Bacterial Communities and Virulence Associated with Pine Wood Nematode Bursaphelenchus xylophilus from Different Pinus spp.
Abstract
1. Introduction
2. Results
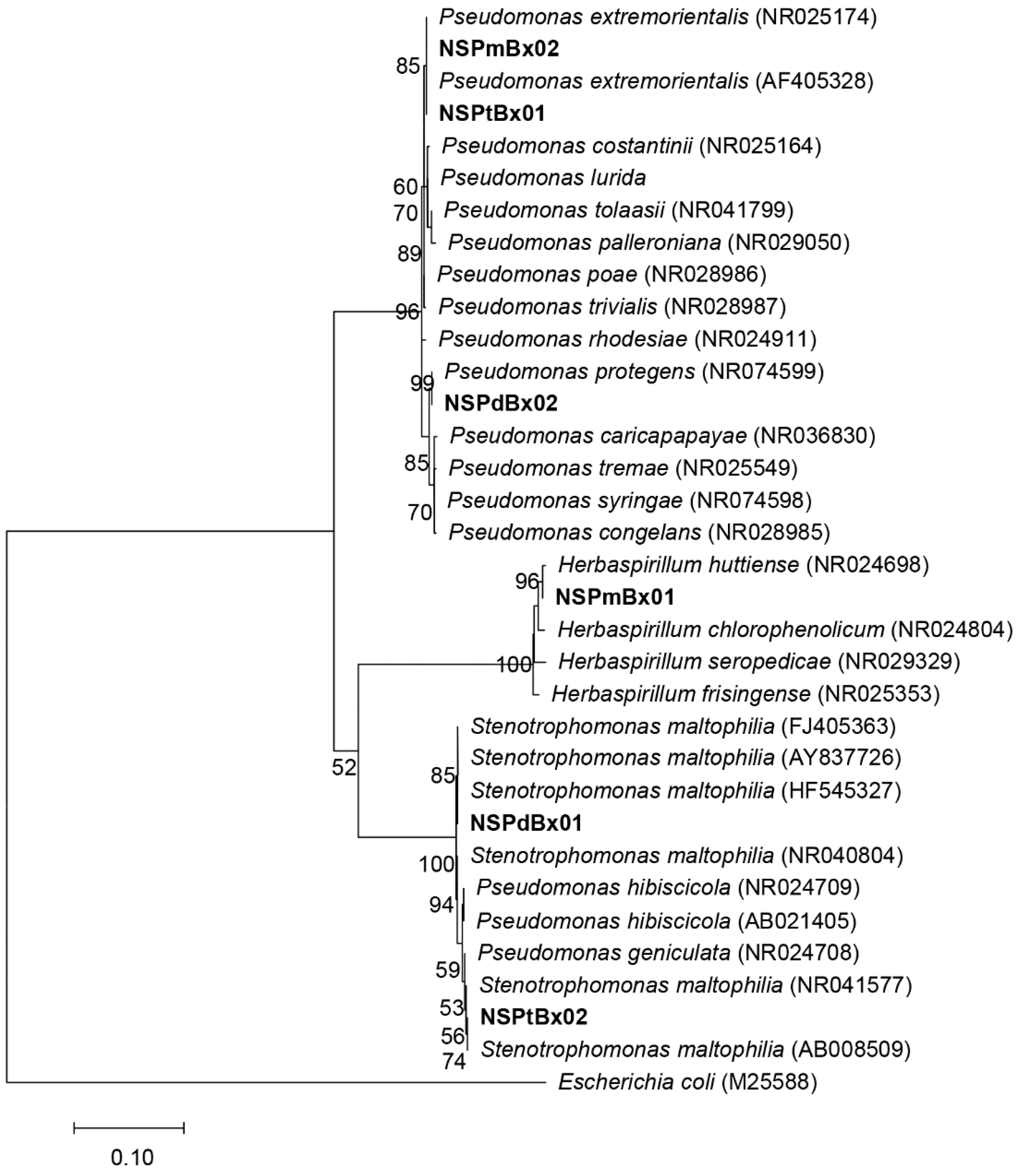
2.1. Culturable Bacteria in PWNs Isolated from Different Pine Species
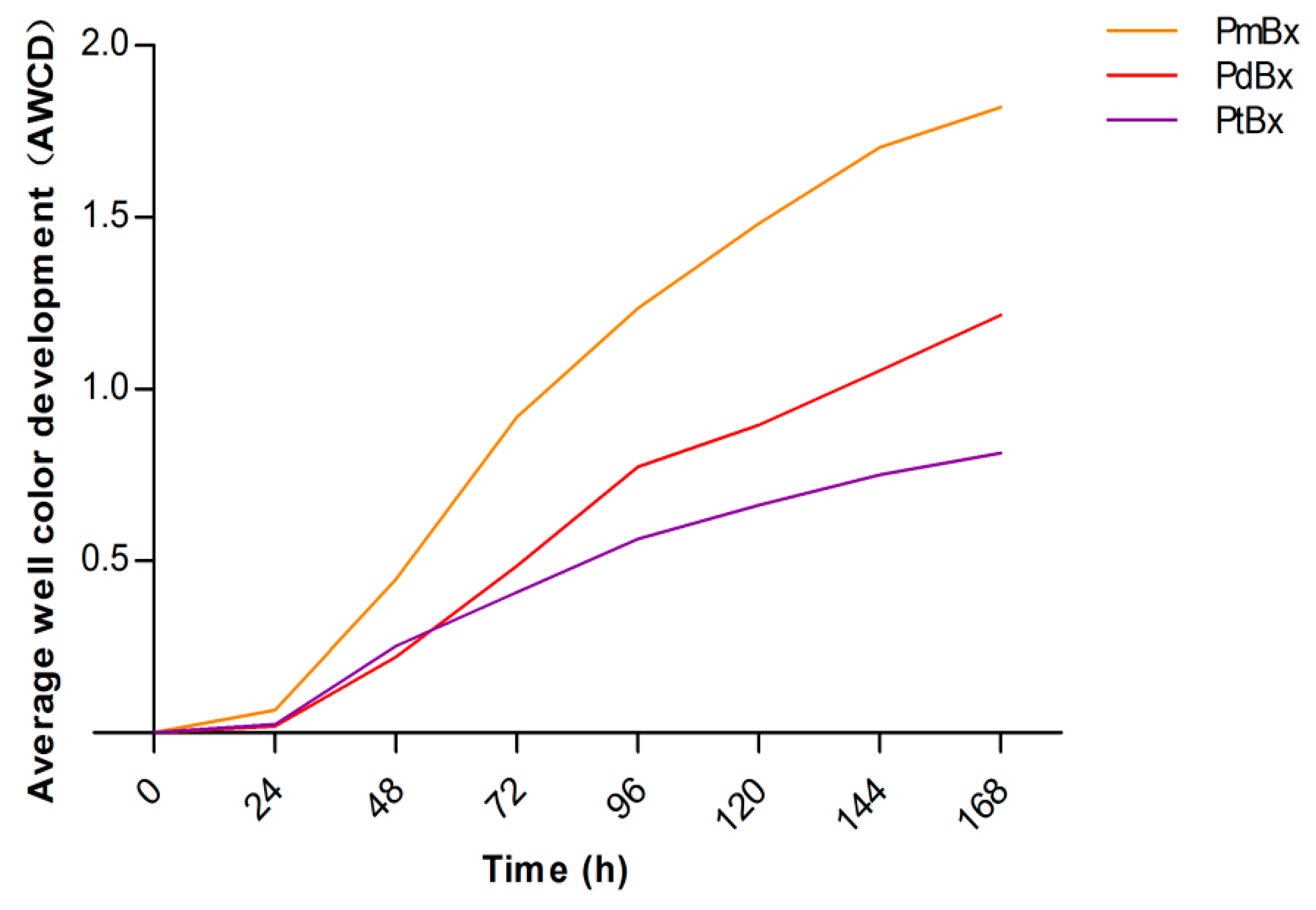
2.2. Carbon Metabolic Activities of Bacteria in PWNs Isolated from Different Pine Species
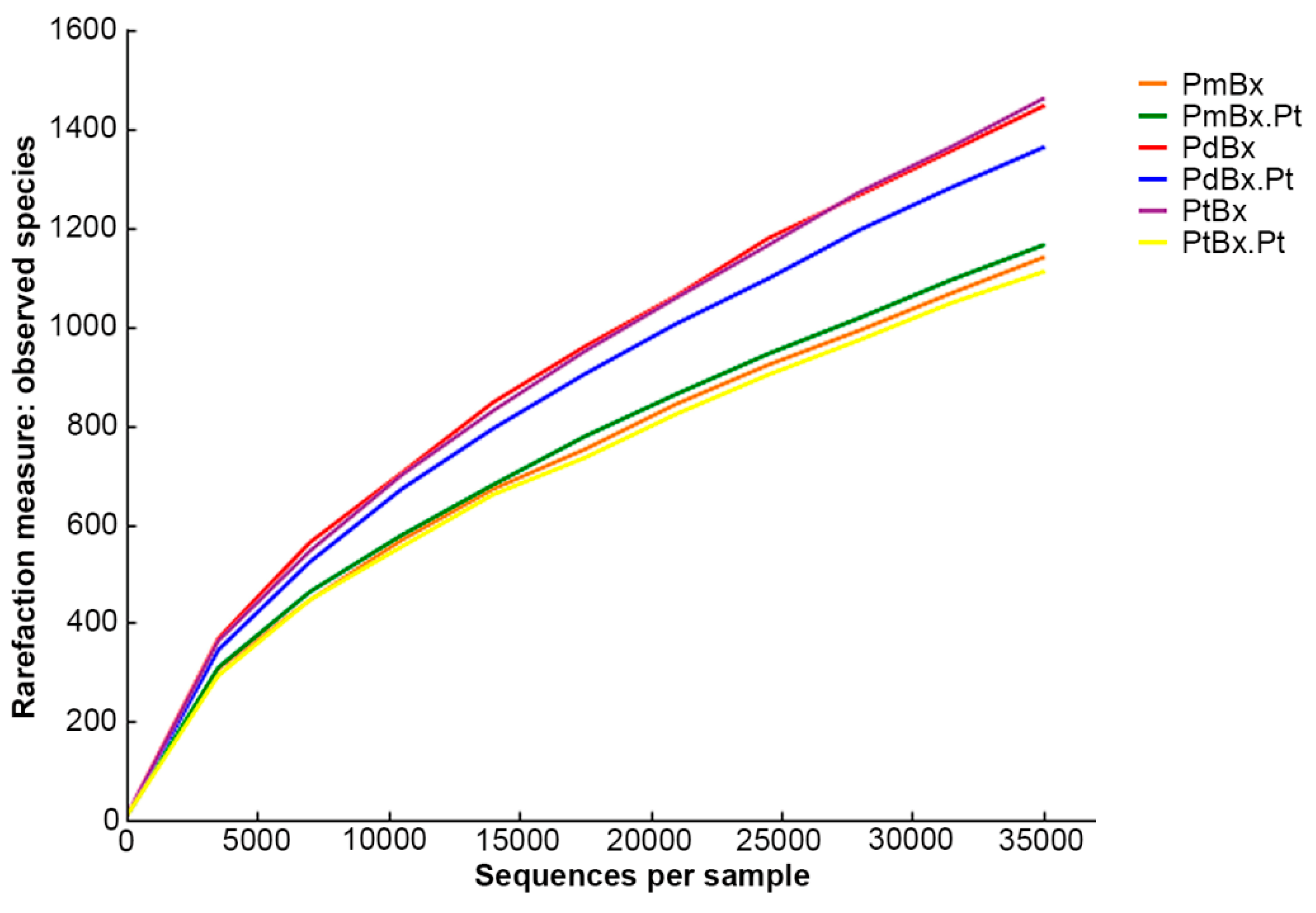
2.3. Diversity and Evenness of Bacterial Communities in PWNs Isolated from Different Pines
2.4. Variation in Carbon Source Utilization by Bacteria from PWNs Isolated from Different Pine Species
2.5. Diversity and Community Structure of Bacteria Associated with PWNs from Different Pine Species after Passage through P. thunbergii
2.6. Virulence of Different B. xylophilus Isolates Following Inoculation of P. thunbergii
3. Discussion
4. Materials and Methods
4.1. Collection and Surface Sterilization of Nematodes
4.2. Isolation and Identification of Culturable Bacteria from Nematodes
4.3. Biolog EcoPlate Measurement of Bacterial Community of Nematodes
4.4. Inoculation of P. thunbergii with B. xylophilus
4.5. High-Throughput Sequencing of Bacterial Community of Nematodes
4.6. Virulence Test of the Nematode Isolates
4.7. Statistical Analysis
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PWN | Pine wood nematode |
| PWD | Pine wilt disease |
| rDNA | Ribosomal deoxyribonucleic acid |
| NA | Nutrient agar |
| NB | Nutrient broth |
| PCR | Polymerase chain reaction |
| NCBI | National center for biotechnology information |
| BLAST | Basic local alignment search tool |
| MEGA | Molecular evolutionary genetic analysis |
| K2P | Kimura 2-parameter |
| AWCD | Average well color development |
| DNA | Deoxyribonucleic acid |
| BFP | Barcoded-tag fusion primer |
| OTU | Operational taxonomic unit |
| RDP | Ribosomal database project |
| DSI | Disease severity index |
| ROS | Reactive oxygen species |
References
- Li, Y.X.; Wang, Y.; Liu, Z.Y.; Wang, X.; Lu, Q.; Jia, X.Z.; Zhang, X.Y. Functional analysis of the venom allergen-like protein gene from pine wood nematode Bursaphelenchus xylophilus using a baculovirus expression system. Physiol. Mol. Plant Pathol. 2016, 93, 58e66. [Google Scholar] [CrossRef]
- Liu, K.C.; Zeng, F.L.; Ben, A.L.; Han, Z.M. Pathogenicity and repulsion for toxin-producing bacteria of dominant bacteria on the surface of American pine wood nematodes. J. Phytopathol. 2017, 165, 580–588. [Google Scholar] [CrossRef]
- Meng, F.L.; Wang, J.; Wang, X.; Li, Y.X.; Zhang, X.Y. Expression analysis of thaumatin-like proteins from Bursaphelenchus xylophilus and Pinus massoniana. Physiol. Mol. Plant Pathol. 2017, 100, 178e184. [Google Scholar] [CrossRef]
- Oku, H.; Shiraishi, T.; Ouchi, S.; Kurozumi, S.; Ohta, H. Pine wilt toxin, the metabolite of a bacterium associated with a nematode. Naturwissenschaften. 1980, 67, 198–199. [Google Scholar] [CrossRef]
- Chai, X.M.; Jiang, P. Occurrence and Control of Pine Wilt Disease; China Agricultural Press: Beijing, China, 2003; pp. 7–9. [Google Scholar]
- Ichihara, Y.; Fukuda, K.; Suzuki, K. Early symptom development and histological changes associated with migration of Bursaphelenchus xylophilus in seedling tissues of Pinus thunbergii. Plant. Dis. 2000, 84, 675–680. [Google Scholar] [CrossRef]
- Wang, H.L.; Han, S.F.; Zhao, B.G. Distribution and pathogenicitiy of bacteria carried by pine wood nematode in epidemic regions and hosts. J. Beijing For. Univ. 2004, 26, 48–53. [Google Scholar]
- Roriz, M.; Santos, C.; Vasconcelos, M.W. Population dynamics of bacteria associated with different strains of the pine wood nematode Bursaphelenchus xylophilus after inoculation in maritime pine (Pinus pinaster). Exp. Parasitol. 2011, 128, 357–364. [Google Scholar] [CrossRef]
- Tian, X.L.; Mao, Z.C.; Chen, G.H.; Xie, B.Y. Ecological relationships between Bursaphelenchus xylophilus and its companion microorganisms. Chin. J. Appl. Ecol. 2011, 22, 810–815. [Google Scholar]
- Nascimento, F.X.; Hasegawa, K.; Mota, M.; Vicente, C.S. Bacterial role in pine wilt disease development—Review and future perspectives. Environ. Microbiol. Rep. 2015, 7, 51–63. [Google Scholar] [CrossRef]
- Hu, K.J.; Wang, Q.L.; Yang, B.J. The pathogenicity of different strains of Bursaphelenchus xylophilus and Bursaphelenchus mucronatus. For. Res. 1994, 7, 381–385. [Google Scholar]
- Han, Z.M.; Hong, Y.D.; Zhao, B.G. A study on pathogenicity of bacteria carried by pine wood nematodes. J. Phytopathol. 2003, 151, 683–689. [Google Scholar] [CrossRef]
- Vicente, C.S.; Nascimento, F.; Espada, M.; Barbosa, P.; Mota, M.; Glick, B.R.; Oliveira, S. Characterization of bacteria associated with pinewood nematode Bursaphelenchus xylophilus. PLoS ONE 2012, 7, e46661. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.; Vicente, C.; Cock, P.; Tavares, M.; Rossi, M.; Hasegawa, K.; Mota, M. From plants to nematodes: Serratia grimesii BXF1 genome reveals an adaptation to the modulation of multi-species interactions. Microb. Genom. 2018, 4. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, F.X.; Espada, M.; Barbosa, P.; Rossi, M.J.; Vicente, C.S.L.; Mota, M. Non-specific transient mutualism between the plant parasitic nematode, Bursaphelenchus xylophilus, and the opportunistic bacterium Serratia quinivorans BXF1, a plant-growth promoting pine endophyte with antagonistic effects. Environ. Microbiol. 2016, 18, 5265–5276. [Google Scholar] [CrossRef] [PubMed]
- He, L.X.; Wu, X.Q.; Xue, Q.; Qiu, X.W. Effects of endobacterium (Stenotrophomonas maltophilia) on pathogenesis-related gene expression of pine wood nematode (Bursaphelenchus xylophilus) and pine wilt disease. Int. J. Mol. Sci. 2016, 17, 778. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.J.; Wu, X.Q.; Xiang, Y.; Fang, X.; Ye, J.R. The effect of endobacteria on the development and virulence of the pine wood nematode, Bursaphelenchus xylophilus. Nematology 2015, 17, 581–589. [Google Scholar] [CrossRef]
- Wu, X.Q.; Yuan, W.M.; Tian, X.J.; Fan, B.; Fang, X.; Ye, J.R.; Ding, X.L. Specific and functional diversity of endophytic bacteria from pine wood nematode Bursaphelenchus xylophilus with different virulence. Int. J. Biol. Sci. 2013, 9, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Wu, X.Q.; Zhou, A.D. Bacterial diversity and community structure in the pine wood nematode Bursaphelenchus xylophilus and B. mucronatus with different virulence by high-throughput sequencing of the 16S rDNA. PLoS ONE 2015, 10, e0137386. [Google Scholar] [CrossRef] [PubMed]
- Proença, D.N.; Grass, G.; Morais, P.V. Understanding pine wilt disease: Roles of the pine endophytic bacteria and of the bacteria carried by the disease-causing pinewood nematode. Microbiologyopen 2017, 6, e00415. [Google Scholar] [CrossRef]
- Qian, W.Q.; Cao, F.X.; Wang, M.; Wang, S.L. The affection of infection of Bursaphenlenchus xylophilus in Pinus massoniana on the content of several kinds of acid and on some enzymes’ activity. Hunan For. Sci. Technol. 2009, 36, 8–10. [Google Scholar]
- He, L.X. Role of nitric oxide and nucleases in different pine species inoculated with a nematode (Bursaphelenchus xylophilus) in their resistance to the infection. Sci. Silva. Sin. 2012, 48, 109–114. [Google Scholar]
- He, L.X.; Wu, X.Q.; Yu, L.Z. The relationship between difference of superoxide anion and lesion in the interaction of different varieties of pines and Bursaphelenchus xylophilus. J. Nanjing For. Univ. 2011, 35, 25–30. [Google Scholar]
- He, L.X.; Wu, X.Q.; Yu, L.Z.; Ji, J.; Ye, J.R. The difference of H2O2 and oxidative enzyme in the interaction of different resistance pines and Bursaphenlenchus xylophilus. J. Nanjing For. Univ. 2010, 34, 13–17. [Google Scholar]
- Chen, S.; Bagdasarian, M.; Walker, E.D. Elizabethkingia anophelis: Molecular manipulation and interactions with mosquito hosts. Appl. Environ. Microb. 2015, 81, 2233–2243. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Y.; Chew, S.C.; Tay, M.; Salido, M.M.; Teo, J.; Lauro, F.M.; Givskov, M.; Yang, L. Complete genome sequence and transcriptomic analysis of the novel pathogen Elizabethkingia anophelis in response to oxidative stress. Genome Biol. Evol. 2015, 7, 1676–1685. [Google Scholar] [CrossRef]
- Ngwa, C.J.; Glockner, V.; Abdelmohsen, U.R.; Scheuermayer, M.; Fischer, R.; Hentschel, U.; Pradel, G. 16S rRNA gene-based identification of Elizabethkingia meningoseptica (Flavobacteriales: Flavobacteriaceae) as a dominant midgut bacterium of the Asian malaria vector Anopheles stephensi (Dipteria: Culicidae) with antimicrobial activities. J. Med. Entomol. 2013, 50, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.D.; Dangl, J.L. The plant immune system. Nature 2006, 444, 323–329. [Google Scholar] [CrossRef]
- Kikuchi, T.; Cotton, J.A.; Dalzell, J.J.; Hasegawa, K.; Kanzaki, N.; McVeigh, P.; Takanashi, T.; Tsai, I.J.; Assefa, S.A.; Cock, P.J.; et al. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011, 7, e1002219. [Google Scholar] [CrossRef]
- Shinya, R.; Morisaka, H.; Kikuchi, T.; Takeuchi, Y.; Ueda, M.; Futai, K. Secretome analysis of the pine wood nematode Bursaphelenchus xylophilus reveals the tangled roots of parasitism and its potential for molecular mimicry. PLoS ONE 2013, 8, e67377. [Google Scholar] [CrossRef]
- Vicente, C.S.; Ikuyo, Y.; Mota, M.; Hasegawa, K. Pine wood nematode-associated bacteria contribute to oxidative stress resistance of Bursaphelenchus xylophilus. BMC Microbiol. 2013, 13, 1–8. [Google Scholar] [CrossRef]
- Cheng, X.Y.; Tian, X.L.; Wang, Y.S.; Lin, R.M.; Mao, Z.C.; Chen, N.; Xie, B.Y. Metagenomic analysis of the pinewood nematode microbiome reveals a symbiotic relationship critical for xenobiotics degradation. Sci. Rep. 2013, 3, 1869. [Google Scholar] [CrossRef] [PubMed]
- Mamiya, Y. Scanning electron microscopy of pine seedling wood tissue sections inoculated with the pine wood nematode Bursaphelenchus xylophilus previously prepared for light microscopy. J. Nematol. 2012, 44, 255–259. [Google Scholar] [PubMed]
- Yan, X.; Cheng, X.Y.; Wang, Y.S.; Luo, J.; Mao, Z.C.; Ferris, V.R.; Xie, B.Y. Comparative transcriptomics of two pathogenic pinewood nematodes yields insights into parasitic adaptation to life on pine hosts. Gene 2012, 505, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.M.; Wu, X.Q.; Ye, J.R.; Tian, X.J. Observation by transmission electron microscope and identification of endophytic bacteria isolated from Bursaphenlenchus xylophilus and B. mucronatus. Acta Microbiol. Sin. 2011, 51, 1071–1077. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Garland, J.L.; Mills, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl Environ. Microb. 1991, 57, 2351–2359. [Google Scholar]
- Hu, J.L.; Lin, X.G.; Wang, J.H.; Dai, J.; Chen, R.R.; Zhang, J.B.; Wong, M.H. Microbial functional diversity, metabolic quotient, and invertase activity of a sandy loam soil as affected by long-term application of organic amendment and mineral fertilizer. J. Soil Sediment. 2011, 11, 271–280. [Google Scholar] [CrossRef]
- Xu, H.Q.; Xiao, R.L.; Song, T.Q.; Luo, W.; Ren, Q.; Huang, Y. Effects of mulching and intercropping on the functional diversity of soil microbial communities in tea plantations. Biodivers. Sci. 2008, 16, 166. [Google Scholar]
- Caporaso, J.G.; Lauber, C.L.; Walters, W.A.; Berg-Lyons, D.; Huntley, J.; Fierer, N.; Owens, S.M.; Betley, J.; Fraser, L.; Bauer, M.; et al. Ultra-high-throughput microbial community analysis on the Illumina HiSeq and MiSeq platforms. ISME J. 2012, 6, 1621–1624. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef] [PubMed]



| B. xylophilus | Diversity Index | |
|---|---|---|
| Shannon-Wiener (H) | Evenness (E) | |
| PmBx | 2.760 ± 0.334 a | 0.086 ± 0.011 b |
| PdBx | 2.965 ± 0.082 a | 0.114 ± 0.003 a |
| PtBx | 2.506 ± 0.043 a | 0.104 ± 0.013 ab |
| B. xylophilus | OTU (97%) | Coverage (C) % | Chao1 (97%) | Shannon-Wiener(H) (97%) |
|---|---|---|---|---|
| PmBx | 1140 | 100 | 3189 | 5.27 |
| PmBx.Pt | 1165 | 100 | 3256 | 5.42 |
| PdBx | 1447 | 100 | 3871 | 6.01 |
| PdBx.Pt | 1363 | 100 | 3785 | 4.74 |
| PtBx | 1462 | 100 | 4178 | 5.55 |
| PtBx.Pt | 1111 | 100 | 2847 | 5.45 |
| Nematode Strains | Infection rates (%) | DSI | ||
|---|---|---|---|---|
| 30 d | 60 d | 30 d | 60 d | |
| PmBx | 60 | 100 | 15 | 65 |
| PmBx.Pt | 80 | 100 | 70 | 100 |
| PdBx | 0 | 40 | 0 | 40 |
| PdBx.Pt | 20 | 80 | 5 | 75 |
| PtBx | 20 | 80 | 10 | 75 |
| PtBx.Pt | 60 | 80 | 60 | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Q.; Xiang, Y.; Wu, X.-Q.; Li, M.-J. Bacterial Communities and Virulence Associated with Pine Wood Nematode Bursaphelenchus xylophilus from Different Pinus spp. Int. J. Mol. Sci. 2019, 20, 3342. https://doi.org/10.3390/ijms20133342
Xue Q, Xiang Y, Wu X-Q, Li M-J. Bacterial Communities and Virulence Associated with Pine Wood Nematode Bursaphelenchus xylophilus from Different Pinus spp. International Journal of Molecular Sciences. 2019; 20(13):3342. https://doi.org/10.3390/ijms20133342
Chicago/Turabian StyleXue, Qi, Yang Xiang, Xiao-Qin Wu, and Ming-Jie Li. 2019. "Bacterial Communities and Virulence Associated with Pine Wood Nematode Bursaphelenchus xylophilus from Different Pinus spp." International Journal of Molecular Sciences 20, no. 13: 3342. https://doi.org/10.3390/ijms20133342
APA StyleXue, Q., Xiang, Y., Wu, X.-Q., & Li, M.-J. (2019). Bacterial Communities and Virulence Associated with Pine Wood Nematode Bursaphelenchus xylophilus from Different Pinus spp. International Journal of Molecular Sciences, 20(13), 3342. https://doi.org/10.3390/ijms20133342





