. Axonal translation of beta-catenin regulates synaptic vesicle dynamics. J. Neurosci. 2013, 33, 5584–5589. [Google Scholar] [CrossRef]
- Costa-Mattioli, M.; Sossin, W.S.; Klann, E.; Sonenberg, N. Translational control of long-lasting synaptic plasticity and memory. Neuron 2009, 61, 10–26. [Google Scholar] [CrossRef] [PubMed]
- Cracco, J.B.; Serrano, P.; Moskowitz, S.I.; Bergold, P.J.; Sacktor, T.C. Protein synthesis-dependent LTP in isolated dendrites of CA1 pyramidal cells. Hippocampus 2005, 15, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.M.; Kayser, M.S.; Bear, M.F. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science 2000, 288, 1254–1257. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Caroni, P. Selective neuronal vulnerability in neurodegenerative diseases: From stressor thresholds to degeneration. Neuron 2011, 71, 35–48. [Google Scholar] [CrossRef] [PubMed]
- Scarnati, M.S.; Kataria, R.; Biswas, M.; Paradiso, K.G. Active presynaptic ribosomes in the mammalian brain, and altered transmitter release after protein synthesis inhibition. Elife 2018, 7, e36697. [Google Scholar] [CrossRef] [PubMed]
- Ping, L.; Duong, D.M.; Yin, L.; Gearing, M.; Lah, J.J.; Levey, A.I.; Seyfried, N.T. Global quantitative analysis of the human brain proteome in Alzheimer’s and Parkinson’s Disease. Sci. Data 2018, 5, 180036. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, S.; Ingolia, N.T. The Growing Toolbox for Protein Synthesis Studies. Trends Biochem. Sci. 2017, 42, 612–624. [Google Scholar] [CrossRef]
- Ingolia, N.T.; Lareau, L.F.; Weissman, J.S. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell 2011, 147, 789–802. [Google Scholar] [CrossRef]
- Pircher, A.; Gebetsberger, J.; Polacek, N. Ribosome-associated ncRNAs: An emerging class of translation regulators. RNA Biol. 2014, 11, 1335–1339. [Google Scholar] [CrossRef]
- Wilson, R.S.; Nairn, A.C. Cell-Type-Specific Proteomics: A Neuroscience Perspective. Proteomes 2018, 6, 51. [Google Scholar] [CrossRef]
- Smellie, R.M.; Mc, I.W.; Davidson, J.N. The incorporation of 15N, 35S and 14C into nucleic acids and proteins of rat liver. Biochim. Biophys. Acta 1953, 11, 559–565. [Google Scholar] [CrossRef]
- Ong, S.E.; Blagoev, B.; Kratchmarova, I.; Kristensen, D.B.; Steen, H.; Pandey, A.; Mann, M. Stable isotope labeling by amino acids in cell culture, SILAC, as a simple and accurate approach to expression proteomics. Mol. Cell Proteom. 2002, 1, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Schwanhausser, B.; Gossen, M.; Dittmar, G.; Selbach, M. Global analysis of cellular protein translation by pulsed SILAC. Proteomics 2009, 9, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Selbach, M.; Schwanhausser, B.; Thierfelder, N.; Fang, Z.; Khanin, R.; Rajewsky, N. Widespread changes in protein synthesis induced by microRNAs. Nature 2008, 455, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Smeets, J.S.J.; Horstman, A.M.H.; Schijns, O.; Dings, J.T.A.; Hoogland, G.; Gijsen, A.P.; Goessens, J.P.B.; Bouwman, F.G.; Wodzig, W.; Mariman, E.C.; et al. Brain tissue plasticity: Protein synthesis rates of the human brain. Brain 2018, 141, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Price, J.C.; Guan, S.; Burlingame, A.; Prusiner, S.B.; Ghaemmaghami, S. Analysis of proteome dynamics in the mouse brain. Proc. Natl. Acad. Sci. USA 2010, 107, 14508–14513. [Google Scholar] [CrossRef] [PubMed]
- McShane, E.; Sin, C.; Zauber, H.; Wells, J.N.; Donnelly, N.; Wang, X.; Hou, J.; Chen, W.; Storchova, Z.; Marsh, J.A.; et al. Kinetic Analysis of Protein Stability Reveals Age-Dependent Degradation. Cell 2016, 167, 803–815.e821. [Google Scholar] [CrossRef]
- Fornasiero, E.F.; Mandad, S.; Wildhagen, H.; Alevra, M.; Rammner, B.; Keihani, S.; Opazo, F.; Urban, I.; Ischebeck, T.; Sakib, M.S.; et al. Precisely measured protein lifetimes in the mouse brain reveal differences across tissues and subcellular fractions. Nat. Commun. 2018, 9, 4230. [Google Scholar] [CrossRef]
- Xu, P.; Tan, H.; Duong, D.M.; Yang, Y.; Kupsco, J.; Moberg, K.H.; Li, H.; Jin, P.; Peng, J. Stable isotope labeling with amino acids in Drosophila for quantifying proteins and modifications. J. Proteome Res. 2012, 11, 4403–4412. [Google Scholar] [CrossRef]
- Dorrbaum, A.R.; Kochen, L.; Langer, J.D.; Schuman, E.M. Local and global influences on protein turnover in neurons and glia. Elife 2018, 7, e34202. [Google Scholar] [CrossRef]
- Cagnetta, R.; Frese, C.K.; Shigeoka, T.; Krijgsveld, J.; Holt, C.E. Rapid Cue-Specific Remodeling of the Nascent Axonal Proteome. Neuron 2018, 99, 29–46.e24. [Google Scholar] [CrossRef] [PubMed]
- Cagnetta, R.; Wong, H.H.; Frese, C.K.; Mallucci, G.R.; Krijgsveld, J.; Holt, C.E. Noncanonical Modulation of the eIF2 Pathway Controls an Increase in Local Translation during Neural Wiring. Mol. Cell 2019, 73, 474–489.e5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, C.S.; Foehr, S.; Garfield, D.A.; Furlong, E.E.; Steinmetz, L.M.; Krijgsveld, J. Ultrasensitive proteome analysis using paramagnetic bead technology. Mol. Syst. Biol. 2014, 10, 757. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.C.; Meier, S.E.; Ingram, A.L.; Abisambra, J.F. PERK-opathies: An Endoplasmic Reticulum Stress Mechanism Underlying Neurodegeneration. Curr. Alzheimer Res. 2016, 13, 150–163. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Lee, J.J.; Link, A.J.; Graumann, J.; Tirrell, D.A.; Schuman, E.M. Labeling, detection and identification of newly synthesized proteomes with bioorthogonal non-canonical amino-acid tagging. Nat. Protoc. 2007, 2, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Link, A.J.; Graumann, J.; Tirrell, D.A.; Schuman, E.M. Selective identification of newly synthesized proteins in mammalian cells using bioorthogonal noncanonical amino acid tagging (BONCAT). Proc. Natl. Acad. Sci. USA 2006, 103, 9482–9487. [Google Scholar] [CrossRef] [PubMed]
- Dieterich, D.C.; Hodas, J.J.; Gouzer, G.; Shadrin, I.Y.; Ngo, J.T.; Triller, A.; Tirrell, D.A.; Schuman, E.M. In situ visualization and dynamics of newly synthesized proteins in rat hippocampal neurons. Nat. Neurosci. 2010, 13, 897–905. [Google Scholar] [CrossRef]
- Tcherkezian, J.; Brittis, P.A.; Thomas, F.; Roux, P.P.; Flanagan, J.G. Transmembrane receptor DCC associates with protein synthesis machinery and regulates translation. Cell 2010, 141, 632–644. [Google Scholar] [CrossRef]
- Hinz, F.I.; Dieterich, D.C.; Schuman, E.M. Teaching old NCATs new tricks: Using non-canonical amino acid tagging to study neuronal plasticity. Curr. Opin. Chem. Boil. 2013, 17, 738–746. [Google Scholar] [CrossRef]
- Liu, H.H.; Cline, H.T. Fragile X Mental Retardation Protein Is Required to Maintain Visual Conditioning-Induced Behavioral Plasticity by Limiting Local Protein Synthesis. J. Neurosci. 2016, 36, 7325–7339. [Google Scholar] [CrossRef]
- Bowling, H.; Bhattacharya, A.; Zhang, G.; Lebowitz, J.Z.; Alam, D.; Smith, P.T.; Kirshenbaum, K.; Neubert, T.A.; Vogel, C.; Chao, M.V.; et al. BONLAC: A combinatorial proteomic technique to measure stimulus-induced translational profiles in brain slices. Neuropharmacology 2016, 100, 76–89. [Google Scholar] [CrossRef] [PubMed]
- Hodas, J.J.; Nehring, A.; Hoche, N.; Sweredoski, M.J.; Pielot, R.; Hess, S.; Tirrell, D.A.; Dieterich, D.C.; Schuman, E.M. Dopaminergic modulation of the hippocampal neuropil proteome identified by bioorthogonal noncanonical amino acid tagging (BONCAT). Proteomics 2012, 12, 2464–2476. [Google Scholar] [CrossRef] [PubMed]
- Schanzenbacher, C.T.; Sambandan, S.; Langer, J.D.; Schuman, E.M. Nascent Proteome Remodeling following Homeostatic Scaling at Hippocampal Synapses. Neuron 2016, 92, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Schanzenbacher, C.T.; Langer, J.D.; Schuman, E.M. Time- and polarity-dependent proteomic changes associated with homeostatic scaling at central synapses. Elife 2018, 7, e33322. [Google Scholar] [CrossRef] [PubMed]
- Schiapparelli, L.M.; McClatchy, D.B.; Liu, H.H.; Sharma, P.; Yates, J.R., 3rd; Cline, H.T. Direct detection of biotinylated proteins by mass spectrometry. J. Proteome Res. 2014, 13, 3966–3978. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Liu, H.H.; Schiapparelli, L.; McClatchy, D.; He, H.Y.; Yates, J.R., 3rd; Cline, H.T. Acute synthesis of CPEB is required for plasticity of visual avoidance behavior in Xenopus. Cell Rep. 2014, 6, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.H.; McClatchy, D.B.; Schiapparelli, L.; Shen, W.; Yates, J.R., 3rd; Cline, H.T. Role of the visual experience-dependent nascent proteome in neuronal plasticity. Elife 2018, 7, e33420. [Google Scholar] [CrossRef] [PubMed]
- McClatchy, D.B.; Ma, Y.; Liu, C.; Stein, B.D.; Martinez-Bartolome, S.; Vasquez, D.; Hellberg, K.; Shaw, R.J.; Yates, J.R., 3rd. Pulsed Azidohomoalanine Labeling in Mammals (PALM) Detects Changes in Liver-Specific LKB1 Knockout Mice. J. Proteome Res. 2015, 14, 4815–4822. [Google Scholar] [CrossRef] [PubMed]
- Calve, S.; Witten, A.J.; Ocken, A.R.; Kinzer-Ursem, T.L. Incorporation of non-canonical amino acids into the developing murine proteome. Sci. Rep. 2016, 6, 32377. [Google Scholar] [CrossRef]
- Evans, H.T.; Benetatos, J.; Van Roijen, M.; Bodea, L.G.; Gotz, J. Decreased synthesis of ribosomal proteins in tauopathy revealed by non-canonical amino acid labelling. EMBO J. 2019, 38, e101174. [Google Scholar] [CrossRef]
- Koren, S.A.; Hamm, M.J.; Meier, S.E.; Weiss, B.E.; Nation, G.K.; Chishti, E.A.; Arango, J.P.; Chen, J.; Zhu, H.; Blalock, E.M.; et al. Tau drives translational selectivity by interacting with ribosomal proteins. Acta Neuropathol. 2019, 137, 571–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Bowling, H.; Hom, N.; Kirshenbaum, K.; Klann, E.; Chao, M.V.; Neubert, T.A. In-depth quantitative proteomic analysis of de novo protein synthesis induced by brain-derived neurotrophic factor. J. Proteome Res. 2014, 13, 5707–5714. [Google Scholar] [CrossRef] [PubMed]
- Bowling, H.; Bhattacharya, A.; Zhang, G.; Alam, D.; Lebowitz, J.Z.; Bohm-Levine, N.; Lin, D.; Singha, P.; Mamcarz, M.; Puckett, R.; et al. Altered steady state and activity-dependent de novo protein expression in fragile X syndrome. Nat. Commun. 2019, 10, 1710. [Google Scholar] [CrossRef] [PubMed]
- Howden, A.J.; Geoghegan, V.; Katsch, K.; Efstathiou, G.; Bhushan, B.; Boutureira, O.; Thomas, B.; Trudgian, D.C.; Kessler, B.M.; Dieterich, D.C.; et al. QuaNCAT: Quantitating proteome dynamics in primary cells. Nat. Methods 2013, 10, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; McClatchy, D.B.; Barkallah, S.; Wood, W.W.; Yates, J.R., 3rd. Quantitative analysis of newly synthesized proteins. Nat. Protoc 2018, 13, 1744–1762. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; McClatchy, D.B.; Barkallah, S.; Wood, W.W.; Yates, J.R., 3rd. HILAQ: A Novel Strategy for Newly Synthesized Protein Quantification. J. Proteome Res. 2017, 16, 2213–2220. [Google Scholar] [CrossRef] [PubMed]
- Beatty, K.E.; Liu, J.C.; Xie, F.; Dieterich, D.C.; Schuman, E.M.; Wang, Q.; Tirrell, D.A. Fluorescence visualization of newly synthesized proteins in mammalian cells. Angew. Chem. Int. Ed. 2006, 45, 7364–7367. [Google Scholar] [CrossRef] [PubMed]
- Ngo, J.T.; Schuman, E.M.; Tirrell, D.A. Mutant methionyl-tRNA synthetase from bacteria enables site-selective N-terminal labeling of proteins expressed in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 4992–4997. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, I.; Marter, K.; Kobler, O.; Niehues, S.; Abele, J.; Muller, A.; Bussmann, J.; Storkebaum, E.; Ziv, T.; Thomas, U.; et al. Cell-selective labelling of proteomes in Drosophila melanogaster. Nat. Commun. 2015, 6, 7521. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Castelao, B.; Schanzenbacher, C.T.; Hanus, C.; Glock, C.; Tom Dieck, S.; Dorrbaum, A.R.; Bartnik, I.; Nassim-Assir, B.; Ciirdaeva, E.; Mueller, A.; et al. Cell-type-specific metabolic labeling of nascent proteomes in vivo. Nat. Biotechnol. 2017, 35, 1196–1201. [Google Scholar] [CrossRef]
- Alvarez-Castelao, B.; Schanzenbacher, C.T.; Langer, J.D.; Schuman, E.M. Cell-type-specific metabolic labeling, detection and identification of nascent proteomes in vivo. Nat. Protoc. 2019, 14, 556–575. [Google Scholar] [CrossRef]
- Krogager, T.P.; Ernst, R.J.; Elliott, T.S.; Calo, L.; Beranek, V.; Ciabatti, E.; Spillantini, M.G.; Tripodi, M.; Hastings, M.H.; Chin, J.W. Labeling and identifying cell-specific proteomes in the mouse brain. Nat. Biotechnol. 2018, 36, 156–159. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Possenti, A.; Freer, R.; Nakano, Y.; Villegas, N.C.H.; Tang, M.; Cauhy, P.V.M.; Lassus, B.A.; Chen, S.; Fowler, S.L.; et al. A tau homeostasis signature is linked with the cellular and regional vulnerability of excitatory neurons to tau pathology. Nat. Neurosci. 2019, 22, 47–56. [Google Scholar] [CrossRef]
- Nathans, D. Puromycin Inhibition of Protein Synthesis: Incorporation of Puromycin into Peptide Chains. Proc. Natl. Acad. Sci. USA 1964, 51, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.K.; Clavarino, G.; Ceppi, M.; Pierre, P. SUnSET, a nonradioactive method to monitor protein synthesis. Nat. Methods 2009, 6, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Prouty, W.F.; Goldberg, A.L. Fate of abnormal proteins in E. coli accumulation in intracellular granules before catabolism. Nat. New Biol. 1972, 240, 147–150. [Google Scholar] [CrossRef]
- Meier, S.; Bell, M.; Lyons, D.N.; Rodriguez-Rivera, J.; Ingram, A.; Fontaine, S.N.; Mechas, E.; Chen, J.; Wolozin, B.; LeVine, H., 3rd; et al. Pathological Tau Promotes Neuronal Damage by Impairing Ribosomal Function and Decreasing Protein Synthesis. J. Neurosci. 2016, 36, 1001–1007. [Google Scholar] [CrossRef]
- Hoeffer, C.A.; Santini, E.; Ma, T.; Arnold, E.C.; Whelan, A.M.; Wong, H.; Pierre, P.; Pelletier, J.; Klann, E. Multiple components of eIF4F are required for protein synthesis-dependent hippocampal long-term potentiation. J. Neurophysiol. 2013, 109, 68–76. [Google Scholar] [CrossRef]
- Starck, S.R.; Green, H.M.; Alberola-Ila, J.; Roberts, R.W. A general approach to detect protein expression in vivo using fluorescent puromycin conjugates. Chem. Biol. 2004, 11, 999–1008. [Google Scholar] [CrossRef]
- Aviner, R.; Geiger, T.; Elroy-Stein, O. Genome-wide identification and quantification of protein synthesis in cultured cells and whole tissues by puromycin-associated nascent chain proteomics (PUNCH-P). Nat. Protoc. 2014, 9, 751–760. [Google Scholar] [CrossRef]
- Jose, L.H.S.; Signer, R.A.J. Cell-type-specific quantification of protein synthesis in vivo. Nat. Protoc. 2019, 14, 441–460. [Google Scholar] [CrossRef] [PubMed]
- Aviner, R.; Geiger, T.; Elroy-Stein, O. Novel proteomic approach (PUNCH-P) reveals cell cycle-specific fluctuations in mRNA translation. Genes Dev. 2013, 27, 1834–1844. [Google Scholar] [CrossRef] [PubMed]
| Methodology | Incubation Time | Considerations | Current Model Utility | Refs. | |
|---|---|---|---|---|---|
| pSILAC: Pulsed Stable Isotopic Labeling of Amino acids | Pulsed isotopic labeling of amino acids | Short to Long | Robust incorporation but generally requires long incubation times. May introduce a bias of tag incorporation. | In vitro | [31,32] |
| BONCAT: Bio-orthogonal Non-Canonical Amino acid Tagging | Non-canonical amino acid incorporation and chemical capture | Short to Medium | Weak incorporation at shorter incubation timescales. Can be adapted for fluorescent detection. Strong MS detection after purification. | In vitro Ex vivo In vivo | [35,36,37,38,39,40,41,42,43,44,45,46,47,49,50] |
| PALM: Pulsed Azidohomoalanine Labeling in Mammals | In vivo BONCAT using AHA-enriched feed | Long | Weak incorporation and requires multi-day diet on enriched feed. Nascent translation can be detected in sub-cellular fractions. | In vivo | [48] |
| BONLAC: Combinatorial BONCAT and pSILAC | Combined pSILAC with BONCAT enrichment | Medium | Enables the robust detection of nascent peptides but with a greater experimental complexity. | In vitro Ex vivo | [41,52,53] |
| mMetRS BONCAT: Mutated Methionyl-tRNA synthetase coupled with BONCAT | BONCAT but with cell-specific expression of expanded tRNAs | Medium | Requires genetic manipulation or viral-mediated genetic transfer but can be adapted for cell-specific investigations of nascent translation. | In vitro In vivo | [59,60,61,62] |
| Puromycin | Puromycin labeling and affinity capture | Short | Requires simple injection followed by affinity capture. Can inhibit translation at high concentrations. | In vitro Ex vivo In vivo | [51,65,67,68] |
| PUNCH-P: Puromycin associated Nascent Chain Proteomics | Puromycin-biotin labeling and chemical capture | Short | Requires tissue homogenization prior to incubation but with strong incorporation. | In vitro In vivo& | [70,72] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koren, S.A.; Gillett, D.A.; D’Alton, S.V.; Hamm, M.J.; Abisambra, J.F. Proteomic Techniques to Examine Neuronal Translational Dynamics. Int. J. Mol. Sci. 2019, 20, 3524. https://doi.org/10.3390/ijms20143524
Koren SA, Gillett DA, D’Alton SV, Hamm MJ, Abisambra JF. Proteomic Techniques to Examine Neuronal Translational Dynamics. International Journal of Molecular Sciences. 2019; 20(14):3524. https://doi.org/10.3390/ijms20143524
Chicago/Turabian StyleKoren, Shon A., Drew A. Gillett, Simon V. D’Alton, Matthew J. Hamm, and Jose F. Abisambra. 2019. "Proteomic Techniques to Examine Neuronal Translational Dynamics" International Journal of Molecular Sciences 20, no. 14: 3524. https://doi.org/10.3390/ijms20143524
APA StyleKoren, S. A., Gillett, D. A., D’Alton, S. V., Hamm, M. J., & Abisambra, J. F. (2019). Proteomic Techniques to Examine Neuronal Translational Dynamics. International Journal of Molecular Sciences, 20(14), 3524. https://doi.org/10.3390/ijms20143524





