Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation
Abstract
1. Introduction
2. Results
3. Discussion
4. Material and Methods
4.1. Animals and Experimental Procedures
4.2. Immunofluorescence Procedure
4.3. Negative Control
4.4. Counting and Statistics
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lang, R.; Gundlach, A.L.; Kofler, B. The galanin peptide family: receptor pharmacology, pleiotropic biological actions, and implications in health and disease. Pharm. Ther. 2007, 115, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Gonkowski, S.; Burliński, P.; Skobowiat, C.; Majewski, M.; Całka, J. Inflammation- and axotomy-induced changes in galanin-like immunoreactive (GAL-LI) nerve structures in the porcine descending colon. Acta Vet. Hung. 2010, 58, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Arciszewski, M.B.; Barabasz, S.; Całka, J. Immunohistochemical localization of galanin receptors (GAL-R1, GAL-R2, and GAL-R3) on myenteric neurons from the sheep and dog stomach. Ann. Anat. 2008, 190, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Kisfalvi Jr, I.; Burghardt, B.; Bálint, A.; Zelles, T.; Vizi, E.S.; Varga, G. Antisecretory effects of galanin and its putative antagonists M15, M35 and C7 in the rat stomach. J. Physiol. Paris 2000, 94, 37–42. [Google Scholar] [CrossRef]
- Tatemoto, K.; Rökaeus, A.; Jörnvall, H.; McDonald, T.J.; Mutt, V. Galanin—A novel biologically active peptide from porcine intestine. Febs Lett. 1983, 164, 124–128. [Google Scholar] [CrossRef]
- Hoyle, C.H.; Burnstock, G. Galanin-like immunoreactivity in enteric neurons of the human colon. J. Anat. 1989, 166, 23–33. [Google Scholar] [PubMed]
- Ekblad, E.; Rökaeus, A.; Håkanson, R.; Sundler, F. Galanin nerve fibers in the rat gut: distribution, origin and projections. Neuroscience 1985, 16, 355–363. [Google Scholar] [CrossRef]
- Anselmi, L.; Stella SLJr Lakhter, A.; Hirano, A.; Tonini, M.; Sternini, C. Galanin receptors in the rat gastrointestinal tract. Neuropeptides 2005, 39, 349–352. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, H.X.; Hökfelt, T. The participation of galanin in pain processing at the spinal level. Trends Pharm. Sci. 2002, 23, 468–474. [Google Scholar] [CrossRef]
- Locker, F.; Lang, A.A.; Koller, A.; Lang, R.; Bianchini, R.; Kofler, B. Galanin modulates human and murine neutrophil activation in vitro. Acta Physiol. 2015, 213, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Pidsudko, Z.; Kaleczyc, J.; Wasowicz, K.; Sienkiewicz, W.; Majewski, M.; Zajac, W.; Lakomy, M. Distribution and chemical coding of intramural neurons in the porcine ileum during proliferative enteropathy. J. Comp. Pathol. 2008, 138, 23–31. [Google Scholar] [CrossRef]
- Szymanska, K.; Makowska, K.; Gonkowski, S. The Influence of High and Low Doses of Bisphenol A (BPA) on the Enteric Nervous System of the Porcine Ileum. Int. J. Mol. Sci. 2018, 19, 917. [Google Scholar] [CrossRef] [PubMed]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Chemical modifications of peptides and their impact on food properties. Chem. Rev. 2011, 111, 7876–7903. [Google Scholar] [CrossRef] [PubMed]
- Attoff, K.; Kertika, D.; Lundqvist, J.; Oredsson, S.; Forsby, A. Acrylamide affects proliferation and differentiation of the neural progenitor cell line C17.2 and the neuroblastoma cell line SH-SY5Y. Toxicol. In Vitro 2016, 35, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Naruszewicz, M.; Zapolska-Downar, D.; Kośmider, A.; Nowicka, G.; Kozłowska-Wojciechowska, M.; Vikström, A.S.; Törnqvist, M. Chronic intake of potato chips in humans increases the production of reactive oxygen radicals by leukocytes and increases plasma C-reactive protein: a pilot study. Am. J. Clin. Nutr. 2009, 89, 773–777. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, A.; Chattopadhyay, R.; Mitra, S.; Crowe, S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014, 94, 329–354. [Google Scholar] [CrossRef] [PubMed]
- Zödl, B.; Schmid, D.; Wassler, G.; Gundacker, C.; Leibetseder, V.; Thalhammer, T.; Ekmekcioglu, C. Intestinal transport and metabolism of acrylamide. Toxicology 2007, 232, 99–108. [Google Scholar] [CrossRef]
- WHO. Health Implications of Acrylamide in Food; FAO/WHO: Geneva, Switzerland, 2002; Available online: http://apps.who.int/iris/handle/10665/42563 (accessed on 15 April 2019).
- Verma, N.; Rettenmeier, A.W.; Schmitz-Spanke, S. Recent advances in the use of Sus scrofa (pig) as a model system for proteomic studies. Proteomics 2011, 11, 776–793. [Google Scholar] [CrossRef]
- Brown, D.R.; Timmermans, J.P. Lessons from the porcine enteric nervous system. Neurogastroenterol. Motil. 2004, 16, 50–54. [Google Scholar] [CrossRef]
- Furness, J.B.; Callaghan, B.P.; Rivera, L.R.; Cho, H.J. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv. Exp. Med. Biol. 2014, 817, 39–71. [Google Scholar]
- Bulc, M.; Palus, K.; Zielonka, Ł.; Gajęcka, M.; Całka, J. Changes in expression of inhibitory substances in the intramural neurons of the stomach following streptozotocin- induced diabetes in the pig. World J. Gastroenterol. 2017, 23, 6088–6099. [Google Scholar] [CrossRef] [PubMed]
- Zalecki, M.; Sienkiewicz, W.; Franke-Radowiecka, A.; Klimczuk, M.; Kaleczyc, J. The Influence of Gastric Antral Ulcerations on the Expression of Galanin and GalR1, GalR2, GalR3 Receptors in the Pylorus with Regard to Gastric Intrinsic Innervation of the Pyloric Sphincter. PLoS ONE. 2016, 11, e0155658. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Całka, J. Neurochemical Plasticity of the Coeliac-Superior Mesenteric Ganglion Complex Neurons Projecting to the Prepyloric Area of the Porcine Stomach following Hyperacidity. Neural Plast. 2016, 2016, 8596214. [Google Scholar] [CrossRef]
- Gańko, M.; Całka, J. Localization and chemical coding of the dorsal motor vagal nucleus (DMX) neurons projecting to the porcine stomach prepyloric area in the physiological state and after stomach partial resection. J. Mol. Neurosci. 2014, 52, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, J.D.; Martz, S.; Gil, V.; Wang, X.Y.; Jimenez, M.; Parsons, S. Two independent networks of interstitial cells of cajal work cooperatively with the enteric nervous system to create colonic motor patterns. Front. Neurosci. 2011, 5, 93. [Google Scholar] [CrossRef]
- Timmermans, J.P.; Hens, J.; Adriaensen, D. Outer submucous plexus: an intrinsic nerve network involved in both secretory and motility processes in the intestine of large mammals and humans. Anat. Rec. 2001, 262, 71–78. [Google Scholar] [CrossRef]
- Schepp, W.; Prinz, C.; Tatge, C.; Håkanson, R.; Schusdziarra, V.; Classen, M. Galanin inhibits gastrin release from isolated rat gastric G-cells. Am. J. Physiol. 1990, 258, G596–G602. [Google Scholar] [CrossRef] [PubMed]
- Delvaux, M.; Botella, A.; Fioramonti, J.; Frexinos, J.; Bueno, L. Galanin induces contraction of isolated cells from circular muscle layer of pig ileum. Regul. Pept. 1991, 32, 369–374. [Google Scholar] [CrossRef]
- Sternini, C.; Anselmi, L.; Guerrini, S.; Cervio, E.; Pham, T.; Balestra, B.; Vicini, R.; Baiardi, P.; D’agostino, G.L.; Tonini, M. Role of galanin receptor 1 in peristaltic activity in the guinea pig ileum. Neuroscience 2004, 125, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Guerrini, S.; Raybould, H.E.; Anselmi, L.; Agazzi, A.; Cervio, E.; Reeve, J.R., Jr.; Tonini, M.; Sternini, C. Role of galanin receptor 1 in gastric motility in rat. Neurogastroenterol. Motil. 2004, 16, 429–438. [Google Scholar] [CrossRef]
- Allescher, H.D.; Daniel, E.E.; Dent, J.; Fox, J.E. Inhibitory function of VIP-PHI and galanin in canine pylorus. Am. J. Physiol. 1989, 256, G789–G797. [Google Scholar] [CrossRef] [PubMed]
- Bauer, F.E.; Zintel, A.; Kenny, M.J.; Calder, D.; Ghatei MABloom, S.R. Inhibitory effect of galanin on postprandial gastrointestinal motility and gut hormone release in humans. Gastroenterology 1989, 97, 260–264. [Google Scholar] [CrossRef]
- Botella, A.; Delvaux, M.; Bueno, L.; Frexinos, J. Intracellular pathways triggered by galanin to induce contraction of pig ileum smooth muscle cells. J. Physiol. 1992, 458, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Makowska, K.; Rytel, L.; Lech, P.; Osowski, A.; Kruminis-Kaszkiel, E.; Gonkowski, S. Cocaine- and amphetamine-regulated transcript (CART) peptide in the enteric nervous system of the porcine esophagus. C. R. Biol. 2018, 341, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Mirabella, N.; Lamanna, C.; Assisi, L.; Botte, V.; Cecio, A. The relationships of nicotinamide adenine dinucleotide phosphate-d to nitric oxide synthase, vasoactive intestinal polypeptide, galanin and pituitary adenylate activating polypeptide in pigeon gut neurons. Neurosci. Lett. 2000, 293, 147–151. [Google Scholar] [CrossRef]
- Biancani, P.; Walsh, J.H.; Behar, J. Vasoactive intestinal polypeptide. A neurotransmitter for lower esophageal sphincter relaxation. J. Clin. Investig. 1984, 73, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Wiley, J.W.; O’Dorisio, T.M.; Owyang, C. Vasoactive intestinal polypeptide mediates cholecystokinin-induced relaxation of the sphincter of Oddi. J. Clin. Investig. 1988, 81, 1920–1924. [Google Scholar] [CrossRef]
- Nishio, H.; Hayashi, Y.; Terashima, S.; Takeuchi, K. Role of endogenous nitric oxide in mucosal defense of inflamed rat stomach following iodoacetamide treatment. Life Sci. 2006, 79, 1523–1530. [Google Scholar] [CrossRef]
- Ekblad, E. CART in the enteric nervous system. Peptides 2006, 27, 2024–2030. [Google Scholar] [CrossRef]
- Kozłowska, A.; Godlewski, J.; Majewski, M. Distribution Patterns of Cocaine- and Amphetamine-Regulated Transcript- and/or Galanin-Containing Neurons and Nerve Fibers Located in the Human Stomach Wall Affected by Tumor. Int. J. Mol. Sci. 2018, 19, 3357. [Google Scholar] [CrossRef]
- Vasina, V.; Barbara, G.; Talamonti, L.; Stanghellini, V.; Corinaldesi, R.; Tonini, M.; De Ponti, F.; De Giorgio, R. Enteric neuroplasticity evoked by inflammation. Auton. Neurosci. 2006, 126–127, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Shipp, A.; Lawrence, G.; Gentry, R.; McDonald, T.; Bartow, H.; Bounds, J.; Macdonald, N.; Clewell, H.; Allen, B.; Van Landingham, C. Acrylamide: review of toxicity data and dose-response analyses for cancer and noncancer effects. Crit. Rev. Toxicol. 2006, 36, 481–608. [Google Scholar] [CrossRef] [PubMed]
- Lo Pachin, R.M. The changing view of acrylamide neurotoxicity. Toxicol. Vitro 2010, 25, 573–579. [Google Scholar]
- Ewaleifoh, O.; Trinh, M.; Griffin, J.W.; Nguyen, T. A novel system to accelerate the progression of nerve degeneration in transgenic mouse models of neuropathies. Exp. Neurol. 2012, 237, 153–159. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Palus, K.; Bulc, M.; Całka, J. Changes in VIP-, SP- and CGRP- like immunoreactivity in intramural neurons within the pig stomach following supplementation with low and high doses of acrylamide. Neurotoxicology 2018, 69, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Palus, K.; Makowska, K.; Całka, J. Acrylamide-induced alterations in the cocaine- and amphetamine-regulated peptide transcript (CART)-like immunoreactivity within the enteric nervous system of the porcine small intestines. Ann. Anat. 2018, 219, 94–101. [Google Scholar] [CrossRef]
- Mahoney, S.A.; Hosking, R.; Farrant, S.; Holmes, F.E.; Jacoby, A.S.; Shine, J.; Iismaa, T.P.; Scott, M.K.; Schmidt, R.; Wynick, D. The second galanin receptor GalR2 plays a key role in neurite outgrowth from adult sensory neurons. J. Neurosci. 2003, 23, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Lourenssen, S.; Miller, K.G.; Blennerhassett, M.G. Discrete responses of myenteric neurons to structural and functional damage by neurotoxins in vitro. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G228–G239. [Google Scholar] [CrossRef]
- Santhanasabapathy, R.; Vasudevan, S.; Anupriya, K.; Pabitha, R.; Sudhandiran, G. Farnesol quells oxidative stress, reactive gliosis and inflammation during acrylamide-induced neurotoxicity: Behavioral and biochemical evidence. Neuroscience 2015, 308, 212–227. [Google Scholar] [CrossRef] [PubMed]
- Matkowskyj, K.; Royan, S.V.; Blunier, A.; Hecht, G.; Rao, M.; Benya, R.V. Age-dependent differences in galanin-dependent colonic fluid secretion after infection with Salmonella typhimurium. Gut 2009, 58, 1201–1206. [Google Scholar] [CrossRef] [PubMed]
- Dallos, A.; Kiss, M.; Polyánka, H.; Dobozy, A.; Kemény, L.; Husz, S. Galanin receptor expression in cultured human keratinocytes and in normal human skin. J. Peripher. Nerv. Syst. 2006, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Pozo, D.; Ganea, D. The significance of vasoactive intestinal peptide in immunomodulation. Pharm. Rev. 2004, 56, 249–290. [Google Scholar] [CrossRef] [PubMed]
- Ganea, D.; Hooper, K.M.; Kong, W. The neuropeptide vasoactive intestinal peptide: direct effects on immune cells and involvement in inflammatory and autoimmune diseases. Acta Physiol. 2015, 213, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Arciszewski, M.B.; Ekblad, E. Effects of vasoactive intestinal peptide and galanin on survival of cultured porcine myenteric neurons. Regul. Pept. 2005, 125, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Sandgren, K.; Ekblad, E. Increased expression of nitric oxide synthase in cultured neurons from adult rat colonic submucous ganglia. Auton. Neurosci. 2004, 114, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Yunker, A.M.; Galligan, J.J. Extrinsic denervation increases myenteric nitric oxide synthase-containing neurons and inhibitory neuromuscular transmission in guinea pig. J. Auton. Nerv. Syst. 1998, 71, 148–158. [Google Scholar] [CrossRef]
- Rychlik, A.; Gonkowski, S.; Nowicki, M.; Calka, J. Inflammatory bowel disease affects density of nitrergic nerve fibers in the mucosal layer of the canine gastrointestinal tract. Can. J. Vet. Res. 2017, 81, 129–136. [Google Scholar]
- Gonkowski, S.; Burlinski, P.; Szwajca, P.; Całka, J. Changes in cocaine- and amphetamine-regulated transcript- like immunoreactive (CART-LI) nerve structures of the porcine descending colon during proliferative enteropathy. Bull. Vet. Inst. Pulawy 2012, 56, 199–203. [Google Scholar] [CrossRef]
- Kasacka, I.; Piotrowska, Z. Evaluation of density and distribution of CART-immunoreactive structures in gastrointestinal tract of hypertensive rats. Biofactors. 2012, 38, 407–415. [Google Scholar] [CrossRef]
- Palus, K.; Bulc, M.; Całka, J. Changes in Somatostatin-Like Immunoreactivity in the Sympathetic Neurons Projecting to the Prepyloric Area of the Porcine Stomach Induced by Selected Pathological Conditions. BioMed Res. Int. 2017, 2017, 9037476. [Google Scholar] [CrossRef]
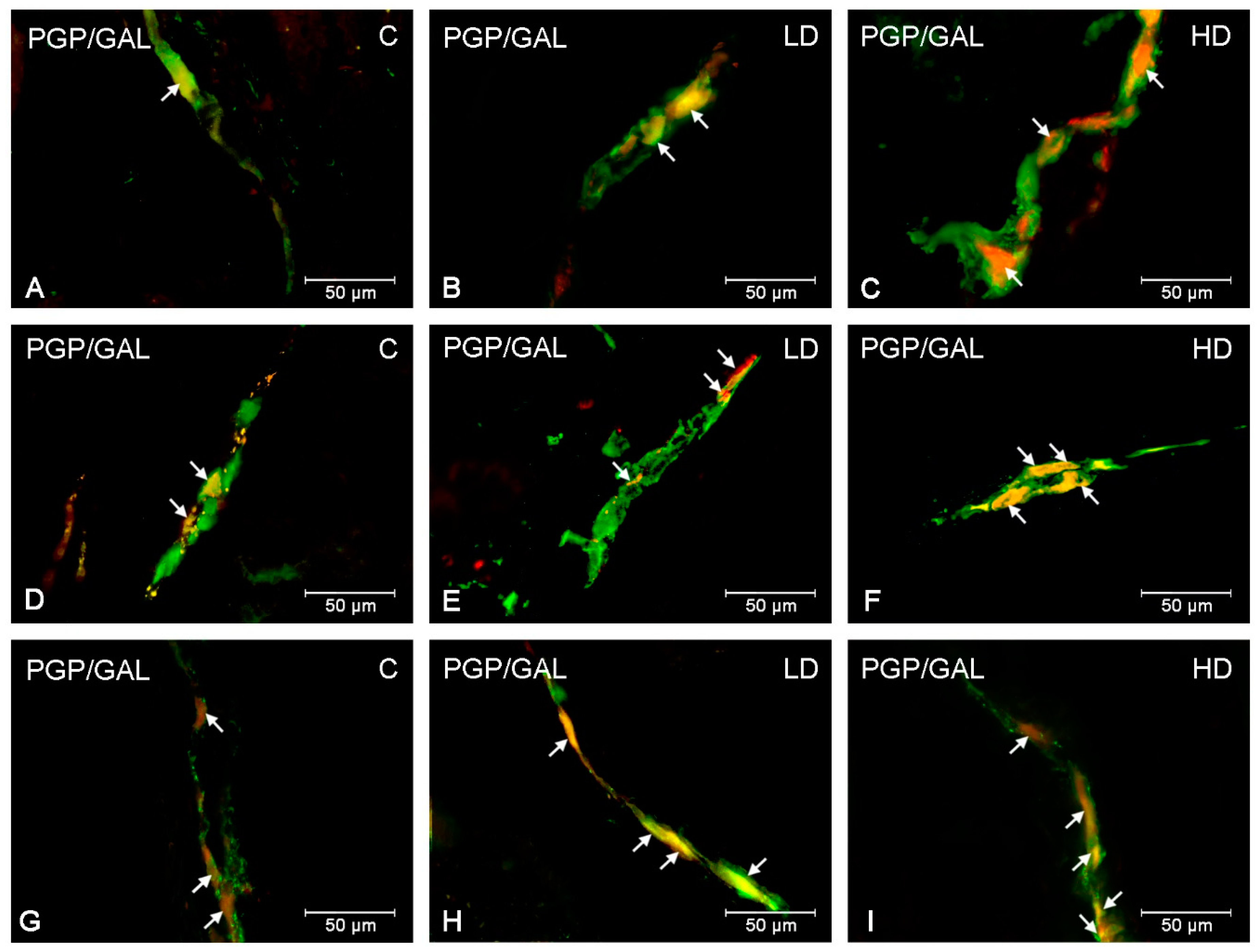
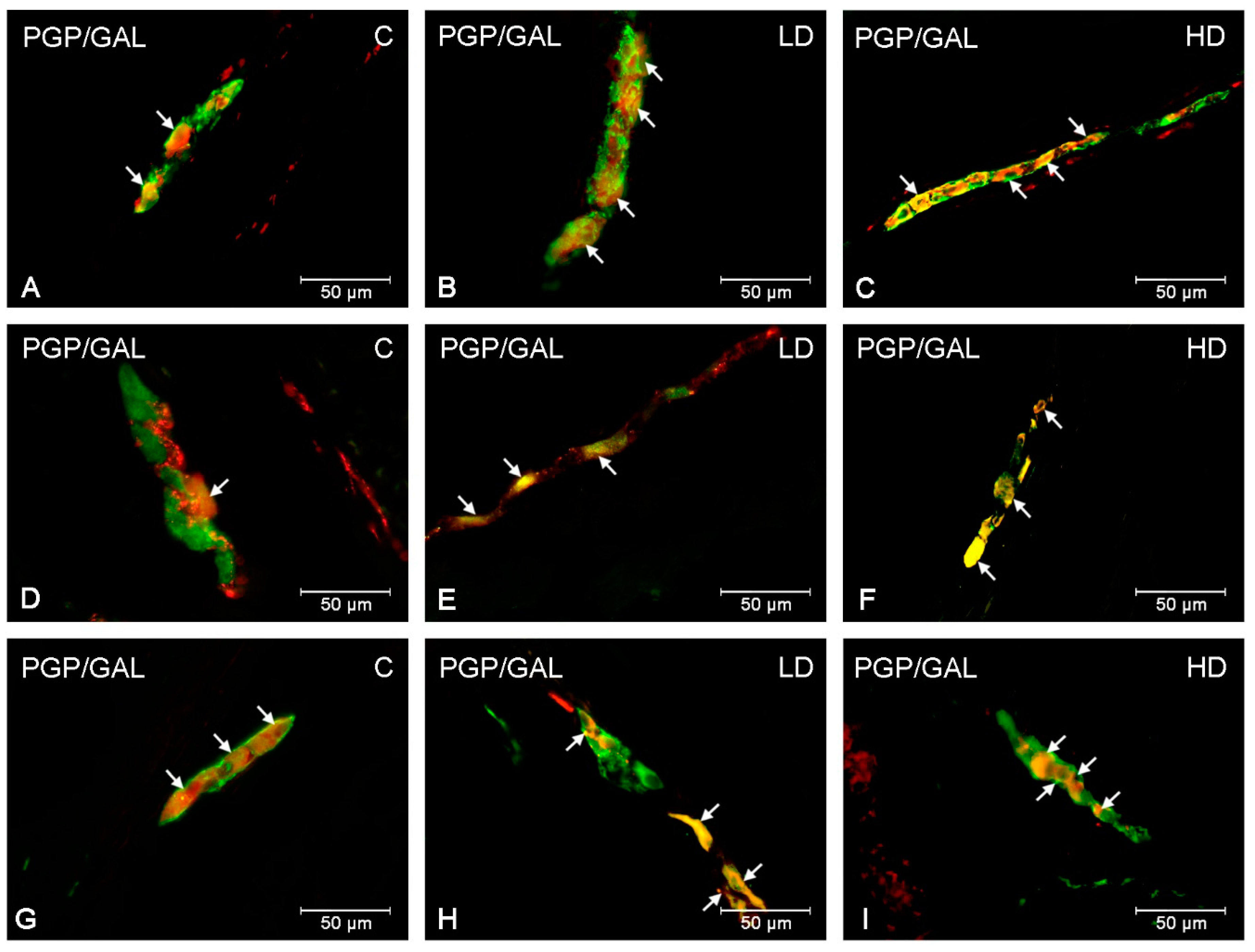
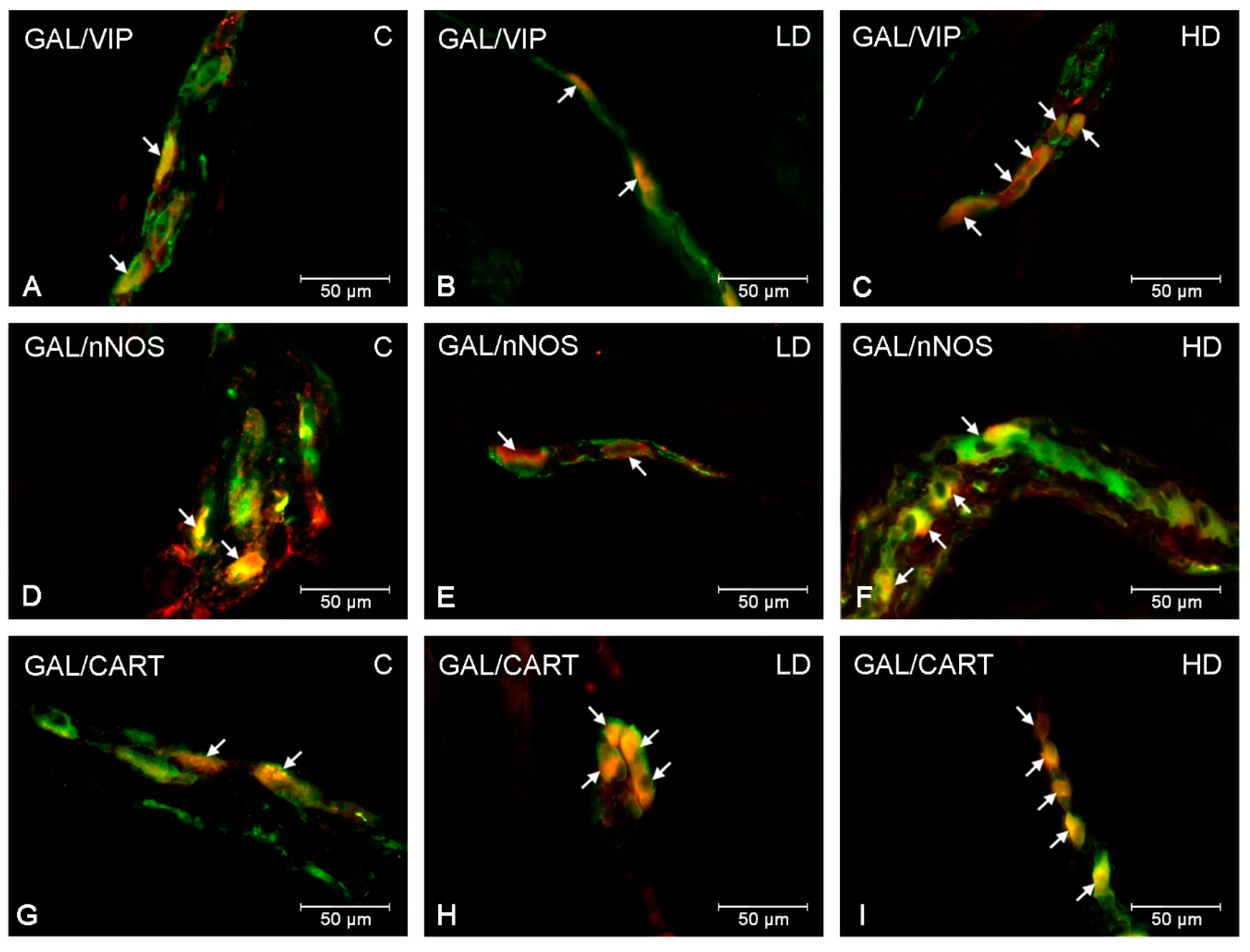
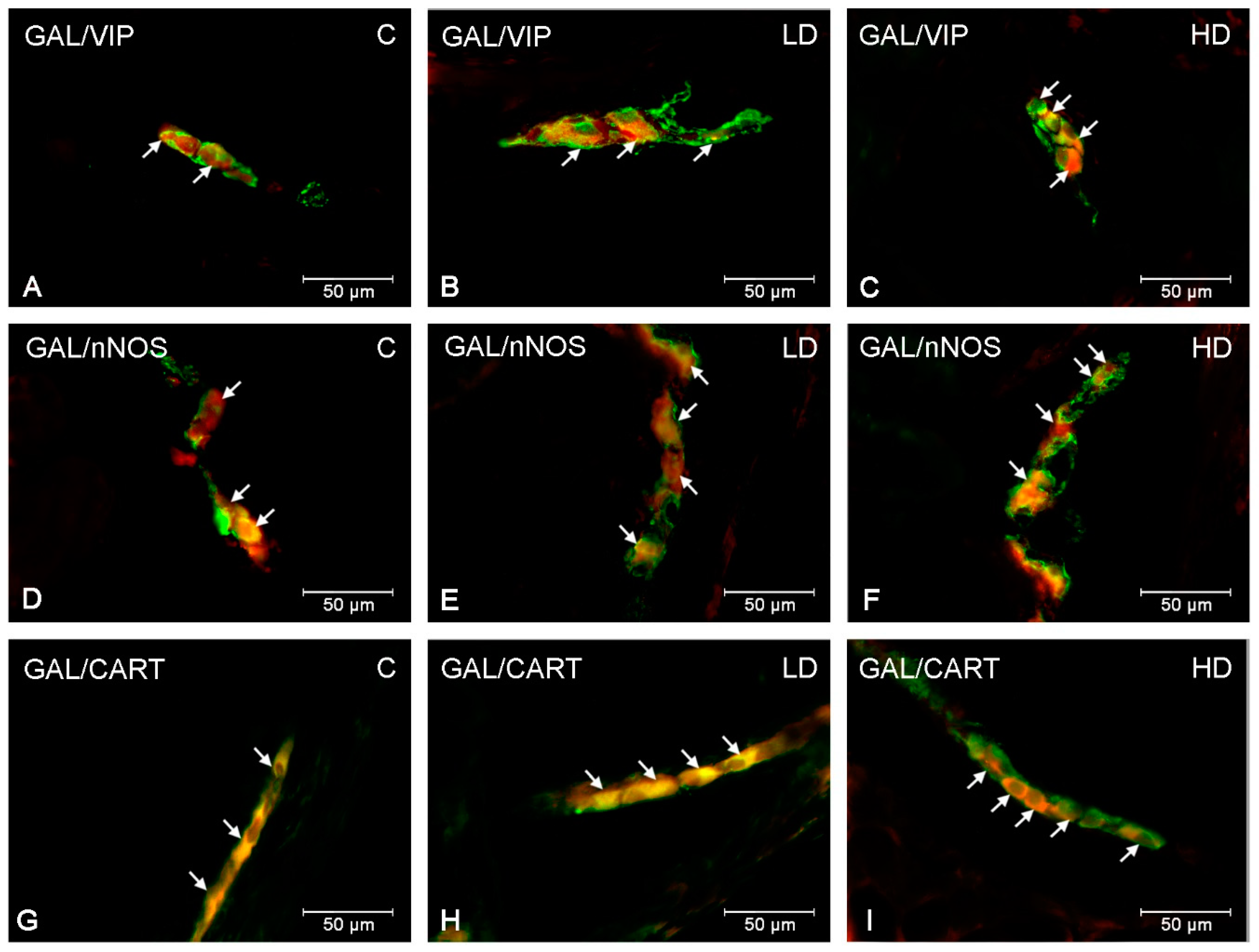
| Part of the Stomach | Cardia | Corpus | Pylorus | ||||||
|---|---|---|---|---|---|---|---|---|---|
| C Group | LD Group | HD Group | C Group | LD Group | HD Group | C Group | LD Group | HD Group | |
| MP | 24.67 ± 0.67 | 29.89 ± 1.08 (**) | 35.35 ± 0.64 (***) | 19.71 ± 0.70 | 27.81 ± 1.23 (***) | 33.55 ± 0.92 (***) | 25.14 ± 1.15 | 33.59 ± 1.17 (***) | 37.14 ± 0.43 (***) |
| SP | 37.36 ± 0.71 | 40.43 ± 0.70 (**) | 46.92 ± 0.42 (***) | 40.92 ± 0.84 | 51.38 ± 1.28 (***) | 58.53 ± 1.63 (***) | 36.87 ± 1.11 | 40.37 ± 0.66 (*) | 45.42 ± 0.58 (***) |
| GAL/VIP | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part of the Stomach | Cardia | Corpus | Pylorus | ||||||
| C Group | LD Group | HD Group | C Group | LD Group | HD Group | C Group | LD Group | HD Group | |
| MP | 46.79 ± 2.18 | 50.28 ± 1.55 | 60.67 ± 2.34 (***) | 43.20 ± 1.33 | 45.17 ± 0.73 | 51.49 ± 0.89 (***) | 47.53 ± 0.67 | 50.57 ± 0.79 | 58.63± 1.72 (***) |
| SP | 48.20 ± 0.89 | 52.51 ± 1.81 | 60.19 ± 2.06 (***) | 39.32 ± 0.69 | 42.34 ± 0.78 (*) | 49.27 ± 0.86 (***) | 48.36 ± 1.37 | 51.48 ± 1.24 | 59.95 ± 1.16 (***) |
| GAL/nNOS | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part of the Stomach | Cardia | Corpus | Pylorus | ||||||
| C Group | LD Group | HD Group | C Group | LD Group | HD Group | C Group | LD Group | HD Group | |
| MP | 33.37 ± 0.79 | 39.28 ± 1.30 (**) | 46.77 ± 1.20 (***) | 47.77 ± 1.22 | 51.76 ± 1.46 | 56.81 ± 1.06 (***) | 32.98 ± 0.51 | 39.50 ± 1.15 (**) | 46.77 ± 1.38 (***) |
| SP | 35.17 ± 0.62 | 39.92 ± 1.40 (*) | 46.76 ± 0.86 (***) | 46.16 ± 1.152 | 50.55 ± 1.23 (*) | 57.23 ± 0.84 (***) | 37.62 ± 1.51 | 42.32 ± 1.01 (*) | 48.03 ± 1.14 (***) |
| GAL/CART | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Part of the Stomach | Cardia | Corpus | Pylorus | ||||||
| C Group | LD Group | HD Group | C Group | LD Group | HD Group | C Group | LD Group | HD Group | |
| MP | 51.68 ± 0.85 | 54.29 ± 1.52 | 66.74 ± 0.48 (***) | 32.25 ± 1.12 | 36.39 ± 1.55 | 40.95 ± 0.76 (***) | 46.29 ± 1.30 | 54.60 ± 0.51 (***) | 56.81± 1.65 (***) |
| SP | 51.34 ± 1.02 | 59.06 ± 1.69 (**) | 63.62 ± 0.97 (***) | 32.41 ± 1.36 | 34.98 ± 0.67 | 40.59 ± 1.46 (***) | 51.30 ± 1.16 | 51.79 ± 1.24 | 54.97 ± 1.23 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palus, K.; Makowska, K.; Całka, J. Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. Int. J. Mol. Sci. 2019, 20, 3345. https://doi.org/10.3390/ijms20133345
Palus K, Makowska K, Całka J. Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. International Journal of Molecular Sciences. 2019; 20(13):3345. https://doi.org/10.3390/ijms20133345
Chicago/Turabian StylePalus, Katarzyna, Krystyna Makowska, and Jarosław Całka. 2019. "Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation" International Journal of Molecular Sciences 20, no. 13: 3345. https://doi.org/10.3390/ijms20133345
APA StylePalus, K., Makowska, K., & Całka, J. (2019). Alterations in Galanin-Like Immunoreactivity in the Enteric Nervous System of the Porcine Stomach Following Acrylamide Supplementation. International Journal of Molecular Sciences, 20(13), 3345. https://doi.org/10.3390/ijms20133345






