Abstract
Cellulosomes are an extracellular supramolecular multienzyme complex that can efficiently degrade cellulose and hemicelluloses in plant cell walls. The structural and unique subunit arrangement of cellulosomes can promote its adhesion to the insoluble substrates, thus providing individual microbial cells with a direct competence in the utilization of cellulosic biomass. Significant progress has been achieved in revealing the structures and functions of cellulosomes, but a knowledge gap still exists in understanding the interaction between cellulosome and lignocellulosic substrate for those derived from biorefinery pretreatment of agricultural crops. The cellulosomic saccharification of lignocellulose is affected by various substrate-related physical and chemical factors, including native (untreated) wood lignin content, the extent of lignin and xylan removal by pretreatment, lignin structure, substrate size, and of course substrate pore surface area or substrate accessibility to cellulose. Herein, we summarize the cellulosome structure, substrate-related factors, and regulatory mechanisms in the host cells. We discuss the latest advances in specific strategies of cellulosome-induced hydrolysis, which can function in the reaction kinetics and the overall progress of biorefineries based on lignocellulosic feedstocks.
1. Introduction
Bioproducts, including biofuels and value-added chemicals derived from renewable resources, provide sustainable alternatives for petroleum-based products which contribute to climate change and energy crisis [1,2]. Among the variety of renewable resources, lignocellulosic biomass is the most abundant and economical carbon source on the earth. The development of bioproducts converted from lignocellulosic biomass should ultimately be essential for sustainable development without threatening food supplies and human survival [3]. However, as a natural protective barrier, the structure of plant cell wall is a recalcitrant network composed of cellulose, hemicellulose, and lignin, which is extremely difficult to degrade into fermentable sugars. Therefore, cellulose degradation and sugar release are becoming the typically rate-limiting factor for lignocellulosic biomass utilization [4]. Various efforts have been paid to gain access and deconstruct fermentable sugars in lignocellulosic biomass. In the existing biorefinery process, commercial exogenous cellulases are employed to hydrolyze lignocellulosic biomass synergistically, whereas the large amount of cellulase consumption would almost counteract its benefit of using low-cost feedstock [5]. Combining microbial enzyme generation, saccharification with fermentation in one-step, the consolidated bioprocessing (CBP) has been accepted as an economically feasible strategy for bioproduct conversion from lignocellulosic biomass [6]. Although some aerobic fungi such as Trichoderma reesei, Aspergillus niger, and T. koningii exhibit potential cellulase extracellular secretion by the common natural habitats of these microorganisms, the requirement of continuous oxygen supply and nutrient competition with other co-cultured microorganisms has limited the possibilities of CBP with fungi [7]. Recently, cellulosomes—the multienzyme complexes produced by certain anaerobic cellulolytic bacteria have gained considerable attention, owing to their specifically design to overcome the natural recalcitrant network consisted by plant cell wall polysaccharides [8,9]. It has been reported that the polycellulosomes are as large as 100 MDa in nature, and the cellulosomes range in mass is 650,000 Da–2.5 MDa [10]. Therefore, as one of the most efficient naturally occurring biocatalysts to degrade lignocellulosic biomass, cellulosomes are potential substitutes for reducing enzyme loading in industrial scale biorefineries.
The supermolecular cellulosome complexes were first described in the cellulolytic thermophilic Clostridium thermocellum in the early 1980s [11]. Generally, cellulosomes consist of non-enzymatic scaffolding proteins associated with a variety of enzymatic subunits that play a decisive role to degrade cellulose and hemicellulose. The architectures and components of the multienzyme systems are various with different bacteria [12]. The main functions of cellulosomes include: (i) improvement of substrate uptake; (ii) tighten the specific interaction with certain substrates; and (iii) synergistic activity and processivity of cellulases [13]. Interestingly, cellulosomes have been verified to degrade not only crystalline cellulose, but also non-crystalline hemicelluloses, or even chitin and pectin [14]. The major producers of cellulosomes can be classified into several genera, i.e., Clostridium, Ruminococcus, Acetivibrio, Bacteroides, and Pseudobacteroides belong to both mesophile and thermophile [5,8,10,15] (Figure 1), but no cellulosome has been identified in microorganism that can grow above 65 °C and in the Archaea. These microorganisms exist in various environmental niches, such as sewage sludge, soil, animal guts, rumen, and wood chip piles. The different microbial sources are constantly observed by characterization and comparison of the cellulosomal enzyme properties.
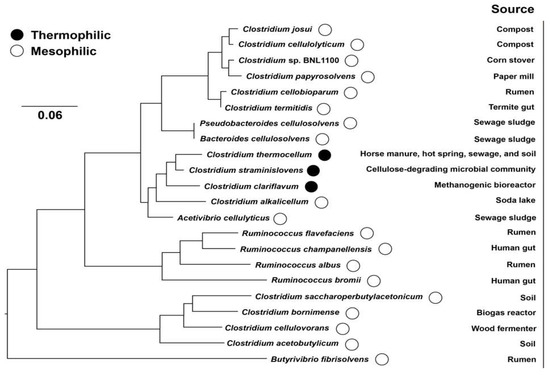
Figure 1.
Phylogenetic tree based on 16S rRNA sequencing of the anaerobic cellulosome-producing bacteria.
In recent times, numerous attempts have been described to improve cellulosomal catalysis by maximizing enzyme activities and/or creating the synergy between cellulosomal hydrolysis and the consequential fermentation [16,17]. Most of the efforts focus on engineering an ideal microorganism for CBP application, although the interaction between cellulosomes and lignocellulosic substrates remains to be clarified. This article presents a review of recent advances involved in properties of cellulosomes with respect of the composition and structural characterization. Moreover, the substrate-related physical and chemical factors affecting cellulosome adsorptions and catalytic activities are discussed in detail. We also describe the enzyme diversity and regulatory mechanisms of cellulosomes, and their latest achievements and limitations in potential CBP of lignocellulosic biomass to bioproducts.
2. Cellulosome Composition and Assembly
The mechanisms of cellulosome assemblies are one of the greatest interests to reveal the structure–function relationship. Efficient degradation of lignocellulosic biomass by cellulosomes requires appropriate composition of enzymes and optimal cellulosome structures. The estimated molecular mass of individual cellulosome produced by different microorganism ranges from 2 × 106 to 6 × 106 [18]. The cellulosome consists of two major components, namely (i) non-enzymatic scaffoldins including enzyme-binding sties named cohesins and carbohydrate-binding module (CBM); (ii) catalytic enzymes with dockerins interacting with cohesins in scaffoldins (Type I interaction) or surface layer homology domain (Type II interaction) [9,10]. Figure 2 shows the assembly of each component of cellulosomes on the cell surface and their possible interactions with lignocellulosic substrate derived after different types of pretreatment processes. These micro-structures can either suspended freely in the liquid (Figure 2a), connect to intermediate scaffodins (Figure 2b), or bind on the bacteria cell wall (Figure 2c). In this review, we summarize the cellulosome-related factors in Section 2 and the substrate-related factors in Section 3.
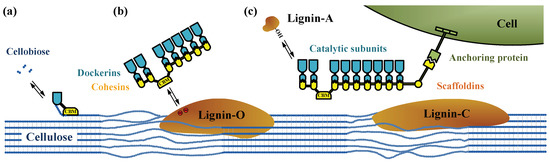
Figure 2.
Cellulosomal assemblies and the hypothetical interactions with pretreated substrates. (a) free enzymes; (b) cell-free scaffodins; and (c) on cell wall. Lignin-O: sulfite treated; Lignin-A: dissolved; and Lignin-C: condensed lignin.
2.1. Scaffoldin
The structure of scaffoldin forms the backbone of the enzymatic subunits, which is assembled by the dockerins. The scaffolding proteins contain one or more cohesin domains (Coh) and binding to substrates via CBM [19]. There are mainly three types of scaffoldin, i.e., primary, anchoring, and adaptor scaffoldins. Among them, the primary scaffoldin is the most common one and contains numerous Cohs that interact with dockerin-containing enzymes [20,21]. Although the mechanism by which a single primary scaffoldin can attach to the cell surface remains unknown, it is deduced that the scaffoldin should play a regulatory role during the assembly of cellulosome by using different substrates [10,21].
2.2. Cohesin–Dockerin Interaction
The cohesin–dockerin interaction can be considered as a mechanism of plug-and-socket in which the cohesin socket is plugged by the dockerin [22]. The various sequences of cohesin and dockerin are associated with the signature sequences of the cellulosomal enzymes [23]. In other words, the heterogenous nature of cellulosomes caused by the interactive variability of cohesin–dockerin pairs with different expressions in cohesin repeats, enzyme connections to the scaffoldins and species-specific variations [24,25,26]. The cohesin–dockerin interaction is known as one of the strongest protein–protein interactions in nature, even approaching the strength between high-affinity antigen and antibody (Ka–1011 M−1) [27,28]. There are three types of cohesin–dockerin interaction have been reported according to sequence homologies of the cohesins and their binding partners, i.e., Type I, II, and III interactions [29,30]. Type I interactions are located between dockerin-containing enzymatic subunits and anchoring scaffoldins. Type II interactions are usually located between anchoring scaffoldins and enzyme-binding primary scaffoldins [9]. In addition, Type III interactions do not interact with either Type I or Type II domains [29]. Type I and Type II interactions are observed in Clostridium spp., while Type III interactions exist in ruminococcal cellulosomes [31].
2.3. CBMs (CBDs)
The cellulosomal CBM also called cellulose binding domain (CBD), belonging to carbohydrate-binding module family 3, is present on scaffoldins that bind the cellulosome tightly to the cellulosic substrate by disrupting its crystal surface at the solid–liquid interface [32,33]. Besides cellulose, some CBMs such as the CBM of Clostridium cellulovorans can also bind to chitin, which has similar crystalline structure to cellulose [34]. Although the CBM is a non-catalytic domain, it brings the cellulosomal enzymes close to its substrate, and therefore making the hydrolysis more efficient compared to free enzymes [35,36,37]. The CBM specific binding with substrate depends on the content and arrangement of amino acids [38]. For instance, cellulosomal CBM recognizes crystalline cellulose as reflected in homologous binding surface, which consists of mostly polar and aromatic side chains [39,40].
2.4. Cellulosomal Enzymes
The cellulosomal enzymes were first described in Clostridium thermocellum by cloning and expressing genomic libraries [41]. Cellulosomes usually exhibit better breakdown of substrates compared to free enzymes owing to their close proximity of the expressed enzymes, which act synergistically [42,43]. In general, the free enzymes depend on a CBM for guiding their catalytic domains to the substrates, whereas a dockerin domain located on the cellulosomal enzymes by which the enzymes are incorporated into the cellulosome complex. In this manner, cellulosomal enzymes contain the catalytic domains assembled by the duplicated dockerins linked to cohesins in scaffoldins via calcium dependent interactions [44,45].
So far, almost all cellulosome producers are characterized to produce large amount of glycoside hydrolase 48 (GH48) exoglucanase, which is crucial for enzymatic activity [46,47]. Intriguingly, not only cellulases but also hemicellulases [48,49,50,51] and other carbohydrate-active cellulosomal enzymes such as ligninases [52,53], pectinases [54,55], mannanases [56,57,58], and chitinases [59,60] were subsequently identified with the cellulosomes. These plant polysaccharide-degrading enzymes are highly complex and diverse, which makes it difficult to understand the mechanisms of protein assemblies and organizations. It has been reported that the complex cellulosomal architecture is responsible for minimizing the diffusion of certain carbohydrates and facilitate their uptakes by the cellulosomal enzymes for complete degradation [9,61]. The expressions and activities of cellulosomal catalytic subunits can be varied according to the substrate availablity [62,63,64]. Cellulosomes with different compositions can be assembled on a microorganism with various enzyme complexes when grew on different carbon sources [65]. Therefore, understanding of the interactions between cellulosomes and the utilized substrates is crucial to reveal the mechanisms of cellulosome expression and apply the potential CBP for bioproducts conversion.
3. Effects of Several Substrate-Related Factors on Cellulosome-Induced Hydrolysis
Despite the cellulosome complex being certainly viewed as an efficient natural system to break down the lignocellulosic biomass, there are several substrate-related factors that determine the reaction kinetics and yield of degradation of plant polysaccharide to its respective short chain monomers. These include: (i) carbon sources, i.e., types and sizes of lignin and hemicelluloses, as well as related complexes in the substrate; (ii) chemical compounds, i.e., inhibitors and/or promoters; and (iii) pretreatment effects, i.e., the extent of lignin and xylan removal, lignin structure, pore volume, or accessible surface area [66]. The interaction between cellulosome and substrate-related factors is shown in Figure 3. The key parameters involved in the hydrolysis system may include two major functions (i.e., substrate-cellulosome interaction and cellulolysis reaction), eight mechanisms (grey circles), and their corresponding biological factors (white circles). Since it is nearly impossible to obtain a homogenous substrate that containing particles in the same size, it is difficult to get a pure cellulosomal enzyme expressed in the same level. All these substrate-related factors are interconnected and any single alteration would affect the others. The performance of pretreatment can directly affect the product yields and kinetics of the downstream processes, i.e., substrate hydrolysis. Some mechanisms of delignification for different pretreatment techniques have been confirmed while many questions still remain unanswered, which is mostly due to high complexity of lignin structure [67,68]. The substrate-related factors are the most sensitive and representative parameters include the substrate accessibility to cellulase [69], enzyme–additive interactions [70], crystallinity [71], and others [66,72].
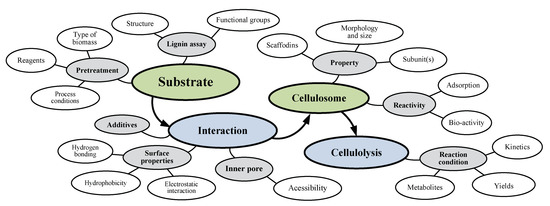
Figure 3.
Interactions between cellulosome and substrate-related factors.
3.1. Effects of Different Carbon Sources
Extracellular carbon sources affect the assembly of the cellulosome by regulating its enzymes and structural compositions, thus ensuring the present of optimal factors to break down the available carbohydrates [73,74,75]. Although approximately 90% of native or treated cellulosic substrates can be degraded by the appropriate cellulosomes, there are still many challenges for effective hydrolysis of different carbon sources by the cellulosome enzyme complexes [76,77].
Several previous studies initially demonstrated alterations of cellulosome compositions upon cultivation of the cellulosome producers on different carbon sources. Han et al. [78] cultured C. cellulovorans ATCC 35296 anaerobically in medium containing 1% (w/v) of Avicel, xylan, pectin and mixed polysaccharides (Avicel/xylan/pectin (3:1:1, by wt)) as carbon substrates, respectively. As a result, the cellulosome population was observed heterogeneously, although the scaffolding protein CbpA, endoglucanase EngE, and cellobiohydrolase ExgS were relatively constant. The cellulase activity was promoted by cellulosome contained CbpA, EngE/EngK, ExgS/EngH, and EngL in cells grew on a mixture of carbon sources, while high xylanase activity was detected in cellulosomes derived from cellulose, pectin and mixed carbon, which had larger amounts of XynB, XynA, and unknown proteins (35–45 kDa). These results indicated that the ratio of cellulosomal subpopulations in C. cellulovorans was controlled by its autogenous regulatory system that make up the cellulosomal population.
Similarly, the cellulosome assembly of C. thermocellum strain DSM 1313 was examined for its response to available sole carbon sources, i.e., glucose, cellobiose, microcrystalline cellulose, alkaline-pretreated switchgrass, alkaline-pretreated corn stover, and dilute acid-pretreated corn stover [79]. Different catalytic and structural subunits (scaffoldins) were finally investigated in the different cellulosome samples. Cellulosomes derived from microcrystalline cellulose and glucose exhibited higher endoglucanase-to-exoglucanase ratios, as well as catalytic subunit-per-scaffoldin ratios compared to the lignocellulose-derived cellulosome types. The results verified glucose- and microcrystalline cellulose-derived cellulosomes were more efficient in their action on carbon sources than other cellulosome samples. Curiously, compared with the cellulosomes of strain C. cellulovorans ATCC 35296 grew on carbon sources such as Avicel, xylan, AXP (Avicel–xylan–pectin, 3:1:1), and cellobiose, the enzyme compositions between Avicel and cellobiose culture were similar and that almost no repression of cellulase enzymes when cells grew on cellobiose [63].
On the other hand, in order to understand the synergistic relationship between cellulosomes and noncellulosomal (hemi)cellulolytic enzymes, changes in mRNA and protein expression were examined with cultures of C. cellulovorans ATCC 35296 grew on cellobiose, cellulose, pectin, xylan, and corn fiber or mixtures, respectively [80]. Expression profiles of both the cellulosome and noncellulosomal enzymes were strongly affected by different carbon sources, whereas cellulosomal proteomes were more affected by the carbon source as compared to noncellulosomal enzymes. Furthermore, Fierobe et al. [81] compared hydrolysis effects of C. cellulolyticum cellulosome and free enzyme systems on recalcitrant substrates and tractable substrates. For the recalcitrant cellulose–Avicel, the presence of a CBM on scaffoldin and enzyme proximity on the organization of cellulosome chimeras contributed almost equally to the elevated action on the recalcitrant substrate, whereas the cellulosome chimeras exhibited little or no advantage over free enzymes on the tractable substrate–bacterial cellulose.
Recently, cellulosomes displayed on the cell surface was compared between cells grew on soluble or recalcitrant insoluble substrates by using C. clariflavum [82]. According to immunolabeling of four cellulosome components: ScaA, ScaB, ScaC, and the most prominent enzyme, GH48, the results explored that the cellulosome producer required closely attached cellulosomes on its surface to break down the highly recalcitrant substrates. How these specific variations occur in response to the available carbon sources? One possibility is that the substrate-induced enzyme expressions determine the amounts of the various cellulosomal enzymes during the cellulosome assembly [77,80,83,84,85]. The other may cause by the specific interactions between the dockerins and their cognate cohesins [10,65,86]. Since certain cohesins can bind to enzymes by the docherins that are absent in other cohesins [10,15], the cellulosomal composition may varies with the enzyme expressions and the interactions of different cohesin–dockerin pairs. Moreover, Nataf et al. [87] revealed the cellulosomal regulatory mechanism at the genomic level. They suggested the cellulosomal genes were regulated via an extracellular sensing mechanism, in which alternative σ factors (i.e., σI1 or σI6) were activated in response to the carbohydrates in the extracellular surroundings.
It is known that the accumulation of carbon monomers such as glucose, cellobiose, as well as some other end products of hydrolysis will inhibit cellulases and decrease glucose yields [88,89,90]. In contrast to aerobic cellulase, kinetics studies related to cellulosome are quite limited owing to the intricate structure and catalytic mechanism. Lin et al. [91] described the utilization of recombinant anchoring cellulosome from B. subtilis W800N strain to degrade the Chlorella lipid-deprived residues. The kinetics parameters of maximum reaction rate (Vmax) and the Michaelis–Menten constant (Km) values displayed a prevailing effect when the cellulosome obtained in the supernatant as compared to the whole cells. Recently, a mathematical model was developed to estimate the inhibitory effect of glucose on cellulosome by using C. thermocellum (Equation (1)) [92]
where C was the glucose concentration, K was the inhibition constant for glucose on cellulosome, v was the rate of the hydrolytic action of cellulosome, and constant A could be deduced from the slope of the straight line, which plotted based on C versus 1/v. It described the relationship between glucose concentration and saccharification rate at a specific glucose concentration or a specific time. Glucose accumulation in a long term is independent to the saccharification rate at a specific time. Hence, methods that can decrease the glucose-induced inhibition on cellulosome should be effective in enhancing cellulose saccharification by the anaerobic cellulosome-producing bacteria [93]. Attempts to eliminate side reactions such as ethanol and CO2 fermentation proved the utilization of certain adsorbents (i.e., activated carbon and biochar) could lower the inhibition of glucose and improve the adsorption of substrates onto cellulosome [92].
3.2. Effects of Different Chemical Compounds
The CBP efficiency can potentially be improved by optimizing cellulosome activity and/or creating the synergy between cellulosomic saccharification and the subsequently fermentation. Various compounds generated by the pretreatment process or derived from the fermentation by-products usually are inhibitory to cell growth and fermentation activity [94,95,96]. These chemical compounds exist in the substrates significantly affect the cellulosome-induced biorefineries based on lignocellulosic biomass. Amongst these, the cellulosome activity of wild-type strain was inhibited by ethanol concentrations above 2% (v/v), whereas those evolved strains remained viable when ethanol concentrations increased up to 8% (v/v). Compared with commercial enzymes, C. thermocellum cellulosomes were generally able to tolerate higher ethanol concentrations [97]. In addition, in regard to the inhibitors released during the pretreatment of lignocellulosic biomass, typically, furfural and phenols are considered unfavorable side-products owing to their inhibitory effects on cell growth [98,99]. The C. thermocellum cellulosomes demonstrated tolerance on certain concentrations of furfural (≤5 mM), p-hydroxybenzoic acid (≤50 mM), and catechol (≤1 mM), respectively [97].
Parsiegla et al. [100] studied the chemical structure of the thiooligosaccharide methyl 4-S-β-cellobiosyl-4-thio-cellobioside (IG4), which performed as an inhibitor to the cellulosome of C. cellulolyticum. The orientation of the inhibitor molecules Inh1 and Inh2 was consistent with a processive action towards the non-reducing ends from the reducing ends of the cellulose chains. Moreover, You et al. [101] assembled a cellulosome-microbe complex ex vivo on the Bacillus subtilis surface, which displayed a mini-scaffoldin bound with three dockerin-containing cellulase components, i.e., endoglucanase Cel5, processive endoglucanase Cel9 and cellobiohydrolase Cel48. The hydrolysis performance indicated that high concentration cellodextrins in the boundary layer would inhibit cellulosome activity more strongly than short chain products because the β-glucosidase without a CBM usually works in the bulk phase [102]. Therefore, cellulosomes that expedite the cellulose bioconversion rate can help to construct CBP microorganisms with improved performance, which is expected to hydrolyze recalcitrant substrate efficiently under low secretory cellulase levels.
On the other hand, the organic acids almost occur as products or by-products in microbial fermentation [103,104], in which both pH changes and anion accumulations occur in the bioreactor. The change in pH will drastically alter cellulosome capability for cellulose digestion [105,106,107]. In order to determine the effects of organic acid anions on cellulosome-induced cellulose hydrolysis, the cellulosomal enzyme activities of C. thermocellum JYT01 were investigated in the presence of formate, acetate, and lactate [97]. Interestingly, although these anions inhibited the cell growth, at the same time these acted as promoters to cellulosome activity at a concentration of formate, acetate, and lactate below 100, 200, and 50 mM, respectively, while negative effect was only observed beyond their critical concentrations. As a result, the promoted Avicel hydrolysis was achieved by supplementing exogenous organic acid anions in a living-cell culture. It presumed that the active domains of certain cellulosome harbor a moiety for specific anion-binding, and -promoting substrate recognitions in the presence of cellulose–anion compounds [97,108].
3.3. Effects of Pretreatment
Lignocellulosic biomass with only exterior surface is not applicable for the microbial digestion, owing to its low accessible surface areas. Pretreatment is considered crucial for valorization of lignocellulosic biomass into value-added bioproducts. The direct physical contact between the cellulosome producers and lignocellulosic surfaces are necessary to start the biocatalysis. Since pretreatment operations change several decisive factors concurrently, and it is hard to predict its effectiveness directly [66,109]. In fact, the effectiveness of pretreatment is usually evaluated by enzymatic hydrolysis or merely based on the yields of target products by fermentation [66]. Generally, the pretreatment process varies depending on the type of lignocellulosic biomass and there is no standalone method can be applied for all feedstock, because this varies with the type of natural biomass [110]. Currently, several available techniques have been developed to remove lignin from lignocellulosic biomass, i.e., acid pretreatment (such as organosolv or sulfite) and alkaline pretreatment (such as ammonia or NaOH). Table 1 compares the effect of different pretreatment on lignin structure and enzymatic hydrolysis. To the best of our knowledge, no special class of cellulases appear in cellulosomes because most of the cellulosomal enzymes belong to the same set of enzyme families as those of free cellulases. The understandings of free enzymes’ efficiency should provide a reliable foundation to evaluate the effects of pretreatment on cellulosomic catalysis.

Table 1.
Alteration of lignin structure during pretreatment and their effects on enzymatic hydrolysis of pretreated substrate
Degradation of lignocellulosic biomass usually involves three steps: (i) enzyme adsorption to the substrate surface, (ii) hydrolysis of the substrate, and (iii) desorption of the cellulase into the liquid [129]. Similar to the microbial degradation, the lignocellulosic pore volume or accessible surface area for the cellulosomic enzymes is among the most affecting factors to the lignocellulose hydrolysis rate and yield. It means that once the diffusion of an enzyme molecule into a pore, the size of the enzymatic component should not be equal to the size of the pore owing to the wall confinement [130]. Moreover, when the pore size of lignocellulosic substrate is narrow, then β-glucosidase would not accompany other groups of cellulosomic cellulases into the pore. In other words, more spaces for synergistic actions between the different groups of cellulosomic cellulases are crucial for efficient hydrolysis of lignocellulosic biomass [66,130,131].
On the other hand, pretreatment is actually an important process to increase the surface area of substrate available for cellulosome. The total accessible surface area of lignocellulosic biomass is the sum of its external and internal surfaces, among which the external surface area depends on the size and shape of the material, while the internal surface area depends on its pore size and distribution [66,132]. Although higher than 90% of the sieved Avicel surface is accessible to free enzymes when it is in an average diameter of 100 μm [133], the large size of cellulosomes will prevent them from accessing many pores of the internal surface area. The presence of multiple enzymes on the cellulosomes can compensate for this limitation of cellulosomes to attack the binding sites in the pores [17,134]. Hence, the digestibility of lignocellulose for cellulosomes is significantly affected by the factor of accessible surface area, which will be gradually increased with the enzymatic hydrolysis caused by the removal of partial cellulose and hemicellulose. Besides the surface area, it is noticed that the cellulosomic hydrolysis rate also depends on the hydrolysis stages [79,135]. The rate of hydrolysis is normally rapid at the beginning stage and it becomes considerably slower during the latter stage, despite the availability of higher surface area. The slower hydrolysis rate should be a result of the higher crystallinity regions of the substrates, deactivation of the hydrolytic enzymes, and the increasing concentrations of the lignin [136,137].
In an attempt to increase the adsorption and hydrolysis rate of cellulosomal enzyme, Moraïs et al. [138] observed the effects of reduced recalcitrance on wheat straw degradation by using native and designer cellulosomes, respectively. Actions of cellulosomes were estimated either directly following the size reduction by mechanical treatment or an additional pretreatment by sodium hypochlorite to reduce the lignin content in order to promote enzymatic hydrolysis. The result without chemical pretreatment demonstrated that there was no significant effect on lignin content of the wheat straw substrate when utilized both the native and designer cellulosomes. Thus, although microbial enzymes demonstrate and ability to solubilize lignin and increase the cellulase access to cellulose [139,140], the cellulosomes hardly reduce cellulose content and/or decompose hemicellulose without prior pretreatment of the lignocellulosic substrate. However, it was reported that the chemical pretreatment of lignocellulose before enzymatic digestion usually generated lignocellulose-derived by-products such as phenolic compounds that would further inhibit the enzymatic saccharification [94,141]. To overcome this barrier, Davidi et al. [142] constructed a cellulosome with extra enzyme activities on lignin. The resultant chimera finally increased two-fold of the reducing sugars derived from wheat straw compared with the designed trivalent cellulosomes lacking the laccase, which can catalyze the oxidation of various phenolic and nonphenolic compounds [142,143].
4. Conclusions
Lignocellulosic biomass is a renewable resource with great potential to facilitate important bioproducts conversion by CBP in the context of biorefineries. However, the low rates and high costs of lignocellulose decomposition are the main barriers to commercialization of this biological conversion processes. To address these barriers, one significant area of heightened research activity is the study of naturally occurring cellulosomes produced by certain anaerobic bacteria. Numerous cellulosome-related investigations have been confirmed at the molecular level such as the structures and functions of cellulosomes, as well as gene modifications to enhance the biocatalysis of the enzyme complex, although the understanding of interactions between cellulosomes and their lignocellulosic substrate are still limited.
In this review, the current understanding relating to several substrate-related physical and chemical factors affecting the activities of cellulosomes are summarized. Different carbon sources play significant impacts on the cellulosomal assembly by regulating the expression of enzyme activities and structural compositions. In addition, cellulosomic enzyme adsorption or desorption is an important biological parameter related to the degradation of the lignocellulosic substrates. Substrate accessibility is another crucial parameter of the lignocellulosic substrate, which is a desirable factor for all pretreatments. External surface area of the lignocellulosic biomass can be increased by the physical size reduction as well as changing of the particle shapes, while increase in the internal surface of the substrates should be followed by typical chemical or even biological pretreatments. Therefore, special attention should be paid to the pretreatment methods utilized for valorization of the lignocellulosic biomass into bioproducts prior to the cellulosomic catalysis.
Author Contributions
Writing—original draft preparation, Y.W.; Writing—review and editing, Y.W., L.L., F.L., M.K.I., C.S.K.L.; Supervision, S.-Y.L.
Funding
This research was funded by the GDAS’ Project of Science and Technology Development (2019GDASYL-0102005), the Hong Kong General Research Fund (15212319), and the Hong Kong Environment and Conservation Fund (ECF 85/2017).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Yamakawa, C.K.; Qin, F.; Mussatto, S.I. Advances and opportunities in biomass conversion technologies and biorefineries for the development of a bio-based economy. Biomass Bioenergy 2018, 119, 54–60. [Google Scholar] [CrossRef]
- Bauer, F.; Coenen, L.; Hansen, T.; McCormick, K.; Voytenko Palgan, Y. Technological innovation systems for biorefineries: A review of the literature. Biofuels Bioprod. Biorefin. 2017, 11, 534–548. [Google Scholar] [CrossRef]
- Periyasamy, K.; Santhalembi, L.; Mortha, G.; Aurousseau, M.; Boyer, A.; Subramanian, S. Bioconversion of lignocellulosic biomass to fermentable sugars by immobilized magnetic cellulolytic enzyme cocktails. Langmuir 2018, 34, 6546–6555. [Google Scholar] [CrossRef] [PubMed]
- Amezcua-Allieri, M.A.; Sánchez-Duran, T.; Aburto, J. Study of chemical and enzymatic hydrolysis of cellulosic material to obtain fermentable sugars. J. Chem. 2017, 2017, 5680105. [Google Scholar] [CrossRef]
- Chi, X.; Li, J.; Wang, X.; Zhang, Y.; Leu, S.; Wang, Y. Bioaugmentation with Clostridium tyrobutyricum to improve butyric acid production through direct rice straw bioconversion. Bioresour. Technol. 2018, 263, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Olson, D.G.; McBride, J.E.; Shaw, A.J.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tashiro, Y.; Sonomoto, K. Fermentative production of lactic acid from renewable materials: Recent achievements, prospects, and limits. J. Biosci. Bioeng. 2015, 119, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, S.P.; Henske, J.K.; O’Malley, M.A. Driving biomass breakdown through engineered cellulosomes. Bioengineered 2015, 6, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Behera, S.; Kumar Sharma, N.; Kumar, S. Bioprospecting thermostable cellulosomes for efficient biofuel production from lignocellulosic biomass. Bioresour. Bioprocess. 2015, 2, 38. [Google Scholar] [CrossRef]
- Doi, R.H.; Kosugi, A.; Murashima, K.; Tamaru, Y.; Han, S.O. Cellulosomes from mesophilic bacteria. J. Bacteriol. 2003, 20, 5907–5914. [Google Scholar] [CrossRef]
- Lamed, R.; Setter, E.; Bayer, E.A. Characterization of a cellulose-binding, cellulase-containing complex in Clostridium thermocellum. J. Bacteriol. 1983, 156, 828–836. [Google Scholar] [PubMed]
- Bayer, E.A.; Lamed, R.; White, B.A.; Flint, H.J. From cellulosomes to cellulosomics. Chem. Rec. 2008, 8, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Desvaux, M. Clostridium cellulolyticum model organism of mesophilic cellulolytic clostridia. FEMS Microbiol. Rev. 2005, 29, 741–764. [Google Scholar] [CrossRef] [PubMed]
- Quiroz-Castañeda, R.E.; Folch-Mallol, J.L. Hydrolysis of biomass mediated by cellulases for the production of sugars. In Sustainable Degradation of Lignocellulosic Biomass Techniques, Applications and Commercialization; Chandel, A., Ed.; InTech: London, UK, 2013; pp. 119–155. [Google Scholar]
- Artzi, L.; Bayer, E.A.; Moraïs, S. Cellulosomes: Bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 2017, 15, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, G.A.L.; Mori, Y.; Kamiya, N. Biomolecular assembly strategies to develop potential artificial cellulosomes. Sustain. Chem. Process. 2014, 2, 19. [Google Scholar] [CrossRef]
- Moraïs, S.; Shterzer, N.; Lamed, R.; Bayer, E.A.; Mizrahi, I. A combined cell-consortium approach for lignocellulose degradation by specialized Lactobacillus plantarum cells. Biotechnol. Biofuel. 2014, 7, 112. [Google Scholar] [CrossRef]
- Béguin, P.; Lemaire, M. The Cellulosome: An exocellular, multiprotein complex specialized in cellulose degradation. Crit. Rev. Biochem. Mol. Biol. 1996, 31, 201–236. [Google Scholar] [CrossRef] [PubMed]
- Mazzoli, R.; Lamberti, C.; Pessione, E. Engineering new metabolic capabilities in bacteria: Lessons from recombinant cellulolytic strategies. Trends Biotechnol. 2012, 30, 111–119. [Google Scholar] [CrossRef]
- Pagès, S.; Bélaïch, A.; Fierobe, H.P.; Tardif, C.; Gaudin, C.; Bélaïch, J.P. Sequence analysis of scaffolding protein CipC and ORFXp, a new cohesin-containing protein in Clostridium cellulolyticum: Comparison of various cohesin domains and subcellular localization of ORFXp. J. Bacteriol. 1999, 181, 1801–1810. [Google Scholar]
- Erickson, S.J.; Duvall, S.W.; Fuller, J.; Schrader, R.; MacLean, P.; Lowe, J.R. Differential associations between maternal scaffolding and toddler emotion regulation in toddlers born preterm and full term. Early Hum. Dev. 2013, 89, 699–704. [Google Scholar] [CrossRef]
- Bayer, E.A.; Bélaïch, J.P.; Shoham, Y.; Lamed, R. The cellulosomes: Multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 2004, 58, 521–554. [Google Scholar] [CrossRef] [PubMed]
- Shoham, Y.; Lamed, R.; Bayer, E.A. The cellulosome concept as an efficient microbial strategy for the degradation of insoluble polysaccharides. Trends Microbiol. 1999, 275, 275–281. [Google Scholar] [CrossRef]
- Bomble, Y.J.; Beckham, G.T.; Matthews, J.F.; Nimlos, M.R.; Himmel, M.E.; Crowley, M.F. Modeling the self-assembly of the cellulosome enzyme complex. J. Biol. Chem. 2011, 286, 5614–5623. [Google Scholar] [CrossRef] [PubMed]
- Koukiekolo, R.; Cho, H.Y.; Kosugi, A.; Inui, M.; Yukawa, H.; Doi, R.H. Degradation of corn fiber by Clostridium cellulovorans cellulases and hemicellulases and contribution of scaffolding protein CbpA. Appl. Environ. Microbiol. 2005, 71, 3504–3511. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fierobe, H.P.; Mingardon, F.; Mechaly, A.; Belaich, A.; Rincon, M.T.; Pages, S.; Lamed, R.; Tardif, C.; Belaich, J.P.; Bayer, E.A. Action of designer cellulosomes on homogeneous versus complex substrates: Controlled incorporation of three distinct enzymes into a defined trifunctional scaffoldin. J. Biol. Chem. 2005, 280, 16325–16334. [Google Scholar] [CrossRef] [PubMed]
- Valbuena, A.; Oroz, J.; Hervas, R.; Manuel Vera, A.; Rodriguez, D.; Menendez, M.; Sulkowska, J.I.; Cieplak, M.; Carrion-Vazquez, M. On the remarkable mechanostability of scaffoldins and the mechanical clamp motif. Proc. Natl. Acad. Sci. USA 2009, 106, 13791–13796. [Google Scholar] [CrossRef]
- Gunnoo, M.; Cazade, P.A.; Galera-Prat, A.; Nash, M.A.; Czjzek, M.; Cieplak, M.; Alvarez, B.; Aguilar, M.; Karpol, A.; Gaub, H.; et al. Nanoscale engineering of designer cellulosomes. Adv. Mater. 2016, 28, 5619–5647. [Google Scholar] [CrossRef]
- Bras, J.L.A.; Alves, V.D.; Carvalho, A.L.; Najmudin, S.; Prates, J.A.M.; Ferreira, L.M.A.; Bolam, D.N.; Romao, M.J.; Gilbert, H.J.; Fontes, C.M.G.A. Novel Clostridium thermocellum Type I cohesin–dockerin complexes reveal a single binding mode. J. Biol. Chem. 2012, 287, 44394–44405. [Google Scholar] [CrossRef]
- Peer, A.; Smith, S.P.; Bayer, E.A.; Lamed, R.; Borovok, I. Noncellulosomal cohesin- and dockerin-like modules in the three domains of life. FEMS Microbiol. Lett. 2009, 291, 1–16. [Google Scholar] [CrossRef]
- Voronov-Goldman, M.; Yaniv, O.; Gul, O.; Yoffe, H.; Salama-Alber, O.; Slutzki, M.; Levy-Assaraf, M.; Jindou, S.; Shimon, L.J.W.; Borovok, I.; et al. Standalone cohesin as a molecular shuttle in cellulosome assembly. FEBS Lett. 2015, 589, 1569–1576. [Google Scholar] [CrossRef]
- Resch, M.G.; Donohoe, B.S.; Baker, J.O.; Decker, S.R.; Bayer, E.A.; Beckham, G.T.; Himmel, M.E. Fungal cellulases and complexed cellulosomal enzymes exhibit synergistic mechanisms in cellulose deconstruction. Energ. Environ. Sci. 2013, 6, 1858–1867. [Google Scholar] [CrossRef]
- Ichikawa, S.; Karita, S.; Kondo, M.; Goto, M. Cellulosomal carbohydrate-binding module from Clostridium josui binds to crystalline and non-crystalline cellulose, and soluble polysaccharides. FEBS Lett. 2011, 588, 3886–3890. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.A.; Takagi, M.; Hashida, S.; Shoseyov, O.; Doi, R.H.; Segel, I.H. Characterization of the cellulose-binding domain of the Clostridium cellulovorans cellulose-binding protein A (CbpA). J. Bacteriol. 1993, 175, 5762–5768. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, Y.; Karita, S.; Ibrahim, A.; Chan, H.; Doi, R.H. A large gene cluster for the Clostridium cellulovorans cellulosome. J. Bacteriol. 2000, 182, 5906–5910. [Google Scholar] [CrossRef] [PubMed]
- Caspi, J.; Irwin, D.; Lamed, R.; Li, Y.; Fierobe, H.P.; Wilson, D.B.; Bayer, E.A. Conversion of Thermobifida fusca free exoglucanases into cellulosomal components: Comparative impact on cellulose-degrading activity. J. Biotechnol. 2008, 135, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Gerngross, U.T.; Romaniec, M.P.; Kobayashi, T.; Huskisson, N.S.; Demain, A.L. Sequencing of a Clostridium thermocellum gene (cipA) encoding the cellulosomal SL-protein reveals an unusual degree of internal homology. Mol. Microbiol. 1993, 10, 1155. [Google Scholar] [CrossRef]
- Yaniv, O.; Jindou, S.; Frolow, F.; Lamed, R.; Bayer, E.A. A simple method for determining specificity of carbohydrate-binding modules for purified and crude insoluble polysaccharide substrates. Methods Mol. Biol. 2012, 908, 101–107. [Google Scholar] [PubMed]
- Boraston, A.B.; Bolam, D.N.; Gilbert, H.J.; Davies, G.J. Carbohydrate-binding modules: Fine-tuning polysaccharide recognition. Biochem. J. 2004, 382, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Shoseyov, O.; Shani, Z.; Levy, I. Carbohydrate binding modules: Biochemical properties and novel applications. Microbiol. Mol. Biol. Rev. 2006, 70, 283–295. [Google Scholar] [CrossRef]
- Hazlewood, G.P.; Romaniec, M.P.M.; Davidson, K.; Grépinet, O.; Béguin, P.; Millet, J.; Raynaud, O.; Aubert, J.P. A catalogue of Clostridium thermocellum endoglucanase, β-glucosidase and xylanase genes cloned in Escherichia coli. FEMS Microbiol. Lett. 1988, 51, 231–236. [Google Scholar] [CrossRef]
- Vazana, Y.; Moraïs, S.; Barak, Y.; Lamed, R.; Bayer, E.A. Interplay between Clostridium thermocellum family 48 and family 9 cellulases in cellulosomal versus noncellulosomal states. Appl. Environ. Microbiol. 2010, 76, 3236–3243. [Google Scholar] [CrossRef] [PubMed]
- Bayer, E.A.; Chanzy, H.; Lamed, R.; Shoham, Y. Cellulose, cellulases and cellulosomes. Curr. Opin. Struct. Biol. 1998, 8, 548–557. [Google Scholar] [CrossRef]
- Bayer, E.A.; Morag, E.; Lamed, R. The cellulosome—A treasure-trove for biotechnology. Trends Biotechnol. 1994, 12, 378–386. [Google Scholar] [CrossRef]
- Ding, S.Y.; Xu, Q.; Crowley, M.; Zeng, Y.; Nimlos, M.; Lamed, R.; Bayer, E.A.; Himmel, M.E. A biophysical perspective on the cellulosome: New opportunities for biomass conversion. Curr. Opin. Biotechnol. 2008, 19, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Morag, E.; Halevy, I.; Bayer, E.A.; Lamed, R. Isolation and properties of a major cellobiohydrolase from the cellulosome of Clostridium thermocellum. J. Bacteriol. 1991, 173, 4155–4162. [Google Scholar] [CrossRef] [PubMed]
- Ravachol, J.; Borne, R.; Meynial-Salles, I.; Soucaille, P.; Pages, S.; Tardif, C. Combining free and aggregated cellulolytic systems in the cellulosome-producing bacterium Ruminiclostridium cellulolyticum. Biotechnol. Biofuels 2015, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Morag, E.; Bayer, E.A.; Lamed, R. Relationship of cellulosomal and noncellulosomal xylanases of Clostridium thermocellum to cellulose-degrading enzymes. J. Bacteriol. 1993, 172, 6098–6105. [Google Scholar] [CrossRef] [PubMed]
- Mohand-Oussaid, O.; Payot, S.; Guedon, E.; Gelhaye, E.; Youyou, A.; Petitdemange, H. The extracellular xylan degradative system in Clostridium cellulolyticum cultivated on xylan: Evidence for cell-free cellulosome production. J. Bacteriol. 1999, 181, 4035–4040. [Google Scholar] [PubMed]
- Maki, M.; Leung, K.T.; Qin, W. The prospects of cellulase-producing bacteria for the bioconversion of lignocellulosic biomass. Int. J. Biol. Sci. 2009, 5, 500–516. [Google Scholar]
- Akinosho, H.; Yee, K.; Close, D.; Ragauskas, A. The emergence of Clostridium thermocellum as a high utility candidate for consolidated bioprocessing applications. Front. Chem. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Yuan, L. Production of multifunctional chimaeric enzymes in plants: A promising approach for degrading plant cell wall from within. Plant Biotechnol. J. 2010, 8, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Cragg, S.M.; Beckham, G.T.; Bruce, N.C.; Bugg, T.D.; Distel, D.L.; Dupree, P.; Etxabe, A.G.; Goodell, B.S.; Jellison, J.; McGeehan, J.E.; et al. Lignocellulose degradation mechanism sacross the tree of life. Curr. Opin. Chem. Biol. 2015, 29, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Fernandes, V.O.; Dias, F.M.V.; Prates, JA.M.; Ferreira, L.M.A.; Fontes, C.M.G.A.; Goyal, A.; Centeno, M.S.J. Role of pectinolytic enzymes identified in Clostridium thermocellum cellulosome. PLoS ONE 2015, 10, e0116787. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, Y.; Doi, R.H. Pectate lyase A, an enzymatic subunit of the Clostridium cellulovorans cellulosome. Proc. Natl. Acad. Sci. USA 2001, 98, 4125–4129. [Google Scholar] [CrossRef] [PubMed]
- Perret, S.; Belaich, A.; Fierobe, H.P.; Belaich, J.P.; Tardif, C. Towards designer cellulosomes in Clostridia: Mannanase enrichment of the cellulosomes produced by Clostridium cellulolyticum. J. Bacteriol. 2004, 186, 6544–6552. [Google Scholar] [CrossRef] [PubMed]
- Sabathé, F.; Bélaïch, A.; Soucaille, P. Characterization of the cellulolytic complex (cellulosome) of Clostridium acetobutylicum. FEMS Microbiol. Lett. 2002, 217, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Kurokawa, J.; Hemjinda, E.; Arai, T.; Karita, S.; Kimura, T.; Sakka, K.; Ohmiya, K. Sequence of the Clostridium thermocellum mannanase gene man26B and characterization of the translated product. Biosci. Biotechnol. Biochem. 2001, 65, 548–554. [Google Scholar] [CrossRef]
- Zverlov, V.V.; Fuchs, K.P.; Schwarz, W.H. Chi18A, the endochitinase in the cellulosome of the thermophilic, cellulolytic bacterium Clostridium thermocellum. Appl. Environ. Microbiol. 2002, 68, 3176–3179. [Google Scholar] [CrossRef] [PubMed]
- Tamaru, Y.; Miyake, H.; Kuroda, K.; Ueda, M.; Doi, R.H. Comparative genomics of the mesophilic cellulosome-producing Clostridium cellulovorans and its application to biofuel production via consolidated bioprocessing. Envion. Technol. 2010, 31, 889–903. [Google Scholar] [CrossRef] [PubMed]
- Vodovnik, M.; Logar, R.M. Cellulosomes-promising supramolecular machines of anaerobic cellulolytic microorganisms. Acta. Chim. Slov. 2010, 57, 767–774. [Google Scholar] [PubMed]
- Gold, N.D.; Martin, V.J.J. Global view of the Clostridium thermocellum cellulosome revealed by quantitative proteomic analysis. J. Bacteriol. 2007, 189, 6787–6795. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Jeon, S.D.; Shim, H.J.; Doi, R.H.; Han, S.O. Cellulosomic profiling produced by Clostridium cellulovorans during growth on different carbon sources explored by the cohesin marker. J. Biotechnol. 2010, 145, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Tsai, S.L.; Goyal, G.; Chen, W. Surface display of a functional minicellulosome by intracellular complementation using a synthetic yeast consortium and its application to cellulose hydrolysis and ethanol production. Appl. Environ. Microbiol. 2010, 76, 7514–7520. [Google Scholar] [CrossRef] [PubMed]
- Artzi, L.; Morag, E.; Barak, Y.; Lamed, R.; Bayer, E.A. Clostridium clariflavum: Key cellulosome players are revealed by proteomic analysis. mBio 2015, 6, e00411-15. [Google Scholar] [CrossRef] [PubMed]
- Leu, S.Y.; Zhu, J.Y. Substrate-related factors affecting enzymatic saccharification of lignocelluloses: Our recent understanding. Bioenergy Res. 2013, 6, 405–415. [Google Scholar] [CrossRef]
- Chan, K.L.; Dong, C.; Wong, M.S.; Kim, L.H.; Leu, S.Y. Plant chemistry associated dynamic modelling to enhance urban vegetation carbon sequestration potential via bioenergy harvesting. J. Clean. Prod. 2018, 197, 1084–1094. [Google Scholar] [CrossRef]
- Sosnowski, P.; Wieczorek, A.; Ledakowicz, S. Anaerobic co-digestion of sewage sludge and organic fraction of municipal solid wastes. Adv. Environ. Res. 2003, 7, 609–616. [Google Scholar] [CrossRef]
- Carrère, H.; Dumas, C.; Battimelli, A.; Batstone, D.J.; Delgenès, J.P.; Steyer, J.P.; Ferrer, I. Pretreatment methods to improve sludge anaerobic degradability: A review. J. Hazard. Mater. 2010, 183, 1–15. [Google Scholar]
- Zhen, G.; Lu, X.; Kato, H.; Zhao, Y.; Li, Y.Y. Overview of pretreatment strategies for enhancing sewage sludge disintegration and subsequent anaerobic digestion: Current advances, full-scale application and future perspectives. Renew. Sustain. Energy Rev. 2017, 69, 559–577. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Leu, S.Y.; Chen, S. Toward a fundamental understanding of cellulase-lignin interactions in the whole slurry enzymatic saccharification process. Biofuels Bioprod. Biorefin. 2016, 10, 648–663. [Google Scholar] [CrossRef]
- Pan, X.; Gilkes, N.; Kadla, J.; Pye, K.; Saka, S.; Gregg, D.; Ehara, K.; Xie, D.; Lam, D.; Saddler, J. Bioconversion of hybrid poplar to ethanol and co-products using an organosolv fractionation process: Optimization of process yields. Biotechnol. Bioeng. 2006, 94, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.; Goodenough, P.W.; Owen, E.; Bhat, M.K. Cellobiose: A true inducer of cellulosome in different strains of Clostridium thermocellum. FEMS Microbiol. Lett. 1993, 111, 73–78. [Google Scholar] [CrossRef][Green Version]
- Bae, J.; Morisaka, H.; Kuroda, K.; Ueda, M. Cellulosome complexes: Natural biocatalysts as arming microcompartments of enzymes. J. Mol. Microbiol. Biotechnol. 2013, 23, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Han, S.O.; Yukawa, H.; Inui, M.; Doi, R.H. Transcription of Clostridium cellulovorans cellulosomal cellulase and hemicellulose genes. J. Bacteriol. 2003, 185, 2520–2527. [Google Scholar] [CrossRef] [PubMed]
- Raman, B.; Pan, C.; Hurst, G.B.; Rodriguez, M.J.; McKeown, C.K.; Lankford, P.K.; Samatova, N.F.; Mielenz, J.R. Impact of pretreated switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: A quantitative proteomic analysis. PLoS ONE 2009, 4, e5271. [Google Scholar] [CrossRef] [PubMed]
- Morisaka, H.; Matsui, K.; Tatsukami, Y.; Kuroda, K.; Miyake, H.; Tamaru, Y.; Ueda, M. Profile of native cellulosomal proteins of Clostridium cellulovorans adapted to various carbon sources. AMB Express 2012, 2, 37. [Google Scholar] [CrossRef] [PubMed]
- Han, S.O.; Yukawa, H.; Inui, M.; Doi, R.H. Effect of carbon source on the cellulosomal subpopulations of Clostridium cellulovorans. Microbiology 2005, 151, 1491–1497. [Google Scholar] [CrossRef][Green Version]
- Yoav, S.; Barak, Y.; Shamshoum, M.; Borovok, I.; Lamed, R.; Dassa, B.; Hadar, Y.; Morag, E.; Bayer, E.A. How does cellulosome composition influence deconstruction of lignocellulosic substrates in Clostridium (Ruminiclostridium) thermocellum DSM 1313? Biotechnol. Biofuels 2017, 10, 222. [Google Scholar] [CrossRef]
- Han, S.O.; Cho, H.Y.; Yukawa, H.; Inui, M.; Doi, R.H. Regulation of expression of cellulosomes and noncellulosomal (hemi)cellulolytic enzymes in Clostridium cellulovorans during growth on different carbon sources. J. Bacteriol. 2004, 188, 4218–4227. [Google Scholar] [CrossRef]
- Fierobe, H.B.; Bayer, E.A.; Tardif, C.; Czjzek, M.; Mechaly, A.; Belaich, A.; Lamed, R.; Shoham, Y.; Belaich, J.P. Degradation of cellulose substrates by cellulosome chimeras. Substrate targeting versus proximity of enzyme components. J. Biol. Chem. 2002, 227, 49621–49630. [Google Scholar] [CrossRef]
- Artzi, L.; Dadosh, T.; Milrot, E.; Moraïs, S.; Levin-Zaidman, S.; Morag, E.; Bayer, E.A. Colocalization and disposition of cellulosomes in Clostridium clariflavum as revealed by correlative superresolution imaging. mBio 2018, 9, e00012-18. [Google Scholar] [CrossRef] [PubMed]
- Han, S.O.; Yukawa, H.; Inui, M.; Doi, R.H. Regulation of expression of cellulosomal cellulase and hemicellulase genes in Clostridium cellulovorans. J. Bacteriol. 2003, 185, 6067–6075. [Google Scholar] [CrossRef] [PubMed]
- Kosugi, A.; Murashima, K.; Doi, R.H. Characterization of xylanolytic enzymes in Clostridium cellulovorans: Expression of xylanase activity dependent on growth substrates. J. Bacteriol. 2001, 183, 7037–7043. [Google Scholar] [CrossRef] [PubMed]
- Murashima, K.; Kosugi, A.; Doi, R.H. Determination of subunit composition of Clostridium cellulovorans cellulosomes that degrade plant cell walls. Appl. Environ. Microbiol. 2002, 68, 1610–1615. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Park, J.S.; Matano, Y.; Doi, R.H. Cohesin–dockerin interactions of cellulosomal subunits of Clostridium cellulovorans. J. Bacteriol. 2001, 183, 5431–5435. [Google Scholar] [CrossRef] [PubMed]
- Nataf, Y.; Bahari, L.; Kahei-Raifer, H.; Borovok, I.; Lamed, R.; Bayer, E.A.; Sonenshein, A.L.; Shoham, Y. Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic polysaccharides via alternative sigma factors. Proc. Natl. Acad. Sci. USA 2010, 107, 18646–18651. [Google Scholar] [CrossRef]
- Qing, Q.; Yang, B.; Wyman, C.E. Xylooligomers are strong inhibitors of cellulose hydrolysis by enzymes. Bioresour. Technol. 2010, 101, 9624–9630. [Google Scholar] [CrossRef]
- Teugjas, H.; Valjamae, P. Product inhibition of cellulases studied with 14C-labeled cellulose substrates. Biotechnol. Biofuels 2013, 6, 104. [Google Scholar] [CrossRef]
- Carere, C.R.; Sparling, R.; Cicek, N.; Levin, D.B. Third generation biofuels via direct cellulose fermentation. Int. J. Mol. Sci. 2008, 9, 1342–1360. [Google Scholar] [CrossRef]
- Lin, C.C.; Kan, S.C.; Yeh, C.W.; Chen, C.I.; Shieh, C.J.; Liu, Y.C. Kinetics Study for the Recombinant Cellulosome to the Degradation of Chlorella Cell Residuals; International Journal of Biological, Biomolecular, Agricultural, Food and Biotechnological Engineering; World Academy of Science Engineering and Technology: Paris, France, 2015; Volume 9, pp. 782–785. [Google Scholar]
- Zhang, P.; Wang, B.; Xiao, Q.; Wu, S. A kinetics modeling study on the inhibition of glucose on cellulosome of Clostridium thermocellum. Bioresour. Technol. 2015, 190, 36–43. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.H.P.; Lynd, L.R. Enzyme–microbe synergy during cellulose hydrolysis by Clostridium thermocellum. Proc. Natl. Acad. Sci. USA 2006, 103, 16165–16169. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Wang, Y.; Zhang, H.; Leu, S.Y. Feasibility of high-concentration cellulosic bioethanol production from undetoxified whole Monterey pine slurry. Bioresour. Technol. 2018, 250, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Weil, J.R.; Dien, B.; Bothast, R.; Hendrickson, R.; Mosier, N.S.; Ladisch, M.R. Removal of fermentation inhibitors formed during pretreatment of biomass by polymeric adsorbents. Ind. Eng. Chem. Res. 2002, 41, 6132–6138. [Google Scholar] [CrossRef]
- Min, S.; Kim, O.J.; Bae, J.; Chung, T.N. Effect of pretreatment with the NADPH xxidase inhibitor Apocynin on the therapeutic efficacy of human placenta-derived mesenchymal stem cells in intracerebral hemorrhage. Intl. J. Mol. Sci. 2018, 19, 3679. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Qin, Y.; Li, Y.; Ji, Y.; Huang, J.; Song, H.; Xu, J. Factors influencing cellulosome activity in consolidated bioprocessing of cellulosic ethanol. Bioresour. Technol. 2010, 101, 9560–9569. [Google Scholar] [CrossRef] [PubMed]
- Duarte, L.; Carvalheiro, F.; Neves, I.; Girio, F. Effects of aliphatic acids, furfural, and phenolic compounds on Debaryomyces hansenii CCMI 941. Appl. Biochem. Biotechnol. 2005, 121, 413–425. [Google Scholar] [CrossRef]
- Guarnieri, M.T.; Franden, M.A.; Johnson, C.W.; Beckham, G.T. Conversion and assimilation of furfural and 5-(hydroxymethyl)furfural by Pseudomonas putida KT2440. Metabol. Eng. Commun. 2017, 4, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Parsiegla, G.; Juy, M.; Reverbel-Leroy, C.; Tardif, C.; Velaich, J.P.; Driguez, H.; Haser, R. The crystal structure of the processive endocellulase CelF of Clostridium cellulolyticum in complex with a thiooligosaccharide inhibitor at 2.0 Å resolution. EMBO J. 1998, 17, 5551–5562. [Google Scholar] [CrossRef] [PubMed]
- You, C.; Zhang, X.Z.; Sathitsukssanoh, N.; Lynd, L.R.; Zhang, Y.H.P. Enhanced microbial utilization of recalcitrant cellulose by an ex vivo cellulosome-microbe complex. Appl. Environ. Microbiol. 2012, 78, 1437–1444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.H.P. Substrate channeling and enzyme complexes for biotechnological applications. Biotechnol. Adv. 2011, 29, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Alonso, S.; Rendueles, M.; Diaz, M. Microbial production of specialty organic acids from renewable and waste materials. Crit. Rev. Biotechol. 2013, 35, 497–513. [Google Scholar] [CrossRef] [PubMed]
- Wackett, L.P. Microbial acid fermentation products: An annotated selection of world wide web sites relevant to the topics in microbial biotechnology. Microb. Biotechnol. 2018, 11, 268–269. [Google Scholar] [CrossRef] [PubMed]
- Zverlov, V.V.; Velikodvorskaya, G.A.; Schwarz, W.H. Two new cellulosome components encoded downstream of celI in the genome of Clostridium thermocellum: The non-processive endoglucanase CelN and the possibly structural protein CseP. Microbiology 2003, 149, 515–524. [Google Scholar] [CrossRef] [PubMed]
- Bras, J.L.; Pinheiro, B.A.; Cameron, K.; Cuskin, F.; Viegas, A.; Najmudin, S.; Bule, P.; Pires, V.M.R.; Romao, M.J.; Bayer, E.A.; et al. Diverse specificity of cellulosome attachment to the bacterial cell surface. Sci. Rep. 2016, 6, 38292. [Google Scholar] [CrossRef] [PubMed]
- Leis, B.; Held, C.; Andreeen, B.; Liebl, W.; Graubner, S.; Schulte, L.P.; Schwarz, W.H.; Zverlov, V.V. Optimizing the composition of a synthetic cellulosome complex for the hydrolysis of softwood pulp: Identification of the enzymatic core functions and biochemical complex characterization. Biotechnol. Biofuels 2018, 11, 220. [Google Scholar] [CrossRef] [PubMed]
- Correia, M.A.; Prates, J.A.; Bras, J.; Fontes, C.M.; Newman, J.A.; Lewis, R.J.; Gilbert, H.J.; Flint, J.E. Crystal structure of a cellulosomal family 3 carbohydrate esterase from Clostridium thermocellum provides insights into the mechanism of substrate recognition. J. Mol. Biol. 2008, 379, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Taherzadeh, M.; Karimi, K. Pretreatment of lignocellulosic wastes to improve ethanol and biogas production: A review. Int. J. Mol. Sci. 2008, 9, 1621–1651. [Google Scholar] [CrossRef]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, C. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Ding, S.Y.; Liu, Y.S.; Zeng, Y.; Himmel, M.E.; Baker, J.O.; Bayer, E.A. How does plant cell wall nanoscale architecture correlate with enzymatic digestibility? Science 2012, 338, 1055–1060. [Google Scholar] [CrossRef]
- Siqueira, G.; Várnai, A.; Ferraz, A.; Milagres, A.M.F. Enhancement of cellulose hydrolysis in sugarcane bagasse by the selective removal of lignin with sodium chlorite. Appl. Energy 2013, 102, 399–402. [Google Scholar] [CrossRef]
- Wallace, J.; Brienzo, M.; García-Aparicio, M.P.; Görgens, J.F. Lignin enrichment and enzyme deactivation as the root cause of enzymatic hydrolysis slowdown of steam pretreated sugarcane bagasse. New Biotechnol. 2016, 33, 361–371. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, L.; Zhou, T.; Wu, Y.; Xu, F. The dual effects of lignin content on enzymatic hydrolysis using film composed of cellulose and lignin as a structure model. Bioresour. Technol. 2016, 200, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Shi, W.; Sun, W.; Li, X.; Wang, F.; Zhao, J.; Qu, Y. Differences in the adsorption of enzymes onto lignins from diverse types of lignocellulosic biomass and the underlying mechanism. Biotechnol. Biofuels 2014, 7, 38. [Google Scholar] [CrossRef] [PubMed]
- Nakagame, S.; Chandra, R.P.; Kadla, J.F.; Saddler, J.N. Enhancing the enzymatic hydrolysis of lignocellulosic biomass by increasing the carboxylic acid content of the associated lignin. Biotechnol. Bioeng. 2011, 108, 538–548. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Huang, Y.; Sun, R.; Tu, M. The strong association of condensed phenolic moieties in isolated lignins with their inhibition of enzymatic hydrolysis. Green Chem. 2016, 18, 4276–4286. [Google Scholar] [CrossRef]
- Yang, Q.; Pan, X. Correlation between lignin physicochemical properties and inhibition to enzymatic hydrolysis of cellulose. Biotechnol. Bioeng. 2016, 113, 1213–1224. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Pareek, N.; Gillgren, T.; Jönsson, L.J. Adsorption of proteins involved in hydrolysis of lignocellulose on lignins and hemicelluloses. Bioresour. Technol. 2013, 148, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Lancefield, C.S.; Panovic, I.; Deuss, P.J.; Bartac, K.; Westwood, N.J. Pre-treatment of lignocellulosic feedstocks using biorenewable alcohols: Towards complete biomass valorisation. Green Chem. 2017, 19, 202–214. [Google Scholar] [CrossRef]
- Kim, J.S.; Lee, Y.Y.; Kim, T.H. A review on alkaline pretreatment technology for bioconversion of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 42–48. [Google Scholar] [CrossRef]
- Sipponen, M.H.; Pihlajaniemi, V.; Pastinen, O.; Laakso, S. Reduction of surface area of lignin improves enzymatic hydrolysis of cellulose from hydrothermally pretreated wheat straw. RSC Adv. 2014, 4, 36591–36596. [Google Scholar] [CrossRef][Green Version]
- Sipponen, M.H.; Rahikainen, J.; Leskinen, T.; Pihlajaniemi, V.; Mattinen, M.L.; Lange, H.; Crestini, C.; Österberg, Ö.M. Structural changes of lignin in biorefinery pretreatments and consequences to enzyme-lignin interactions. Nord. Pulp Pap. Res. J. 2017, 32, 550–571. [Google Scholar] [CrossRef]
- Huang, C.; He, J.; Min, D.; Lai, C.; Yong, Q. Understanding the nonproductive enzyme adsorption and physicochemical properties of residual lignins in moso bamboo pretreated with sulfuric acid and kraft pulping. Appl. Biochem. Biotechnol. 2016, 180, 1508–1523. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Gwak, K.S.; Treasure, T.; Jameel, H.; Chang, H.M.; Park, S. Effect of lignin chemistry on the enzymatic hydrolysis of woody biomass. ChemSusChem 2014, 7, 1942–1950. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Hu, F.; Sannigrahi, P.; Jung, S.; Ragauskas, A.J.; Wyman, C.E. Carbohydrate derived-pseudo-lignin can retard cellulose biological conversion. Biotechnol. Bioeng. 2013, 110, 737–753. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.; Tanner, D.; Sørensen, H.R.; Meyer, A.S. New degradation compounds from lignocellulosic biomass pretreatment: Routes for formation of potent oligophenolic enzyme inhibitors. Green Chem. 2017, 19, 464–473. [Google Scholar] [CrossRef]
- Taherzadeh, M.J.; Karimi, K. Enzyme-based hydrolysis processes for ethanol from lignocellulosic materials: A review. Bioresources 2007, 2, 707–738. [Google Scholar]
- Bubner, P.; Dohr, J.; Plank, H.; Mayrhofer, C.; Nidetzky, B. Cellulases dig deep: In Situ observation of the mesoscopic structural dynamics of enzymatic cellulose degradation. J. Biol. Chem. 2012, 287, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Karimi, K.; Taherzadeh, M.J. A critical review on analysis in pretreatment of lignocelluloses: Degree of polymerization, adsorption/desorption, and accessibility. Bioresour. Technol. 2016, 203, 348–356. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Kumar, R.; Karimi, K. Pretreatment of lignocellulosic biomass. In Lignocellulose-Based Bioproducts; Karimi, K., Ed.; Springer International Publishing: Berlin, Germany, 2015; Volume 1, pp. 85–154. [Google Scholar]
- Neuman, R.P.; Walker, L.P. Solute exclusion from cellulose in packed columns: Experimental investigation and pore volume measurements. Biotechnol. Bioeng. 1992, 40, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Resch, M.G.; Podkaminer, K.; Yang, S.; Baker, J.O.; Donohoe, B.S.; Wilson, C.; Klingeman, D.M.; Olson, D.G.; Decker, S.R.; et al. Dramatic performance of Clostridium thermocellum explained by its wide range of cellulase modalities. Sci. Adv. 2016, 2, e1501254. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.B. Demonstration of the importance for cellulose hydrolysis of CelS, the most abundant cellulosomal cellulase in Clostridium thermocellum. Proc. Natl. Acad. Sci. USA 2010, 107, 17855–17856. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gao, G.; Li, Y.; Yang, L.; Liang, Y.; Jin, H.; Han, W.; Feng, Y.; Zhang, Z.; Wang, J. Cloning, expression, and characterization of a thermophilic endoglucanase, AcCel12B from Acidothermus cellulolyticus 11B. Int. J. Mol. Sci. 2015, 16, 25080–25095. [Google Scholar] [CrossRef] [PubMed]
- Khodaverdi, M.; Jeihanipour, A.; Karimi, K.; Taherzadeh, M.J. Kinetic modeling of rapid enzymatic hydrolysis of crystalline cellulose after pretreatment by NMMO. J. Ind. Microbiol. Biotechnol. 2012, 39, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Moraïs, S.; Morag, E.; Barak, Y.; Goldman, D.; Hadar, Y.; Lamed, R.; Shoham, Y.; Wilson, D.B.; Bayer, E.A. Deconstruction of lignocellulose into soluble sugars by native and designer cellulosomes. mBio 2012, 3, e00508-12. [Google Scholar] [CrossRef] [PubMed]
- Keller, F.A.; Hamilton, J.E.; Nguyen, Q.A. Microbial pretreatment of biomass: Potential for reducing severity of thermochemical biomass pretreatment. Appl. Biochem. Biotechnol. 2003, 105, 27–41. [Google Scholar] [CrossRef]
- Shi, J.; Sharma-Shivappa, R.; Chinn, M.; Howell, N. Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenergy 2009, 33, 88–96. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of lignocellulose: Formation of inhibitory byproducts and strategies for minimizing their effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef]
- Davidi, L.; Moraïs, S.; Artzi, L.; Knop, D.; Hadar, Y.; Arfi, Y.; Bayer, E.A. Toward combined delignification and saccharification of wheat straw by a laccase-containing designer cellulosome. Proc. Natl. Acad. Sci. USA 2016, 113, 10854–10859. [Google Scholar] [CrossRef]
- Glazunova, O.A.; Moiseenko, K.V.; Kamenihina, I.A.; Isaykina, T.U.; Yaropolov, A.I.; Fedorova, T.V. Laccases with variable properties from different strains of Steccherinum ochraceum: Does glycosylation matter? Int. J. Mol. Sci. 2019, 20, 2008. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).