Glucagon-Like Peptide-1 Receptor Agonist Attenuates Autophagy to Ameliorate Pulmonary Arterial Hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3β Pathways
Abstract
1. Introduction
2. Results
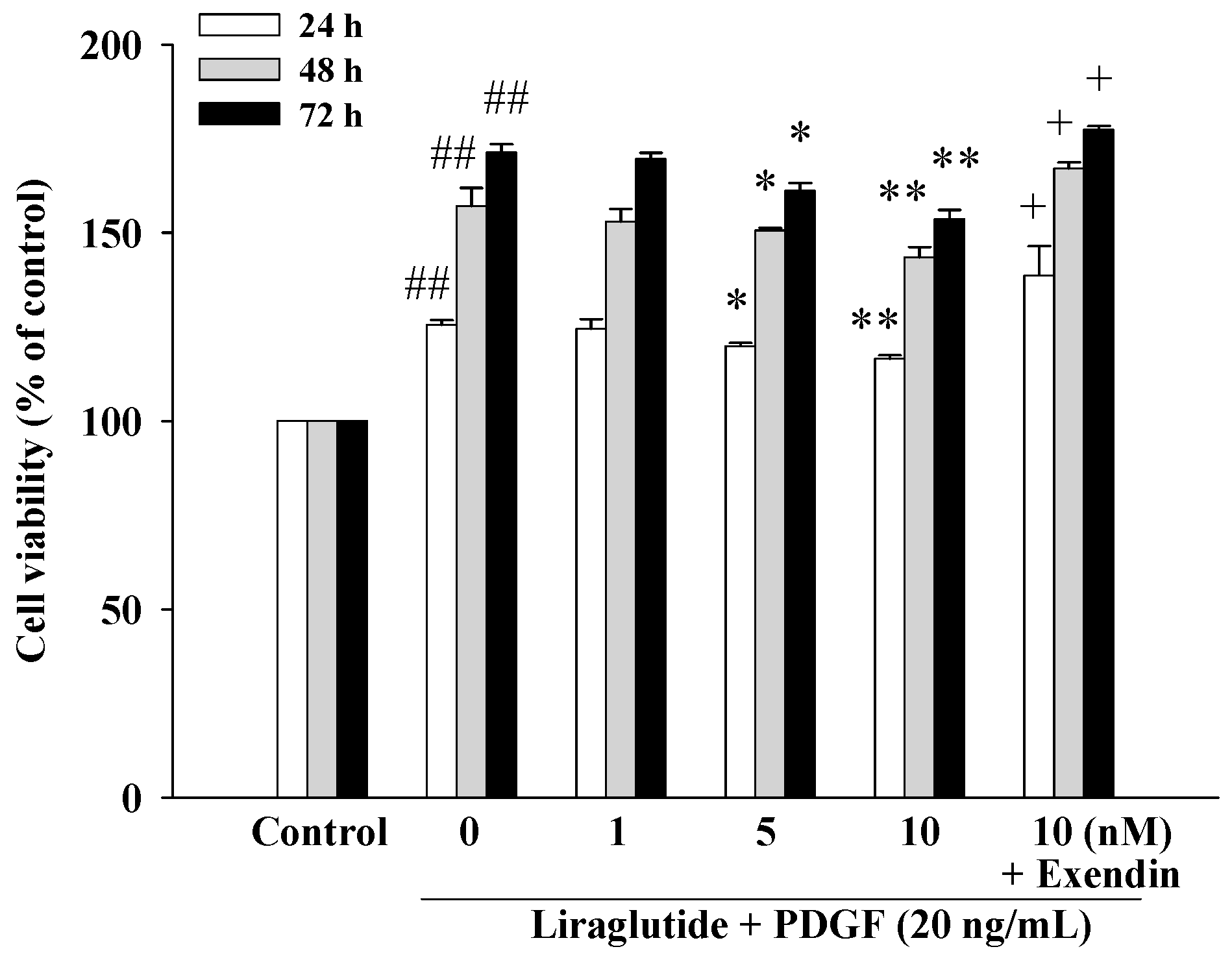
2.1. Liraglutide Inhibited Platelet-Derived Growth Factor BB (PDGF-BB)-Induced PASMC Proliferation
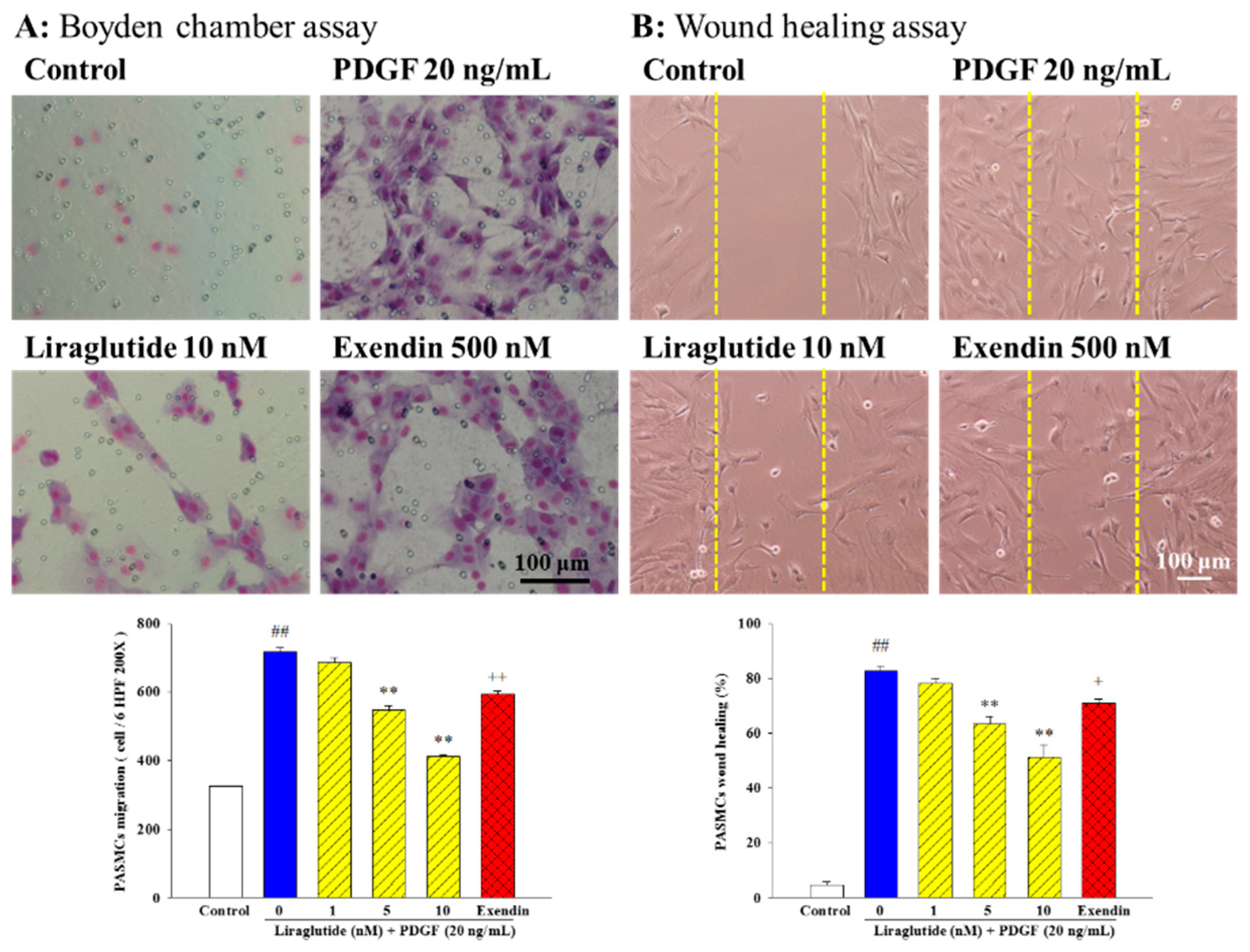
2.2. Liraglutide Inhibited PDGF-BB-Induced PASMC Migration and Motility
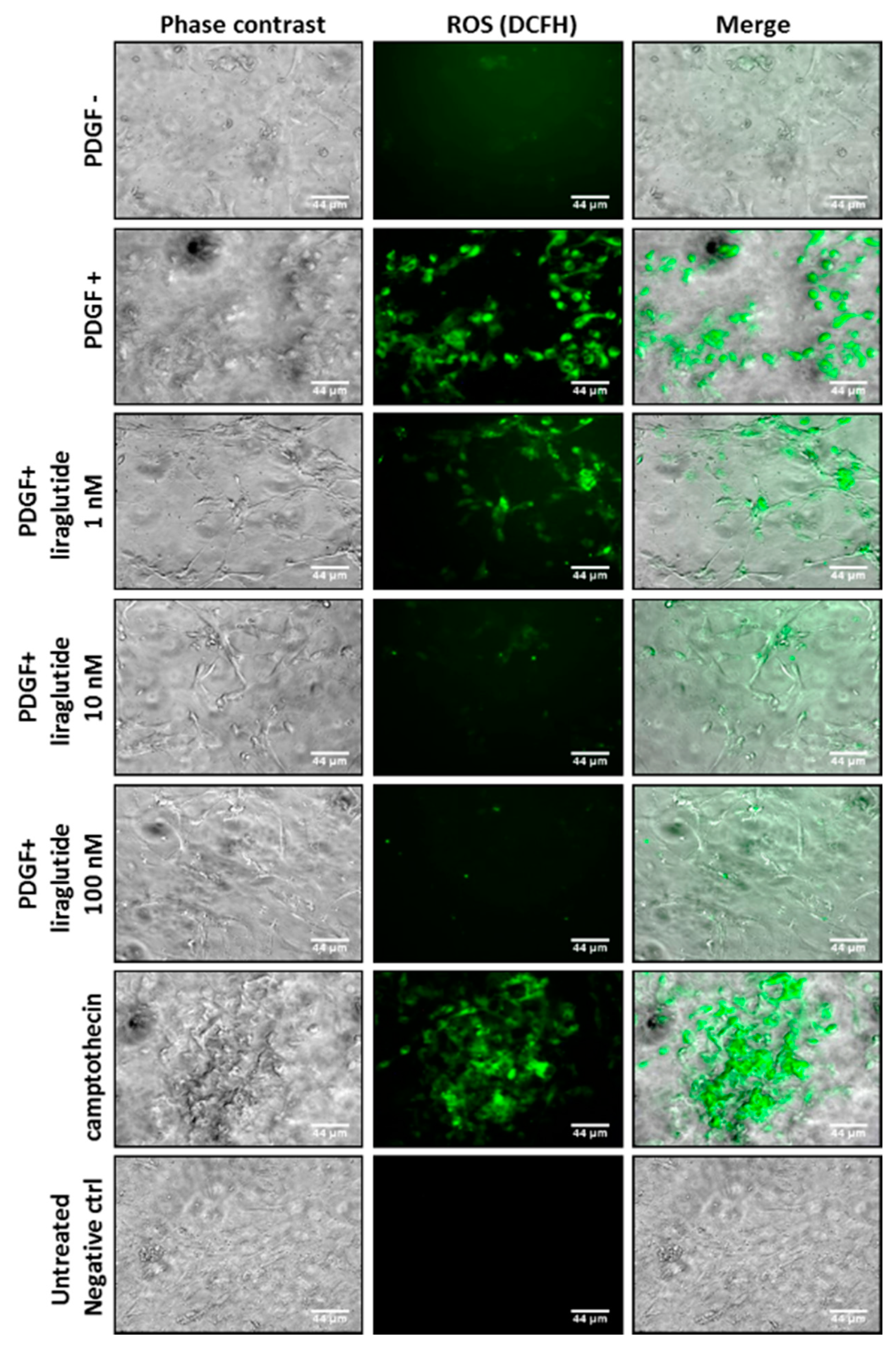
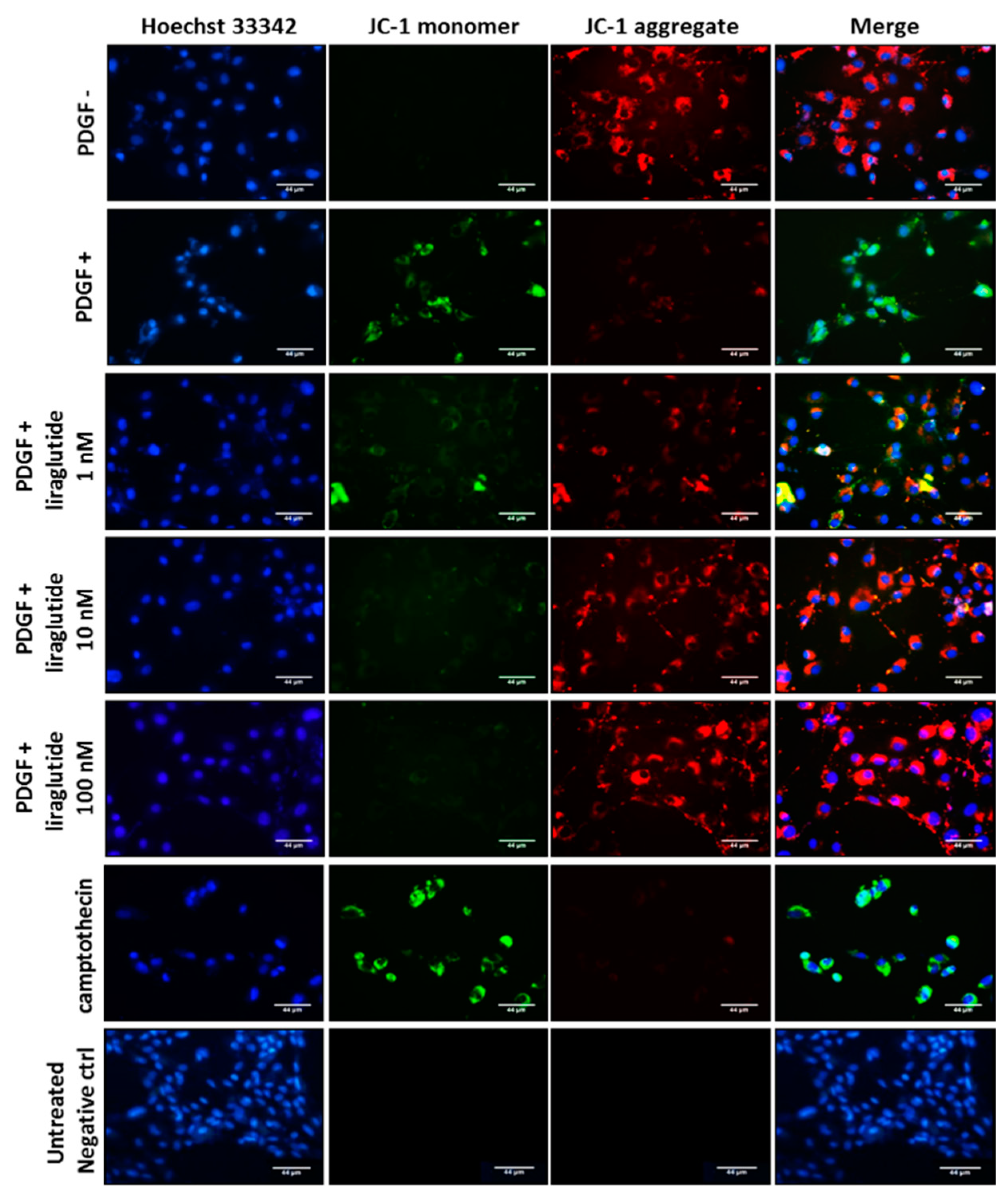
2.3. Liraglutide Attenuated PDGF-BB-Induced Mitochondrial ROS Generation
2.4. Liraglutide Attenuated PDGF-BB-Induced Cell Apoptosis
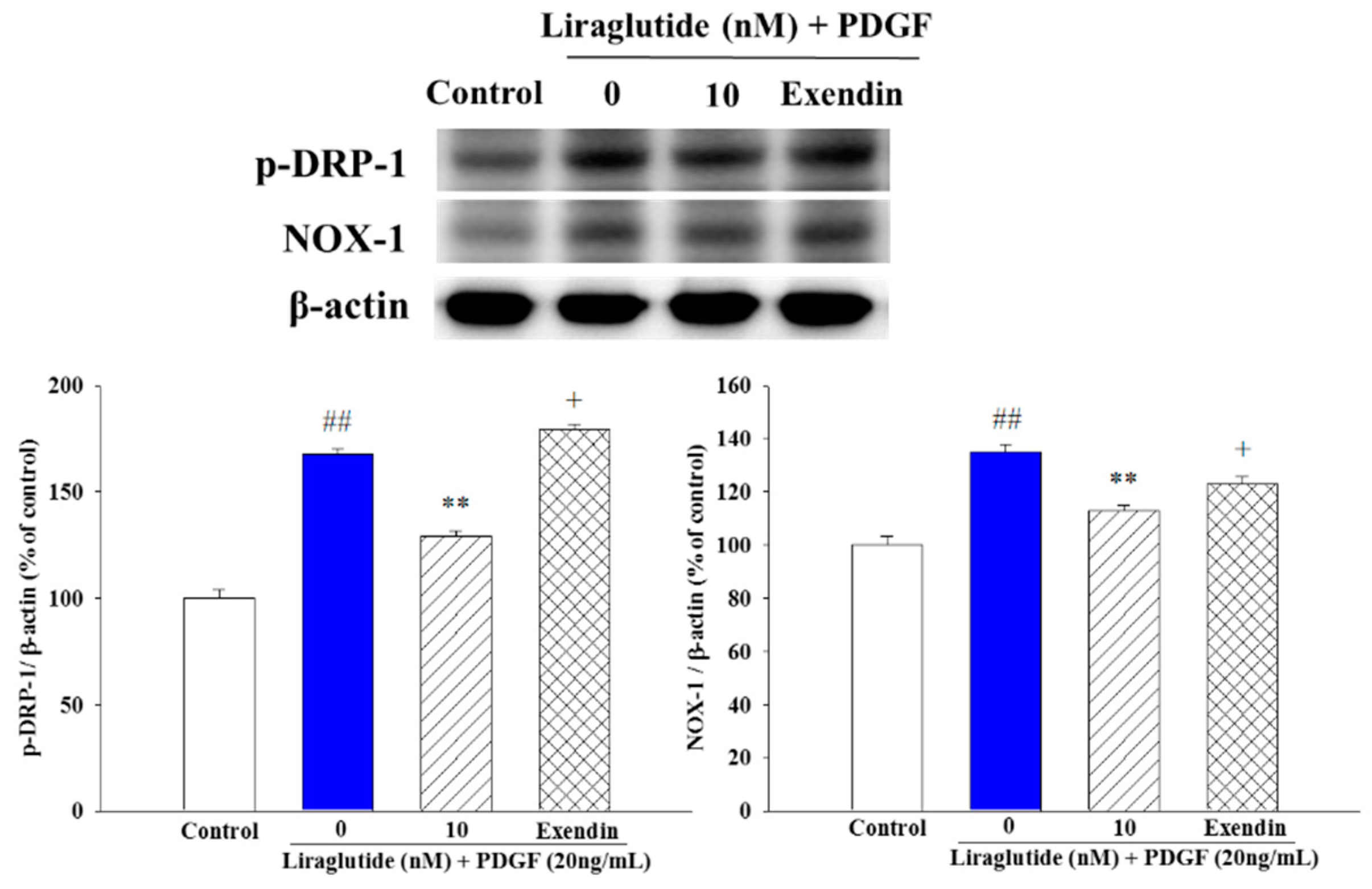
2.5. Liraglutide Attenuated PDGF-BB-Induced NADPH Oxidase 1 (NOX1) Expression and Mitochondrial Fission in the PASMCs
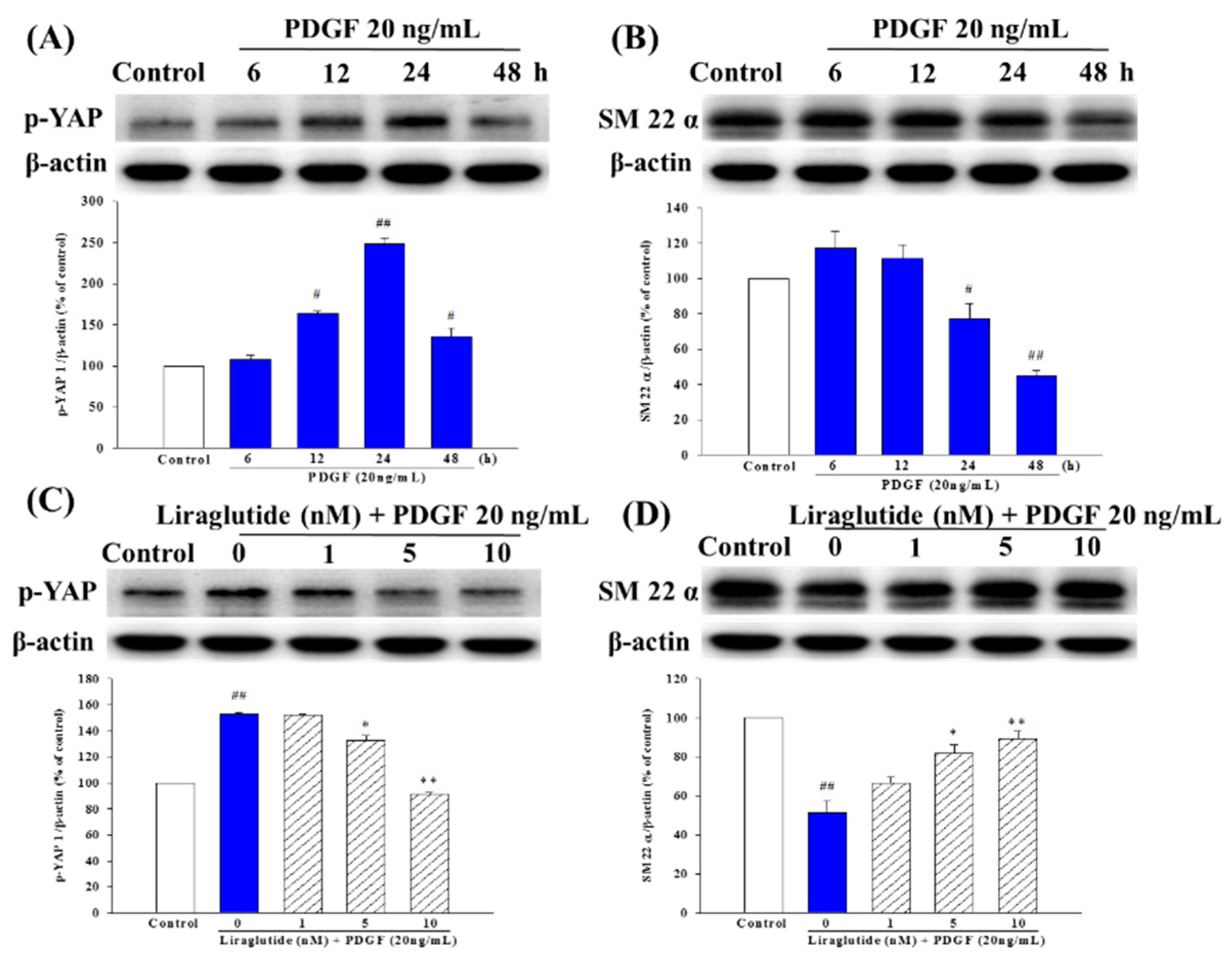
2.6. Liraglutide Inhibited PDGF-BB-Induced Phenotype Switching in the PASMCs
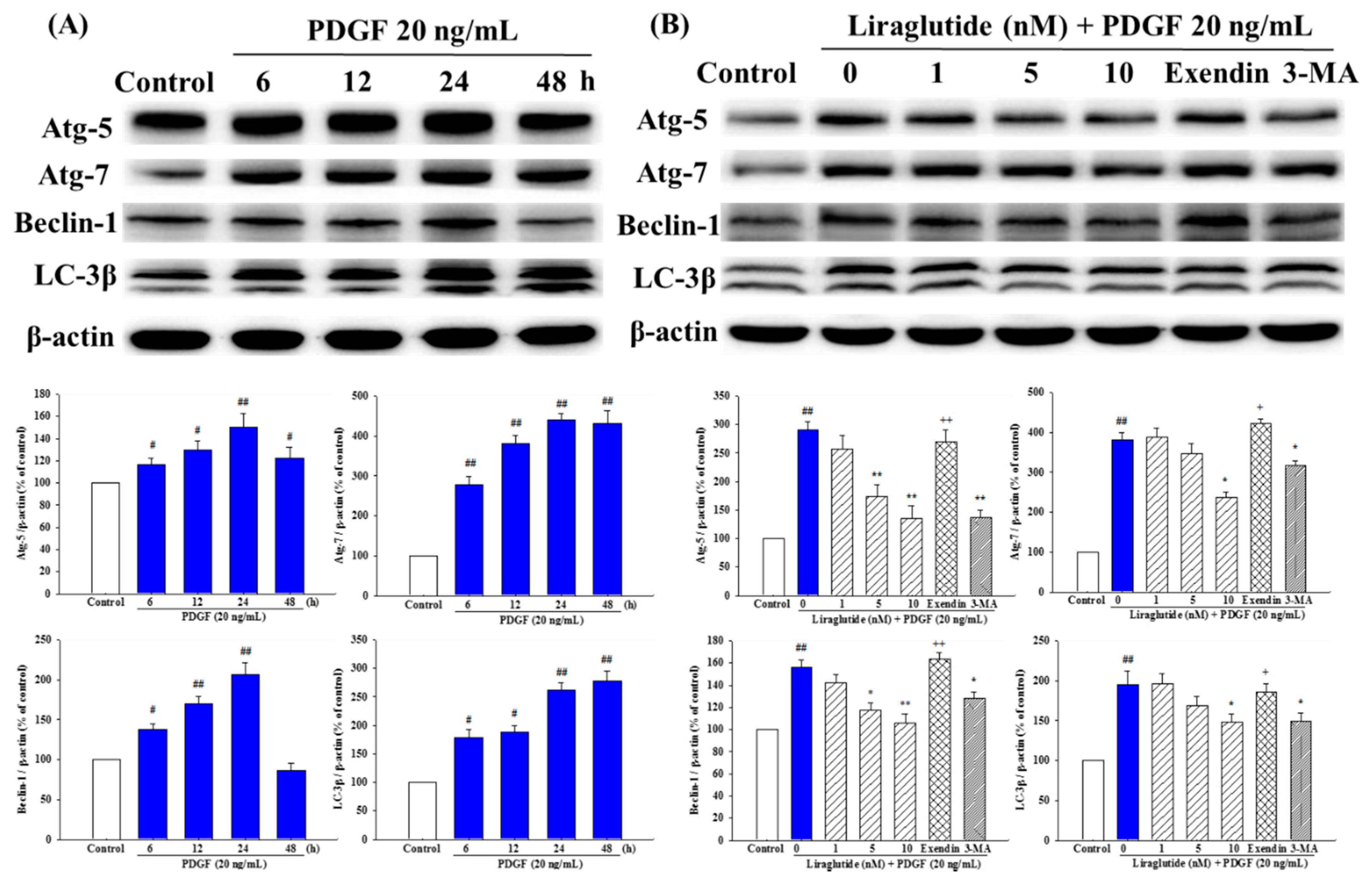
2.7. Liraglutide Attenuated PDGF-BB-Induced Autophagy in the PASMCs
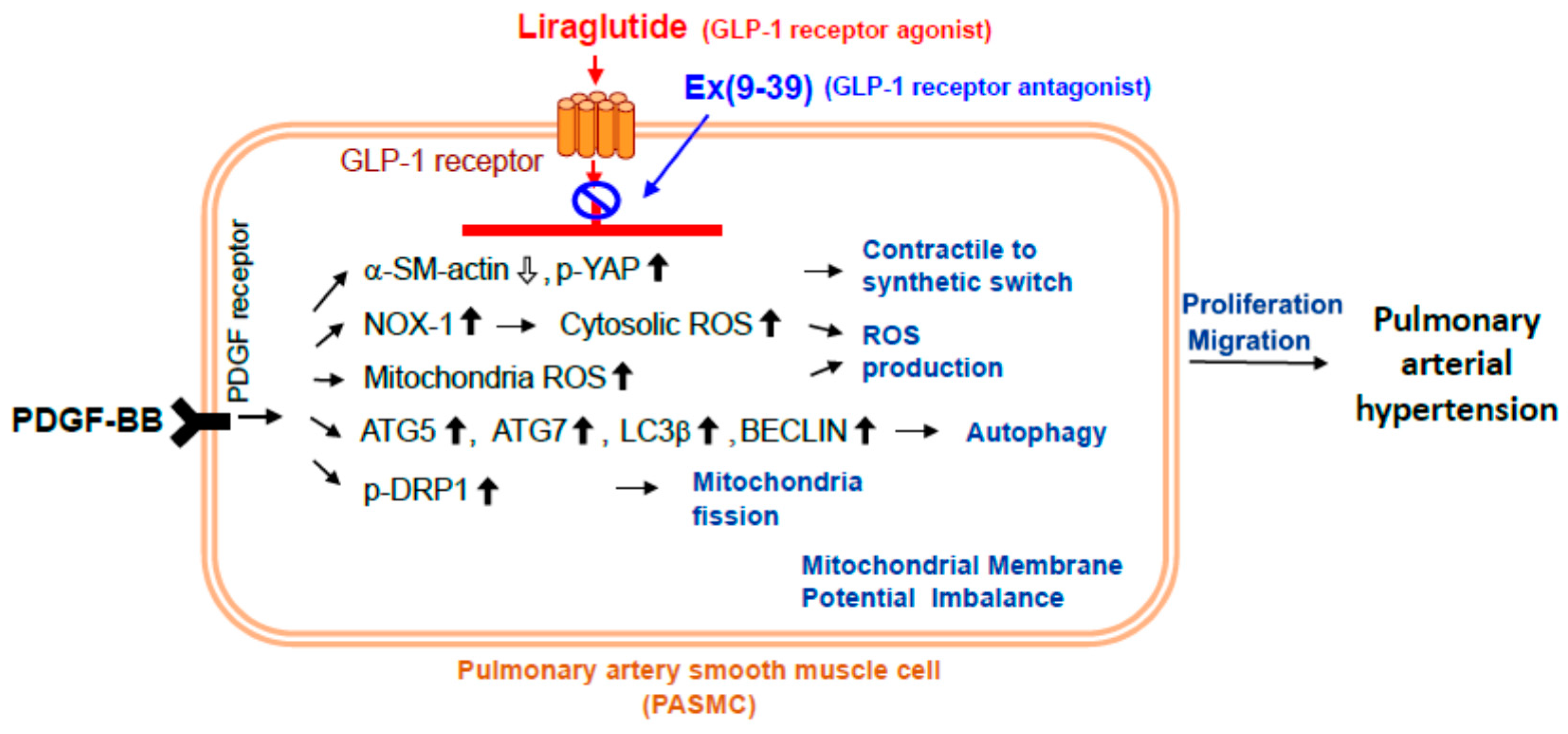
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Preparation of PASMCs
4.3. Cell Proliferation Assay
4.4. Determination of Cell Migration
4.5. Autophagy Assay
4.6. Western Blot Analysis
4.7. Determination of Intracellular ROS
4.8. Determination of Mitochondrial Membrane Potential
4.9. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Halliwell, B. Reactive oxygen species and the central nervous system, in Free Radicals in the Brain. J. Neurochem. 1992, 59, 1609–1623. [Google Scholar] [CrossRef] [PubMed]
- McBride, H.M.; Neuspiel, M.; Wasiak, S. Mitochondria: More than just a powerhouse. Curr. Biol. 2006, 16, R551–R560. [Google Scholar] [CrossRef] [PubMed]
- Zemirli, N.; Morel, E.; Molino, D. Mitochondrial Dynamics in Basal and Stressful Conditions. Int. J. Mol. Sci. 2018, 19, 564. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, M.R.; Nabavi, S.F.; Habtemariam, S.; Erdogan, O.I.; Daglia, M.; Nabavi, S.M. The effects of baicalein and baicalin on mitochondrial function and dynamics: A review. Pharmacol. Res. 2015, 100, 296–308. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kaarniranta, K.; Kauppinen, A. Mitochondrial dysfunction and oxidative stress activate inflammasomes: Impact on the aging process and age-related diseases. Cell Mol. Life Sci. 2012, 69, 2999–3013. [Google Scholar] [CrossRef] [PubMed]
- Arruda, A.P.; Pers, B.M.; Parlakgül, G.; Güney, E.; Inouye, K.; Hotamisligil, G.S. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat. Med. 2014, 20, 1427–1435. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural. Regen. Res. 2013, 8, 2003. [Google Scholar] [PubMed]
- Huang, M.L.; Chiang, S.; Kalinowski, D.S.; Bae, D.H.; Sahni, S.; Richardson, D.R. The role of the antioxidant response in mitochondrial dysfunction in degenerative diseases: Cross-talk between antioxidant defense, autophagy, and apoptosis. Oxid. Med. Cell Longev. 2019, 2019, 6392763. [Google Scholar] [CrossRef]
- Pajares, M.; Cuadrado, A.; Engedal, N.; Jirsova, Z.; Cahova, M. The Role of Free Radicals in Autophagy Regulation: Implications for Ageing. Oxid. Med. Cell Longev. 2018, 2018, 2450748. [Google Scholar] [CrossRef]
- Yu, L.; Chen, Y.; Tooze, S.A. Autophagy pathway: Cellular and molecular mechanisms. Autophagy 2018, 14, 207–215. [Google Scholar] [CrossRef]
- Mizushima, N. A brief history of autophagy from cell biology to physiology and disease. Nat. Cell Biol. 2018, 20, 521. [Google Scholar] [CrossRef] [PubMed]
- Ferrucci, M.; Biagioni, F.; Ryskalin, L.; Limanaqi, F.; Gambardella, S.; Frati, A.; Fornai, F. Ambiguous Effects of Autophagy Activation Following Hypoperfusion/Ischemia. Int. J. Mol. Sci. 2018, 19, 2756. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Pascual, C.; Klionsky, D.J. Autophagy: Machinery and regulation. Microb. Cell. 2016, 3, 588. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Potus, F.; Wu, D.; Dasgupta, A.; Chen, K.H.; Mewburn, J.; Lima, P.; Archer, S.L. Increased Drp1-Mediated Mitochondrial Fission Promotes Proliferation and Collagen Production by Right Ventricular Fibroblasts in Experimental Pulmonary Arterial Hypertension. Front. Physiol. 2018, 9, 828. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, F.K.; Sposito, A.C. Cardiovascular effects of glucagon-like peptide 1 (GLP-1) receptor agonists. Cardiovasc. Diabetol. 2014, 13, 142. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Jelinek, H.F.; Al-Aubaidy, H. Glucagon like peptide-1 and its receptor agonists: Their roles in management of Type 2 diabetes mellitus. Diabetes Metab. Syndr. 2016, 11, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Lovshin, J.; Cherney, D. GLP-1R Agonists and Endothelial Dysfunction: More Than Just Glucose Lowering? Diabetes 2015, 64, 2319–2321. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; She, M.; Xu, M.; Chen, H.; Li, J.; Chen, X.; Zheng, D.; Liu, J.; Chen, S.; Zhu, J.; et al. GLP-1 treatment protects endothelial cells from oxidative stress-induced autophagy and endothelial dysfunction. Int. J. Biol. Sci. 2018, 14, 1696–1708. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Tsai, K.B.; Hsu, J.H.; Shin, S.J.; Wu, J.R.; Yeh, J.L. Liraglutide prevents and reverses monocrotaline-induced pulmonary arterial hypertension by suppressing ET-1 and enhancing eNOS/sGC/PKG pathways. Sci. Rep. 2016, 6, 31788. [Google Scholar] [CrossRef]
- Torres, G.; Morales, P.E.; García-Miguel, M.; Norambuena-Soto, I.; Cartes-Saavedra, B.; Vidal-Peña, G.; Moncada-Ruff, D.; Sanhueza-Olivares, F.; San, M.A.; Chiong, M. Glucagon-like peptide-1 inhibits vascular smooth muscle cell dedifferentiation through mitochondrial dynamics regulation. Biochem. Pharmacol. 2016, 104, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chan, D.C. Mitochondrial Dynamics in Regulating the Unique Phenotypes of Cancer and Stem Cells. Cell Metab. 2017, 26, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Okerson, T.; Yan, P.; Stonehouse, A.; Brodows, R. Effects of exenatide on systolic blood pressure in subjects with type 2 diabetes. Am. J. Hypertens. 2010, 23, 334–339. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Chen, L.; Ji, Q.; Liu, X.; Ma, J.; Tandon, N.; Bhattacharyya, A.; Kumar, A.; Kim, K.W.; Yoon, K.H.; et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: A 16-week, randomized, double-blind, active control trial(*). Diabetes Obes. Metab. 2011, 13, 81–88. [Google Scholar] [PubMed]
- Yanai, H.; Adachi, H.; Hamasaki, H.; Masui, Y.; Yoshikawa, R.; Moriyama, S.; Mishima, S.; Sako, A. Effects of 6-month sitagliptin treatment on glucose and lipid metabolism, blood pressure, body weight and renal function in type 2 diabetic patients: A chart-based analysis. J. Clin. Med. Res. 2012, 4, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Maiuri, M.C.; Zalckvar, E.; Kimchi, A.; Kroemer, G. Self-eating and self-killing: Crosstalk between autophagy and apoptosis. Nat. Rev. Mol. Cell Biol. 2007, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Moscat, J.; Diaz-Meco, M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009, 37, 1001–1004. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, A.D.; Kimchi, A. Life in the balance-a mechanistic view of the crosstalk between autophagy and apoptosis. J. Cell Sci. 2012, 125, 52596–52598. [Google Scholar] [CrossRef]
- Schaaf, M.B.; Houbaert, D.; Meçe, O.; Agostinis, P. Autophagy in endothelial cells and tumor angiogenesis. Cell Death Differ. 2019, 26, 6656–6679. [Google Scholar] [CrossRef]
- Denton, D.; Kumar, S. Autophagy-dependent cell death. Cell Death Differ. 2019, 26, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.H.; Lam, H.C.; Jin, Y.; Kim, H.P.; Cao, J.; Lee, S.J.; Ifedigbo, E.; Parameswaran, H.; Ryter, S.W.; Choi, A.M. Autophagy protein microtubule-associated protein 1 light chain-3B (LC3B) activates extrinsic apoptosis during cigarette smoke-induced emphysema. Proc. Natl Acad. Sci. USA 2010, 107, 18880–18885. [Google Scholar] [CrossRef] [PubMed]
- Agbani, E.; Coats, P.; Wadsworth, R.M. Threshold of peroxynitrite cytotoxicity in bovine pulmonary artery endothelial and smooth muscle cells. Toxicol. In Vitro 2011, 25, 1680–1686. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakao, S.; Tanabe, N.; Tatsumi, K. The estrogen paradox in pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2010, 299, L435–L438. [Google Scholar] [CrossRef] [PubMed]
- Perros, F.; Montani, D.; Dorfmüller, P.; Durand-Gasselin, I.; Tcherakian, C.; Le Pavec, J.; Mazmanian, M.; Fadel, E.; Mussot, S.; Mercier, O.; et al. Platelet-derived growth factor expression and function in idiopathic pulmonary arterial hypertension. Am. J. Respir. Crit. Care Med. 2008, 178, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Salabei, J.K.; Hill, B.G. Autophagic regulation of smooth muscle cell biology. Redox Biol. 2015, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Meng, N.; Gong, Y.; Mu, X.; Wang, Y.H.; Su, L.; Jiang, C.S.; Zhang, H. Novel Role of Heterogeneous Nuclear Ribonucleoprotein E1 in Regulation of Apoptosis and Autophagy by a Triazole Derivative in Vascular Endothelial Cells. Int. J. Biol. Sci. 2019, 15, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Yaïci, A.; Sztrymf, B.; Montani, D. Pulmonary hypertension: From genetics to treatments. Rev. Pneumol. Clin. 2004, 60, 196–201. [Google Scholar] [CrossRef]
- Fraidenburg, D.R.; Yuan, J.X. Hungry for more: Autophagy in the pathogenesis of pulmonary arterial hypertension. Circ. Res. 2013, 112, 1091–1093. [Google Scholar] [CrossRef]
- Lee, S.J.; Smith, A.; Guo, L.; Alastalo, T.P.; Li, M.; Sawada, H.; Liu, X.; Chen, Z.H.; Ifedigbo, E.; Jin, Y.; et al. Autophagic protein LC3B confers resistance against hypoxia-induced pulmonary hypertension. Am. J. Respir. Crit. Care Med. 2011, 83, 649–658. [Google Scholar] [CrossRef]
- Wesselborg, S.; Stork, B. Autophagy signal transduction by ATG proteins: From hierarchies to networks. Cell Mol. Life Sci. 2015, 72, 4721–4757. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571. [Google Scholar] [CrossRef] [PubMed]
- Long, L.; Yang, X.; Southwood, M.; Lu, J.; Marciniak, S.J.; Dunmore, B.J.; Morrell, N.W. Chloroquine prevents progression of experimental pulmonary hypertension via inhibition of autophagy and lysosomal bone morphogenetic protein type II receptor degradation. Circ. Res. 2013, 112, 1159–1170. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.Y.; Li, Y.B.; Cao, M.M.; Wang, Y. Liraglutide Improves the Survival of INS-1 Cells by Promoting Macroautophagy. Int. J. Endocrinol. Metab. 2013, 11, 184. [Google Scholar]
- Graf, M.R.; Jia, W.; Johnson, R.S.; Dent, P.; Mitchell, C.; Loria, R.M. Autophagy and the functional roles of Atg5 and beclin-1 in the anti-tumor effects of 3beta androstene 17alpha diol neuro-steroid on malignant glioma cells. J. Steroid. Biochem. Mol. Biol. 2009, 115, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Jiang, H.; Zhang, Y.; Ma, Y.; Li, L.; Wu, J. Liraglutide Enhances Autophagy and Promotes Pancreatic β Cell Proliferation to Ameliorate Type 2 Diabetes in High-Fat-Fed and Streptozotocin-Treated Mice. Med. Sci. Monit. 2018, 24, 2310–2316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Du, J.; Zhu, E.; Zhang, J.; Han, J.; Zhao, W.; Sun, B.; Tian, D. Liraglutide suppresses proliferation and induces adipogenic differentiation of 3T3-L1 cells via the Hippo-YAP signaling pathway. Mol. Med. Rep. 2018, 17, 4499–4507. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Wang, W.-T.; Lee, S.-S.; Kuo, Y.-R.; Wang, Y.-C.; Yen, S.-J.; Lee, M.-Y.; Yeh, J.-L. Glucagon-Like Peptide-1 Receptor Agonist Attenuates Autophagy to Ameliorate Pulmonary Arterial Hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3β Pathways. Int. J. Mol. Sci. 2019, 20, 3435. https://doi.org/10.3390/ijms20143435
Wu Y-C, Wang W-T, Lee S-S, Kuo Y-R, Wang Y-C, Yen S-J, Lee M-Y, Yeh J-L. Glucagon-Like Peptide-1 Receptor Agonist Attenuates Autophagy to Ameliorate Pulmonary Arterial Hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3β Pathways. International Journal of Molecular Sciences. 2019; 20(14):3435. https://doi.org/10.3390/ijms20143435
Chicago/Turabian StyleWu, Yi-Chia, Wei-Ting Wang, Su-Shin Lee, Yur-Ren Kuo, Ya-Chin Wang, Shih-Jung Yen, Mei-Yueh Lee, and Jwu-Lai Yeh. 2019. "Glucagon-Like Peptide-1 Receptor Agonist Attenuates Autophagy to Ameliorate Pulmonary Arterial Hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3β Pathways" International Journal of Molecular Sciences 20, no. 14: 3435. https://doi.org/10.3390/ijms20143435
APA StyleWu, Y.-C., Wang, W.-T., Lee, S.-S., Kuo, Y.-R., Wang, Y.-C., Yen, S.-J., Lee, M.-Y., & Yeh, J.-L. (2019). Glucagon-Like Peptide-1 Receptor Agonist Attenuates Autophagy to Ameliorate Pulmonary Arterial Hypertension through Drp1/NOX- and Atg-5/Atg-7/Beclin-1/LC3β Pathways. International Journal of Molecular Sciences, 20(14), 3435. https://doi.org/10.3390/ijms20143435





